Serum Levels of Copper and Zinc and Survival in Breast Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Approval and Informed Consent
2.3. Analytical Procedures
2.4. Statistical Analysis
3. Results
3.1. Mean Plasma Zinc and Copper Levels in the Study Group
3.2. Cox Regression Models for Overall and Breast Cancer-Specific Survival
3.3. Overall and Breast Cancer Specific Survival by Quartiles of Zinc
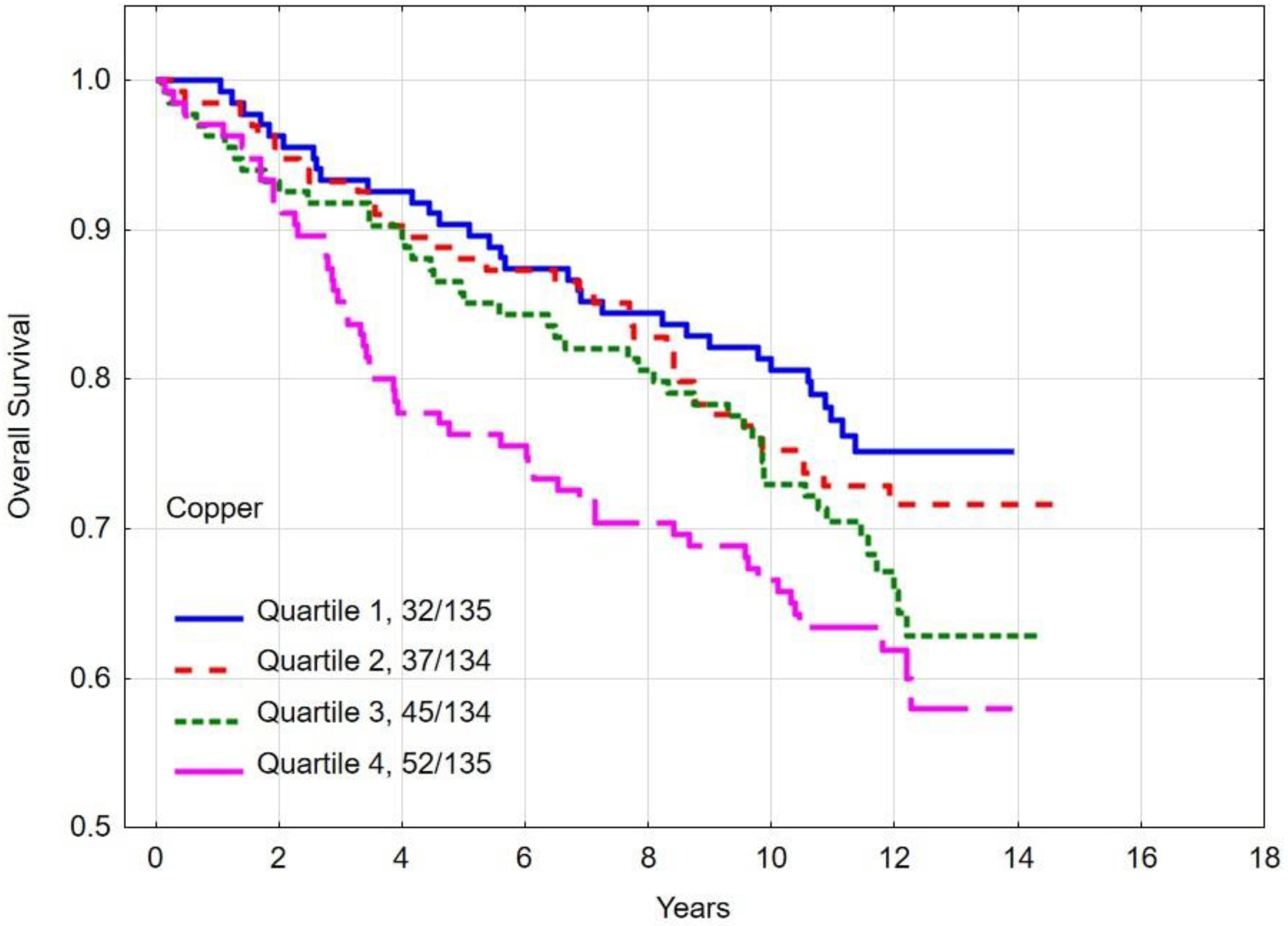
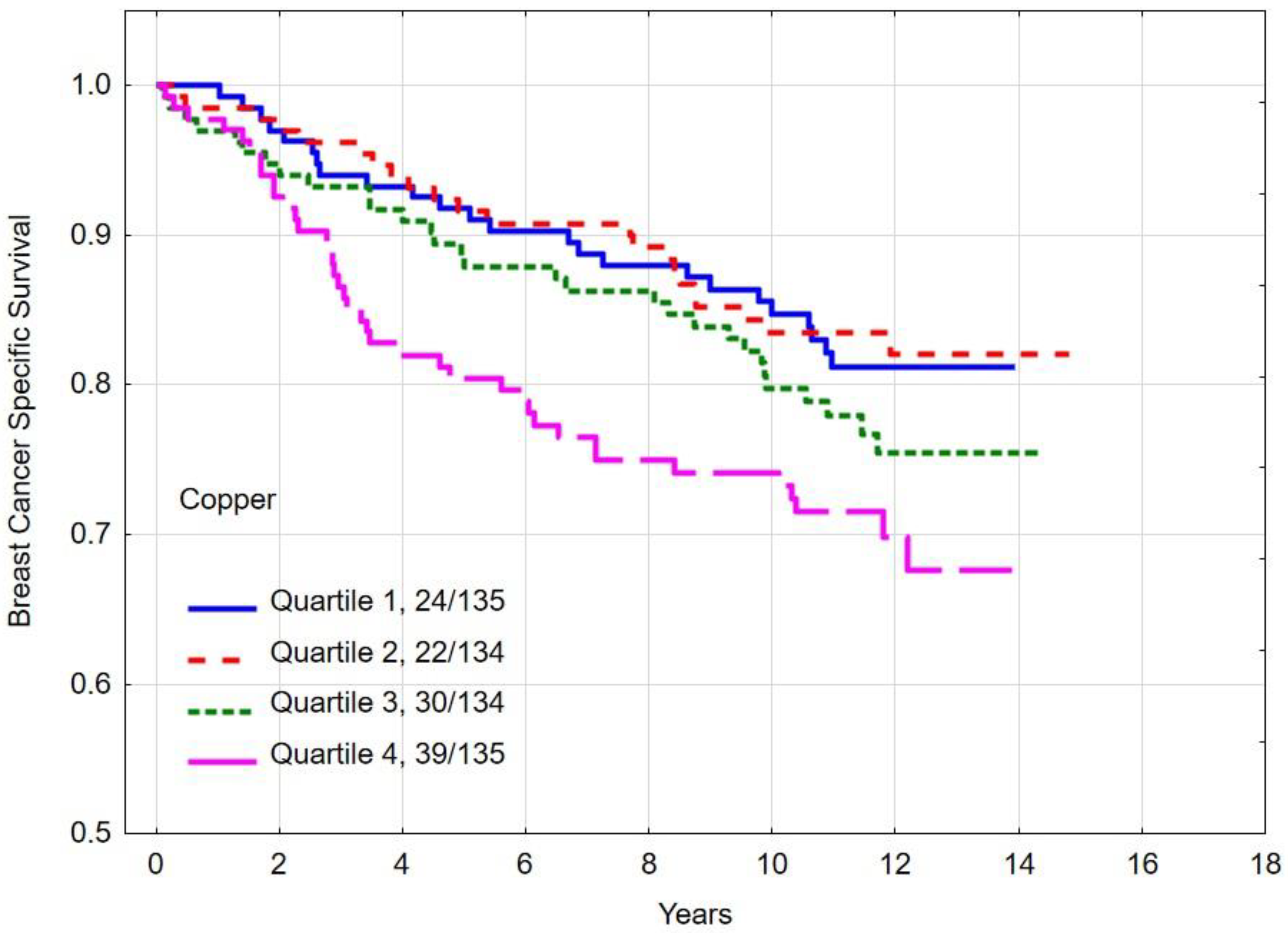
3.4. Overall and Breast Cancer-Specific Survival by Quartiles of Copper
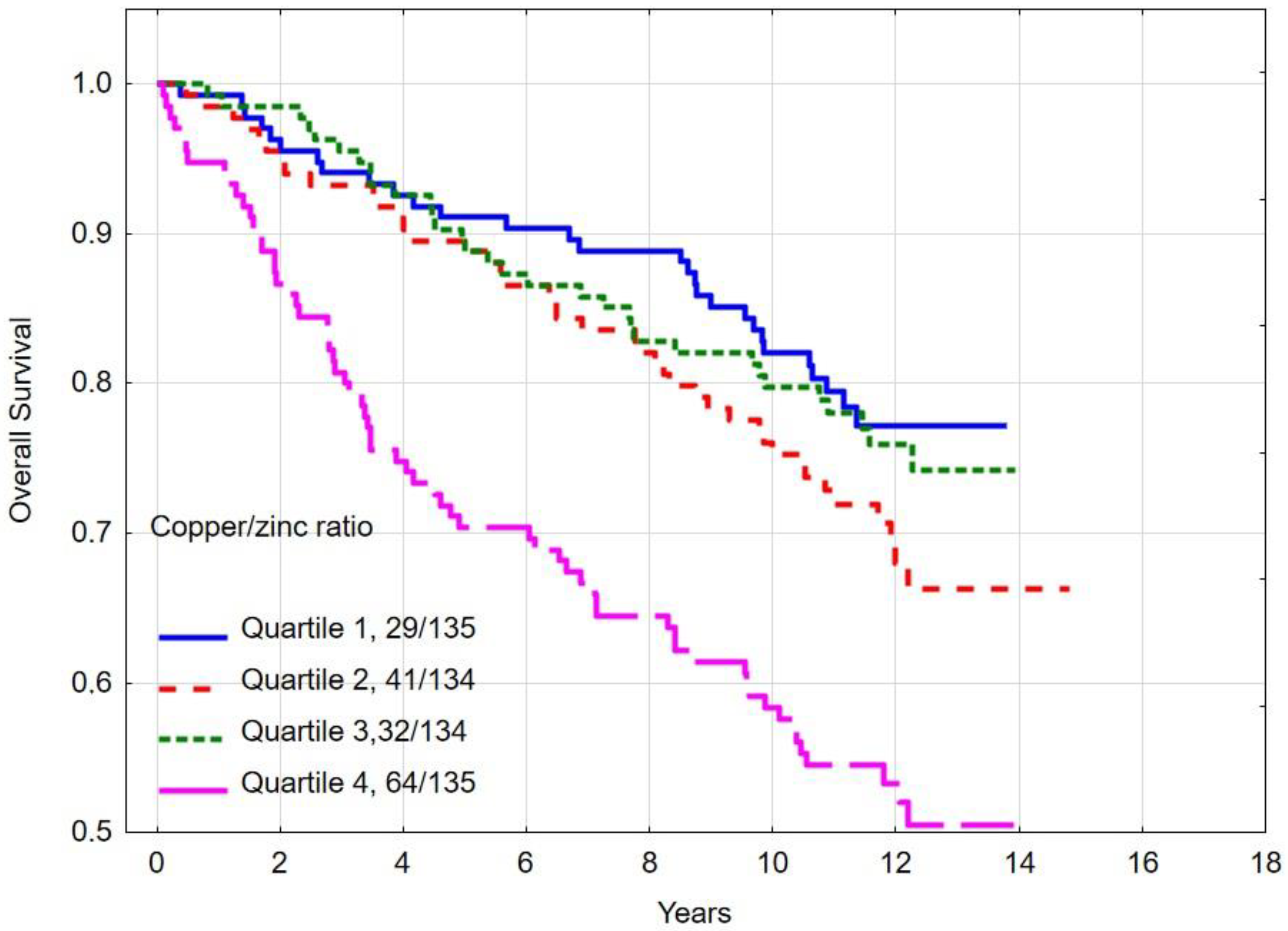
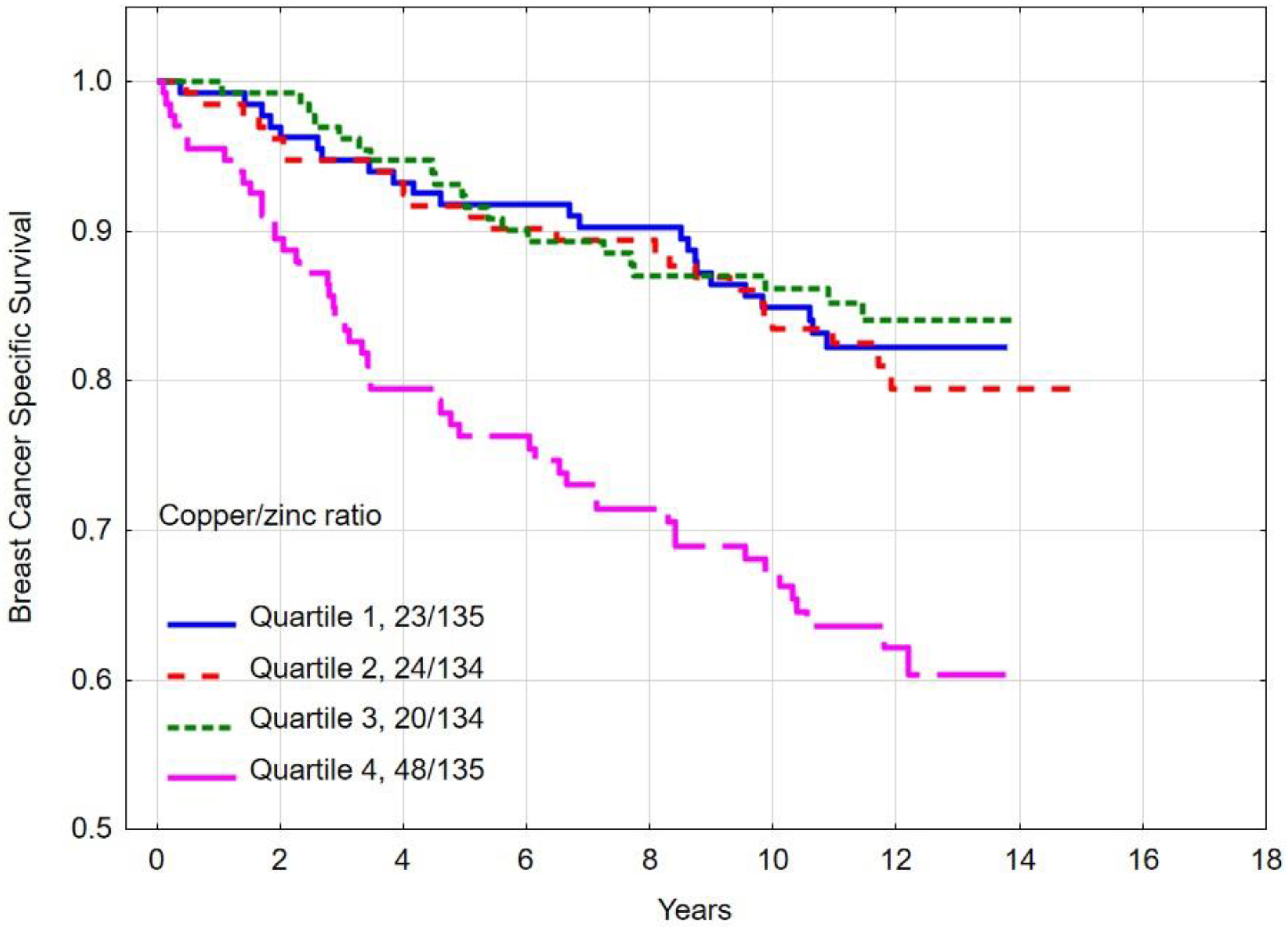
3.5. Overall and Breast Cancer-Specific Survival by Quartiles of Copper/Zinc (Cu/Zn) Ratio
3.6. The Most Important Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lappano, R.; Malaguarnera, R.; Belfiore, A.; Maggiolini, M. Recent advances on the stimulatory effects of metals in breast cancer. Mol. Cell. Endocrinol. 2017, 457, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Sandsveden, M.; Nilsson, E.; Borgquist, S.; Rosendahl, A.H.; Manjer, J. Prediagnostic serum selenium levels in relation to breast cancer survival and tumor characteristics. Int. J. Cancer 2020, 147, 2424–2436. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Bergkvist, L.; Wolk, A. Selenium intake and breast cancer mortality in a cohort of Swedish women. Breast Cancer Res. Treat. 2012, 134, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Dębniak, T.; Falco, M.; Kładny, J.; et al. Serum selenium levels predict survival after breast cancer. Breast Cancer Res. Treat. 2018, 167, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Szwiec, M.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Dębniak, T.; Jakubowska, A.; Lener, M.; Falco, M.; et al. Serum Selenium Level Predicts 10-Year Survival after Breast Cancer. Nutrients 2021, 13, 953. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: Anupdate. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Hennigar, S.R.; Kelley, A.M.; McClung, J.P. Metallothionein and zinc transporter expression in circulating human blood cells as biomarkers of zinc status: A systematic review. Adv. Nutr. 2016, 7, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Gumulec, J.; Masarik, M.; Krizkova, S.; Adam, V.; Hubalek, J.; Hrabeta, J.; Eckschlager, T.; Stiborova, M.; Kizek, R. Insight to physiology and pathology of zinc (II) ions and their actions in breast and prostate carcinoma. Curr. Med. Chem. 2011, 33, 5041–5051. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.L.; McCormick, N.H.; Velasquez, V.; Lopez, V. Zinc in specialized secretory tissues: Roles in the pancreas, prostate andmammary gland. Adv. Nutr. 2011, 2, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Kelleher, S.L. Cellular mechanisms of zinc dysregulation: A perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients 2012, 4, 875–903. [Google Scholar] [CrossRef]
- Taylor, C.G.; Giesbrecht, J.A. Dietary zinc deficiency and expression of lmphocyte signal transduction proteins. Can. J. Physiol. Pharmacol. 2000, 78, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Kiouri, D.P.; Tsoupra, E.; Peana, M.; Perlepes, S.P.; Stefanidou, M.E.; Chasapis, C.T. Multifunctional role of zinc in human health: An update. EXCLI J. 2023, 22, 809–827. [Google Scholar] [PubMed]
- Jouybari, L.; Kiani, F.; Akbari, A.; Sanagoo, A.; Sayehmiri, F.; Aaseth, J.; Chartrand, M.S.; Sayehmiri, K.; Chirumbolo, S.; Bjørklund, G. A meta-analysis of zinc levels in breast cancer. J. Trace Elem. Med. Biol. 2019, 56, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Pasha, Q.; Malik, S.A.; Shaheen, N.; Shah, M.H. Comparison of trace elements in the scalp hair of malignant and benign breast lesions versus healthy women. Biol. Trace Elem. Res. 2010, 134, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Saleh, F.; Behbehani, A.; Asfar, S.; Khan, I.; Ibrahim, G. Abnormal blood levels of trace elements and metals, DNA damage, and breast cancer in the state of Kuwait. Biol. Trace Elem. Res. 2011, 141, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.F.; Lu, P.; Zeng, L.; Yang, Y.H.; Luo, J.; Yang, Y.W.; Wang, D. Serum total oxidant/antioxidant status and trace element levels in breast cancer patients. Int.J. Clin. Oncol. 2012, 17, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, V.; Sathisha, T.G.; Kasturi, K.; Mallika, D.S.; Amos, S.J.; Ragunatha, S. Serum levels of metal ions in female patients with breast cancer. J. Clin. Diagn. Res. 2015, 9, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Geraki, K.; Farquharson, M.; Bradley, D. Concentrations of Fe, Cu and Zn in breast tissue: A synchrotron XRF study. Phys. Med. Biol. 2002, 47, 2327. [Google Scholar] [CrossRef] [PubMed]
- Zowczak, M.; Iskra, M.; Torliński, L.; Cofta, S. Analysis of serum copper and zinc concentrations in cancer patients. Biol. Trace Elem. Res. 2001, 82, 1–8. [Google Scholar] [CrossRef]
- Ionescu, J.G.; Novotny, J.; Stejskal, V.; Lätsch, A.; Blaurock-Busch, E.; Eisenmann-Klein, M. Increased levels of transition metals in breast cancer tissue. Neuro Endocrinol. Lett. 2006, 27, 36–39. [Google Scholar]
- Cui, Y.; Vogt, S.; Olson, N.; Glass, A.G.; Rohan, T.E. Levels of zinc, selenium, calcium, and iron inbenign breast tissue and risk of subsequent breast cancer, cancer epidemiol. Cancer Epidemiol. Biomark. Prev. 2007, 8, 1682–1685. [Google Scholar] [CrossRef] [PubMed]
- Magalhăes, T.; Becker, M.; Carvalho, M.L.; Bohlen, A. Study of Br, Zn, Cu and Fe concentrations in healthy and cancer breast tissues by TXRF. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 1473–1479. [Google Scholar] [CrossRef]
- Arinola, O.; Charles-Davies, M. Micronutrient levels in the plasma of Nigerian females with breast cancer. Afr. J. Biotechnol. 2008, 7, 1620–1623. [Google Scholar] [CrossRef][Green Version]
- Al-Ebraheem, A.; Farquharson, M.J.; Ryan, E. The evaluation of biologically importanttrace metals in liver, kidney and breast tissue. Appl. Radiat. Isot. 2009, 67, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fu, J.; Wang, Y.; Liao, C.; Tao, Y.; Jiang, G. Use of scalp hair as indicator of human exposure to heavy metals in an electronic waste recycling area. Environ. Pollut. 2009, 157, 2445–2451. [Google Scholar] [CrossRef] [PubMed]
- Joo, N.S.; Kim, S.M.; Jung, Y.S.; Kim, K.M. Hair iron and other minerals’ level in breast cancer patients. Biol. Trace Elem. Res. 2009, 129, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Hyun-Jeong, Y.; Yun-Sang, Y.; Soo-Hwan, J.; Yong-Sik, E.; Nam-Seok, J. The relationship between hair zinc and body mass index in breast cancer patients, Korean. J. Fam. Med. 2010, 31, 607–612. [Google Scholar]
- Cihan, C.Y.B.; Sözen, S.; Yıldırım, S.Ö. Trace elements and heavy metals in hair of stage III breast Cancer patients. Biol. Trace Elem. Res. 2011, 144, 360–379. [Google Scholar] [CrossRef] [PubMed]
- Sarita, P. Analysis of trace elements in blood sera of breast cancer patients by particle induced X-ray emission. J. Radioanal. Nucl. Chem. 2012, 294, 355–361. [Google Scholar] [CrossRef]
- Gholizadeh, N.; Kabiri, Z.; Kakuee, O.; Saleh-Kotahi, M.; Changizi, V.; Fathollahi, V.; Oliaiy, P.; Omranipour, R. Feasibility of breast cancer screening by PIXE analysis of hair. Biol. Trace Elem. Res. 2013, 153, 105–110. [Google Scholar] [CrossRef]
- Rehman, S.; Husnain, S.M. A probable risk factor of female breast cancer: Study on benign and malignant breast tissue samples. Biol. Trace Elem. Res. 2014, 157, 24–29. [Google Scholar] [CrossRef]
- Borges de Araújo, C.G.; Oliveira do Nascimento Holanda, A.; de Souza Rocha, C.V.; Soaresdo Nascimento, A.P.; Simplício Revoredo, C.M.; Borges da Silva, B.; do Nascimento Nogueira, N.; do Nascimento Marreiro, D. Relationship between zincemia, superoxide dismutase activity and marker of oxidative stress in women with breast cancer. Nutr. Hosp. 2015, 32, 785–791. [Google Scholar] [PubMed]
- Karki, K.; Pande, D.; Negi, R.; Khanna, S.; Khanna, R.S.; Khanna, H.D. Correlation of serum toll like receptor 9 and trace elements with lipid peroxidation in the patients of breast diseases. J. Trace Elem. Med. Biol. 2015, 30, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.K.; Kazi, T.G.; Afridi, H.I.; Talpur, F.N. Interaction between carcinogenie and anti-carcinogenic trace elements in the scalp hair samples of different types of Pakistani female cancerpatients. Clin. Chim. Acta 2015, 439, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Finney, L.; Vogt, S.; Fukai, T.; Glesne, D. Copper and angiogenesis: Unravelling a relationship key to cancer progression. Clin. Exp. Pharmacol. Physiol. 2009, 36, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Samuni, A.; Chevion, M.; Czapski, G. Unusual copper-induced sensitization of the biological damage due to superoxide radicals. J. Biol. Chem. 1981, 24, 12632–12635. [Google Scholar] [CrossRef]
- Martin, M.B.; Reiter, R.; Pham, T.; Avellanet, Y.R.; Camara, J.; Lahm, M.; Pentecost, E.; Pratap, K.; Gilmore, B.A.; Divekar, S.; et al. Estrogen-like activity of metals in Mcf-7 breast Cancer cells. Endocrinology 2003, 144, 2425–2436. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Zhang, M.; Xie, P.; Wang, S.; Wang, Y. Comprehensive analysis of cuproptosis-relatedgenes and tumor microenvironment infiltration characterization in breast cancer. Front. Immunol. 2022, 13, 978909. [Google Scholar] [CrossRef]
- Choi, R.; Kim, M.J.; Sohn, I.; Kim, S.; Kim, I.; Ryu, J.M.; Choi, H.J.; Kim, J.M.; Lee, S.K.; Yu, J.; et al. Serum Trace Elements and Their Associations with Breast Cancer Subgroups in Korean Breast Cancer Patients. Nutrients 2018, 11, 37. [Google Scholar] [CrossRef]
- Osredkar, J.; Sustar, N. Copper and zinc, biological role and significance of copper/zinc Imbalance. J. Clin. Toxicol. 2011, S3, 001. [Google Scholar] [CrossRef]
- Gurer-Orhan, H.; Ince, E.; Konyar, D.; Saso, L.; Suzen, S. The Role of Oxidative Stress Modulators in Breast Cancer. Curr. Med. Chem. 2018, 25, 4084–4101. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Cai, Q.; Shu, X.O.; Nechuta, S.J. The role of biomarkers of oxidative stress in brest cancer risk and prognosis: A systematic review of the epidemiologic literature. J. Womens Health 2017, 26, 467–482. [Google Scholar] [CrossRef] [PubMed]
- Mezzetti, A.; Pierdomenico, S.D.; Costantini, F.; Romano, F.; De Cesare, D.; Cuccurullo, F.; Imbastaro, T.; Riario-Sforza, G.; Di Giacomo, F.; Zuliani, G.; et al. Copper/zinc ratio and systemic oxidant load: Effect of aging and aging-related degenerative diseases. Free Radic. Biol. Med. 1998, 25, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zeng, J.W.; Ma, Q.; Zhang, S.; Tang, J.; Feng, J.F. Serum copper and zinc levels in brest cancer: A meta-analysis. J. Trace Elem. Med. Biol. 2020, 62, 126629. [Google Scholar] [CrossRef]
- Fang, A.P.; Chen, P.Y.; Wang, X.Y.; Liu, Z.Y.; Zhang, D.M.; Luo, Y.; Liao, G.-C.; Long, J.-A.; Zhong, R.-H.; Zhou, Z.-G.; et al. Serum copper and zinclevels at diagnosis and hepatocellular carcinoma survival in the Guangdong Liver Cancer Cohort. Int. J. Cancer 2019, 11, 2823–2832. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Suzuki, K.; Sasaki, R.; Otani, M.; Aoki, K. Mortality rates from cancer or all causes and SOD activity level and Zn/Cu ratio in peripheral blood: Population based follow-up study. J. Epidemiol. 2002, 12, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, Y.; Demircan, K.; Rosendahl, A.H.; Borgquist, S.; Sandsveden, M.; Manjer, J. Zinc and breast cancer survival: A prospective cohort study of dietary intake and serum levels. Nutrients 2022, 14, 2575. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, Y.; Demircan, K.; Vallon-Christersson, J.; Malmberg, M.; Saal, L.H.; Rydén, L.; Borg, Å.; Schomburg, L.; Sandsveden, M.; Manjer, J. Serum copper, zinc and copper/zinc ratio in relation to survival after breast cancer diagnosis: A prospective multicenter cohort study. Redox Biol. 2023, 63, 102728. [Google Scholar] [CrossRef]
- Górski, B.; Byrski, T.; Huzarski, T.; Jakubowska, A.; Menkiszak, J.; Gronwald, J.; Pluzańska, A.; Bebenek, M.; Fischer-Maliszewska, L.; Grzybowska, E.; et al. Founder mutations in the BRCA1 gene in Polish families with breast-ovarian cancer. Am. J. Hum. Genet. 2000, 66, 1963–1968. [Google Scholar] [CrossRef]
- Lubiński, J.; Lener, M.R.; Marciniak, W.; Pietrzak, S.; Derkacz, R.; Cybulski, C.; Gronwald, J.; Dębniak, T.; Jakubowska, A.; Huzarski, T.; et al. Serum Essential Elements and Survival after Cancer Diagnosis. Nutrients 2023, 15, 2611. [Google Scholar] [CrossRef]
- Yildiz, A.; Kaya, Y.; Tanriverdi, D. Effect of interaction between selenium and zinc on DNA repair in association with cancer prevention. J. Cancer Prev. 2019, 24, 146–154. [Google Scholar] [CrossRef]
- Guo, C.H.; Chen, P.C.; Yeh, M.S.; Hsiung, D.Y.; Wang, C.L. Cu/Zn ratios are associated with nutritional status, oxidative stress, inflammation, and immune abnormalities in patients on peritoneal dialysis. Clin. Biochem. 2011, 44, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2022, 83, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Morales, S.; Pérez-Amado, C.J.; Langley, E.; Hidalgo-Miranda, A. Overview of mitochondrial germline variants and mutations in human disease: Focus on breast cancer (Review). Int. J. Oncol. 2018, 53, 923–936. [Google Scholar]
- Lutsenko, S. Human copper homeostasis: A network of interconnected pathways. Curr. Opin. Chem. Biol. 2010, 14, 211–217. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Ribeiro, S.M.; Braga, C.B.; Peria, F.M.; Domenici, F.A.; Martinez, E.Z.; Feres, O.; da Rocha, J.J.; da Cunha, S.F. Effect of Zinc Supplementation on Antioxidant Defenses and Oxidative Stress Markers in Patients Undergoing Chemotherapy for Colorectal Cancer: A Placebo-Controlled, Prospective Randomized Trial. Biol. Trace Elem. Res. 2016, 169, 8–16. [Google Scholar] [CrossRef]


| Risk Factor | n | % | Mean Zinc Level [µg/L] | SD | p | Mean Copper Level [µg/L] | SD | p | |
|---|---|---|---|---|---|---|---|---|---|
| All | 538 | 100 | 847.3 | ±129.2 | 1152.0 | ±217.6 | |||
| Age range (mean, SD) | 25–92 (57.2 ± 12.1) | n/a | |||||||
| ≤50 | 140 | 26.0 | 854.4 | ±125.3 | 0.45 | 1143.3 | ±263.1 | 0.58 | |
| >50 | 398 | 74.0 | 844.8 | ±130.6 | 1155.1 | ±199.4 | |||
| BRCA1 mutation | |||||||||
| Yes | 62 | 11.5 | 840.1 | ±115.6 | 0.64 | 1138.1 | ±185.9 | 0.59 | |
| No | 476 | 88.5 | 848.3 | ±130.9 | 1153.9 | ±221.5 | |||
| Tumor size [cm] | |||||||||
| ≤2.0 | 316 | 58.8 | 846.9 | ±127.3 | 0.40 | 1142.1 | ±226.2 | 0.26 | |
| 2.1–5.0 | 185 | 34.4 | 855.8 | ±129.1 | 1161.1 | ±197.4 | |||
| ≥5.1 | 12 | 2.2 | 807.7 | ±131.2 | 1234.3 | ±297.4 | |||
| Missing | 25 | 4.6 | 808.5 | ±149.2 | 1170.9 | ±206.8 | |||
| Lymph node status | |||||||||
| Positive | 194 | 36.1 | 847.1 | ±122.0 | 0.79 | 1190.8 | ±226.0 | 0.002 | |
| Negative | 331 | 61.5 | 850.3 | ±133.2 | 1130.5 | ±211.2 | |||
| Missing | 13 | 2.4 | 774.9 | ±115.2 | 1123.3 | ±178.1 | |||
| ER status | |||||||||
| Positive | 378 | 70.3 | 844.1 | ±129.6 | 0.26 | 1142.7 | ±212.1 | 0.11 | |
| Negative | 152 | 28.3 | 858.0 | ±126.9 | 1176.2 | ±231.7 | |||
| Missing | 8 | 1.4 | 797.2 | ±150.8 | 1131.5 | ±183.6 | |||
| PR status | |||||||||
| Positive | 343 | 63.8 | 844.2 | ±132.0 | 0.33 | 1142.8 | ±212.2 | 0.14 | |
| Negative | 174 | 32.3 | 856.0 | ±124.9 | 1173.1 | ±229.2 | |||
| Missing | 21 | 3.9 | 825.5 | ±118.6 | 1128.5 | ±200.9 | |||
| HER2 status | |||||||||
| Positive | 89 | 16.5 | 864.7 | ±146.6 | 0.20 | 1148.4 | ±219.9 | 0.78 | |
| Negative | 423 | 78.7 | 845.5 | ±125.7 | 1155.5 | ±218.3 | |||
| Missing | 26 | 4.8 | 817.8 | ±118.7 | 1107.8 | ±199.4 | |||
| Hormone therapy; ER(+) | |||||||||
| Yes | 361 | 95.5 | 845.6 | ±128.8 | 0.29 | 1145.1 | ±211.5 | 0.32 | |
| No | 17 | 4.5 | 811.9 | ±145.9 | 1092.9 | ±224.0 | |||
| Radiotherapy | |||||||||
| Yes | 330 | 61.3 | 852.1 | ±130.2 | 0.28 | 1149.7 | ±214.6 | 0.74 | |
| No | 193 | 35.9 | 839.5 | ±126.2 | 1156.1 | ±224.4 | |||
| Missing | 15 | 2.8 | 842.5 | ±149.8 | 1151.6 | ±206.3 | |||
| Chemotherapy | |||||||||
| Yes | 292 | 54.2 | 849.6 | ±126.9 | 0.86 | 1169.5 | ±232.9 | 0.03 | |
| No | 230 | 42.8 | 847.7 | ±131.9 | 1128.7 | ±197.5 | |||
| Missing | 16 | 3.0 | 799.6 | ±130.5 | 1167.4 | ±179.9 | |||
| Type of surgery | |||||||||
| Mastectomy | 356 | 66.2 | 846.8 | ±123.2 | 0.39 | 1158.8 | ±220.7 | 0.14 | |
| Lumpectomy | 163 | 30.3 | 857.2 | ±137.5 | 1128.5 | ±208.3 | |||
| Missing | 19 | 3.5 | 772.5 | ±147.3 | 1228.1 | ±220.6 | |||
| Vital status | |||||||||
| Alive | 372 | 69.1 | 860.4 | ±130.9 | 1139.4 | ±214.9 | |||
| Dead | 166 | 30.9 | 818.0 | ±120.7 | <0.001 | 1180.3 | ±221.5 | 0.04 | |
| Dead of breast cancer | 115 | 21.4 | 815.5 | ±116.4 | 0.003 | 1187.9 | ±239.7 | 0.05 | |
| Dead of other cancers | 13 | 2.4 | 826.8 | ±105.7 | 0.56 | 1122.5 | ±222.0 | 0.62 | |
| Dead of any cancers | 128 | 23.8 | 816.7 | ±115.1 | 0.002 | 1181.3 | ±237.9 | 0.08 | |
| Smoking status | |||||||||
| Yes, current | 116 | 21.6 | 847.8 | ±126.8 | 0.26 | 1188.5 | ±237.4 | 0.05 | |
| Yes, past | 140 | 26.0 | 861.3 | ±135.6 | 1155.0 | ±231.1 | |||
| Never | 273 | 50.7 | 839.6 | ±123.0 | 1130.6 | ±196.5 | |||
| Missing | 9 | 1.7 | 860.0 | ±223.5 | 1287.3 | ±265.6 | |||
| Quartile | Number of Patients | Zinc μg/L | Copper μg/L | Cu/Zn Ratio |
|---|---|---|---|---|
| 1 | 135 | 525.2–762.7 | 658.4–1012.4 | 0.769–1.160 |
| 2 | 134 | 762.8–839.7 | 1012.7–1122.3 | 1.161–1.321 |
| 3 | 134 | 840.2–919.6 | 1122.8–1254.6 | 1.322–1.562 |
| 4 | 135 | 920.3–1498.1 | 1255.3–2153.1 | 1.563–3.003 |
| Risk Factor | All-Cause Mortality | Breast Cancer-Specific Mortality | |||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | ||||
| Age | |||||||
| ≤50 | 1.00 | Reference | 1.00 | Reference | |||
| ≥51 | 1.33 | (0.91–1.93) | 0.14 | 0.96 | (0.64–1.45) | 0.86 | |
| BRCA1 mutation | |||||||
| No | 1.00 | Reference | 1.00 | Reference | |||
| Yes | 1.18 | (0.71–1.94) | 0.52 | 0.70 | (0.36–1.39) | 0.31 | |
| Lymph node status | |||||||
| Negative | 1.00 | Reference | 1.00 | Reference | |||
| Positive | 2.56 | (1.87–3.51) | <0.001 | 3.51 | (2.38–5.18) | <0.001 | |
| ER status | |||||||
| Negative | 1.00 | Reference | 1.00 | Reference | |||
| Positive | 1.18 | (0.82–1.69) | 0.37 | 1.11 | (0.74–1.67) | 0.93 | |
| PR status | |||||||
| Negative | 1.00 | Reference | 1.00 | Reference | |||
| Positive | 1.28 | (0.90–1.83) | 0.17 | 0.98 | (0.65–1.48) | 0.93 | |
| HER2 status | |||||||
| Negative | 1.00 | Reference | 1.00 | Reference | |||
| Positive | 1.06 | (0.70–1.60) | 0.79 | 0.85 | (0.52–1.39) | 0.53 | |
| Tumor size [cm] | |||||||
| 0–1.9 | 1.00 | Reference | 1.00 | Reference | |||
| 2.0–4.9 | 1.94 | (1.38–2.71) | <0.001 | 2.30 | (1.51–3.47) | <0.001 | |
| ≥5.0 | 6.14 | (3.15–11.9) | <0.001 | 7.75 | (3.62–16.6) | <0.001 | |
| Radiotherapy | |||||||
| No | 1.00 | Reference | 1.00 | Reference | |||
| Yes | 0.95 | (0.68–1.32) | 0.40 | 0.84 | (0.56–1.27) | 0.42 | |
| Chemotherapy | |||||||
| No | 1.00 | Reference | 1.00 | Reference | |||
| Yes | 1.27 | (0.91–1.96) | 0.15 | 1.78 | (1.17–2.67) | 0.007 | |
| Type of surgery | |||||||
| Lumpectomy | 1.00 | Reference | 1.00 | Reference | |||
| Mastectomy | 1.99 | (1.34–2.96) | <0.001 | 2.24 | (1.36–3.70) | 0.001 | |
| Hormone therapy (only ER positive) | |||||||
| No | 1.00 | Reference | 1.00 | Reference | |||
| Yes | 1.03 | (0.42–2.53) | 0.94 | 1.22 | (0.44–3.33) | 0.70 | |
| Smoking | |||||||
| Never | 1.00 | Reference | 1.00 | Reference | |||
| Yes, past | 0.82 | (0.56–1.19) | 0.52 | 0.77 | (0.49–1.21) | 0.26 | |
| Yes, current | 0.88 | (0.59–1.30) | 0.88 | 0.76 | (0.42–1.16) | 0.17 | |
| Zinc quartile | |||||||
| Quartile 4 | 1.00 | Reference | 1.00 | Reference | |||
| Quartile 1 | 1.91 | (1.23–2.98) | 0.004 | 1.98 | (1.16–3.38) | 0.01 | |
| Quartile 2 | 1.56 | (0.99–2.47) | 0.06 | 1.59 | (0.91–2.76) | 0.10 | |
| Quartile 3 | 1.20 | (0.74–1.93) | 0.46 | 1.25 | (0.70–2.22) | 0.45 | |
| Copper quartile | |||||||
| Quartile 1 | 1.00 | Reference | 1.00 | Reference | |||
| Quartile 2 | 1.18 | (0.73–1.89) | 0.50 | 0.94 | (0.52–1.67) | 0.82 | |
| Quartile 3 | 1.47 | (0.93–2.31) | 0.10 | 1.31 | (0.76–2.24) | 0.33 | |
| Quartile 4 | 1.87 | (1.20–2.90) | 0.005 | 1.85 | (1.11–3.08) | 0.02 | |
| Copper/Zinc ratio quartile | |||||||
| Quartile 1 | 1.00 | Reference | 1.00 | Reference | |||
| Quartile 2 | 1.46 | (0.91–2.35) | 0.12 | 1.08 | (0.61–1.91) | 0.79 | |
| Quartile 3 | 1.10 | (0.67–1.82) | 0.70 | 0.87 | (0.48–1.59) | 0.66 | |
| Quartile 4 | 2.72 | (1.75–4.21) | <0.001 | 2.55 | (1.55–4.19) | <0.001 | |
| Risk Factor | All-Cause Mortality | Breast Cancer-Specific Mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| At Risk (n) | Events (n) | HR (95% CI) | p a | Events (n) | HR (95% CI) | p b | ||||
| Zinc quartile | ||||||||||
| Quartile 4 | 135 | 31 | 1.00 | Reference | 21 | 1.00 | Reference | |||
| Quartile 1 | 134 | 53 | 1.74 | (1.07–2.81) | 0.02 | 37 | 1.85 | (1.02–3.37) | 0.04 | |
| Quartile 2 | 134 | 45 | 1.48 | (0.91–2.41) | 0.11 | 31 | 1.512 | (0.83–2.78) | 0.18 | |
| Quartile 3 | 135 | 37 | 1.12 | (0.68–1.85) | 0.65 | 26 | 1.11 | (0.60–2.07) | 0.74 | |
| Copper quartile | ||||||||||
| Quartile 1 | 135 | 32 | 1.00 | Reference | 24 | 1.00 | Reference | |||
| Quartile 2 | 134 | 37 | 1.15 | (0.70–1.91) | 0.58 | 22 | 0.97 | (0.52–1.80) | 0.91 | |
| Quartile 3 | 134 | 45 | 1.34 | (0.82–2.18) | 0.23 | 30 | 1.14 | (0.63–2.06) | 0.67 | |
| Quartile 4 | 135 | 52 | 1.49 | (0.92–2.42) | 0.10 | 39 | 1.37 | (0.77–2.44) | 0.28 | |
| Copper/zinc ratio quartile | ||||||||||
| Quartile 1 | 135 | 29 | 1.00 | Reference | 23 | 1.00 | Reference | |||
| Quartile 2 | 134 | 41 | 1.56 | (0.93–2.62) | 0.09 | 24 | 1.18 | (0.63–2.20) | 0.61 | |
| Quartile 3 | 134 | 32 | 1.37 | (0.80–2.34) | 0.25 | 20 | 1.07 | (0.56–2.07) | 0.83 | |
| Quartile 4 | 135 | 64 | 2.26 | (1.38–3.69) | 0.001 | 48 | 2.07 | (1.17–3.66) | 0.01 | |
| Risk Factor | Overall Survival (OS) | Breast Cancer-Specific Survival | |||
|---|---|---|---|---|---|
| 10 Year (%) | Log-Rank Test | 10 Year (%) | Log-Rank Test | ||
| All | 73.9 | p | 80.5 | p | |
| Zinc quartile | |||||
| Quartile 1 | 64.9 | 0.006 a | 73.8 | 0.04 b | |
| Quartile 2 | 72.2 | 80.3 | |||
| Quartile 3 | 78.2 | 83.7 | |||
| Quartile 4 | 79.9 | 84.3 | |||
| Zinc quartile | |||||
| Quartile 2–4 | 76.8 | 0.01 | 82.7 | 0.03 | |
| Quartile 1 | 64.9 | 73.8 | |||
| Copper quartile | |||||
| Quartile 1 | 80.6 | 0.03 c | 84.8 | 0.02 d | |
| Quartile 2 | 75.2 | 83.4 | |||
| Quartile 3 | 72.9 | 79.7 | |||
| Quartile 4 | 66.5 | 74.1 | |||
| Copper quartile | |||||
| Quartile 1–3 | 76.3 | 0.01 | 82.7 | 0.007 | |
| Quartile 4 | 66.5 | 74.1 | |||
| Copper/zinc ratio quartile | |||||
| Quartile 1 | 82.1 | <0.001 e | 84.9 | <0.001 f | |
| Quartile 2 | 75.3 | 83.5 | |||
| Quartile 3 | 79.8 | 86.2 | |||
| Quartile 4 | 58.3 | 67.2 | |||
| Copper/zinc ratio quartile | |||||
| Quartile 1–3 | 79.0 | <0.001 | 84.9 | <0.001 | |
| Quartile 4 | 58.3 | 67.2 | |||
| Risk Factor | All-Cause Mortality | Breast Cancer-Specific Mortality | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) Multivariate Analysis | p | 10-Year Survival (%) | p | HR (95% CI) Multivariate Analysis | p | 10-Year Survival (%) | p | ||||
| Zinc quartile | |||||||||||
| Quartile 4 | 1.00 | Reference | 79.9 | 0.004 | 1.00 | Reference | 84.3 | 0.009 | |||
| Quartile 1 | 1.74 | (1.07–2.81) | 0.02 | 64.9 | 1.85 | (1.02–3.37) | 0.04 | 73.8 | |||
| Copper/zinc ratio quartile | |||||||||||
| Quartile 1 | 1.00 | Reference | 82.1 | <0.001 | 1.00 | Reference | 84.9 | <0.001 | |||
| Quartile 4 | 2.26 | (1.38–3.69) | 0.001 | 58.3 | 2.07 | (1.17–3.66) | 0.01 | 67.2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwiec, M.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Dębniak, T.; Jakubowska, A.; Lener, M.R.; Falco, M.; et al. Serum Levels of Copper and Zinc and Survival in Breast Cancer Patients. Nutrients 2024, 16, 1000. https://doi.org/10.3390/nu16071000
Szwiec M, Marciniak W, Derkacz R, Huzarski T, Gronwald J, Cybulski C, Dębniak T, Jakubowska A, Lener MR, Falco M, et al. Serum Levels of Copper and Zinc and Survival in Breast Cancer Patients. Nutrients. 2024; 16(7):1000. https://doi.org/10.3390/nu16071000
Chicago/Turabian StyleSzwiec, Marek, Wojciech Marciniak, Róża Derkacz, Tomasz Huzarski, Jacek Gronwald, Cezary Cybulski, Tadeusz Dębniak, Anna Jakubowska, Marcin R. Lener, Michał Falco, and et al. 2024. "Serum Levels of Copper and Zinc and Survival in Breast Cancer Patients" Nutrients 16, no. 7: 1000. https://doi.org/10.3390/nu16071000
APA StyleSzwiec, M., Marciniak, W., Derkacz, R., Huzarski, T., Gronwald, J., Cybulski, C., Dębniak, T., Jakubowska, A., Lener, M. R., Falco, M., Kładny, J., Baszuk, P., Kotsopoulos, J., Narod, S. A., & Lubiński, J. (2024). Serum Levels of Copper and Zinc and Survival in Breast Cancer Patients. Nutrients, 16(7), 1000. https://doi.org/10.3390/nu16071000







