Reply to Lee, S.Y. Comment on “Krikorian et al. Early Intervention in Cognitive Aging with Strawberry Supplementation. Nutrients 2023, 15, 4431”
Conflicts of Interest
References
- Lee, S.Y. Comment on Krikorian et al. Early Intervention in Cognitive Aging with Strawberry Supplementation. Nutrients 2023, 15, 4431. Nutrients 2024, 16, 824. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Summer, S.S. Early Intervention in Cognitive Aging with Strawberry Supplementation. Nutrients 2023, 15, 4431. [Google Scholar] [CrossRef] [PubMed]
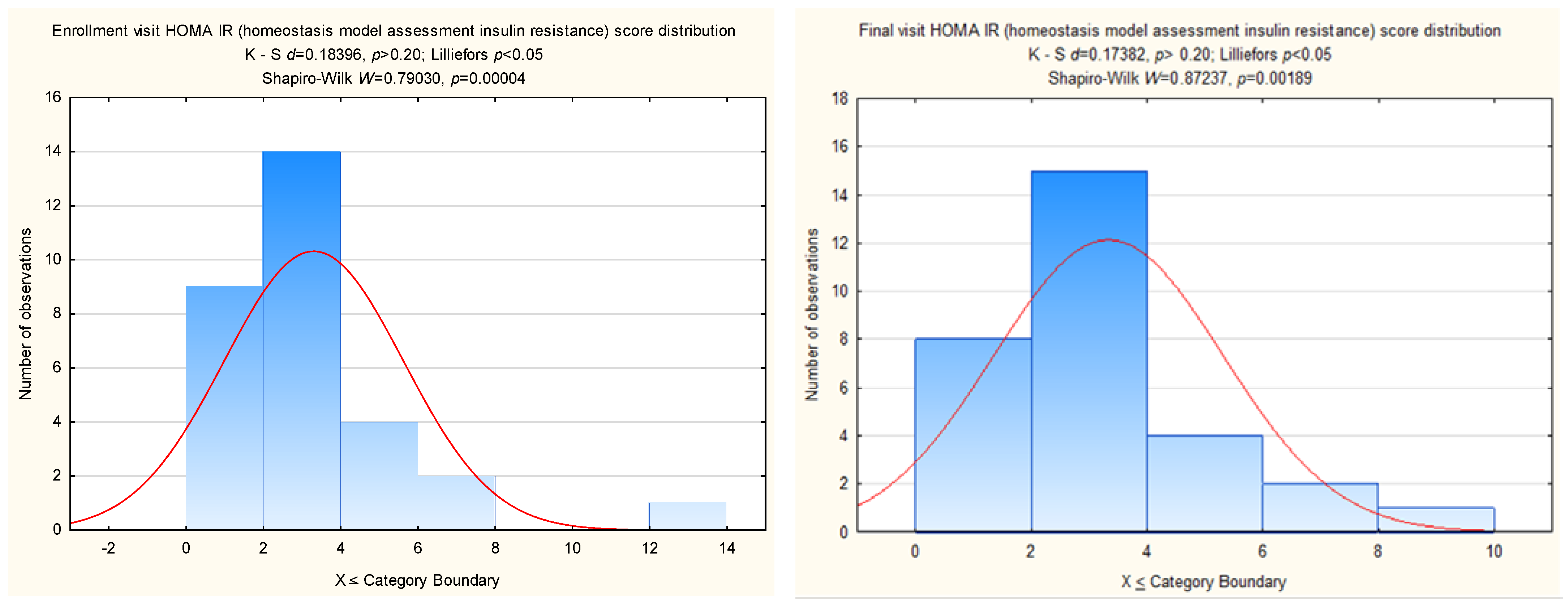
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Sheeber, L.B.; Sorensen, E.D.; Howe, S.R. Data Analytic Techniques for Treatment Outcome Studies with Pretest/Posttest Measurements: An Extensive Primer. J. Psychiatr. Res. 1996, 30, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Ahles, S.; Joris, P.J.; Plat, J. Effects of Berry Anthocyanins on Cognitive Performance, Vascular Function and Cardiometabolic Risk Markers: A Systematic Review of Randomized Placebo-Controlled Intervention Studies in Humans. Int. J. Mol. Sci. 2021, 22, 6482. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krikorian, R.; Welge, J. Reply to Lee, S.Y. Comment on “Krikorian et al. Early Intervention in Cognitive Aging with Strawberry Supplementation. Nutrients 2023, 15, 4431”. Nutrients 2024, 16, 825. https://doi.org/10.3390/nu16060825
Krikorian R, Welge J. Reply to Lee, S.Y. Comment on “Krikorian et al. Early Intervention in Cognitive Aging with Strawberry Supplementation. Nutrients 2023, 15, 4431”. Nutrients. 2024; 16(6):825. https://doi.org/10.3390/nu16060825
Chicago/Turabian StyleKrikorian, Robert, and Jeffrey Welge. 2024. "Reply to Lee, S.Y. Comment on “Krikorian et al. Early Intervention in Cognitive Aging with Strawberry Supplementation. Nutrients 2023, 15, 4431”" Nutrients 16, no. 6: 825. https://doi.org/10.3390/nu16060825
APA StyleKrikorian, R., & Welge, J. (2024). Reply to Lee, S.Y. Comment on “Krikorian et al. Early Intervention in Cognitive Aging with Strawberry Supplementation. Nutrients 2023, 15, 4431”. Nutrients, 16(6), 825. https://doi.org/10.3390/nu16060825





