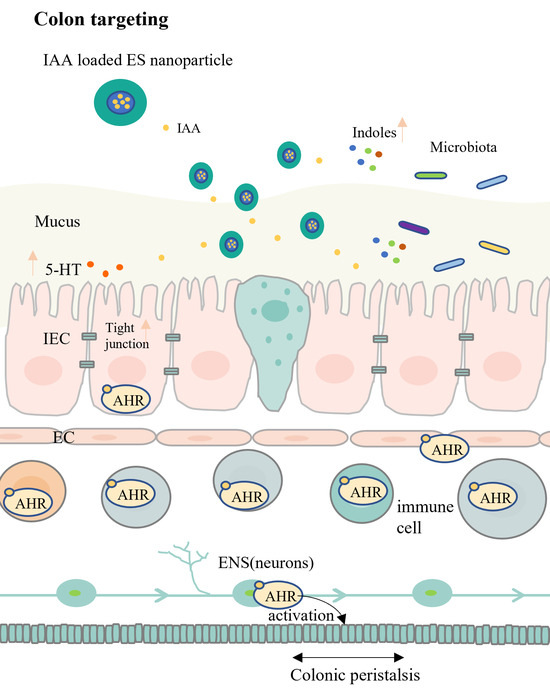
Colon-Targeted Delivery of Indole Acetic Acid Helps Regulate Gut Motility by Activating the AHR Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanoparticles
2.3. Characterisation of the Nanoparticles
2.4. Encapsulation and Loading Efficiencies of the Nanoparticles
2.5. In Vitro Release of IAA from the Nanoparticles
2.6. In Vivo Release and Biodistribution of IAA
2.7. Treatment Study Design
2.8. Assay of ITT
2.9. Quantitative Polymerase Chain Reaction (PCR)
2.10. Western Blot Analysis
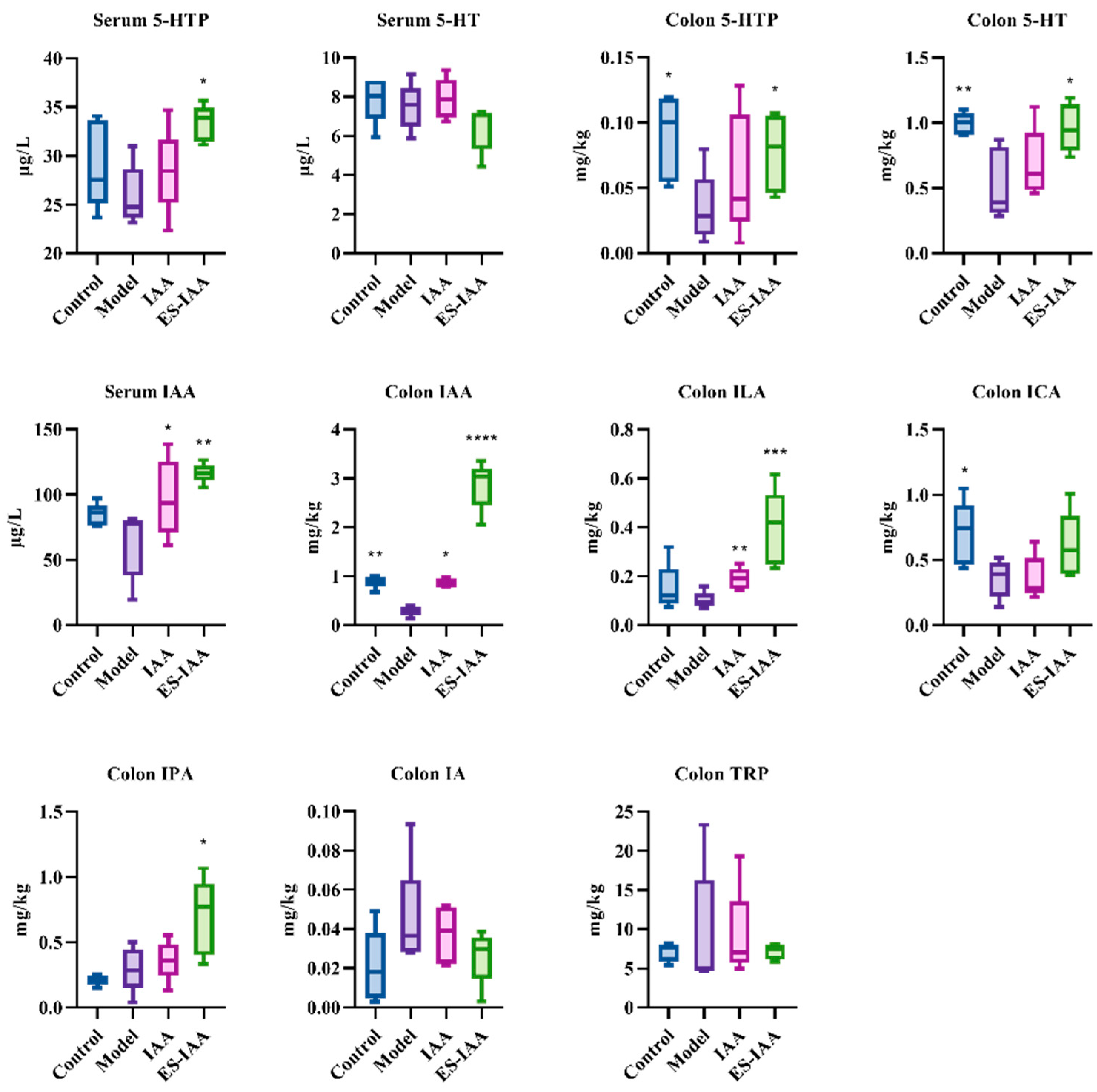
2.11. Ultrahigh-Performance Liquid Chromatography–Mass Spectrometry (UPLC-MS) Analysis of Tryptophan Metabolites
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characterisation of Nanoparticles
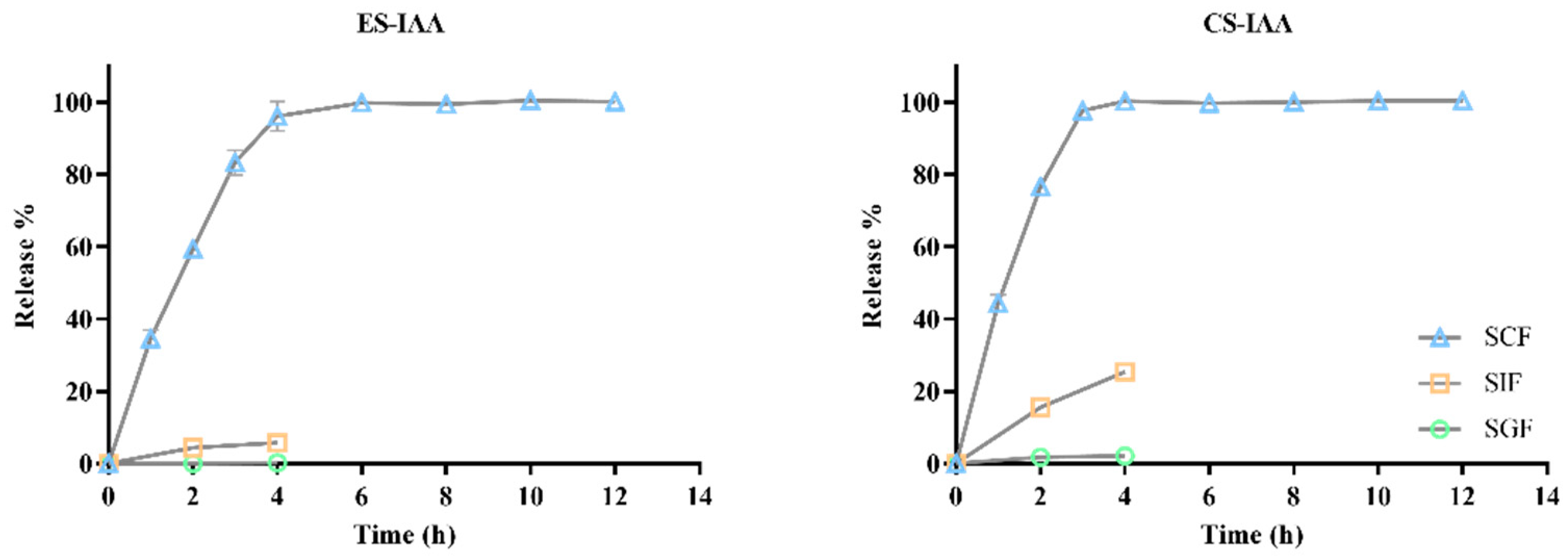
3.2. In Vitro Release of IAA-Loaded Nanoparticles
3.3. In Vivo Release and Biodistribution of IAA
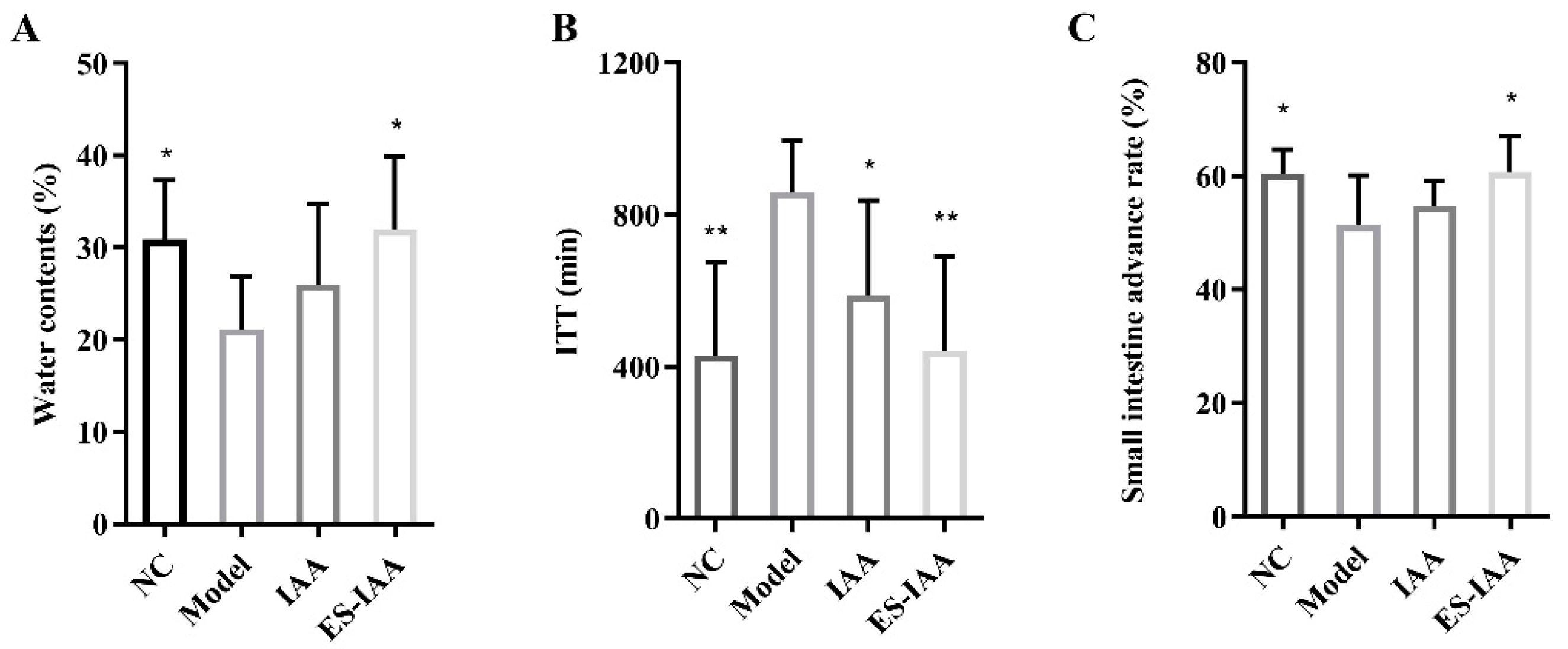
3.4. Effects of IAA Colonic Delivery on Gut Motility
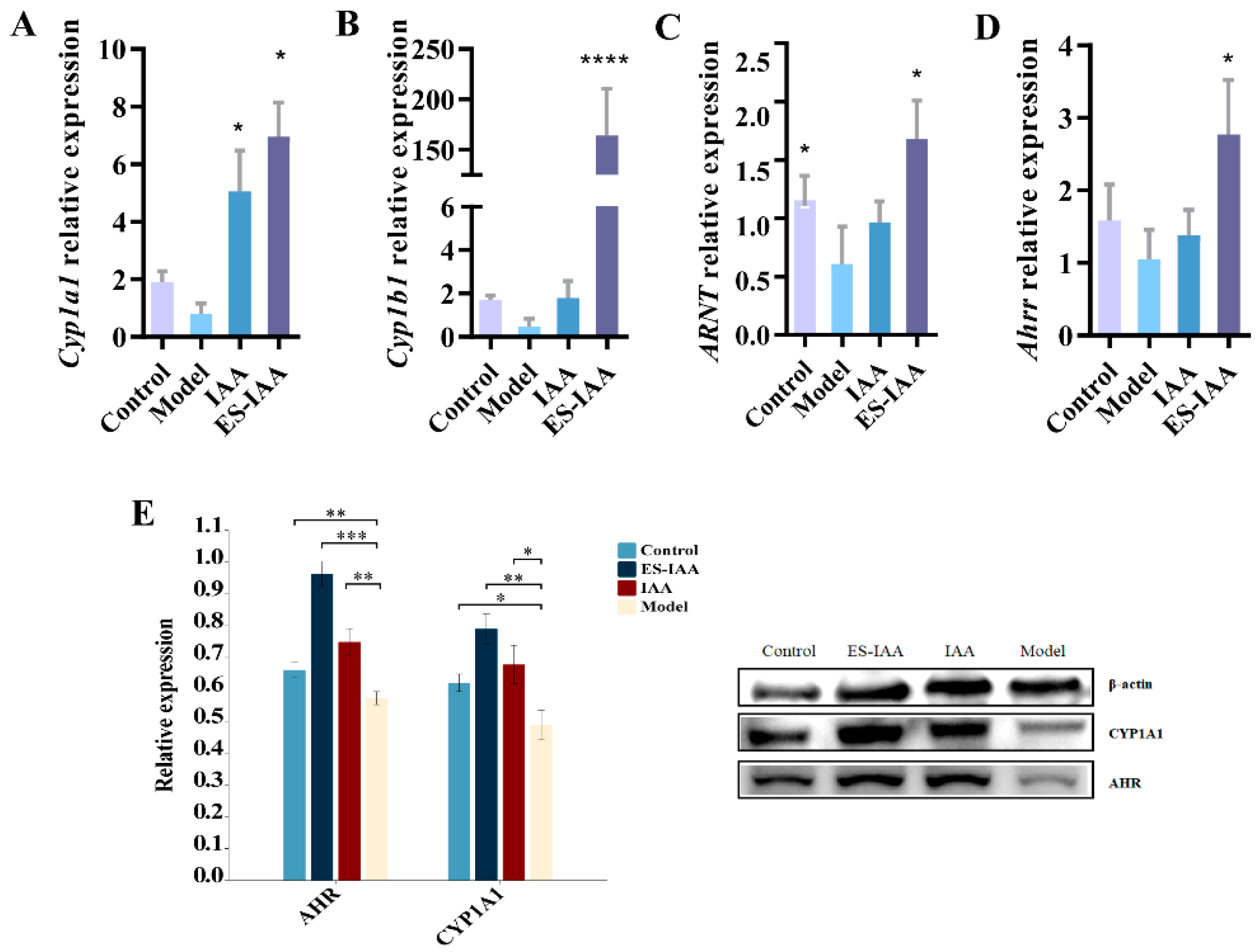
3.5. Regulation of Gut Movement through AHR Activation
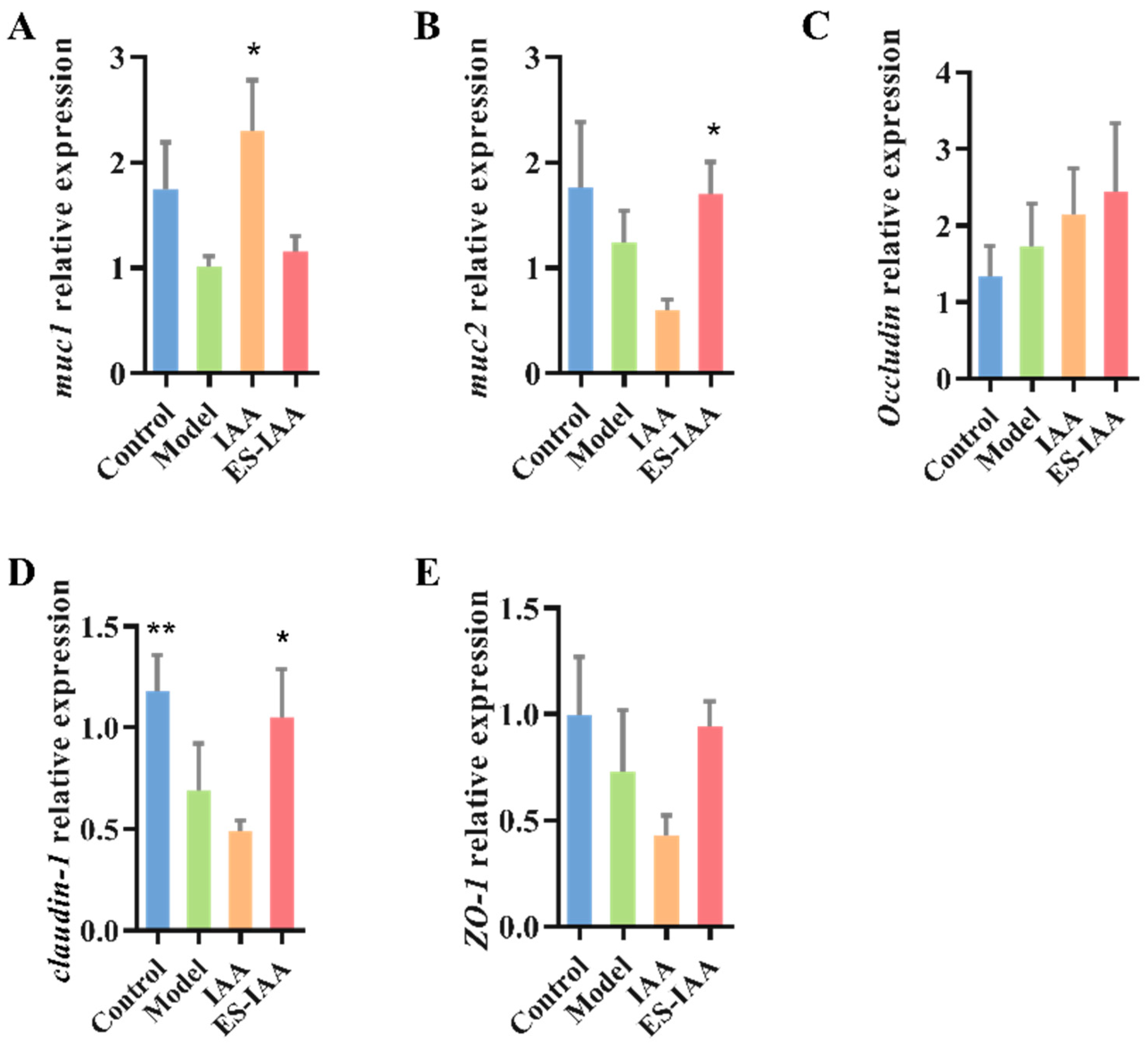
3.6. mRNA Expression Levels of Mucin and Tight Junction (TJ)-Related Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Rolig, A.S.; Mittge, E.K.; Ganz, J.; Troll, J.V.; Melancon, E.; Wiles, T.J.; Alligood, K.; Stephens, W.Z.; Eisen, J.S.; Guillemin, K. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol. 2017, 15, e2000689. [Google Scholar]
- Marrero, D.; Pujol-Vila, F.; Vera, D.; Gabriel, G.; Illa, X.; Elizalde-Torrent, A.; Alvarez, M.; Villa, R. Gut-on-a-chip: Mimicking and monitoring the human intestine. Biosens. Bioelectron. 2021, 181, 113156. [Google Scholar]
- Sensoy, I. A review on the food digestion in the digestive tract and the used in vitro models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [PubMed]
- Pan, R.; Wang, L.; Xu, X.; Chen, Y.; Wang, H.; Wang, G.; Zhao, J.; Chen, W. Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients 2022, 14, 3704. [Google Scholar]
- Camilleri, M. Gastrointestinal motility disorders in neurologic disease. J. Clin. Investig. 2021, 131, e143771. [Google Scholar] [PubMed]
- Waclawiková, B.; Codutti, A.; Alim, K.; El Aidy, S. Gut microbiota-motility interregulation: Insights from in vivo, ex vivo and in silico studies. Gut Microbes 2022, 14, 1997296. [Google Scholar] [CrossRef]
- Dimidi, E.; Christodoulides, S.; Scott, S.M.; Whelan, K. Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv. Nutr. Int. Rev. J. 2017, 8, 484–494. [Google Scholar]
- Ge, X.; Ding, C.; Zhao, W.; Xu, L.; Tian, H.; Gong, J.; Zhu, M.; Li, J.; Li, N. Antibiotics-induced depletion of mice microbiota induces changes in host serotonin biosynthesis and intestinal motility. J. Transl. Med. 2017, 15, 13. [Google Scholar] [CrossRef]
- Obata, Y.; Castaño, Á.; Boeing, S.; Bon-Frauches, A.C.; Fung, C.; Fallesen, T.; de Agüero, M.G.; Yilmaz, B.; Lopes, R.; Huseynova, A.; et al. Neuronal programming by microbiota regulates intestinal physiology. Nature 2020, 578, 284–289. [Google Scholar]
- Agus, A.; Planchais, J.; Sokol, H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar]
- Wong, C.B.; Tanaka, A.; Kuhara, T.; Xiao, J.-Z. Potential effects of indole-3-lactic acid, a metabolite of human bifidobacteria, on NGF-induced neurite outgrowth in PC12 cells. Microorganisms 2020, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tian, P.; Wang, Z.; Pan, R.; Shang, K.; Wang, G.; Zhao, J.; Chen, W. Indole acetic acid exerts anti-depressive effects on an animal model of chronic mild stress. Nutrients 2022, 14, 5019. [Google Scholar] [PubMed]
- Fujii-Kuriyama, Y.; Mimura, J. Molecular mechanisms of AhR functions in the regulation of cytochrome P450 genes. Biochem. Biophys. Res. Commun. 2005, 338, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, B.; Cheng, W.-H.; Wang, Q. Preparation, characterization and evaluation of selenite-loaded chitosan/TPP nanoparticles with or without zein coating. Carbohydr. Polym. 2010, 82, 942–951. [Google Scholar]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar]
- Khalaf, E.M.; Abood, N.A.; Atta, R.Z.; Ramírez-Coronel, A.A.; Alazragi, R.; Parra, R.M.R.; Abed, O.H.; Abosaooda, M.; Jalil, A.T.; Mustafa, Y.F.; et al. Recent progressions in biomedical and pharmaceutical applications of chitosan nanoparticles: A comprehensive review. Int. J. Biol. Macromol. 2023, 231, 123354. [Google Scholar]
- Makhlof, A.; Tozuka, Y.; Takeuchi, H. Design and evaluation of novel pH-sensitive chitosan nanoparticles for oral insulin delivery. Eur. J. Pharm. Sci. 2011, 42, 445–451. [Google Scholar] [CrossRef]
- Vemula, S.K. Formulation and pharmacokinetics of colon-specific double-compression coated mini-tablets: Chronopharmaceutical delivery of ketorolac tromethamine. Int. J. Pharm. 2015, 491, 35–41. [Google Scholar] [CrossRef]
- Xiao, B.; Si, X.; Zhang, M.; Merlin, D. Oral administration of pH-sensitive curcumin-loaded microparticles for ulcerative colitis therapy. Colloids Surf. B Biointerfaces 2015, 135, 379–385. [Google Scholar]
- Sood, A.; Dev, A.; Mohanbhai, S.J.; Shrimali, N.; Kapasiya, M.; Kushwaha, A.C.; Roy Choudhury, S.; Guchhait, P.; Karmakar, S. Disulfide-bridged chitosan-eudragit S-100 nanoparticles for colorectal cancer. ACS Appl. Nano Mater. 2019, 2, 6409–6417. [Google Scholar] [CrossRef]
- Pereira, F.M.; Melo, M.N.; Santos, Á.K.M.; Oliveira, K.V.; Diz, F.M.; Ligabue, R.A.; Morrone, F.B.; Severino, P.; Fricks, A.T. Hyaluronic acid-coated chitosan nanoparticles as carrier for the enzyme/prodrug complex based on horseradish peroxidase/indole-3-acetic acid: Characterization and potential therapeutic for bladder cancer cells. Enzym. Microb. Technol. 2021, 150, 109889. [Google Scholar]
- Wen, P.; Feng, K.; Yang, H.; Huang, X.; Zong, M.-H.; Lou, W.-Y.; Li, N.; Wu, H. Electrospun core-shell structured nanofilm as a novel colon-specific delivery system for protein. Carbohydr. Polym. 2017, 169, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-R.; Miao, H.; Deng, D.-Q.; Vaziri, N.D.; Li, P.; Zhao, Y.-Y. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol. Life Sci. 2021, 78, 909–922. [Google Scholar]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar]
- Zhang, Z.; Mu, X.; Cao, Q.; Shi, Y.; Hu, X.; Zheng, H. Honeybee gut Lactobacillus modulates host learning and memory behaviors via regulating tryptophan metabolism. Nat. Commun. 2022, 13, 2037. [Google Scholar]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.; Sherr, D.; Yarmush, M.; Alaniz, R.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar] [CrossRef]
- John, J.; Blogg, C.; Murray, F.; Schwetz, B.; Gehring, P.J. Teratogenic effects of the plant hormone indole-3-acetic acid in mice and rats. Teratology 1979, 19, 321–324. [Google Scholar] [CrossRef]
- Barden, T.C. Indoles: Industrial, Agricultural and Over-the-Counter Uses. In Heterocyclic Scaffolds II: Reactions and Applications of Indoles; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Ergin, A.; Sezgin Bayindir, Z.; Yüksel, N. Characterization and optimization of colon targeted S-adenosyl-L-methionine loaded chitosan nanoparticles. J. Res. Pharm. 2019, 23, 914–926. [Google Scholar]
- Kulkarni, N.; Jain, P.; Shindikar, A.; Suryawanshi, P.; Thorat, N. Advances in the colon-targeted chitosan based multiunit drug delivery systems for the treatment of inflammatory bowel disease. Carbohydr. Polym. 2022, 288, 119351. [Google Scholar] [PubMed]
- Sadik, A.; Patterson, L.F.S.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 is a metabolic immune checkpoint that activates the AHR and promotes tumor progression. Cell 2020, 182, 1252–1270.e34. [Google Scholar] [PubMed]
- Stockinger, B.; Shah, K.; Wincent, E. AHR in the intestinal microenvironment: Safeguarding barrier function. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 559–570. [Google Scholar] [PubMed]
- Spencer, N.J.; Keating, D.J. Role of 5-HT in the enteric nervous system and enteroendocrine cells. Br. J. Pharmacol. 2022, early view. [Google Scholar] [CrossRef]


| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Cyp1a1 | GCTGATGGCAGAGGTTG | ACGGAGGATAGGAATGAAG |
| Cyp1b1 | CCGAAAAGAAGGCGACTGG | TGCACATCCGGGTATCTGGTAAAG |
| ARNT | AGAGACTTGCCAGGGAAAATCATA | TTTCGAGCCAGGGCACTACAGG |
| Ahrr | AAAGTCAGCATCCCTCCTTG | CCCATCAGATCCTTTGGATG |
| Occludin | AATGTAGAGAAAGGTCCTGGTG | CCTTTAATTCCTGCACCA |
| Muc1 | TACCTACCACACTCACGGAC | TCCTACAAGTTGGCCGAAG |
| Muc2 | CGCCAATTACGCTGAACACT | CCTCGTTGTTCTGACAGTTGC |
| Claudin-1 | ACGCAGGAGCCTCGCCCCGCAGCTGCA | CAGCCAAGGCCTGCATAGCCATGG |
| ZO-1 | CACACGATGCTCAGAGACGAAGG | CTGTATGGTGGCTGCTCAAGGTC |
| Actb | AGCCATGTACGTAGCCATCC | CTCTCAGCTGTGGTGGTGAA |
| CS | Ratio | ES 1 | d (nm) | PDI | Zeta (mV) | EE% | DL% |
|---|---|---|---|---|---|---|---|
| <200 mPa·s | 9:1 | - | 196.10 ± 13.48 | 0.46 ± 0.03 | +40.80 ± 1.39 | 71.06 ± 4.57 | 9.58 ± 0.62 |
| 100 kDa | 9:1 | - | 186.70 ± 14.96 | 0.44 ± 0.05 | +41.37 ± 1.06 | 74.67 ± 2.95 | 10.07 ± 0.40 |
| 150 kDa | 9:1 | - | 201.23 ± 24.51 | 0.43 ± 0.03 | +42.57 ± 3.90 | 80.17 ± 4.94 | 10.81 ± 0.62 |
| 150 kDa | 4.5:1 | - | 185.83 ± 21.53 | 0.48 ± 0.02 | +40.80 ± 1.67 | 71.94 ± 2.79 | 18.18 ± 0.70 |
| 150 kDa | 4.5:1 | + | 297.33 ± 12.70 | 0.54 ± 0.10 | +37.53 ± 0.66 | 83.15 ± 0.83 | 16.03 ± 0.16 |
| 150 kDa | 3:1 | + | 301.43 ± 11.72 | 0.55 ± 0.01 | +38.53 ± 1.44 | 66.3 ± 6.25 | 16.15 ± 1.52 |
| 150 kDa | - | + | 251.83 ± 8.21 | 0.50 ± 0.05 | +33.63 ± 0.94 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Pan, R.; Mei, L.; Tian, P.; Wang, L.; Zhao, J.; Chen, W.; Wang, G. Colon-Targeted Delivery of Indole Acetic Acid Helps Regulate Gut Motility by Activating the AHR Signaling Pathway. Nutrients 2023, 15, 4282. https://doi.org/10.3390/nu15194282
Chen Y, Pan R, Mei L, Tian P, Wang L, Zhao J, Chen W, Wang G. Colon-Targeted Delivery of Indole Acetic Acid Helps Regulate Gut Motility by Activating the AHR Signaling Pathway. Nutrients. 2023; 15(19):4282. https://doi.org/10.3390/nu15194282
Chicago/Turabian StyleChen, Ying, Ruili Pan, Liya Mei, Peijun Tian, Linlin Wang, Jianxin Zhao, Wei Chen, and Gang Wang. 2023. "Colon-Targeted Delivery of Indole Acetic Acid Helps Regulate Gut Motility by Activating the AHR Signaling Pathway" Nutrients 15, no. 19: 4282. https://doi.org/10.3390/nu15194282
APA StyleChen, Y., Pan, R., Mei, L., Tian, P., Wang, L., Zhao, J., Chen, W., & Wang, G. (2023). Colon-Targeted Delivery of Indole Acetic Acid Helps Regulate Gut Motility by Activating the AHR Signaling Pathway. Nutrients, 15(19), 4282. https://doi.org/10.3390/nu15194282