Glycomacropeptide as an Efficient Agent to Fight Pathophysiological Mechanisms of Metabolic Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Glycomacropeptide
2.2. Animals
2.3. Glucose and Lipid Homeostasis
2.4. Analytical Methods
2.5. Histological Analyses
2.6. RNA Isolation, Reverse Transcription and Quantitative PCR Analyses
2.7. Western Blot Analysis
2.8. Glutathione Peroxidase (GPx) Activity
2.9. Free Fatty Acid Analysis
2.10. Metagenomic DNA Extraction
2.11. 16S rRNA V6 Library Construction for Ion Torrent Sequencing and Analysis
2.12. Short Chain Fatty Acid Analysis
2.13. General Statistical Analysis
2.14. Microbiome Statistical Analysis
3. Results
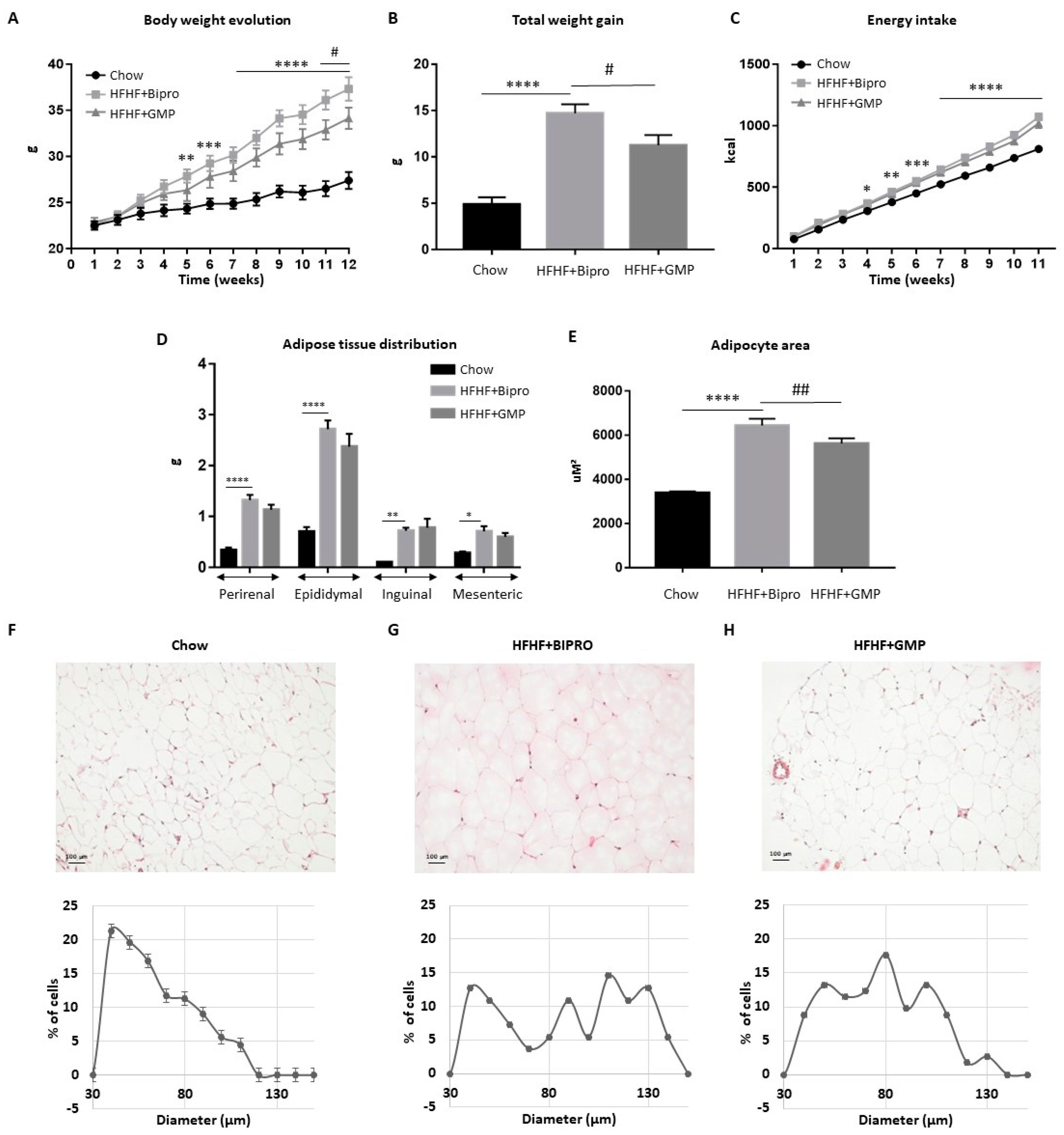
3.1. GMP Supplementation Prevents Excessive Weight Gain and Adipocyte Hypertrophy
3.2. GMP Supplementation Alleviates Systemic Insulin Resistance and Inflammation
3.3. GMP Impact on Gut Morphology and Permeability
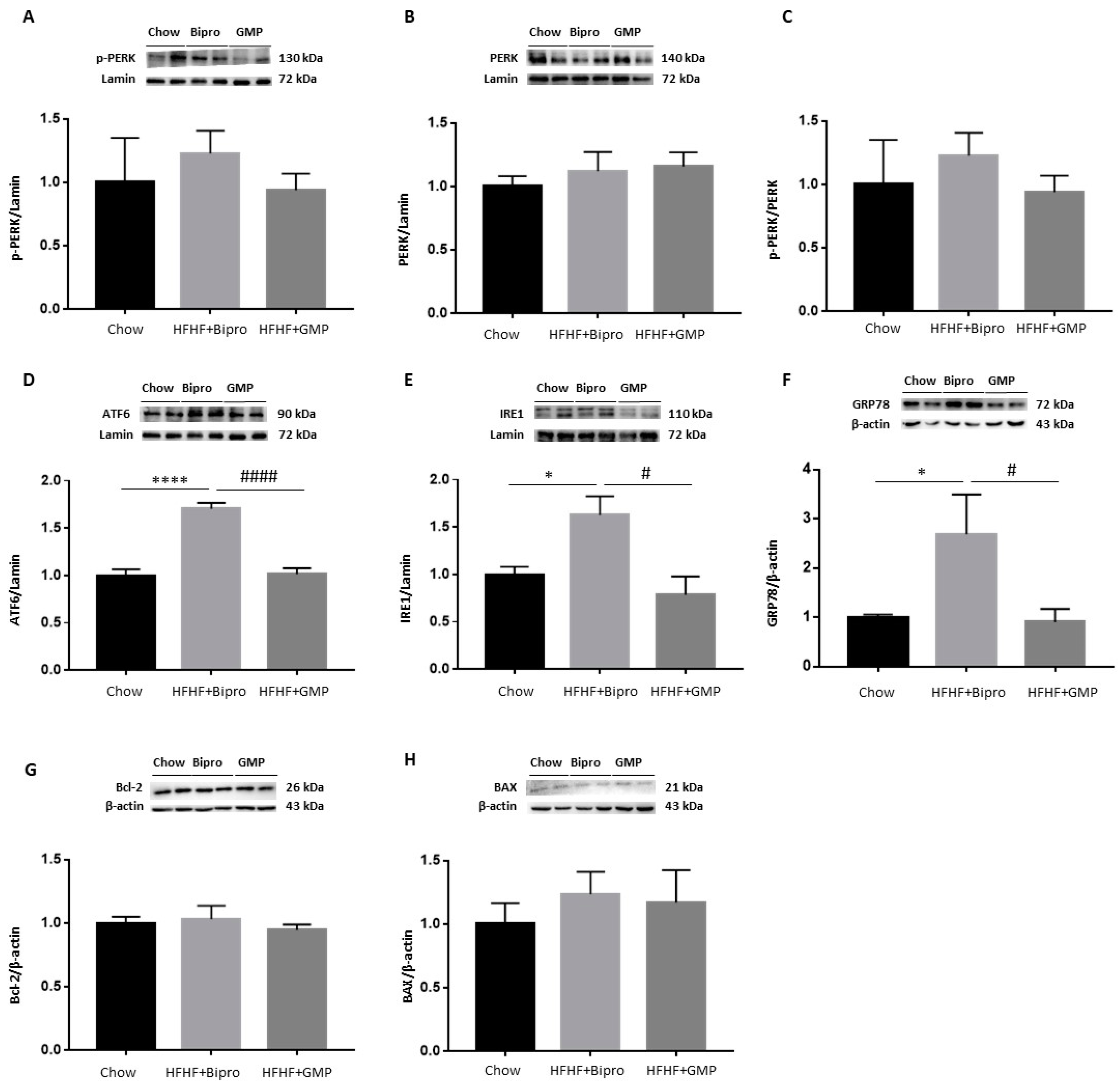
3.4. GMP Fights Gut Inflammation, Abnormal Redox State and ER Stress
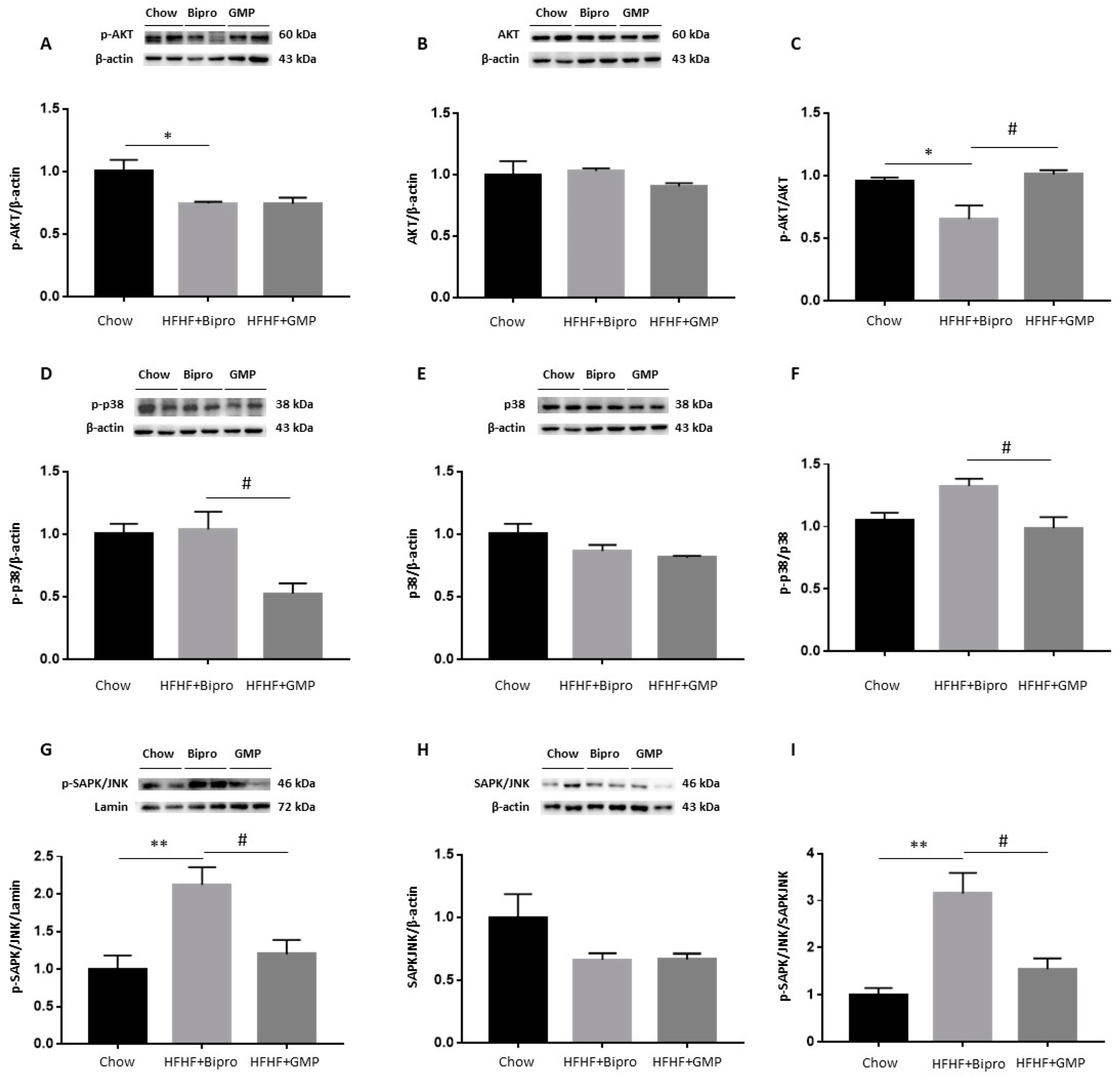
3.5. Attenuation of Intestinal Insulin Resistance by GMP
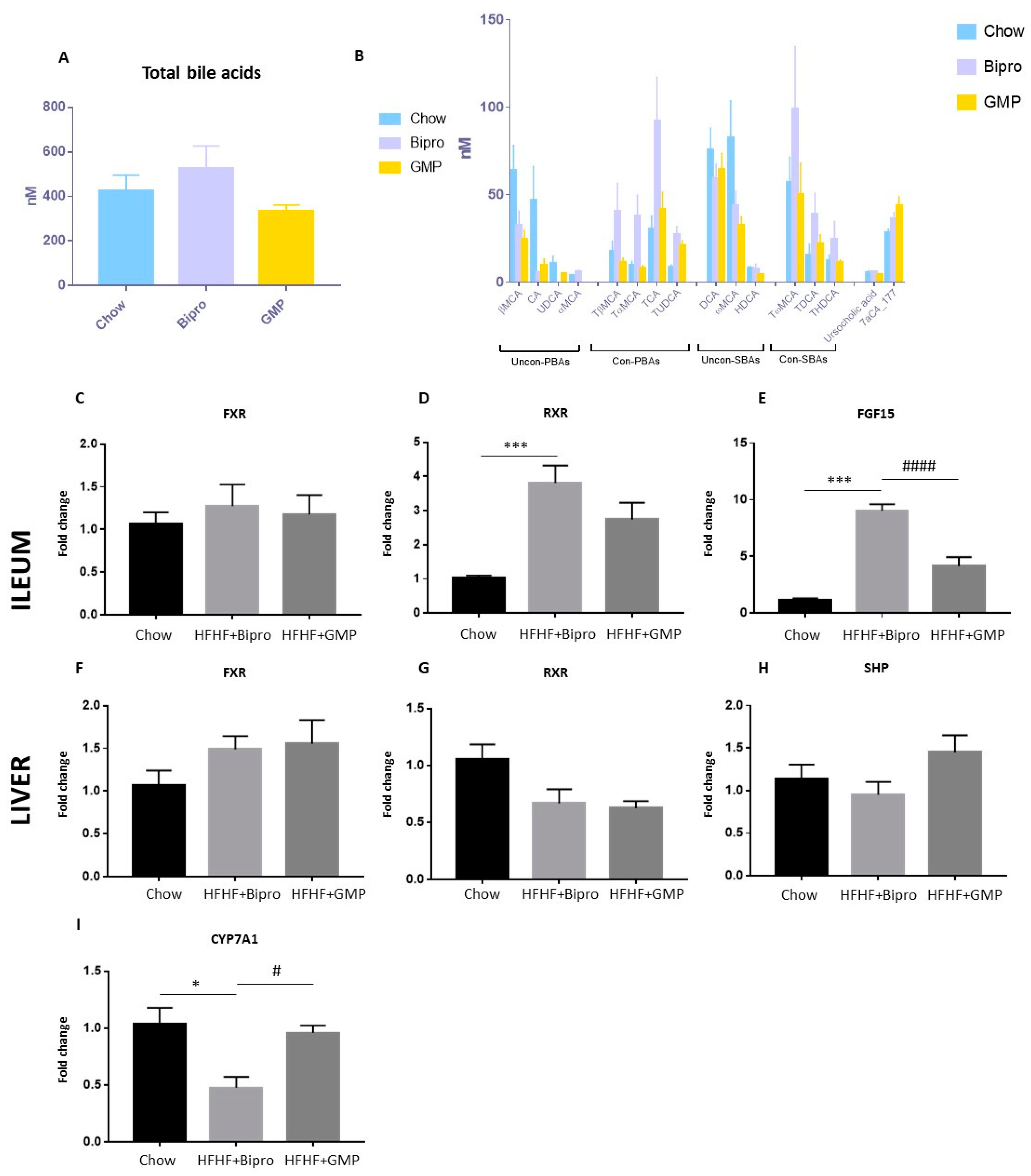
3.6. Bile Acid Metabolism Shift in Gut–Liver Axis in Response to GMP Administration
3.7. Impact of GMP on NAFLD
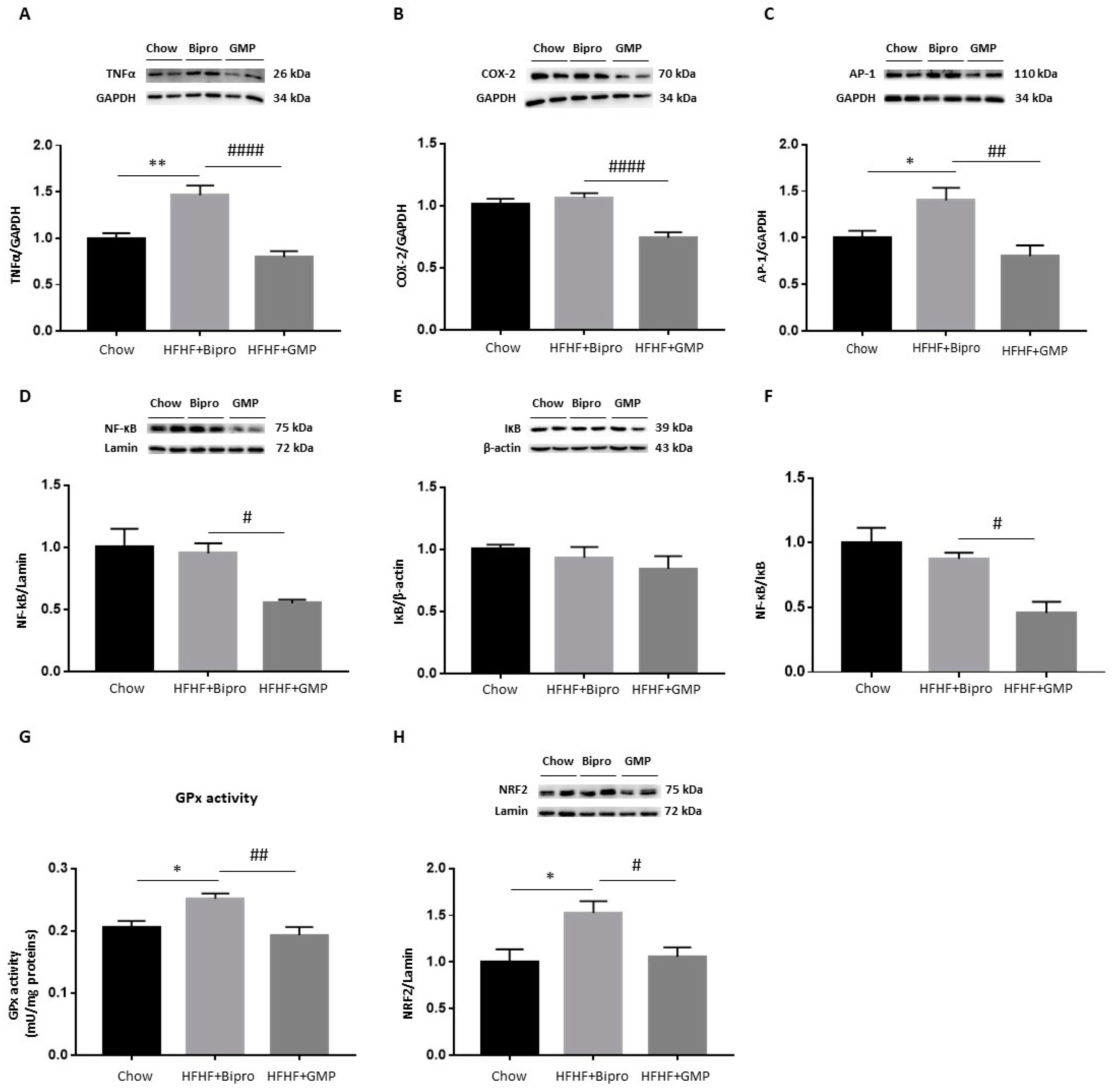
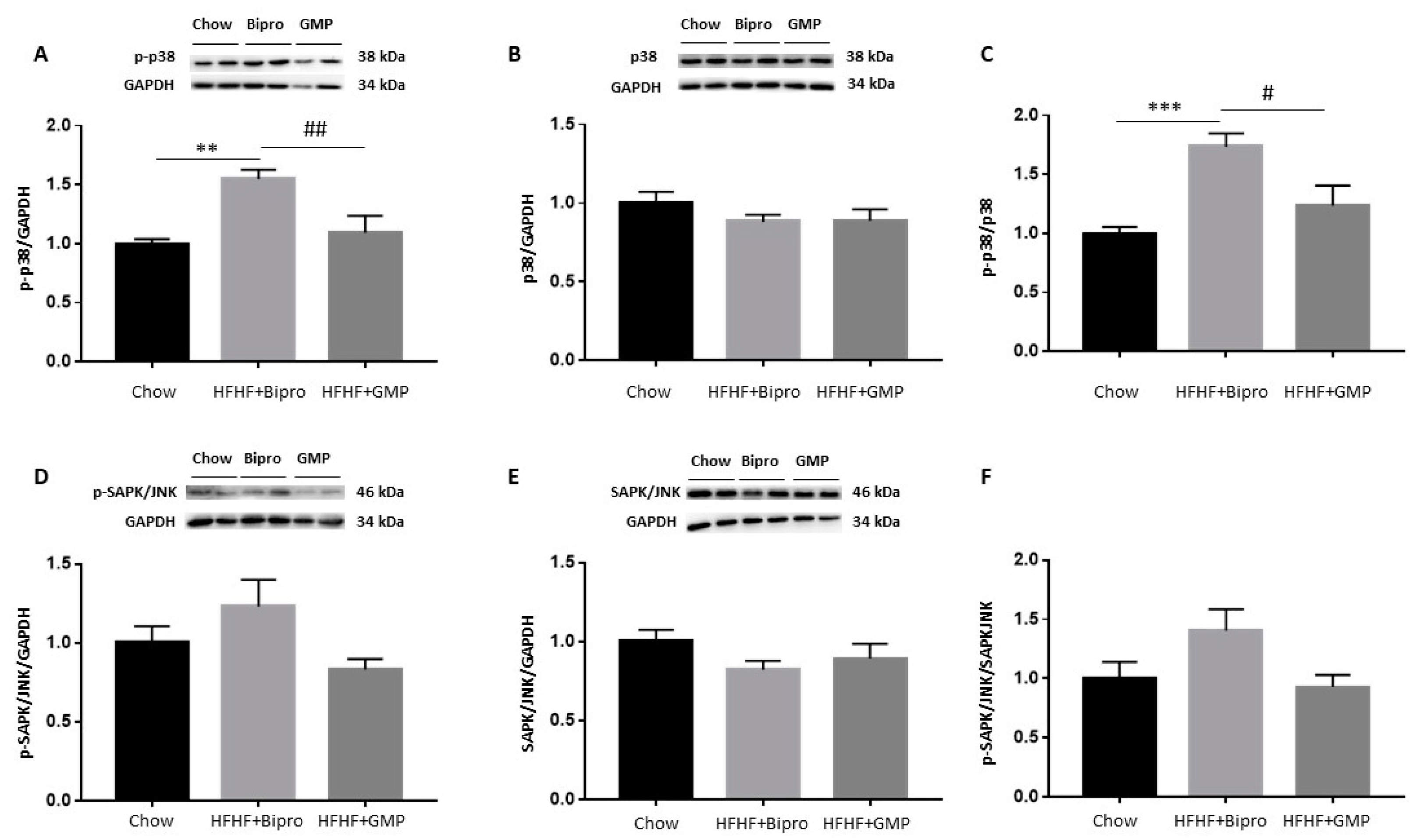
3.8. GMP Attenuates Hepatic Inflammation and OxS
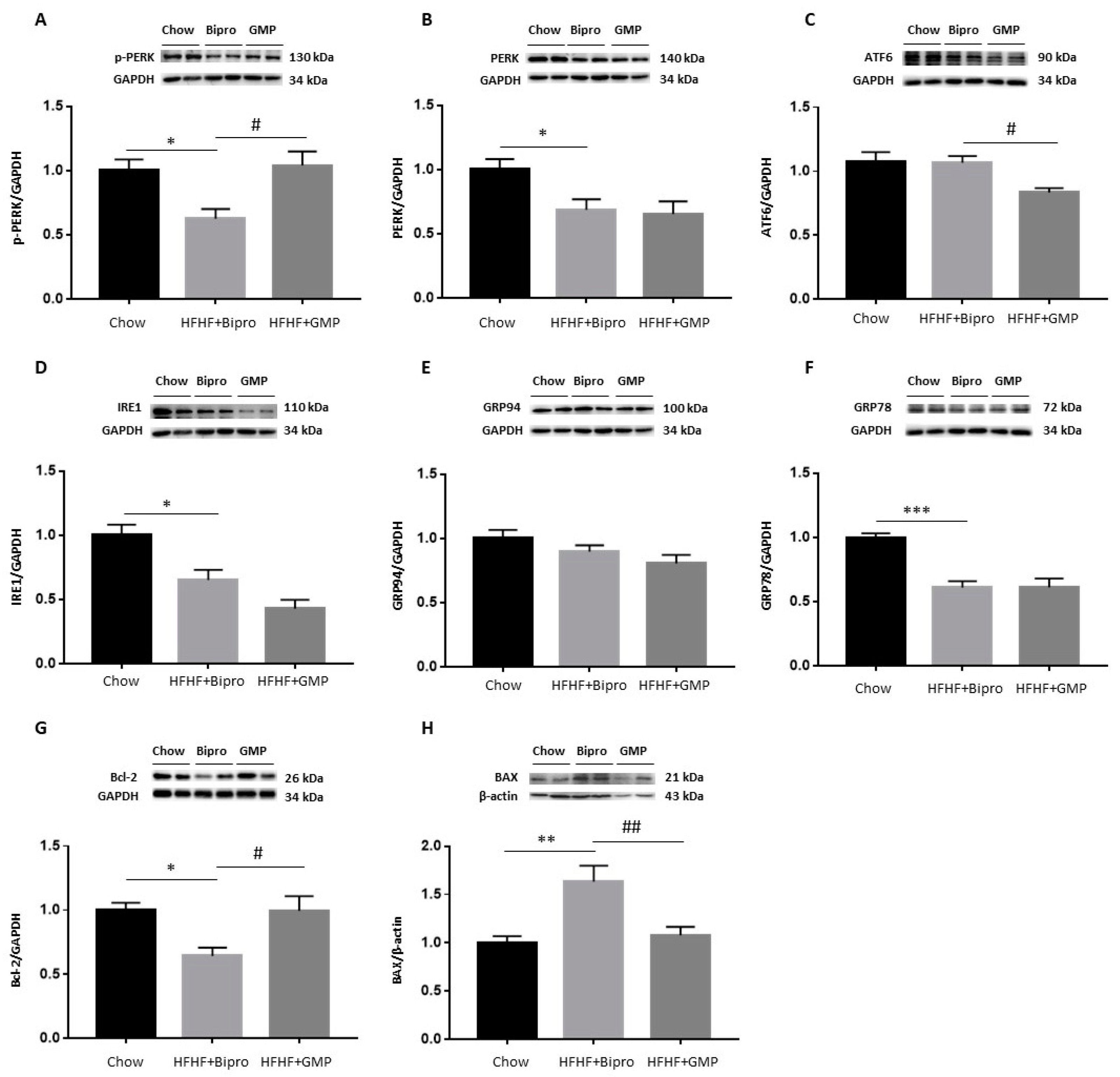
3.9. Regulation of ER Stress and Apoptosis in the Liver by GMP
3.10. Modulation of Insulin Signalling Cascade by GMP in the Liver
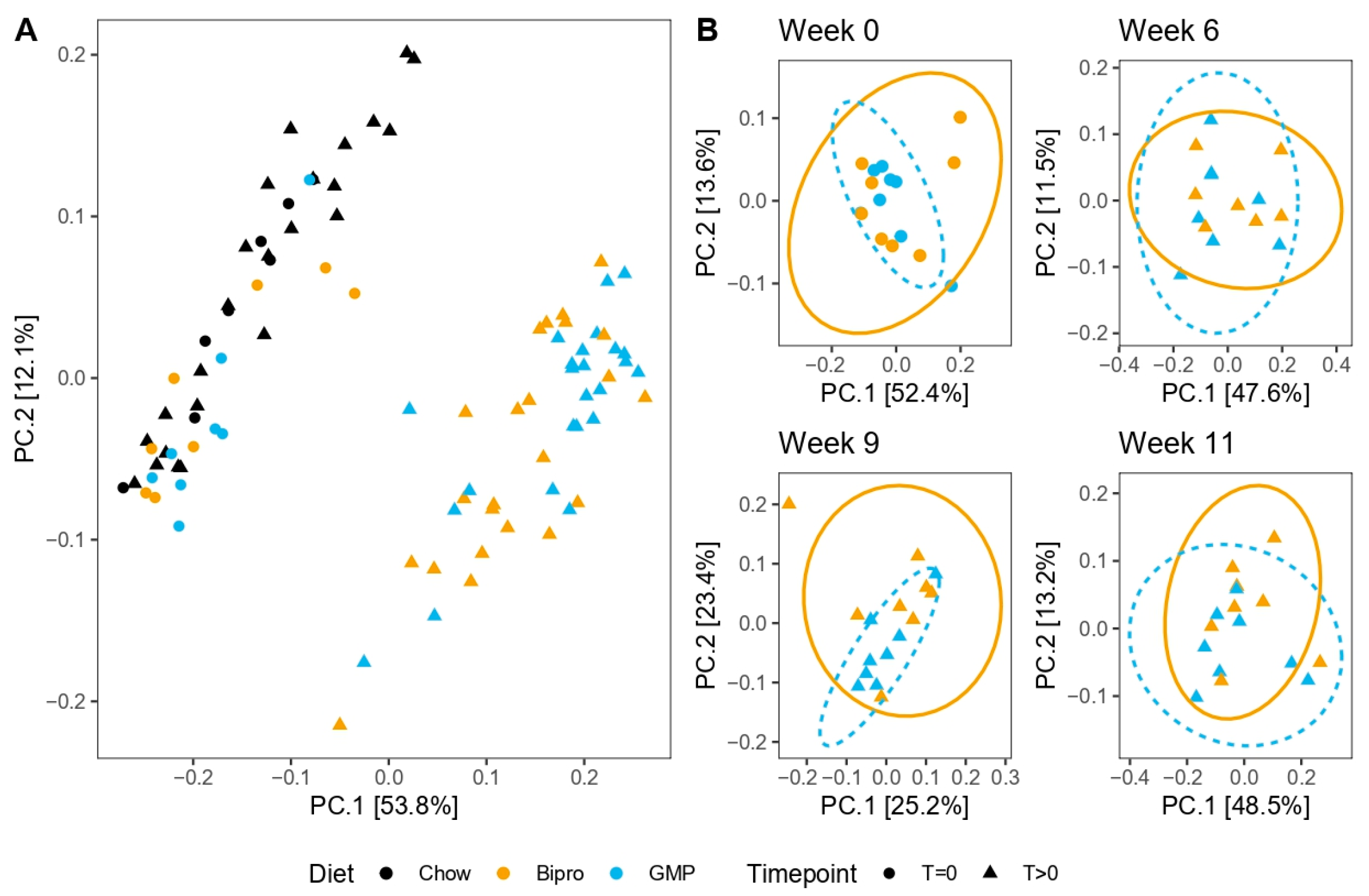
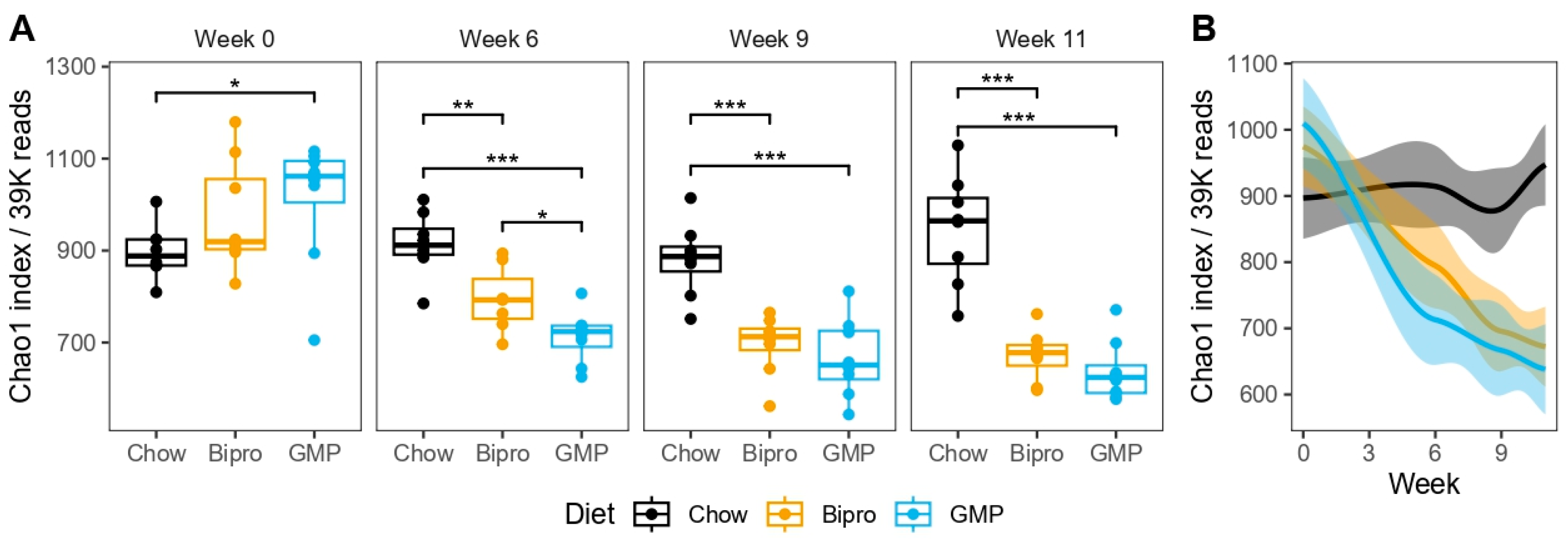
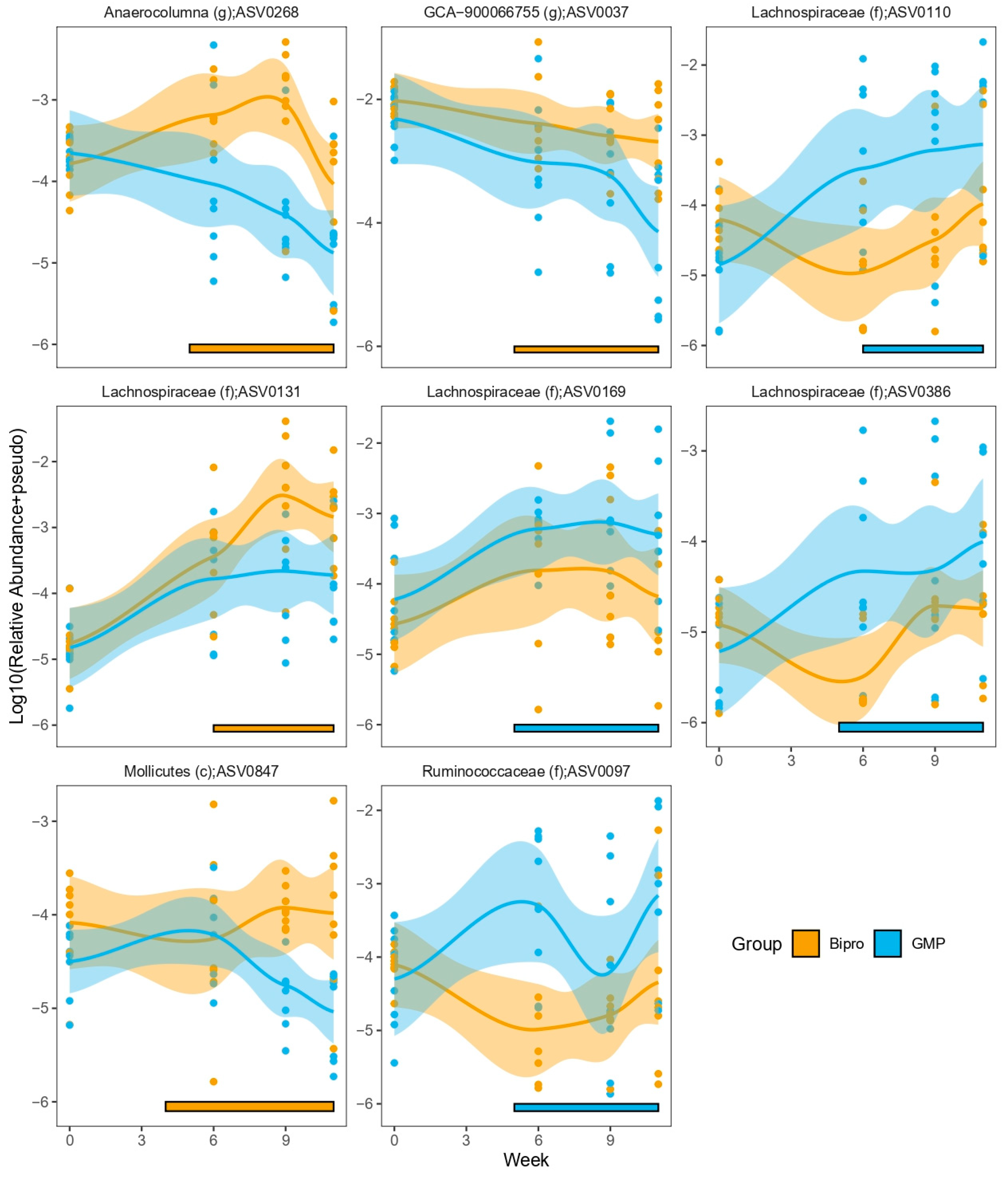
3.11. HFHF Diet Dramatically Impacts Microbiota Composition, While GMP Has Subtle Effects on Microbial Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Geographic distribution of metabolic syndrome and its components in the general adult pop-ulation: A meta-analysis of global data from 28 million individuals. Diabetes Res. Clin. Pract. 2022, 188, 109924. [Google Scholar] [CrossRef]
- Saklayen, M.G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Mendrick, D.L.; Diehl, A.M.; Topor, L.S.; Dietert, R.R.; Will, Y.; La Merrill, M.A.; Bouret, S.; Varma, V.; Hastings, K.L.; Schug, T.T.; et al. Metabolic syndrome and associated diseases: From the bench to the clinic. Toxicol. Sci. 2018, 162, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Lillich, F.F.; Imig, J.D.; Proschak, E. Multi-target approaches in metabolic syndrome. Front. Pharmacol. 2021, 11, 554961. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Boba, A.; Czemplik, M.; Kulma, A.; Wojtasik, W. Influence of the bioactive diet components on the gene expression regulation. Nutrients 2021, 13, 3673. [Google Scholar] [CrossRef]
- Pandita, D.; Pandita, A. Omics Technology for the Promotion of Nutraceuticals and Functional Foods. Front. Physiol. 2022, 13, 817247. [Google Scholar] [CrossRef]
- Samtiya, M.; Samtiya, S.; Badgujar, P.C.; Puniya, A.K.; Dhewa, T.; Aluko, R.E. Health-Promoting and Therapeutic Attributes of Milk-Derived Bioactive Peptides. Nutrients 2022, 14, 3001. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, D.; Mohapatra, S.; Misra, S.; Sahu, P. Milk derived bioactive peptides and their impact on human health—A review. Saudi J. Biol. Sci. 2016, 23, 577–583. [Google Scholar] [CrossRef]
- Singh, K.S.; Singh, B.P.; Rokana, N.; Singh, N.; Kaur, J.; Singh, A.; Panwar, H. Bio-therapeutics from human milk: Prospects and perspectives. J. Appl. Microbiol. 2021, 131, 2669–2687. [Google Scholar] [CrossRef]
- Nielsen, S.D.-H.; Liang, N.; Rathish, H.; Kim, B.J.; Lueangsakulthai, J.; Koh, J.; Qu, Y.; Schulz, H.-J.; Dallas, D.C. Bioactive milk peptides: An updated comprehensive overview and database. Crit. Rev. Food Sci. Nutr. 2023, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Foisy-Sauvé, M.; Ahmarani, L.; Delvin, E.; Sané, A.T.; Spahis, S.; Levy, E. Glycomacropeptide Prevents Iron/Ascorbate-Induced Oxidative Stress, Inflammation and Insulin Sensitivity with an Impact on Lipoprotein Production in Intestinal Caco-2/15 Cells. Nutrients 2020, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Sauvé, M.F.; Feldman, F.; Koudoufio, M.; Ould-Chikh, N.-E.-H.; Ahmarani, L.; Sane, A.; N’timbane, T.; El-Jalbout, R.; Patey, N.; Spahis, S.; et al. Glycomacropeptide for Management of Insulin Resistance and Liver Metabolic Perturbations. Biomedicines 2021, 9, 1140. [Google Scholar] [CrossRef] [PubMed]
- Fändriks, L. Roles of the gut in the metabolic syndrome: An overview. J. Intern. Med. 2017, 281, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.C.; Daoust, L.; Boutkrabt, L.; Pilon, G.; Varin, T.; Dudonné, S.; Levy, É.; Marette, A.; Roy, D.; Desjardins, Y. Wild blueberry proanthocyanidins shape distinct gut microbiota profile and influence glucose homeostasis and intestinal phenotypes in high-fat high-sucrose fed mice. Sci. Rep. 2020, 10, 2217. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Marzullo, P.; Di Renzo, L.; Pugliese, G.; De Siena, M.; Barrea, L.; Muscogiuri, G.; Colao, A.; Savastano, S.; Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. From obesity through gut microbiota to cardiovascular diseases: A dangerous journey. Int. J. Obes. Suppl. 2020, 10, 35–49. [Google Scholar] [CrossRef]
- Wang, P.-X.; Deng, X.-R.; Zhang, C.-H.; Yuan, H.-J. Gut microbiota and metabolic syndrome. Chin. Med. J. 2020, 133, 808–816. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Heimann, E.; Nyman, M.; Pålbrink, A.-K.; Lindkvist-Petersson, K.; Degerman, E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef]
- Brück, W.M.; Redgrave, M.; Tuohy, K.M.; Lönnerdal, B.; Graverholt, G.; Hernell, O.; Gibson, G.R. Effects of bovine α-lactalbumin and casein glycomacropeptide–enriched infant formulae on faecal microbiota in healthy term infants. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 673–679. [Google Scholar] [CrossRef]
- Chabance, B.; Marteau, P.; Rambaud, J.; Migliore-Samour, D.; Boynard, M.; Perrotin, P.; Guillet, R.; Jollès, P.; Fiat, A. Casein peptide release and passage to the blood in humans during digestion of milk or yogurt. Biochimie 1998, 80, 155–165. [Google Scholar] [CrossRef]
- O’Riordan, N.; Kane, M.; Joshi, L.; Hickey, R.M. Structural and functional characteristics of bovine milk protein glycosylation. Glycobiology 2014, 24, 220–236. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhan, B.; Chang, R.; Du, M.; Mao, X. Antidiabetic effect of casein glycomacropeptide hydrolysates on high-fat diet and STZ-induced diabetic mice via regulating insulin signaling in skeletal muscle and modulating gut microbiota. Nutrients 2020, 12, 220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, C.; Barbier, O.; Smalling, R.; Tsuchiya, H.; Lee, S.; Delker, D.; Zou, A.; Hagedorn, C.H.; Wang, L. Bcl2 is a critical regulator of bile acid homeostasis by dictating Shp and lncRNA H19 function. Sci. Rep. 2016, 6, 20559. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, G.R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959, 234, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Auclair, N.; Sané, A.T.; Ahmarani, L.; Ould-Chikh, N.-E.; Patey, N.; Beaulieu, J.-F.; Delvin, E.; Spahis, S.; Levy, E. High-fat diet reveals the impact of Sar1b defects on lipid and lipoprotein profile and cholesterol metabolism. J. Lipid Res. 2023, 64, 100423. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, M.; Campion, J.; Muñoz-Barrutia, A.; Boqué, N.; Moreno, H.; Martínez, J.A.; Milagro, F.; Ortiz-De-Solórzano, C. Adiposoft: Automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.W.; Lee, M.; Song, J.W.; Kim, K.; Lee, J.; Yang, J.; Lee, S.H.; Kim, I.Y.; Choi, J.-H.; Seong, J.K. Fas mutation reduces obesity by increasing IL-4 and IL-10 expression and promoting white adipose tissue browning. Sci. Rep. 2020, 10, 12001. [Google Scholar] [CrossRef] [PubMed]
- Parlee, S.D.; Lentz, S.I.; Mori, H.; MacDougald, O.A. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014, 537, 93–122. [Google Scholar] [PubMed]
- Spahis, S.; Vanasse, M.; Bélanger, S.A.; Ghadirian, P.; Grenier, E.; Levy, E. Lipid profile, fatty acid composition and pro- and anti-oxidant status in pediatric patients with attention-deficit/hyperactivity disorder. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Butcher, J.; Kearns, M.M.; Lamba, S.; Liang, J.; Stintzi, A.; Ismail, N. Effects of pair-housing pubertal and adult male and female mice on LPS-induced age-dependent immune responses: A potential role for the gut microbiota. Brain Behav. Immun. 2023, 110, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample in-ference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Mirarab, S.; Nguyen, N.; Warnow, T. SEPP: SATé-enabled phylogenetic placement. In Biocomputing 2012; World Scientific: Singapore, 2012; pp. 247–258. [Google Scholar]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef]
- Davis, N.M.; Proctor, D.M.; Holmes, S.P.; Relman, D.A.; Callahan, B.J. Simple statistical identification and removal of con-taminant sequences in marker-gene and metagenomics data. Microbiome 2018, 6, 226. [Google Scholar] [CrossRef]
- Oksanen, J. Vegan: Community Ecology Package. 2010. Available online: http://vegan.r-forge.r-project.org/ (accessed on 18 September 2023).
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Paulson, J.N.; Stine, O.C.; Bravo, H.C.; Pop, M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef]
- Lee, J.; Ozcan, U. Unfolded protein response signaling and metabolic diseases. J. Biol. Chem. 2014, 289, 1203–1211. [Google Scholar] [CrossRef]
- Xu, S.-P.; Mao, X.-Y.; Cheng, X.; Chen, B. Ameliorating effects of casein glycomacropeptide on obesity induced by high-fat diet in male Sprague-Dawley rats. Food Chem. Toxicol. 2013, 56, 1–7. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, H.; Zhu, C.; Yan, Y.; Pang, G. Anti-apoptotic effects of milk-derived casein glycomacropeptide on mice with ulcerative colitis. Food Agric. Immunol. 2014, 25, 453–466. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, C.; Ming, Z.; Cao, J.; Yan, Y.; Zhao, P.; Pang, G.; Deng, Z.; Yao, Y.; Chen, Q. Molecular mechanisms by which casein glycomacropeptide maintains internal homeostasis in mice with experimental ulcerative colitis. PLoS ONE 2017, 12, e0181075. [Google Scholar] [CrossRef] [PubMed]
- Després, J.-P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Han, T.S.; Lean, M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016, 5, 2048004016633371. [Google Scholar] [CrossRef]
- Xu, S.; Mao, X.; Ren, F.; Che, H. Attenuating effect of casein glycomacropeptide on proliferation, differentiation, and lipid accumulation of in vitro Sprague-Dawley rat preadipocytes. J. Dairy Sci. 2011, 94, 676–683. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef] [PubMed]
- Lemmer, I.L.; Willemsen, N.; Hilal, N.; Bartelt, A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol. Metab. 2021, 47, 101169. [Google Scholar] [CrossRef] [PubMed]
- Lebeaupin, C.; Vallée, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef] [PubMed]
- Ghezzal, S.; Postal, B.G.; Quevrain, E.; Brot, L.; Seksik, P.; Leturque, A.; Thenet, S.; Carrière, V. Palmitic acid damages gut epithelium integrity and initiates inflammatory cytokine production. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2020, 1865, 158530. [Google Scholar] [CrossRef] [PubMed]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Rutkowski, D.T. Liver function and dysfunction—A unique window into the physiological reach of ER stress and the unfolded protein response. FEBS J. 2019, 286, 356–378. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, D.T.; Kaufman, R.J. That which does not kill me makes me stronger: Adapting to chronic ER stress. Trends Biochem. Sci. 2007, 32, 469–476. [Google Scholar] [CrossRef]
- Hetz, C. The unfolded protein response: Controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Alam Riaz, T.; Kim, H.-R.; Chae, H.-J. The aftermath of the interplay between the endoplasmic reticulum stress response and redox signaling. Exp. Mol. Med. 2021, 53, 151–167. [Google Scholar] [CrossRef]
- Sasako, T.; Ohsugi, M.; Kubota, N.; Itoh, S.; Okazaki, Y.; Terai, A.; Kubota, T.; Yamashita, S.; Nakatsukasa, K.; Kamura, T.; et al. Hepatic Sdf2l1 controls feeding-induced ER stress and regulates metabolism. Nat. Commun. 2019, 10, 947. [Google Scholar] [CrossRef]
- Guillou, H.; Zadravec, D.; Martin, P.G.; Jacobsson, A. The key roles of elongases and desaturases in mammalian fatty acid metabolism: Insights from transgenic mice. Prog. Lipid Res. 2010, 49, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.E.; Murali, S.; Chaves, I.Z.; Suen, G.; Ney, D.M. Glycomacropeptide Impacts Amylin-Mediated Satiety, Postprandial Markers of Glucose Homeostasis, and the Fecal Microbiome in Obese Postmenopausal Women. J. Nutr. 2023, 153, 1915–1929. [Google Scholar] [CrossRef] [PubMed]
- Montanari, C.; Ceccarani, C.; Corsello, A.; Zuvadelli, J.; Ottaviano, E.; Cas, M.D.; Banderali, G.; Zuccotti, G.; Borghi, E.; Verduci, E. Glycomacropeptide Safety and Its Effect on Gut Microbiota in Patients with Phenylketonuria: A Pilot Study. Nutrients 2022, 14, 1883. [Google Scholar] [CrossRef] [PubMed]
- Wernlund, P.G.; Hvas, C.L.; Dahlerup, J.F.; Bahl, M.I.; Licht, T.R.; Knudsen, K.E.B.; Agnholt, J.S. Casein glycomacropeptide is well tolerated in healthy adults and changes neither high-sensitive C-reactive protein, gut microbiota nor faecal butyrate: A restricted randomised trial. Br. J. Nutr. 2021, 125, 1374–1385. [Google Scholar] [CrossRef] [PubMed]
- Ntemiri, A.; Ribière, C.; Stanton, C.; Ross, R.P.; O’connor, E.M.; O’toole, P.W. Retention of microbiota diversity by lactose-free milk in a mouse model of elderly gut microbiota. J. Agric. Food Chem. 2019, 67, 2098–2112. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Shuai, M.; Zhang, Z.; Jiang, Z.; Fu, Y.; Shen, L.; Zheng, J.-S.; Chen, Y.-M. Temporal relationship among adiposity, gut microbiota, and insulin resistance in a longitudinal human cohort. BMC Med. 2022, 20, 171. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The controversial role of human gut lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Meehan, C.J.; Beiko, R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Sobh, M.; Montroy, J.; Daham, Z.; Sibbald, S.; Lalu, M.; Stintzi, A.; Mack, D.; A Fergusson, D. Tolerability and SCFA production after resistant starch supplementation in humans: A systematic review of randomized controlled studies. Am. J. Clin. Nutr. 2022, 115, 608–618. [Google Scholar] [CrossRef]
- Dobranowski, P.A.; Stintzi, A. Resistant starch, microbiome, and precision modulation. Gut Microbes 2021, 13, 1926842. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, I.; Stull, N.D.; Tersey, S.A.; Mirmira, R.G. Phenotypic sexual dimorphism in response to dietary fat manipulation in C57BL/6J mice. J. Diabetes Its Complicat. 2021, 35, 107795. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, U.S.; Walden, T.B.; Carlsson, P.-O.; Jansson, L.; Phillipson, M. Female mice are protected against high-fat diet in-duced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Kim, B.J.; Qu, Y.; Huang, H.; Dallas, D.C. Top-Down Glycopeptidomics Reveals Intact Glycomacropeptide Is Digested to a Wide Array of Peptides in Human Jejunum. J. Nutr. 2022, 152, 429–438. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sauvé, M.F.; Feldman, F.; Sané, A.T.; Koudoufio, M.; Patey, N.; Spahis, S.; Butcher, J.; Duan, H.; Figeys, D.; Marcil, V.; et al. Glycomacropeptide as an Efficient Agent to Fight Pathophysiological Mechanisms of Metabolic Syndrome. Nutrients 2024, 16, 871. https://doi.org/10.3390/nu16060871
Sauvé MF, Feldman F, Sané AT, Koudoufio M, Patey N, Spahis S, Butcher J, Duan H, Figeys D, Marcil V, et al. Glycomacropeptide as an Efficient Agent to Fight Pathophysiological Mechanisms of Metabolic Syndrome. Nutrients. 2024; 16(6):871. https://doi.org/10.3390/nu16060871
Chicago/Turabian StyleSauvé, Mathilde Foisy, Francis Feldman, Alain Théophile Sané, Mireille Koudoufio, Natalie Patey, Schohraya Spahis, James Butcher, Haonan Duan, Daniel Figeys, Valérie Marcil, and et al. 2024. "Glycomacropeptide as an Efficient Agent to Fight Pathophysiological Mechanisms of Metabolic Syndrome" Nutrients 16, no. 6: 871. https://doi.org/10.3390/nu16060871
APA StyleSauvé, M. F., Feldman, F., Sané, A. T., Koudoufio, M., Patey, N., Spahis, S., Butcher, J., Duan, H., Figeys, D., Marcil, V., Stintzi, A., & Levy, E. (2024). Glycomacropeptide as an Efficient Agent to Fight Pathophysiological Mechanisms of Metabolic Syndrome. Nutrients, 16(6), 871. https://doi.org/10.3390/nu16060871





