The Role of Acyl-CoA Synthetase 1 in Bioactive Lipid Accumulation and the Development of Hepatic Insulin Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Plasmids and In Vivo shRNA Plasmids Injection
2.3. Lipid Measurements
2.3.1. Sphingolipids
2.3.2. Diacylglycerols
2.3.3. Long-Chain Acyl-CoA
2.3.4. Plasma FFA, Hepatic Triacylglycerols and Liver Glycogen Content
2.4. Liver Glucose-6-Phosphate (G6P) Content and Estimation of Hepatic Gluconeogenesis
2.5. Protein Expression
2.6. Real-Time PCR
2.7. Plasma Insulin and Glucose Concentration
2.8. OGTT
2.9. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR)
2.10. Protein Concentration
2.11. Statistical Analysis
3. Results
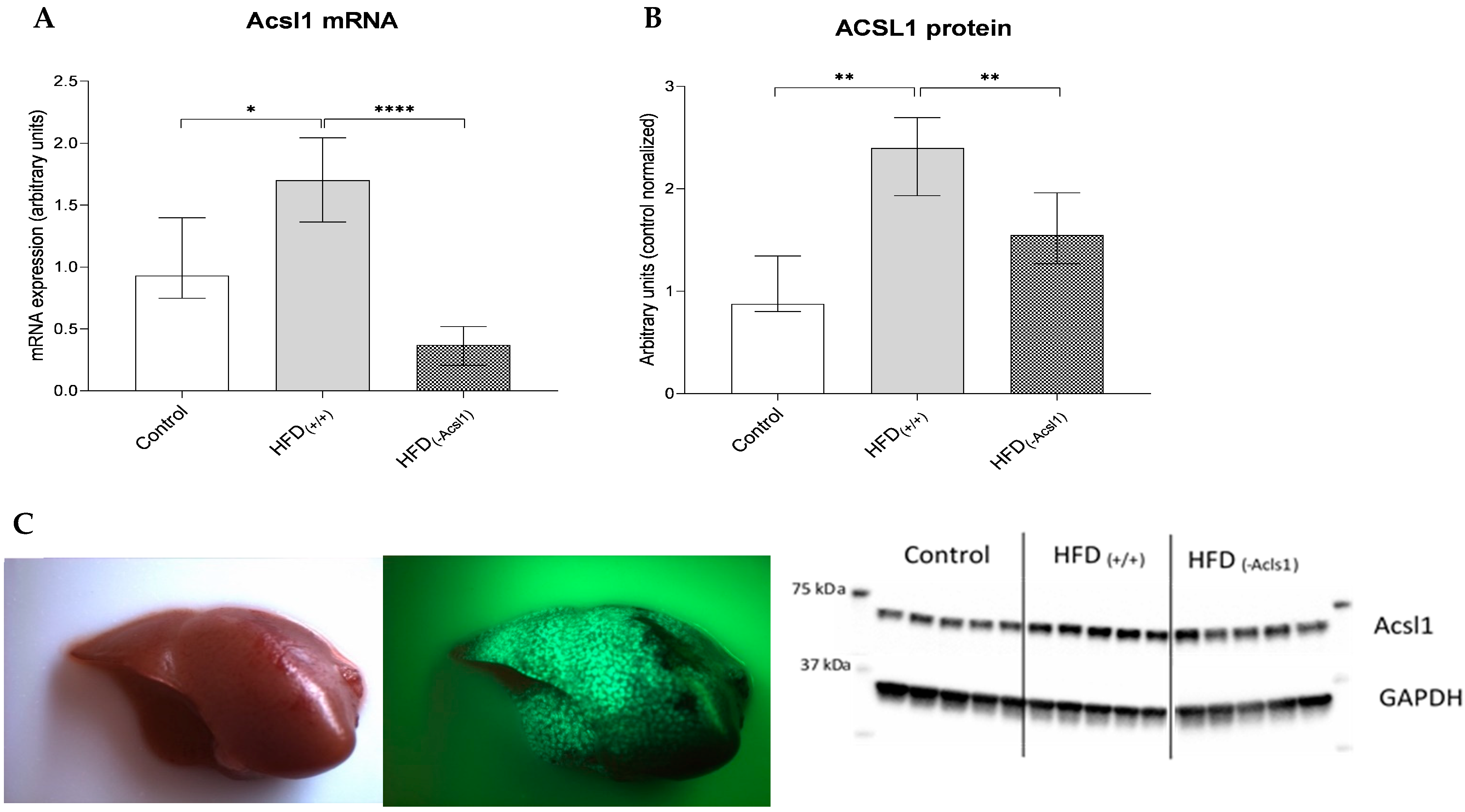
3.1. Effect of HFD Diet and Silencing on Hepatic Expression of ACSL1 Gene
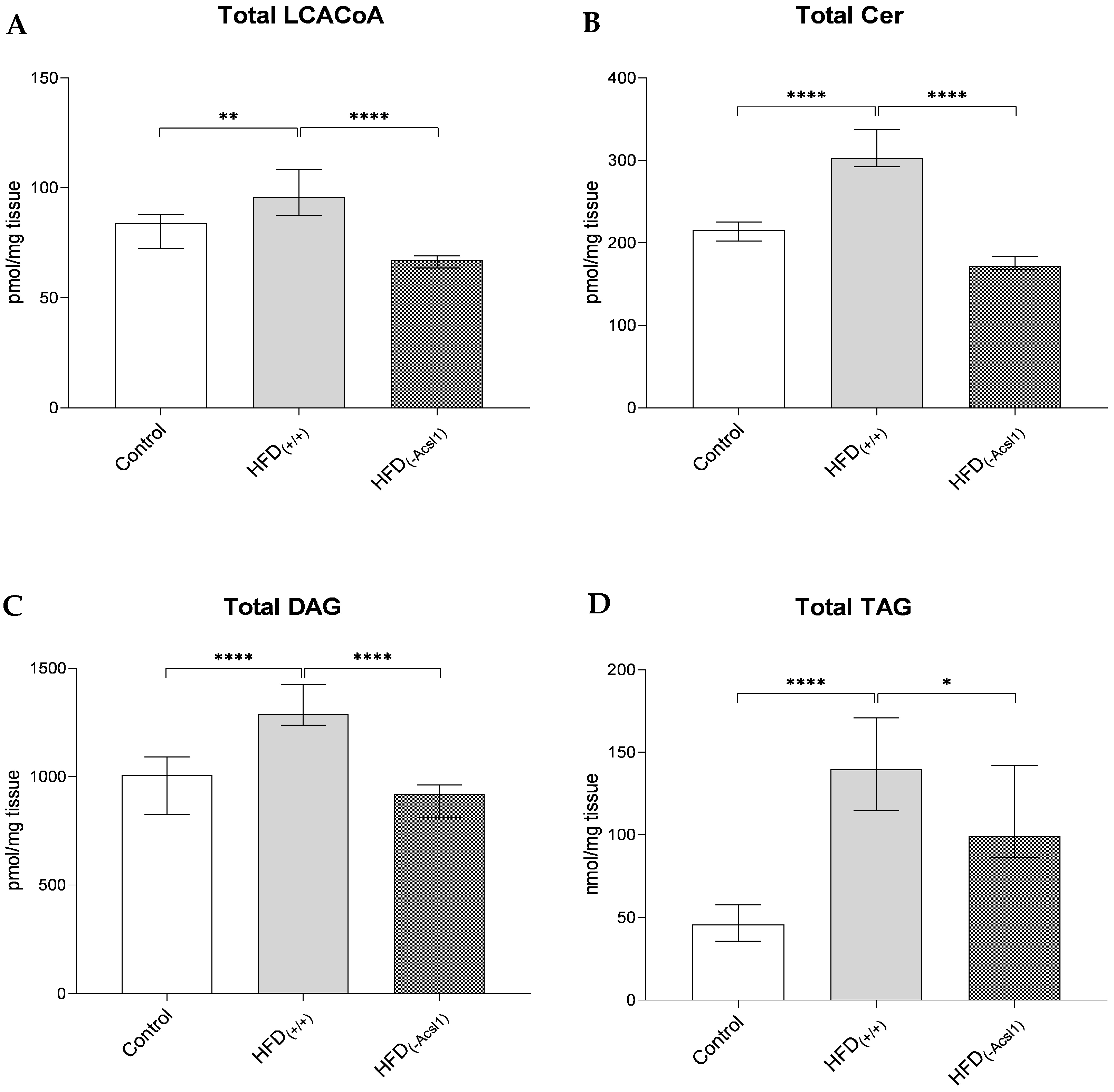
3.2. Plasma FFA Concentration and Hepatic Lipid Content
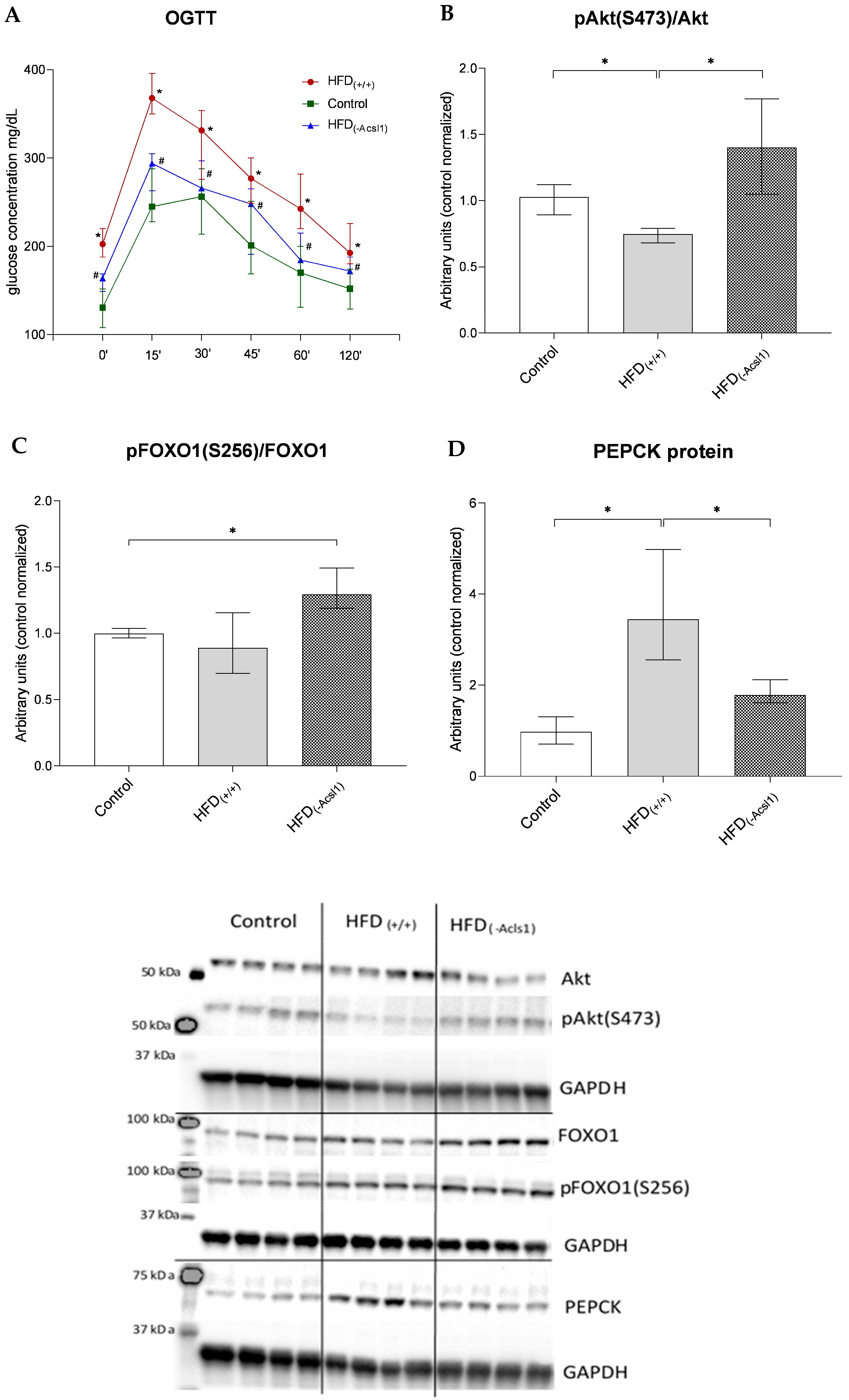
3.3. Hepatic Response to Insulin
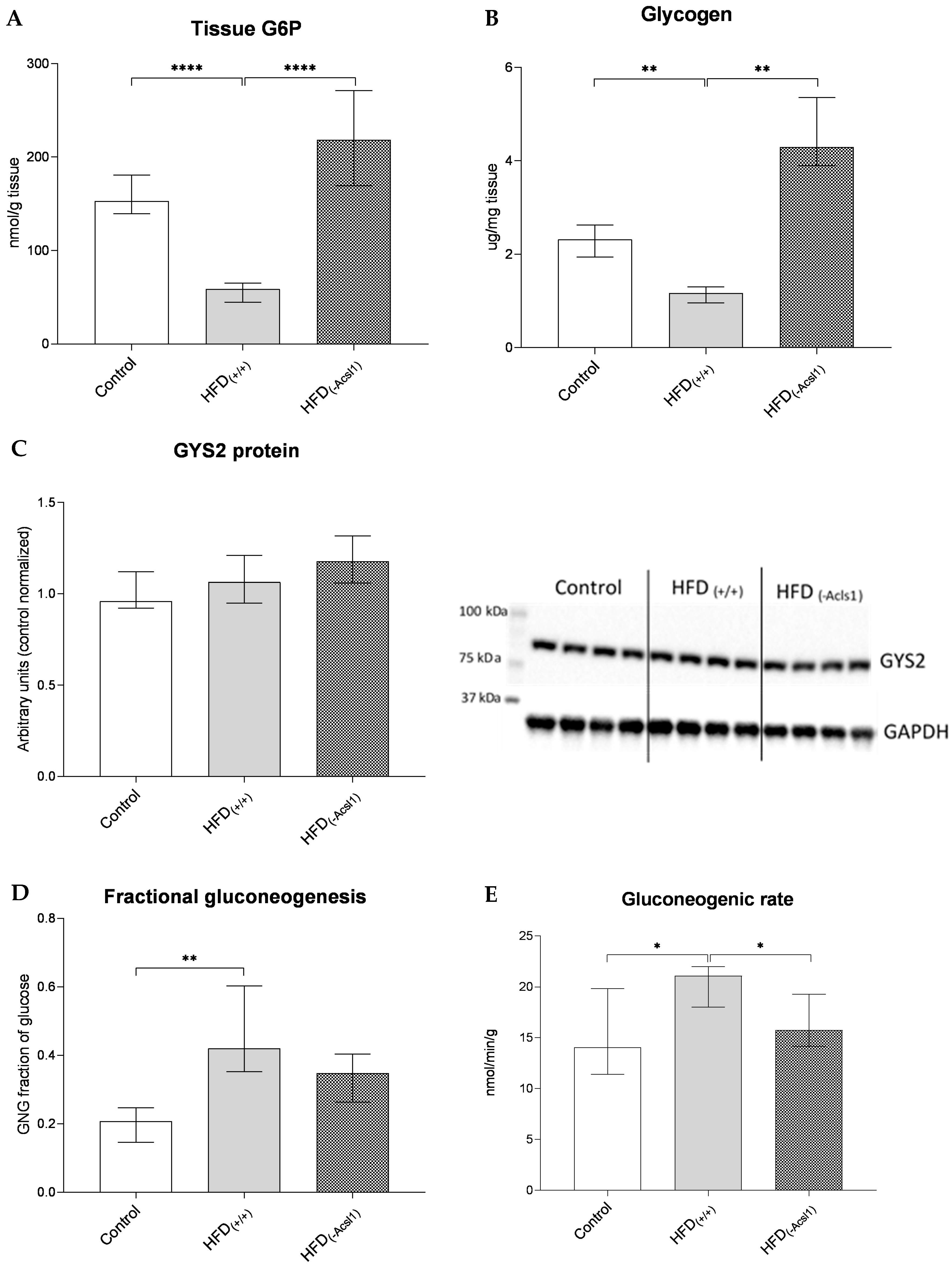
3.4. Hepatic Glucose Metabolism and Indices of Insulin Resistance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boden, G. Obesity and free fatty acids. Endocrinol. Metab. Clin. N. Am. 2008, 37, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Boden, G. Fatty acid-induced inflammation and insulin resistance in skeletal muscle and liver. Curr. Diab. Rep. 2006, 6, 177–181. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Regulation of fat metabolism in skeletal muscle. Ann. N. Y. Acad. Sci. 2002, 967, 217–235. [Google Scholar] [CrossRef]
- Li, L.O.; Ellis, J.M.; Paich, H.A.; Wang, S.; Gong, N.; Altshuller, G.; Thresher, R.J.; Koves, T.R.; Watkins, S.M.; Muoio, D.M.; et al. Liver-specific loss of long chain acyl-CoA synthetase-1 decreases triacylglycerol synthesis and beta-oxidation and alters phospholipid fatty acid composition. J. Biol. Chem. 2009, 284, 27816–27826. [Google Scholar] [CrossRef]
- Ye, J.M.; Dzamko, N.; Cleasby, M.E.; Hegarty, B.D.; Furler, S.M.; Cooney, G.J.; Kraegen, E.W. Direct demonstration of lipid sequestration as a mechanism by which rosiglitazone prevents fatty-acid-induced insulin resistance in the rat: Comparison with metformin. Diabetologia 2004, 47, 1306–1313. [Google Scholar] [CrossRef]
- Zabielski, P.; Hady, H.R.; Chacinska, M.; Roszczyc, K.; Gorski, J.; Blachnio-Zabielska, A.U. The effect of high fat diet and metformin treatment on liver lipids accumulation and their impact on insulin action. Sci. Rep. 2018, 8, 7249. [Google Scholar] [CrossRef]
- Zabielski, P.; Daniluk, J.; Hady, H.R.; Markowski, A.R.; Imierska, M.; Górski, J.; Blachnio-Zabielska, A.U. The effect of high-fat diet and inhibition of ceramide production on insulin action in liver. J. Cell Physiol. 2019, 234, 1851–1861. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol. Sci. 2017, 38, 649–665. [Google Scholar] [CrossRef]
- Perry, R.J.; Samuel, V.T.; Petersen, K.F.; Shulman, G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature 2014, 510, 84–91. [Google Scholar] [CrossRef]
- Galbo, T.; Shulman, G.I. Lipid-induced hepatic insulin resistance. Aging 2013, 5, 582–583. [Google Scholar] [CrossRef] [PubMed]
- Folli, F.; Saad, M.J.; Backer, J.M.; Kahn, C.R. Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. J. Biol. Chem. 1992, 267, 22171–22177. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Kondo, T.; Sajan, M.; Luo, J.; Bronson, R.; Asano, T.; Farese, R.; Cantley, L.C.; Kahn, C.R. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 2006, 3, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Ogawa, W.; Akimoto, K.; Inoue, H.; Miyake, K.; Furukawa, K.; Hayashi, Y.; Iguchi, H.; Matsuki, Y.; Hiramatsu, R.; et al. PKClambda in liver mediates insulin-induced SREBP-1c expression and determines both hepatic lipid content and overall insulin sensitivity. J. Clin. Investig. 2003, 112, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Schmoll, D.; Walker, K.S.; Alessi, D.R.; Grempler, R.; Burchell, A.; Guo, S.; Walther, R.; Unterman, T.G. Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. Evidence for insulin response unit-dependent and -independent effects of insulin on promoter activity. J. Biol. Chem. 2000, 275, 36324–36333. [Google Scholar] [CrossRef] [PubMed]
- Barthel, A.; Schmoll, D.; Krüger, K.D.; Bahrenberg, G.; Walther, R.; Roth, R.A.; Joost, H.G. Differential regulation of endogenous glucose-6-phosphatase and phosphoenolpyruvate carboxykinase gene expression by the forkhead transcription factor FKHR in H4IIE-hepatoma cells. Biochem. Biophys. Res. Commun. 2001, 285, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Balram, A.; Thapa, S.; Chatterjee, S. Glycosphingolipids in Diabetes, Oxidative Stress, and Cardiovascular Disease: Prevention in Experimental Animal Models. Int. J. Mol. Sci. 2022, 23, 15442. [Google Scholar] [CrossRef]
- Aiston, S.; Hampson, L.J.; Arden, C.; Iynedjian, P.B.; Agius, L. The role of protein kinase B/Akt in insulin-induced inactivation of phosphorylase in rat hepatocytes. Diabetologia 2006, 49, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Rajas, F.; Gautier-Stein, A.; Mithieux, G. Glucose-6 Phosphate, A Central Hub for Liver Carbohydrate Metabolism. Metabolites 2019, 9, 282. [Google Scholar] [CrossRef]
- Baskaran, S.; Roach, P.J.; DePaoli-Roach, A.A.; Hurley, T.D. Structural basis for glucose-6-phosphate activation of glycogen synthase. Proc. Natl. Acad. Sci. USA 2010, 107, 17563–17568. [Google Scholar] [CrossRef]
- Bollen, M.; Keppens, S.; Stalmans, W. Specific features of glycogen metabolism in the liver. Biochem. J. 1998, 336 Pt 1, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Rivera, A.; Meza-Ríos, A.; Chagoya de Sánchez, V.; Velasco-Loyden, G.; García-Benavides, L.; Jave-Suarez, L.F.; Monroy-Ramirez, H.C.; Santos-García, A.; Armendáriz-Borunda, J.; Sandoval-Rodríguez, A. Hydrodynamics-based liver transfection achieves gene silencing of CB1 using short hairpin RNA plasmid in cirrhotic rats. PLoS ONE 2020, 15, e0228729. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Sun, R.; Huang, Q.; Tian, Z. Technical Improvement and Application of Hydrodynamic Gene Delivery in Study of Liver Diseases. Front. Pharmacol. 2017, 8, 591. [Google Scholar] [CrossRef] [PubMed]
- Blachnio-Zabielska, A.U.; Persson, X.M.; Koutsari, C.; Zabielski, P.; Jensen, M.D. A liquid chromatography/tandem mass spectrometry method for measuring the in vivo incorporation of plasma free fatty acids into intramyocellular ceramides in humans. Rapid Commun. Mass Spectrom. 2012, 26, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Blachnio-Zabielska, A.U.; Zabielski, P.; Jensen, M.D. Intramyocellular diacylglycerol concentrations and [U-13C]palmitate isotopic enrichment measured by LC/MS/MS. J. Lipid Res. 2013, 54, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Blachnio-Zabielska, A.U.; Koutsari, C.; Jensen, M.D. Measuring long-chain acyl-coenzyme A concentrations and enrichment using liquid chromatography/tandem mass spectrometry with selected reaction monitoring. Rapid Commun. Mass Spectrom. 2011, 25, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Minkler, P.E.; Kerner, J.; Ingalls, S.T.; Hoppel, C.L. Novel isolation procedure for short-, medium-, and long-chain acyl-coenzyme A esters from tissue. Anal. Biochem. 2008, 376, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Young, L.E.A.; Brizzee, C.O.; Macedo, J.K.A.; Murphy, R.D.; Contreras, C.J.; DePaoli-Roach, A.A.; Roach, P.J.; Gentry, M.S.; Sun, R.C. Accurate and sensitive quantitation of glucose and glucose phosphates derived from storage carbohydrates by mass spectrometry. Carbohydr. Polym. 2020, 230, 115651. [Google Scholar] [CrossRef] [PubMed]
- Zabielski, P.; Błachnio-Zabielska, A.U.; Wójcik, B.; Chabowski, A.; Górski, J. Effect of plasma free fatty acid supply on the rate of ceramide synthesis in different muscle types in the rat. PLoS ONE 2017, 12, e0187136. [Google Scholar] [CrossRef]
- Blachnio-Zabielska, A.U.; Chacinska, M.; Vendelbo, M.H.; Zabielski, P. The Crucial Role of C18-Cer in Fat-Induced Skeletal Muscle Insulin Resistance. Cell Physiol. Biochem. 2016, 40, 1207–1220. [Google Scholar] [CrossRef]
- Antoniewicz, M.R.; Kelleher, J.K.; Stephanopoulos, G. Measuring deuterium enrichment of glucose hydrogen atoms by gas chromatography/mass spectrometry. Anal. Chem. 2011, 83, 3211–3216. [Google Scholar] [CrossRef]
- Hachey, D.L.; Parsons, W.R.; McKay, S.; Haymond, M.W. Quantitation of monosaccharide isotopic enrichment in physiologic fluids by electron ionization or negative chemical ionization GC/MS using di-O-isopropylidene derivatives. Anal. Chem. 1999, 71, 4734–4739. [Google Scholar] [CrossRef]
- Millard, P.; Delépine, B.; Guionnet, M.; Heuillet, M.; Bellvert, F.; Létisse, F. IsoCor: Isotope correction for high-resolution MS labeling experiments. Bioinformatics 2019, 35, 4484–4487. [Google Scholar] [CrossRef]
- Haymond, M.W.; Sunehag, A.L. The reciprocal pool model for the measurement of gluconeogenesis by use of [U-(13)C]glucose. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E140–E145. [Google Scholar] [CrossRef]
- Chung, S.T.; Chacko, S.K.; Sunehag, A.L.; Haymond, M.W. Measurements of Gluconeogenesis and Glycogenolysis: A Methodological Review. Diabetes 2015, 64, 3996–4010. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.; Sevillano, J.; de Castro, J.; Herrera, E.; Ramos, M.P. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1269–E1276. [Google Scholar] [CrossRef] [PubMed]
- London, A.; Lundsgaard, A.M.; Kiens, B.; Bojsen-Møller, K.N. The Role of Hepatic Fat Accumulation in Glucose and Insulin Homeostasis-Dysregulation by the Liver. J. Clin. Med. 2021, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Nishimuta, S.; Fukumura, Y.; Michinaga, S.; Egusa, Y.; Hase, T.; Terada, T.; Sakurai, F.; Mizuguchi, H.; Tomita, K.; et al. Liver-specific overexpression of lipoprotein lipase improves glucose metabolism in high-fat diet-fed mice. PLoS ONE 2022, 17, e0274297. [Google Scholar] [CrossRef]
- Singh, A.B.; Dong, B.; Xu, Y.; Zhang, Y.; Liu, J. Identification of a novel function of hepatic long-chain acyl-CoA synthetase-1 (ACSL1) in bile acid synthesis and its regulation by bile acid-activated farnesoid X receptor. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2019, 1864, 358–371. [Google Scholar] [CrossRef]
- Tsuru, H.; Osaka, M.; Hiraoka, Y.; Yoshida, M. HFD-induced hepatic lipid accumulation and inflammation are decreased in Factor D deficient mouse. Sci. Rep. 2020, 10, 17593. [Google Scholar] [CrossRef]
- Saxena, R.; Nassiri, M.; Yin, X.M.; Morral, N. Insights from a high-fat diet fed mouse model with a humanized liver. PLoS ONE 2022, 17, e0268260. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Xu, J.W.; Li, S.; Ng, X.E.; Tung, Y.T. Effects of exercise on high-fat diet-induced non-alcoholic fatty liver disease and lipid metabolism in ApoE knockout mice. Nutr. Metab. 2022, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Longato, L.; Tong, M.; Wands, J.R.; de la Monte, S.M. High fat diet induced hepatic steatosis and insulin resistance: Role of dysregulated ceramide metabolism. Hepatol. Res. 2012, 42, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Kowalski, G.M.; Leslie, S.J.; Risis, S.; Yang, C.; Lee-Young, R.S.; Babb, J.R.; Meikle, P.J.; Lancaster, G.I.; Henstridge, D.C.; et al. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 2013, 56, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Rizza, R.A. Pathogenesis of fasting and postprandial hyperglycemia in type 2 diabetes: Implications for therapy. Diabetes 2010, 59, 2697–2707. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Krssak, M.; Brehm, A.; Bernroider, E.; Anderwald, C.; Nowotny, P.; Dalla Man, C.; Cobelli, C.; Cline, G.W.; Shulman, G.I.; Waldhäusl, W.; et al. Alterations in postprandial hepatic glycogen metabolism in type 2 diabetes. Diabetes 2004, 53, 3048–3056. [Google Scholar] [CrossRef]
- von Wilamowitz-Moellendorff, A.; Hunter, R.W.; García-Rocha, M.; Kang, L.; López-Soldado, I.; Lantier, L.; Patel, K.; Peggie, M.W.; Martínez-Pons, C.; Voss, M.; et al. Glucose-6-phosphate-mediated activation of liver glycogen synthase plays a key role in hepatic glycogen synthesis. Diabetes 2013, 62, 4070–4082. [Google Scholar] [CrossRef]
| Control | HFD(+/+) | HFD(−Acsl1) | |
|---|---|---|---|
| Fasting glucose (mg/dL) (n = 10) | 130.5 (109.5–149.8) | 202.5 (191.8–217.8) * | 182.5 (167.8–189.0) # |
| Fasting insulin (μIU/mL) (n = 10) | 7.15 (5.60–11.33) | 25.25 (13.33–33.30) * | 15.00 (11.10–16.73) # |
| HOMA-IR (n = 10) | 0.40 (0.200–0.700) | 2.25 (1.08–2.58) * | 1.00 (0.88–1.33) # |
| Total FFAs (nmol/mL) (n = 10) | 220.9 (195.0–232.5) | 488.3 (465.3–521.6) * | 415.3 (382.4–446.7) # |
| Baseline body (g) (n = 10) | 17.45 (16.70–18.73) | 18.25 (17.48–9.13) | 19.53 (18.7–20.04) |
| End-point body weights (g) (n = 10) | 27.18 (26.64–28.36) | 30.75 (28.89–34.15) | 32.40 (30.64–35.53) |
| LCACoA | Control | HFD(+/+) | HFD(−Acsl1) |
|---|---|---|---|
| C14:0 | 1.55 (1.38–1.75) | 1.93 (1.70–2.27) * | 1.50 (1.40–1.67) # |
| C16:0 | 9.44 (8.71–10.90) | 16.32 (13.12–17.74) * | 9.03 (8.17–11.00) # |
| C16:1 | 10.37 (9.00–11.30) | 3.93 (3.51–4.44) * | 3.18 (2.68–3.39) # |
| C18:0 | 13.72 (11.68–14.36) | 18.85 (13.32–19.84) * | 10.16 (9.20–11.12) # |
| C18:1 | 15.20 (13.81–17.83) | 18.01 (13.41–18.79) | 10.59 (9.11–10.92) # |
| C18:2 | 29.65 (27.34–33.50) | 38.39 (35.45–44.53) * | 31.37 (28.38–33.41) # |
| C20:0 | 1.44 (1.05–1.51) | 1.64 (1.44–1.78) | 1.23 (1.11–1.36) # |
| Ceramide | Control | HFD(+/+) | HFD(−Acsl1) |
|---|---|---|---|
| d18:1/C14:0 | 0.043 (0.035–0.049) | 0.038 (0.034–0.041) | 0.031 (0.029–0.033) # |
| d18:1/C16:0 | 6.83 (6.56–7.66) | 7.14 (6.89–7.54) | 7.25 (6.89–7.81) |
| d18:1/C18:0 | 1.50 (1.36–1.62) | 3.28 (2.80–3.57) * | 2.26 (2.00–2.26) # |
| d18:1/C18:1 | 0.148 (0.136–0.173) | 0.2817 (0.267–0.316) * | 0.212 (0.192–0.233) # |
| d18:1/C20:0 | 4.24 (3.91–4.83) | 12.48 (11.66–13.61) * | 9.82 (8.21–10.62) # |
| d18:1/C22:0 | 75.42 (60.19–82.90) | 133.8 (129.5–176.0) * | 72.40 (64.88–80.42) # |
| d18:1/C24:0 | 45.97 (41.58–51.69) | 51.24 (47.99–53.49) | 32.04 (31.22–34.04) # |
| d18:1/C24:1 | 80.60 (74.60–84.78) | 87.24 (85.16–91.60) * | 47.93 (43.14–55.22) # |
| Diacylglycerol | Control | HFD(+/+) | HFD(−Acsl1) |
|---|---|---|---|
| C14:0/14:0 | 0.66 (0.58–0.71) | 0.58 (0.51–0.69) | 0.61(0.49–0.70) |
| C16:0/16:0 | 16.65 (12.76–17.50) | 19.48 (17.67–26.94) * | 8.17 (6.99–10.04) # |
| C16:0/18:0 | 3.670 (3.50–4.37) | 10.08 (8.88–11.65) * | 9.23 (7.82–10.57) |
| C16:0/18:1 | 395.3 (305.7–446.8) | 513.5 (460.2–576.3) * | 273.6 (217.7–335.2) # |
| C16:0/18:2 | 182.3 (166.1–207.7) | 229.7 (210.4–251.4) * | 177.1 (160.6–203.4) # |
| C18:0/18:0 | 1.76 (1.62–2.06) | 3.81 (3.12–4.22) * | 2.71 (2.53–3.40) # |
| C18:0/18:1 | 17.79 (15.82–19.33) | 42.85 (39.36–48.57) * | 35.28 (31.85–37.41) # |
| C18:0/18:2 | 16.40 (11.92–19.91) | 40.90 (36.92–52.96) * | 35.01 (32.44–39.59) # |
| C18:1/18:1 | 258.0 (194.2–300.9) | 292.5 (274.3–315.2) | 223.5 (196.6–254.9) # |
| C18:2/18:2 | 73.13 (66.38–77.92) | 105.0 (84.48–108.5) * | 76.21 (63.68–81.41) # |
| C18:0/20:4 | 30.23 (28.20–33.92) | 49.47 (40.20–54.59) * | 39.28 (34.90–44.30) # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zabielski, P.; Imierska, M.; Roszczyc-Owsiejczuk, K.; Kuźmicki, M.; Rogalski, P.; Daniluk, J.; Błachnio-Zabielska, A.U. The Role of Acyl-CoA Synthetase 1 in Bioactive Lipid Accumulation and the Development of Hepatic Insulin Resistance. Nutrients 2024, 16, 1003. https://doi.org/10.3390/nu16071003
Zabielski P, Imierska M, Roszczyc-Owsiejczuk K, Kuźmicki M, Rogalski P, Daniluk J, Błachnio-Zabielska AU. The Role of Acyl-CoA Synthetase 1 in Bioactive Lipid Accumulation and the Development of Hepatic Insulin Resistance. Nutrients. 2024; 16(7):1003. https://doi.org/10.3390/nu16071003
Chicago/Turabian StyleZabielski, Piotr, Monika Imierska, Kamila Roszczyc-Owsiejczuk, Mariusz Kuźmicki, Paweł Rogalski, Jarosław Daniluk, and Agnieszka U. Błachnio-Zabielska. 2024. "The Role of Acyl-CoA Synthetase 1 in Bioactive Lipid Accumulation and the Development of Hepatic Insulin Resistance" Nutrients 16, no. 7: 1003. https://doi.org/10.3390/nu16071003
APA StyleZabielski, P., Imierska, M., Roszczyc-Owsiejczuk, K., Kuźmicki, M., Rogalski, P., Daniluk, J., & Błachnio-Zabielska, A. U. (2024). The Role of Acyl-CoA Synthetase 1 in Bioactive Lipid Accumulation and the Development of Hepatic Insulin Resistance. Nutrients, 16(7), 1003. https://doi.org/10.3390/nu16071003






