Serum 25-Hydroxyvitamin D Status and Vitamin D Supplements Use Are Not Associated with Low Back Pain in the Large UK Biobank Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Vitamin D Status
2.3. Vitamin D Supplements Use
2.4. Low Back Pain
2.5. Covariates
2.6. In- and Exclusion Criteria
- Individuals with a history of dorsalgia diagnoses other than LBP in the primary care data (diagnosed using the ICD-10: M54.0–54.4, 54.6–54.9) before the baseline assessment (n = 19,763);
- Self-reported back pain in the last month in the questionnaire (n = 45,920) or missing information about this question (n = 282) unless LBP was diagnosed in the primary care data;
- History of LBP diagnosis in the primary care data but no current symptoms reported in the question about LBP in the last month (n = 6847).
2.7. Statistical Analyses
2.7.1. General Remarks
2.7.2. Covariates Selection
2.7.3. Association between Vitamin D Status and LBP
2.7.4. The Association between Vitamin D Supplement Use and LBP
2.7.5. Subgroup Analyses
3. Results
3.1. Baseline Overview of the Study Population
3.2. Covariates Associated with LBP at Baseline
3.3. Association of 25(OH)D Status with LBP
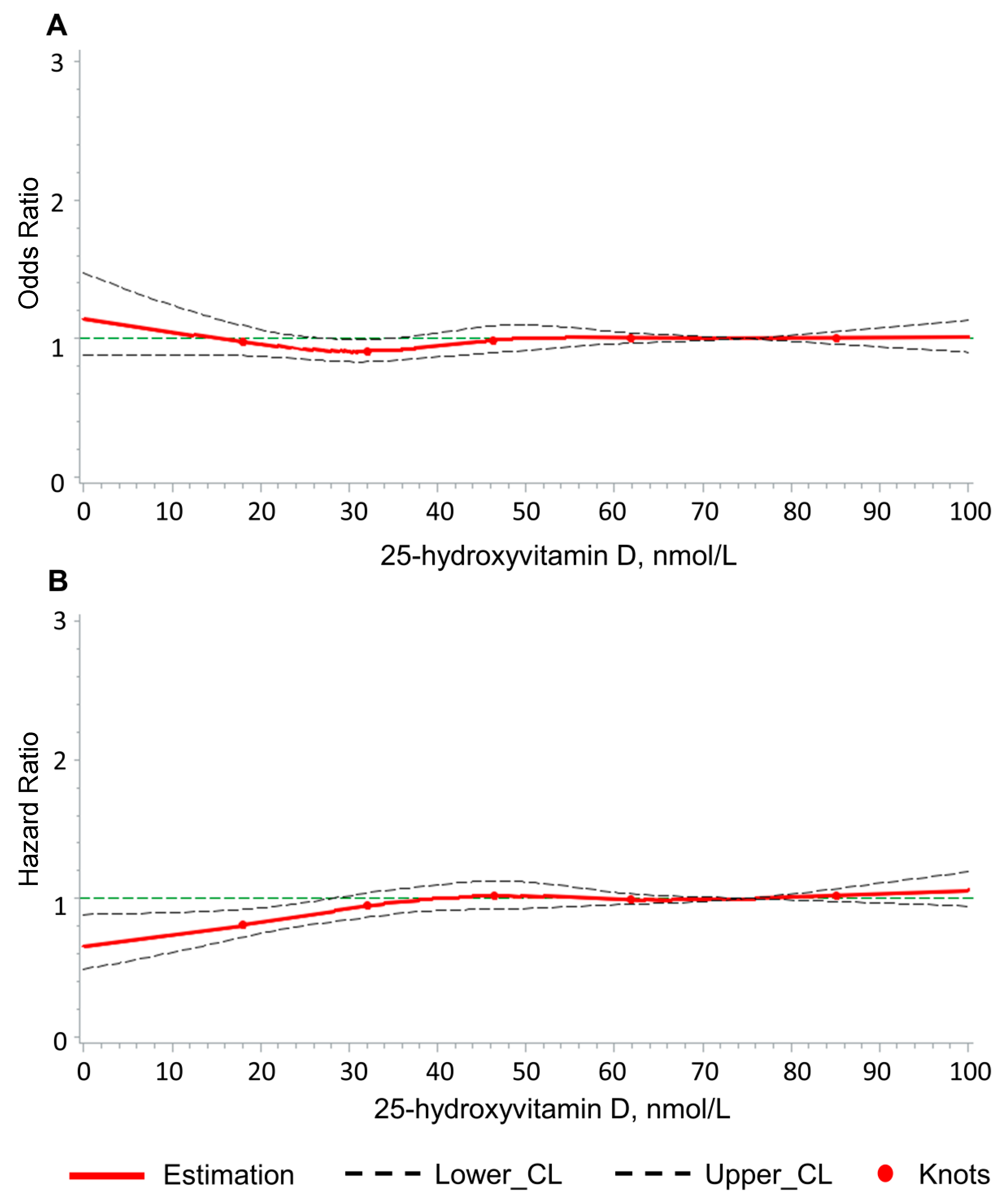
3.4. Dose–Response Association of 25(OH)D Concentration with LBP
3.5. Association of Vitamin D Supplements Use and LBP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 25(OH)D | 25-hydroxyvitamin D |
| BMI | body mass index |
| CHD | coronary heart disease |
| CI | confidence interval |
| EMIS | Egton Medical Information Systems |
| GP | general practitioner |
| HR | hazard ratio |
| ICD-10 | International Statistical Classification of Diseases |
| IQR | interquartile range |
| LBP | low back pain |
| N.A. | not applicable |
| NHS | National Health Service |
| OTC | over-the-counter |
| RCS | restricted cubic splines |
| Ref | reference |
| RIQAS | Randox International Quality Assessment Scheme |
| SAS | Statistical Analysis System |
| UK | United Kingdom |
| VIF | variation inflation factor |
References
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Gonzalez, L. Is vitamin D deficiency a major global public health problem? J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 138–145. [Google Scholar] [CrossRef]
- Prentice, A. Vitamin D deficiency: A global perspective. Nutr. Rev. 2008, 66 (Suppl. S2), S153–S164. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.M.; Botelho, P.B.; Ribeiro, H. Vitamin D and musculoskeletal health: Outstanding aspects to be considered in the light of current evidence. Endocr. Connect. 2022, 11, e210596. [Google Scholar] [CrossRef] [PubMed]
- Plotnikoff, G.A.; Quigley, J.M. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin. Proc. 2003, 78, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Helde-Frankling, M.; Björkhem-Bergman, L. Vitamin D in Pain Management. Int. J. Mol. Sci. 2017, 18, 2170. [Google Scholar] [CrossRef]
- Habib, A.M.; Nagi, K.; Thillaiappan, N.B.; Sukumaran, V.; Akhtar, S. Vitamin D and Its Potential Interplay with Pain Signaling Pathways. Front. Immunol. 2020, 11, 820. [Google Scholar] [CrossRef]
- Wu, Z.; Malihi, Z.; Stewart, A.W.; Lawes, C.M.; Scragg, R. The association between vitamin D concentration and pain: A systematic review and meta-analysis. Public Health Nutr. 2018, 21, 2022–2037. [Google Scholar] [CrossRef]
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef]
- Institute for Health Metrics and Evaluation. Global Burden of Disease Results 2023. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 20 April 2023).
- Ge, L.; Pereira, M.J.; Yap, C.W.; Heng, B.H. Chronic low back pain and its impact on physical function, mental health, and health-related quality of life: A cross-sectional study in Singapore. Sci. Rep. 2022, 12, 20040. [Google Scholar] [CrossRef]
- Scott, N.A.; Moga, C.; Harstall, C. Managing low back pain in the primary care setting: The know-do gap. Pain. Res. Manag. 2010, 15, 392–400. [Google Scholar] [CrossRef]
- International Association for the Study of Pain. Low Back Pain Fact Sheet 2023. Available online: https://www.iasp-pain.org/resources/fact-sheets/low-back-pain/ (accessed on 6 February 2024).
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef]
- Zadro, J.; Shirley, D.; Ferreira, M.; Carvalho-Silva, A.P.; Lamb, S.E.; Cooper, C.; Ferreira, P.H. Mapping the Association between Vitamin D and Low Back Pain: A Systematic Review and Meta-Analysis of Observational Studies. Pain. Physician 2017, 20, 611–640. [Google Scholar] [CrossRef]
- Heuch, I.; Heuch, I.; Hagen, K.; Mai, X.M.; Langhammer, A.; Zwart, J.A. Is there an association between vitamin D status and risk of chronic low back pain? A nested case-control analysis in the Nord-Trøndelag Health Study. BMJ Open. 2017, 7, e018521. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, R.; He, Y.; Zhu, T.; Zhang, W. Causal effect of serum 25-hydroxyvitamin D levels on low back pain: A two-sample mendelian randomization study. Front. Genet. 2022, 13, 1001265. [Google Scholar] [CrossRef]
- Zadro, J.R.; Shirley, D.; Ferreira, M.; Carvalho Silva, A.P.; Lamb, S.E.; Cooper, C.; Ferreira, P.H. Is Vitamin D Supplementation Effective for Low Back Pain? A Systematic Review and Meta-Analysis. Pain. Physician 2018, 21, 121–145. [Google Scholar] [CrossRef]
- UK Biobank. UK Biobank Showcase User Guide: Getting Started: UK Biobank. 2017. Available online: https://biobank.ndph.ox.ac.uk/showcase/ (accessed on 21 July 2021).
- Elliott, P.; Peakman, T.C. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 2008, 37, 234–244. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- UK Biobank. UK Biobank Primary Care Linked Data: UK Biobank 2019. Available online: https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/primary_care_data.pdf (accessed on 21 July 2021).
- Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. The National Academies Collection: Reports funded by National Institutes of Health. In Dietary Reference Intakes for Calcium and Vitamin D; Ross, A.C., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Eds.; National Academies Press (US): Washington, DC, USA, 2011. [Google Scholar]
- Fry, D.; Almond, R.; Moffat, S.; Gordon, M.; Singh, P. UK Biobank Biomarker Project Companion Document to Accompany Serum Biomarker Data: UK Biobank. 2019. Available online: https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf (accessed on 23 July 2021).
- Sha, S.; Nguyen, T.M.N.; Kuznia, S.; Niedermaier, T.; Zhu, A.; Brenner, H.; Schöttker, B. Real-world evidence for the effectiveness of vitamin D supplementation in reduction of total and cause-specific mortality. J. Intern. Med. 2023, 293, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Ciaffi, J.; Meliconi, R.; Landini, M.P.; Mancarella, L.; Brusi, V.; Faldini, C.; Ursini, F. Seasonality of Back Pain in Italy: An Infodemiology Study. Int. J. Environ. Res. Public Health 2021, 18, 1325. [Google Scholar] [CrossRef]
- Hyppönen, E.; Power, C. Hypovitaminosis D in British adults at age 45 y: Nationwide cohort study of dietary and lifestyle predictors. Am. J. Clin. Nutr. 2007, 85, 860–868. [Google Scholar] [CrossRef]
- Roggio, F.; Musumeci, G. Does Back Pain Go on Holiday in the Summer? J. Funct. Morphol. Kinesiol. 2022, 7, 75. [Google Scholar] [CrossRef]
- EMIS. Primary Care: EMIS Health. 2021. Available online: https://www.emishealth.com/primary-care (accessed on 23 July 2023).
- Miller, R.G. Simultaneous Statistical Inference, 2nd ed.; Springer: New York, NY, USA, 1981. [Google Scholar]
- Sterne, J.A.C.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef]
- Yuan, Y.C. Multiple Imputation for Missing Data: Concepts and New Development (Version 9.0); SAS Institute Inc.: Cary, NC, USA, 2011; Available online: https://facweb.cdm.depaul.edu/sjost/csc423/documents/multipleimputation.pdf (accessed on 18 February 2024).
- UCLA. Regression with SAS Chapter 2—Regression Diagnostics: UCLA Advanced Research Computing—Statistical Methods and Data Analytics. 2023. Available online: https://stats.oarc.ucla.edu/sas/webbooks/reg/chapter2/regressionwith-saschapter-2-regression-diagnostics/ (accessed on 16 April 2023).
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Park, H.-J.; Choi, J.-Y.; Lee, W.M.; Park, S.-M. Prevalence of chronic low back pain and its associated factors in the general population of South Korea: A cross-sectional study using the National Health and Nutrition Examination Surveys. J. Orthop. Surg. Res. 2023, 18, 29. [Google Scholar] [CrossRef]

| Variables | Cross-Sectional Analysis (N = 135,934) | Longitudinal Analysis (N = 130,843) |
|---|---|---|
| N (%)/Median (IQR) | N (%)/Median (IQR) | |
| Female sex, n (%) | 73,427 (54.0) | 70,690 (54.0) |
| Age (years), median (IQR) | 58 (50; 63) | 58 (50; 63) |
| BMI, n (%) | ||
| <25 | 46,625 (34.3) | 45,351 (34.6) |
| 25–<30 | 57,440 (42.3) | 55,317 (42.3) |
| ≥30 | 31,368 (23.1) | 29,703 (22.7) |
| Smoking, n (%) | ||
| Never | 76,907 (56.6) | 74,471 (56.9) |
| Ever | 58,990 (43.4) | 56,337 (43.1) |
| Hypertension, n (%) | 35,014 (25.8) | 33,359 (25.5) |
| Diabetes, n (%) | 6286 (4.6) | 5957 (4.6) |
| CHD, n (%) | 5797 (4.3) | 5429 (4.2) |
| Lifetime history of depression, n (%) | 14,614 (10.8) | 13,842 (10.6) |
| No. of chronic diseases, median (IQR) | 1 (0; 3) | 1 (0; 3) |
| Lifetime history of musculoskeletal diseases, n (%) | 72,784 (53.5) | 68,524 (52.4) |
| Lifetime history of injury to the abdomen, lower back, lumbar spine and pelvis, n (%) | 2674 (2.0) | 2384 (1.8) |
| Low back pain in the month before enrolment, n (%) | 5091 (3.8) | N.A. |
| Low back pain during follow-up, n (%) | N.A. | 4288 (3.3) |
| 25(OH)D concentration (nmol/L), median (IQR) | 46.3 (32; 61.9) | 46.4 (32; 61.9) |
| Vitamin D status, n (%) | ||
| Deficiency (<30 nmol/L) | 29,419 (21.6) | 28,216 (21.6) |
| Insufficiency (30–<50 nmol/L) | 46,949 (34.5) | 45,208 (34.6) |
| Sufficiency (≥50 nmol/L) | 59,566 (43.8) | 57,419 (43.9) |
| Vitamin D intake, n (%) | ||
| No | 103,710 (76.3) | 99,886 (76.3) |
| Multivitamins ± minerals | 26,807 (19.7) | 25,792 (19.7) |
| Vitamin D | 5417 (4.0) | 5165 (3.8) |
| Vitamin D Status | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deficiency | Insufficiency | Sufficiency | ||||||||
| Ncase (%) | OR/HR (95% CI) | p-Value a | Ncase (%) | OR/HR (95% CI) | p-Value a | Ncase (%) | OR/HR (95% CI) | |||
| Cross-sectional analyses | ||||||||||
| Adjusted for age & sex | 1203 (4.1) | 1.13 (1.05, 1.22) | 0.0008 | 1741 (3.7) | 1.03 (0.96, 1.10) | 0.42 | 2147 (3.6) | Ref | ||
| Adjusted for all covariates b | 1203 (4.1) | 0.95 (0.87, 1.03) | 0.21 | 1741 (3.7) | 0.97 (0.91, 1.04) | 0.38 | 2147 (3.6) | Ref | ||
| Longitudinal analyses | ||||||||||
| Adjusted for age & sex | 874 (3.1) | 0.93 (0.86, 1.01) | 0.07 | 1533 (3.4) | 1.03 (0.96, 1.10) | 0.45 | 1881 (3.3) | Ref | ||
| Adjusted for all covariates b | 874 (3.1) | 0.87 (0.79, 0.95) | 0.0032 | 1533 (3.4) | 1.00 (0.93, 1.07) | 0.96 | 1881 (3.3) | Ref | ||
| Vitamin Use | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-Users | Multivitamin Users | Vitamin D Users | ||||||||
| Ncase (%) | OR/HR (95%CI) | Ncase (%) | OR/HR (95%CI) | p-Value a | Ncase (%) | OR/HR (95%CI) | p-Value a | |||
| Cross-sectional analyses | ||||||||||
| Adjusted for age & sex | 3824 (3.7) | Ref | 1015 (3.8) | 1.03 (0.96, 1.11) | 0.42 | 252 (4.7) | 1.29 (1.13, 1.47) | 0.0001 | ||
| Adjusted for all covariates b | 3824 (3.7) | Ref | 1015 (3.8) | 0.97 (0.90, 1.05) | 0.45 | 252 (4.7) | 0.99 (0.86, 1.14) | 0.87 | ||
| Longitudinal analyses | ||||||||||
| Adjusted for age & sex | 3269 (3.3) | Ref | 853 (3.3) | 1.01 (0.94, 1.09) | 0.69 | 166 (3.2) | 0.99 (0.85, 1.16) | 0.90 | ||
| Adjusted for all covariates b | 3269 (3.3) | Ref | 853 (3.3) | 0.99 (0.91, 1.07) | 0.74 | 166 (3.2) | 0.93 (0.80, 1.09) | 0.38 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, S.; Chen, L.-J.; Brenner, H.; Schöttker, B. Serum 25-Hydroxyvitamin D Status and Vitamin D Supplements Use Are Not Associated with Low Back Pain in the Large UK Biobank Cohort. Nutrients 2024, 16, 806. https://doi.org/10.3390/nu16060806
Sha S, Chen L-J, Brenner H, Schöttker B. Serum 25-Hydroxyvitamin D Status and Vitamin D Supplements Use Are Not Associated with Low Back Pain in the Large UK Biobank Cohort. Nutrients. 2024; 16(6):806. https://doi.org/10.3390/nu16060806
Chicago/Turabian StyleSha, Sha, Li-Ju Chen, Hermann Brenner, and Ben Schöttker. 2024. "Serum 25-Hydroxyvitamin D Status and Vitamin D Supplements Use Are Not Associated with Low Back Pain in the Large UK Biobank Cohort" Nutrients 16, no. 6: 806. https://doi.org/10.3390/nu16060806
APA StyleSha, S., Chen, L.-J., Brenner, H., & Schöttker, B. (2024). Serum 25-Hydroxyvitamin D Status and Vitamin D Supplements Use Are Not Associated with Low Back Pain in the Large UK Biobank Cohort. Nutrients, 16(6), 806. https://doi.org/10.3390/nu16060806






