Impact of the Mediterranean Diet on the Gut Microbiome of a Well-Defined Cohort of Healthy Individuals
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection and Classification
2.3. Microbiome-Related Definitions
2.4. Sample Processing
2.5. Data Analysis
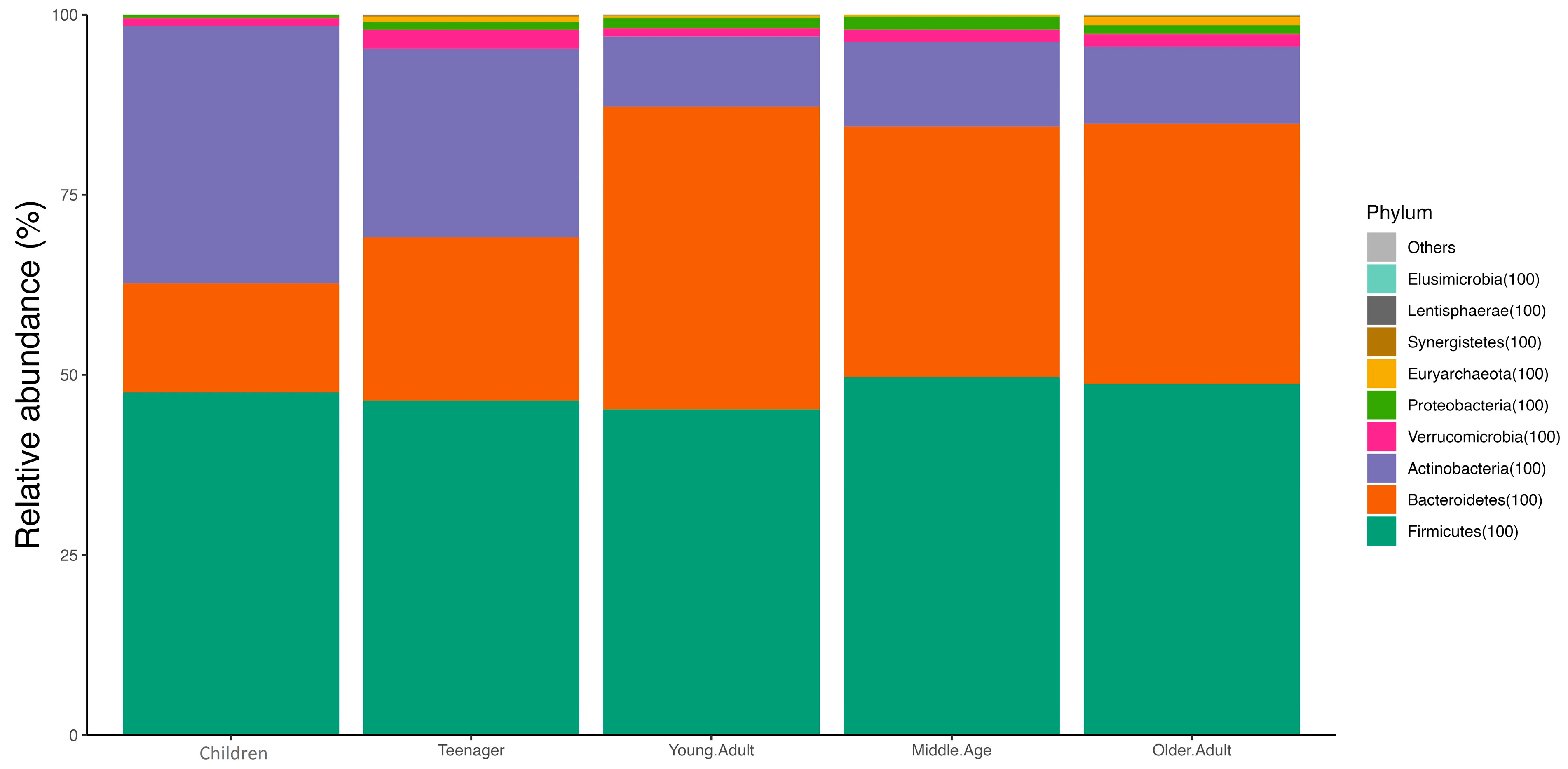
3. Results
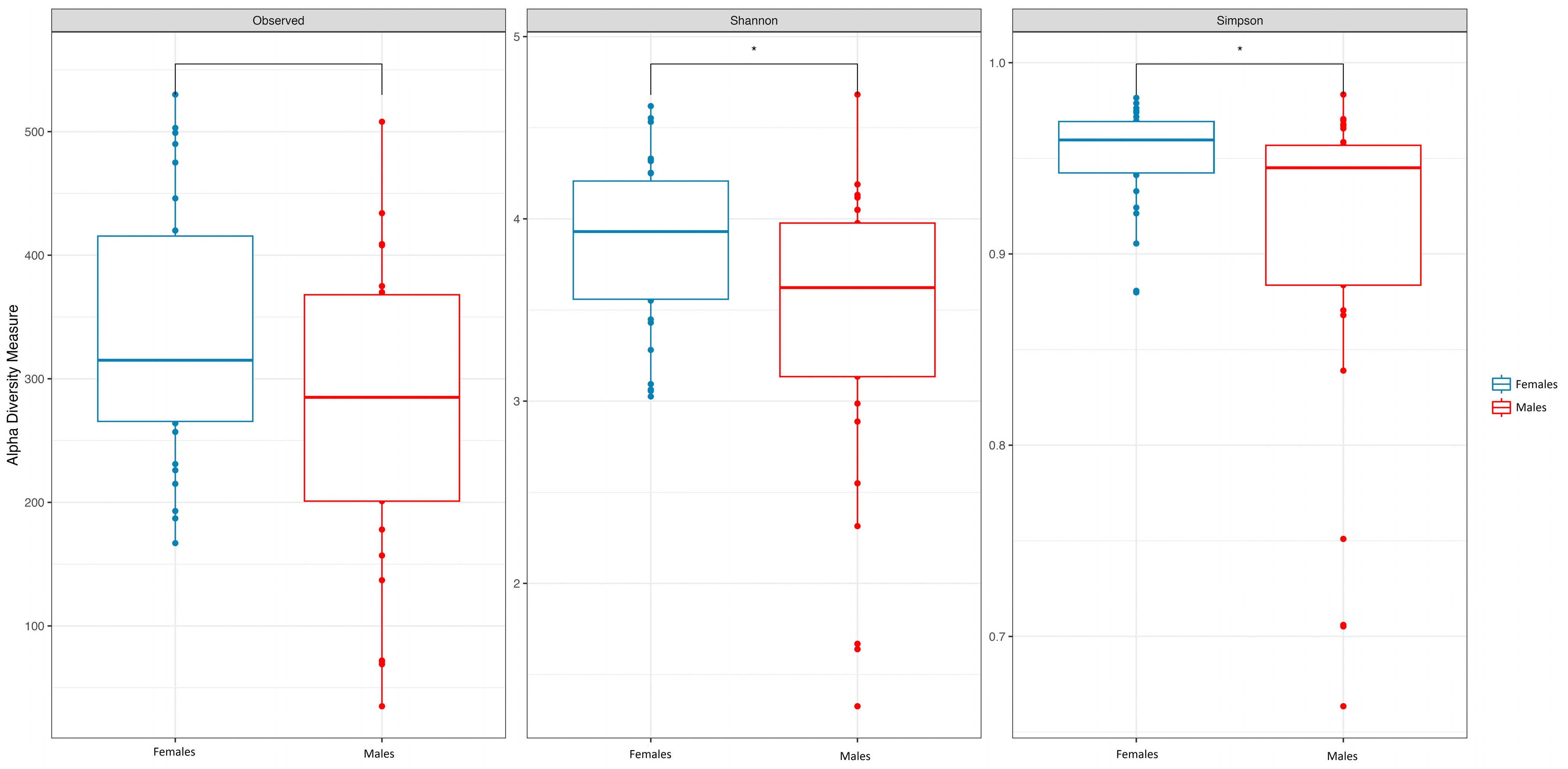
3.1. Age and Sex
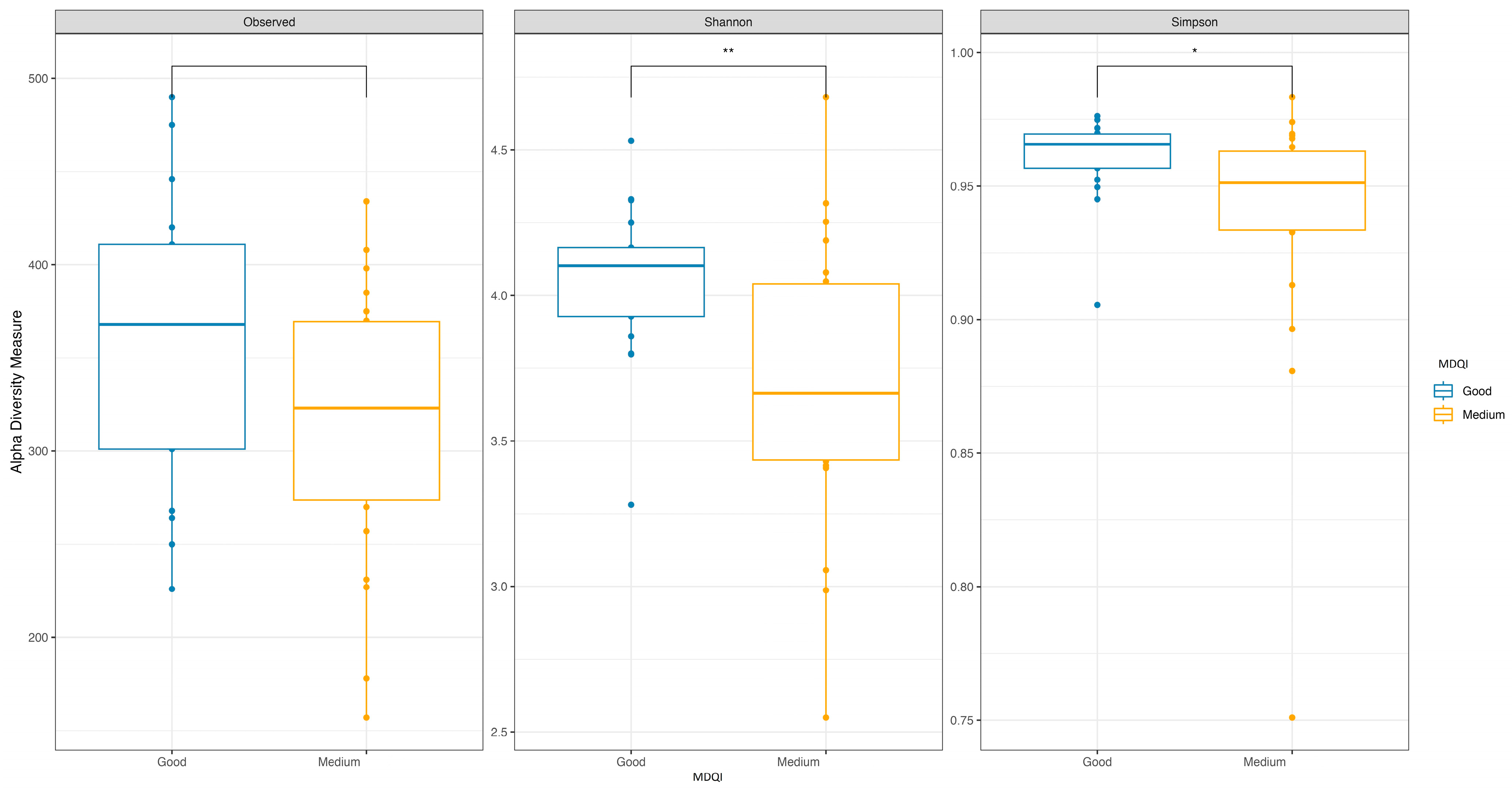
3.2. Adherence to the Mediterranean Diet
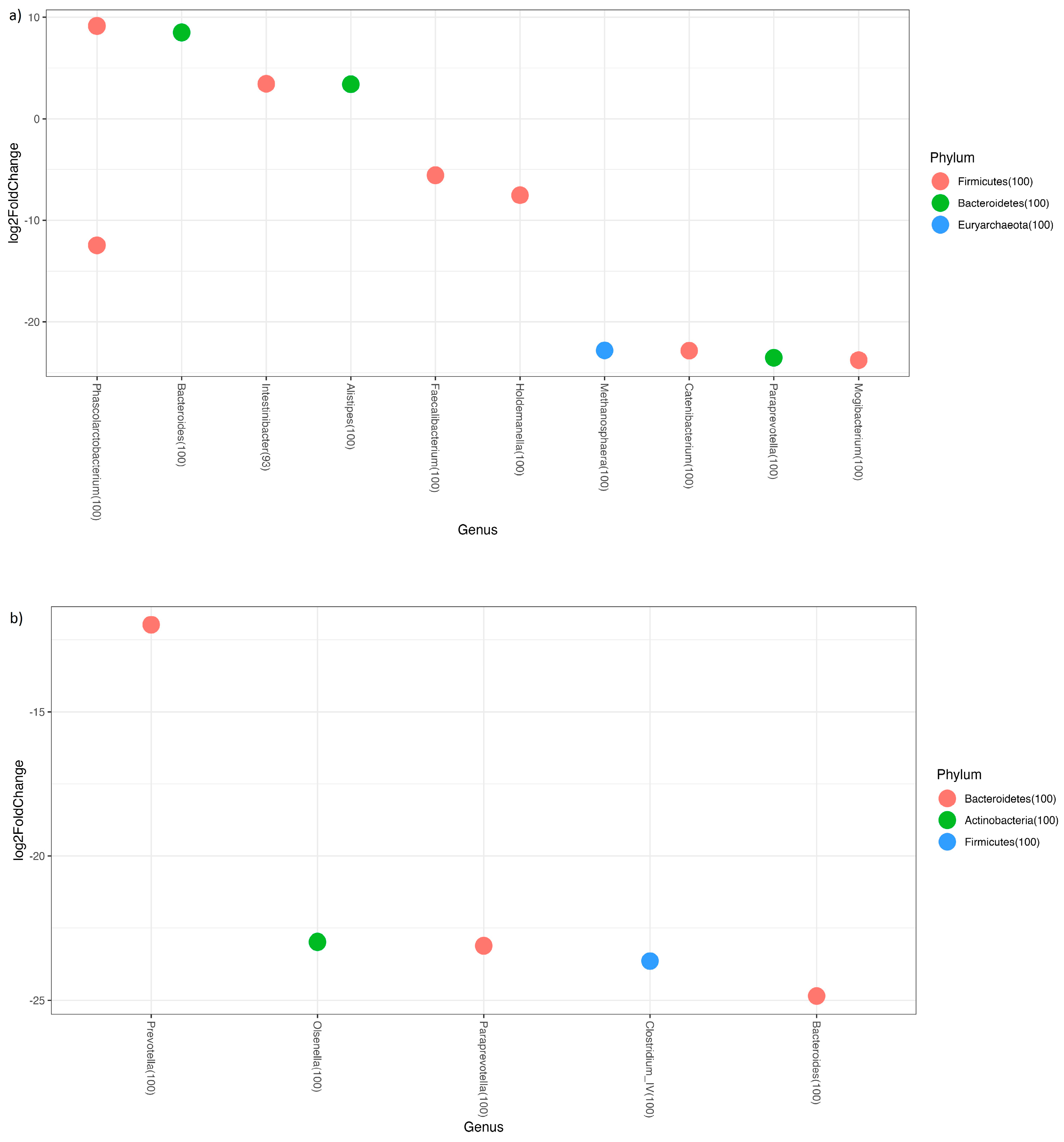
3.3. Predicted Functional Metagenome Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belizário, J.E.; Napolitano, M. Human microbiomes and their roles in dysbiosis, common diseases, and novel therapeutic approaches. Front. Microbiol. 2015, 6, 1050. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Solch, R.J.; Aigbogun, J.O.; Voyiadjis, A.G.; Talkington, G.M.; Darensbourg, R.M.; O’Connell, S.; Pickett, K.M.; Perez, S.R.; Maraganore, D.M. Mediterranean diet adherence, gut microbiota, and Alzheimer’s or Parkinson’s disease risk: A systematic review. J. Neurol. Sci. 2022, 434, 120166. [Google Scholar] [CrossRef]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef]
- Haidar, Y.M.; Cosman, B.C. Obesity epidemiology. Clin. Colon. Rectal Surg. 2011, 24, 205–210. [Google Scholar] [CrossRef]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef]
- Holesh, J.E.; Aslam, S.; Martin, A. Physiology, Carbohydrates. In StatPearls; StatPearls Publishing LLC: Treasure Island, FL, USA, 2024. [Google Scholar]
- Jahan-Mihan, A.; Luhovyy, B.L.; El Khoury, D.; Anderson, G.H. Dietary proteins as determinants of metabolic and physiologic functions of the gastrointestinal tract. Nutrients 2011, 3, 574–603. [Google Scholar] [CrossRef]
- Promintzer, M.; Krebs, M. Effects of dietary protein on glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 463–468. [Google Scholar] [CrossRef]
- Hoffer, L.J.; Bistrian, B.R. Appropriate protein provision in critical illness: A systematic and narrative review. Am. J. Clin. Nutr. 2012, 96, 591–600. [Google Scholar] [CrossRef]
- Delimaris, I. Adverse Effects Associated with Protein Intake above the Recommended Dietary Allowance for Adults. ISRN Nutr. 2013, 2013, 126929. [Google Scholar] [CrossRef]
- Bordoni, L.; Zec, M.; Naumovski, N.; Sergi, D. Editorial: The role of dietary fatty acids in metabolic health. Front. Physiol. 2023, 14, 1211151. [Google Scholar] [CrossRef]
- Sergi, D.; Luscombe-Marsh, N.; Heilbronn, L.K.; Birch-Machin, M.; Naumovski, N.; Lionetti, L.; Proud, C.G.; Abeywardena, M.Y.; O’Callaghan, N. The Inhibition of Metabolic Inflammation by EPA Is Associated with Enhanced Mitochondrial Fusion and Insulin Signaling in Human Primary Myotubes. J. Nutr. 2021, 151, 810–819. [Google Scholar] [CrossRef]
- Unger, R.H. Lipotoxic diseases. Annu. Rev. Med. 2002, 53, 319–336. [Google Scholar] [CrossRef]
- Penney, N.; Barton, W.; Posma, J.M.; Darzi, A.; Frost, G.; Cotter, P.D.; Holmes, E.; Shanahan, F.; O’Sullivan, O.; Garcia-Perez, I. Investigating the Role of Diet and Exercise in Gut Microbe-Host Cometabolism. mSystems 2020, 5, e00677-20. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A.; et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Clauss, M.; Gérard, P.; Mosca, A.; Leclerc, M. Interplay Between Exercise and Gut Microbiome in the Context of Human Health and Performance. Front. Nutr. 2021, 8, 637010. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef]
- De Filippis, F.; Pellegrini, N.; Vannini, L.; Jeffery, I.B.; La Storia, A.; Laghi, L.; Serrazanetti, D.I.; Di Cagno, R.; Ferrocino, I.; Lazzi, C.; et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016, 65, 1812–1821. [Google Scholar] [CrossRef]
- Gutiérrez-Díaz, I.; Fernández-Navarro, T.; Salazar, N.; Bartolomé, B.; Moreno-Arribas, M.V.; de Andres-Galiana, E.J.; Fernández-Martínez, J.L.; de Los Reyes-Gavilán, C.G.; Gueimonde, M.; González, S. Adherence to a Mediterranean Diet Influences the Fecal Metabolic Profile of Microbial-Derived Phenolics in a Spanish Cohort of Middle-Age and Older People. J. Agric. Food Chem. 2017, 65, 586–595. [Google Scholar] [CrossRef]
- Mitsou, E.K.; Kakali, A.; Antonopoulou, S.; Mountzouris, K.C.; Yannakoulia, M.; Panagiotakos, D.B.; Kyriacou, A. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br. J. Nutr. 2017, 117, 1645–1655. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of Mediterranean Diet on Human Gut Microbiota. Nutrients 2020, 13, 7. [Google Scholar] [CrossRef]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M.; Speranza, B.; Racioppo, A.; Altieri, C.; Bevilacqua, A. How Diet and Physical Activity Modulate Gut Microbiota: Evidence, and Perspectives. Nutrients 2022, 14, 2456. [Google Scholar] [CrossRef]
- Bach, A.; Serra-Majem, L.; Carrasco, J.L.; Roman, B.; Ngo, J.; Bertomeu, I.; Obrador, B. The use of indexes evaluating the adherence to the Mediterranean diet in epidemiological studies: A review. Public Health Nutr. 2006, 9, 132–146. [Google Scholar] [CrossRef]
- Garcia-Mantrana, I.; Selma-Royo, M.; Alcantara, C.; Collado, M.C. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front. Microbiol. 2018, 9, 890. [Google Scholar] [CrossRef]
- Gerber, M. Qualitative methods to evaluate Mediterranean diet in adults. Public Health Nutr. 2006, 9, 147–151. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-Item Mediterranean Diet Assessment Tool and Obesity Indexes among High-Risk Subjects: The PREDIMED Trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- Vioque, J.; Gonzalez, L. Validity of a food frequency questionnaire (preliminary results). Eur. J. Cancer Prev. 1991, 1, 19. [Google Scholar] [CrossRef]
- López, J.V. Validez de la evaluación de la ingesta dietética. In Nutrición y Salud Pública: Métodos, Bases Científicas y Aplicaciones; Masson: Spain, 2006; pp. 199–210. [Google Scholar]
- Whittaker, R.J.; Willis, K.J.; Field, R. Scale and species richness: Towards a general, hierarchical theory of species diversity. J. Biogeogr. 2001, 28, 453–470. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017, 66, 515–522. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the Genetic Basis of Fibrolytic Specialization by Lachnospiraceae and Ruminococcaceae in Diverse Gut Communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Steinmeyer, S.; Lee, K.; Jayaraman, A.; Alaniz, R.C. Microbiota metabolite regulation of host immune homeostasis: A mechanistic missing link. Curr. Allergy Asthma Rep. 2015, 15, 24. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Chávez-Carbajal, A.; Nirmalkar, K.; Pérez-Lizaur, A.; Hernández-Quiroz, F.; Ramírez-Del-Alto, S.; García-Mena, J.; Hernández-Guerrero, C. Gut Microbiota and Predicted Metabolic Pathways in a Sample of Mexican Women Affected by Obesity and Obesity Plus Metabolic Syndrome. Int. J. Mol. Sci. 2019, 20, 438. [Google Scholar] [CrossRef]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef]
- Walker, A.W.; Ince, J.; Duncan, S.H.; Webster, L.M.; Holtrop, G.; Ze, X.; Brown, D.; Stares, M.D.; Scott, P.; Bergerat, A.; et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. Isme J. 2011, 5, 220–230. [Google Scholar] [CrossRef]
- Martínez, I.; Lattimer, J.M.; Hubach, K.L.; Case, J.A.; Yang, J.; Weber, C.G.; Louk, J.A.; Rose, D.J.; Kyureghian, G.; Peterson, D.A.; et al. Gut microbiome composition is linked to whole grain-induced immunological improvements. Isme J. 2013, 7, 269–280. [Google Scholar] [CrossRef]
- Kostic, A.D.; Gevers, D.; Siljander, H.; Vatanen, T.; Hyötyläinen, T.; Hämäläinen, A.M.; Peet, A.; Tillmann, V.; Pöhö, P.; Mattila, I.; et al. The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host Microbe 2015, 17, 260–273. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Kameyama, K.; Itoh, K. Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef]
- Marik, P.E.; Varon, J. Omega-3 dietary supplements and the risk of cardiovascular events: A systematic review. Clin. Cardiol. 2009, 32, 365–372. [Google Scholar] [CrossRef]
- Liu, J.; Ma, D.W. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients 2014, 6, 5184–5223. [Google Scholar] [CrossRef]
- Noriega, B.S.; Sanchez-Gonzalez, M.A.; Salyakina, D.; Coffman, J. Understanding the Impact of Omega-3 Rich Diet on the Gut Microbiota. Case Rep. Med. 2016, 2016, 3089303. [Google Scholar] [CrossRef]
- De Weirdt, R.; Van de Wiele, T. Micromanagement in the gut: Microenvironmental factors govern colon mucosal biofilm structure and functionality. NPJ Biofilms Microbiomes 2015, 1, 15026. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Ridlon, J.M.; Hylemon, P.B.; Thacker, L.R.; Heuman, D.M.; Smith, S.; Sikaroodi, M.; Gillevet, P.M. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G168–G175. [Google Scholar] [CrossRef]
- Antharam, V.C.; Li, E.C.; Ishmael, A.; Sharma, A.; Mai, V.; Rand, K.H.; Wang, G.P. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J. Clin. Microbiol. 2013, 51, 2884–2892. [Google Scholar] [CrossRef]
- Morotomi, M.; Nagai, F.; Sakon, H.; Tanaka, R. Paraprevotella clara gen. nov., sp. nov. and Paraprevotella xylaniphila sp. nov., members of the family ‘Prevotellaceae’ isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2009, 59, 1895–1900. [Google Scholar] [CrossRef]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 2017, 7, 2594. [Google Scholar] [CrossRef]
- Zhang, L.; Ouyang, Y.; Li, H.; Shen, L.; Ni, Y.; Fang, Q.; Wu, G.; Qian, L.; Xiao, Y.; Zhang, J.; et al. Metabolic phenotypes and the gut microbiota in response to dietary resistant starch type 2 in normal-weight subjects: A randomized crossover trial. Sci. Rep. 2019, 9, 4736. [Google Scholar] [CrossRef]
- Zhang, L.; Zi, L.; Kuang, T.; Wang, K.; Qiu, Z.; Wu, Z.; Liu, L.; Liu, R.; Wang, P.; Wang, W. Investigating causal associations among gut microbiota, metabolites, and liver diseases: A Mendelian randomization study. Front. Endocrinol. 2023, 14, 1159148. [Google Scholar] [CrossRef]
- Hu, C.; Niu, X.; Chen, S.; Wen, J.; Bao, M.; Mohyuddin, S.G.; Yong, Y.; Liu, X.; Wu, L.; Yu, Z.; et al. A Comprehensive Analysis of the Colonic Flora Diversity, Short Chain Fatty Acid Metabolism, Transcripts, and Biochemical Indexes in Heat-Stressed Pigs. Front. Immunol. 2021, 12, 717723. [Google Scholar] [CrossRef]
- Forbes, J.D.; Chen, C.Y.; Knox, N.C.; Marrie, R.A.; El-Gabalawy, H.; de Kievit, T.; Alfa, M.; Bernstein, C.N.; Van Domselaar, G. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome 2018, 6, 221. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Wang, J.; Qie, J.; Zhu, D.; Zhang, X.; Zhang, Q.; Xu, Y.; Wang, Y.; Mi, K.; Pei, Y.; Liu, Y.; et al. The landscape in the gut microbiome of long-lived families reveals new insights on longevity and aging—Relevant neural and immune function. Gut Microbes 2022, 14, 2107288. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, Y.; He, W.; Tian, Z.; Lin, J.; Liu, Z.; Li, Y.; Chen, M.; Han, S.; Liang, J.; et al. Gut Microbiota and Metabolite Changes in Patients With Ulcerative Colitis and Clostridioides difficile Infection. Front. Microbiol. 2022, 13, 802823. [Google Scholar] [CrossRef]
- Morales, C.; Rojas, G.; Rebolledo, C.; Rojas-Herrera, M.; Arias-Carrasco, R.; Cuadros-Orellana, S.; Maracaja-Coutinho, V.; Saavedra, K.; Leal, P.; Lanas, F.; et al. Characterization of microbial communities from gut microbiota of hypercholesterolemic and control subjects. Front. Cell Infect. Microbiol. 2022, 12, 943609. [Google Scholar] [CrossRef]
- Liang, W.; Yang, Y.; Wang, H.; Yu, X.; Lu, Y.; Shen, S.; Teng, L. Gut microbiota shifts in patients with gastric cancer in perioperative period. Medicine 2019, 98, e16626. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Yang, F.; Hu, Q.; Xiong, D. Correlation between gut bacteria Phascolarctobacterium and exogenous metabolite α-linolenic acid in T2DM: A case-control study. Ann. Transl. Med. 2022, 10, 1056. [Google Scholar] [CrossRef]
- Uema, T.; Millman, J.F.; Okamoto, S.; Nakamura, T.; Yamashiro, K.; Uehara, M.; Honma, K.I.; Miyazato, M.; Ashikari, A.; Saito, S.; et al. Profile of gut microbiota and serum metabolites associated with metabolic syndrome in a remote island most afflicted by obesity in Japan. Sci. Rep. 2022, 12, 17292. [Google Scholar] [CrossRef]
- Therdtatha, P.; Song, Y.; Tanaka, M.; Mariyatun, M.; Almunifah, M.; Manurung, N.E.P.; Indriarsih, S.; Lu, Y.; Nagata, K.; Fukami, K.; et al. Gut Microbiome of Indonesian Adults Associated with Obesity and Type 2 Diabetes: A Cross-Sectional Study in an Asian City, Yogyakarta. Microorganisms 2021, 9, 897. [Google Scholar] [CrossRef]
- Gomez-Arango, L.F.; Barrett, H.L.; Wilkinson, S.A.; Callaway, L.K.; McIntyre, H.D.; Morrison, M.; Dekker Nitert, M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut Microbes 2018, 9, 189–201. [Google Scholar] [CrossRef]
- Astbury, S.; Atallah, E.; Vijay, A.; Aithal, G.P.; Grove, J.I.; Valdes, A.M. Lower gut microbiome diversity and higher abundance of proinflammatory genus Collinsella are associated with biopsy-proven nonalcoholic steatohepatitis. Gut Microbes 2020, 11, 569–580. [Google Scholar] [CrossRef]
- Li, Y.; Fu, R.; Li, R.; Zeng, J.; Liu, T.; Li, X.; Jiang, W. Causality of gut microbiome and hypertension: A bidirectional mendelian randomization study. Front. Cardiovasc. Med. 2023, 10, 1167346. [Google Scholar] [CrossRef]
- Neumann, A.; Björck, L.; Frick, I.M. Finegoldia magna, an Anaerobic Gram-Positive Bacterium of the Normal Human Microbiota, Induces Inflammation by Activating Neutrophils. Front. Microbiol. 2020, 11, 65. [Google Scholar] [CrossRef]
- Peters, B.A.; Dominianni, C.; Shapiro, J.A.; Church, T.R.; Wu, J.; Miller, G.; Yuen, E.; Freiman, H.; Lustbader, I.; Salik, J.; et al. The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome 2016, 4, 69. [Google Scholar] [CrossRef]
- Qin, Q.; Yan, S.; Yang, Y.; Chen, J.; Yan, H.; Li, T.; Gao, X.; Wang, Y.; Li, A.; Wang, S.; et al. The Relationship Between Osteoporosis and Intestinal Microbes in the Henan Province of China. Front. Cell Dev. Biol. 2021, 9, 752990. [Google Scholar] [CrossRef]
- Gryaznova, M.V.; Solodskikh, S.A.; Panevina, A.V.; Syromyatnikov, M.Y.; Dvoretskaya, Y.D.; Sviridova, T.N.; Popov, E.S.; Popov, V.N. Study of microbiome changes in patients with ulcerative colitis in the Central European part of Russia. Heliyon 2021, 7, e06432. [Google Scholar] [CrossRef]
- Ternes, D.; Karta, J.; Tsenkova, M.; Wilmes, P.; Haan, S.; Letellier, E. Microbiome in Colorectal Cancer: How to Get from Meta-omics to Mechanism? Trends Microbiol. 2020, 28, 401–423. [Google Scholar] [CrossRef]
- Derrien, M.; Turroni, F.; Ventura, M.; van Sinderen, D. Insights into endogenous Bifidobacterium species in the human gut microbiota during adulthood. Trends Microbiol. 2022, 30, 940–947. [Google Scholar] [CrossRef]
- Piñero, F.; Vazquez, M.; Baré, P.; Rohr, C.; Mendizabal, M.; Sciara, M.; Alonso, C.; Fay, F.; Silva, M. A different gut microbiome linked to inflammation found in cirrhotic patients with and without hepatocellular carcinoma. Ann. Hepatol. 2019, 18, 480–487. [Google Scholar] [CrossRef]
- Jiang, Z.; Mou, Y.; Wang, H.; Li, L.; Jin, T.; Wang, H.; Liu, M.; Jin, W. Causal effect between gut microbiota and pancreatic cancer: A two-sample Mendelian randomization study. BMC Cancer 2023, 23, 1091. [Google Scholar] [CrossRef]
- Zhang, S.; Ning, R.; Zeng, B.; Deng, F.; Kong, F.; Guo, W.; Zhao, J.; Li, Y. Gut Microbiota Composition and Metabolic Potential of Long-Living People in China. Front. Aging Neurosci. 2022, 14, 820108. [Google Scholar] [CrossRef] [PubMed]
- Van Hul, M.; Le Roy, T.; Prifti, E.; Dao, M.C.; Paquot, A.; Zucker, J.D.; Delzenne, N.M.; Muccioli, G.; Clément, K.; Cani, P.D. From correlation to causality: The case of Subdoligranulum. Gut Microbes 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Li, T.; Feng, Y.; Wang, C.; Shi, T.; Abudurexiti, A.; Zhang, M.; Gao, F. Assessment of causal associations among gut microbiota, metabolites, and celiac disease: A bidirectional Mendelian randomization study. Front. Microbiol. 2023, 14, 1087622. [Google Scholar] [CrossRef]
- Zhuang, P.; Zhang, Y.; Shou, Q.; Li, H.; Zhu, Y.; He, L.; Chen, J.; Jiao, J. Eicosapentaenoic and Docosahexaenoic Acids Differentially Alter Gut Microbiome and Reverse High-Fat Diet-Induced Insulin Resistance. Mol. Nutr. Food Res. 2020, 64, e1900946. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Han, F.; Wang, Q.; Wang, Y.; Dai, X.; Zhu, M. Recent functional insights into the magic role of (p)ppGpp in growth control. Comput. Struct. Biotechnol. J. 2023, 21, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Quinn, P.J.; Yan, A. Kdo2 -lipid A: Structural diversity and impact on immunopharmacology. Biol. Rev. Camb. Philos. Soc. 2015, 90, 408–427. [Google Scholar] [CrossRef] [PubMed]
- Defois, C.; Ratel, J.; Garrait, G.; Denis, S.; Le Goff, O.; Talvas, J.; Mosoni, P.; Engel, E.; Peyret, P. Food Chemicals Disrupt Human Gut Microbiota Activity And Impact Intestinal Homeostasis As Revealed By In Vitro Systems. Sci. Rep. 2018, 8, 11006. [Google Scholar] [CrossRef]
- Lamichhane, S.; Sen, P.; Alves, M.A.; Ribeiro, H.C.; Raunioniemi, P.; Hyötyläinen, T.; Orešič, M. Linking Gut Microbiome and Lipid Metabolism: Moving beyond Associations. Metabolites 2021, 11, 55. [Google Scholar] [CrossRef]
- Murray, M.; Barlow, C.K.; Blundell, S.; Buecking, M.; Gibbon, A.; Goeckener, B.; Kaminskas, L.M.; Leitner, P.; Selby-Pham, S.; Sinclair, A.; et al. Demonstrating a link between diet, gut microbiota and brain: (14)C radioactivity identified in the brain following gut microbial fermentation of (14)C-radiolabeled tyrosine in a pig model. Front. Nutr. 2023, 10, 1127729. [Google Scholar] [CrossRef]




| FEMALES (N 31) | MALES (N 29) | TOTAL (N 60) | p-Value | |
|---|---|---|---|---|
| AGE Median (IQR) | 37.00 (28.50, 54.50) | 26.00 (19.00, 36.00) | 31.00 (24.00, 49.75) | 0.006 |
| Diversity Index | ||||
| SOBS Median (IQR) | 318.55 (261.16, 409.64) | 278.66 (193.37, 366.72) | 312.02 (225.66, 381.65) | 0.052 |
| Inv.Simpson Median (IQR) | 24.74 (17.37, 32.50) | 17.62 (8.57, 23.59) | 21.04 (13.03, 30.21) | 0.015 |
| Shannon Median (IQR) | 3.93 (3.55, 4.20) | 3.63 (3.14, 3.94) | 3.74 (3.38, 4.10) | 0.023 |
| Pielou Median (IQR) | 0.68 (0.62, 0.71) | 0.64 (0.58, 0.68) | 0.67 (0.61, 0.70) | 0.033 |
| Enterotype (ET) | 0.334 | |||
| N-Miss | 1 | 5 | 6 | |
| ET_Bacteroides | 16 (53.3%) | 9 (37.5%) | 25 (46.3%) | |
| ET_Firmicutes | 6 (20.0%) | 4 (16.7%) | 10 (18.5%) | |
| ET_Prevotella | 8 (26.7%) | 11 (45.8%) | 19 (35.2%) |
| GOOD ADHERENCE (N 17) | MEDIUM ADHERENCE (N 22) | TOTAL (N 39) | p-Value | |
|---|---|---|---|---|
| Age Median (IQR) | 48.00 (28.00, 55.00) | 30.00 (26.00, 48.75) | 34.00 (26.00, 54.50) | 0.122 |
| Sex | 0.065 | |||
| Females | 12 (70.6%) | 9 (40.9%) | 21 (53.8%) | |
| Males | 5 (29.4%) | 13 (59.1%) | 18 (46.2%) | |
| Med-DQI Median (IQR) | 4.00 (3.00, 4.00) | 7.00 (6.00, 7.00) | 5.00 (4.00, 7.00) | <0.001 |
| Enterotypes (ETs) | 0.945 | |||
| ET_Bacteroides | 6 (35.3%) | 8 (36.4%) | 14 (35.9%) | |
| ET_Firmicutes | 4 (23.5%) | 6 (27.3%) | 10 (25.6%) | |
| ET_Prevotella | 7 (41.2%) | 8 (36.4%) | 15 (38.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Cuesta, S.; Lozano García, N.; Rodríguez-Fernández, S.; Fernández-Avila, A.I.; Bermejo, J.; Fernández-Avilés, F.; Muñoz, P.; Bouza, E.; Reigadas, E. Impact of the Mediterranean Diet on the Gut Microbiome of a Well-Defined Cohort of Healthy Individuals. Nutrients 2024, 16, 793. https://doi.org/10.3390/nu16060793
Vázquez-Cuesta S, Lozano García N, Rodríguez-Fernández S, Fernández-Avila AI, Bermejo J, Fernández-Avilés F, Muñoz P, Bouza E, Reigadas E. Impact of the Mediterranean Diet on the Gut Microbiome of a Well-Defined Cohort of Healthy Individuals. Nutrients. 2024; 16(6):793. https://doi.org/10.3390/nu16060793
Chicago/Turabian StyleVázquez-Cuesta, Silvia, Nuria Lozano García, Sara Rodríguez-Fernández, Ana I. Fernández-Avila, Javier Bermejo, Francisco Fernández-Avilés, Patricia Muñoz, Emilio Bouza, and Elena Reigadas. 2024. "Impact of the Mediterranean Diet on the Gut Microbiome of a Well-Defined Cohort of Healthy Individuals" Nutrients 16, no. 6: 793. https://doi.org/10.3390/nu16060793
APA StyleVázquez-Cuesta, S., Lozano García, N., Rodríguez-Fernández, S., Fernández-Avila, A. I., Bermejo, J., Fernández-Avilés, F., Muñoz, P., Bouza, E., & Reigadas, E. (2024). Impact of the Mediterranean Diet on the Gut Microbiome of a Well-Defined Cohort of Healthy Individuals. Nutrients, 16(6), 793. https://doi.org/10.3390/nu16060793







