Differences in Plasma 25-Hydroxyvitamin D Levels at Diagnosis of Celiac Disease and Type 1 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Vitamin D Assessment
2.3. Other Clinical Variables
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (25-OHD) | 25-hydroxy vitamin D |
| (25-OHD3) | 25-hydroxy vitamin D |
| CG | Control group |
| CD | Celiac disease |
| CLIA | Chemiluminescent immunoassay |
| CYP27B1 | 1α-hydroxylase |
| DC | Dendritic cell |
| ESPGHAN | European Society for Paediatric Gastroenterology Hepatology and Nutrition |
| IQR | Interquartile range |
| ISPAD | International Society of Pediatric and Adolescent Diabetes |
| T1D | Type 1 diabetes |
References
- Fasano, A.; Catassi, C. Celiac Disease. N. Engl. J. Med. 2012, 367, 2419–2426. [Google Scholar] [CrossRef]
- Green, P.H.R.; Cellier, C. Celiac Disease. N. Engl. J. Med. 2007, 357, 1731–1743. [Google Scholar] [CrossRef]
- Mahmud, F.H.; Murray, J.A.; Kudva, Y.C.; Zinsmeister, A.R.; Dierkhising, R.A.; Lahr, B.D.; Dyck, P.J.; Kyle, R.A.; El-Youssef, M.; Burgart, L.J.; et al. Celiac Disease in Type 1 Diabetes Mellitus in a North American Community: Prevalence, Serologic Screening, and Clinical Features. Mayo Clin. Proc. 2005, 80, 1429–1434. [Google Scholar] [CrossRef]
- Primavera, M.; Giannini, C.; Chiarelli, F. Prediction and Prevention of Type 1 Diabetes. Front. Endocrinol. 2020, 11, 248. [Google Scholar] [CrossRef]
- Lionetti, E.; Catassi, C. New Clues in Celiac Disease Epidemiology, Pathogenesis, Clinical Manifestations, and Treatment. Int. Rev. Immunol. 2011, 30, 219–231. [Google Scholar] [CrossRef]
- Sahebari, M.; Nabavi, N.; Salehi, M. Correlation between Serum 25(OH)D Values and Lupus Disease Activity: An Original Article and a Systematic Review with Meta-Analysis Focusing on Serum VitD Confounders. Lupus 2014, 23, 1164–1177. [Google Scholar] [CrossRef]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef]
- Shen, L.; Zhuang, Q.S.; Ji, H.F. Assessment of Vitamin D Levels in Type 1 and Type 2 Diabetes Patients: Results from Metaanalysis. Mol. Nutr. Food Res. 2016, 60, 1059–1067. [Google Scholar] [CrossRef]
- Duan, S.; Lv, Z.; Fan, X.; Wang, L.; Han, F.; Wang, H.; Bi, S. Vitamin D Status and the Risk of Multiple Sclerosis: A Systematic Review and Meta-Analysis. Neurosci. Lett. 2014, 570, 108–113. [Google Scholar] [CrossRef]
- Feng, R.; Li, Y.; Li, G.; Li, Z.; Zhang, Y.; Li, Q.; Sun, C. Lower Serum 25 (OH) D Concentrations in Type 1 Diabetes: A Meta-Analysis. Diabetes Res. Clin. Pract. 2015, 108, e71–e75. [Google Scholar] [CrossRef]
- Sadeghian, M.; Saneei, P.; Siassi, F.; Esmaillzadeh, A. Vitamin D Status in Relation to Crohn’s Disease: Meta-Analysis of Observational Studies. Nutrition 2016, 32, 505–514. [Google Scholar] [CrossRef]
- Lionetti, E.; Galeazzi, T.; Dominijanni, V.; Acquaviva, I.; Catassi, G.N.; Iasevoli, M.; Malamisura, B.; Catassi, C. Lower Level of Plasma 25-Hydroxyvitamin D in Children at Diagnosis of Celiac Disease Compared with Healthy Subjects: A Case-Control Study. J. Pediatr. 2021, 228, 132–137.e1. [Google Scholar] [CrossRef]
- Mathieu, C.; Gysemans, C.; Giulietti, A.; Bouillon, R. Vitamin D and Diabetes. Diabetologia 2005, 48, 1247–1257. [Google Scholar] [CrossRef]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does Vitamin D Play a Role in Autoimmune Endocrine Disorders? A Proof of Concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Pedro, J.I.S.-; Bilbao, J.R.; Perez de Nanclares, G.; Vitoria, J.C.; Martul, P.; Castaño, L. Heterogeneity of Vitamin D Receptor Gene Association with Celiac Disease and Type 1 Diabetes Mellitus. Autoimmunity 2005, 38, 439–444. [Google Scholar] [CrossRef]
- Rewers, M.; Ludvigsson, J. Environmental Risk Factors for Type 1 Diabetes. Lancet 2016, 387, 2340–2348. [Google Scholar] [CrossRef]
- Unalp-Arida, A.; Ruhl, C.E.; Choung, R.S.; Brantner, T.L.; Murray, J.A. Lower Prevalence of Celiac Disease and Gluten-Related Disorders in Persons Living in Southern vs Northern Latitudes of the United States. Gastroenterology 2017, 152, 1922–1932.e2. [Google Scholar] [CrossRef]
- Assa, A.; Waisbourd-Zinman, O.; Daher, S.; Shamir, R. Birth Month as a Risk Factor for the Diagnosis of Celiac Disease Later in Life: A Population-Based Study. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 367–370. [Google Scholar] [CrossRef]
- Tanpowpong, P.; Obuch, J.C.; Jiang, H.; McCarty, C.E.; Katz, A.J.; Leffler, D.A.; Kelly, C.P.; Weir, D.C.; Leichtner, A.M.; Camargo, C.A. Multicenter Study on Season of Birth and Celiac Disease: Evidence for a New Theoretical Model of Pathogenesis. J. Pediatr. 2013, 162, 501–504. [Google Scholar] [CrossRef]
- Lebwohl, B.; Green, P.H.R.; Murray, J.A.; Ludvigsson, J.F. Season of Birth in a Nationwide Cohort of Coeliac Disease Patients. Arch. Dis. Child 2013, 98, 48–51. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G.; Ramagopalan, S. The Month of Birth Effect in Multiple Sclerosis: Systematic Review, Meta-Analysis and Effect of Latitude. J. Neurol. Neurosurg. Psychiatry 2013, 84, 427–432. [Google Scholar] [CrossRef]
- Torkildsen; Grytten, N.; Aarseth, J.; Myhr, K.M.; Kampman, M.T. Month of Birth as a Risk Factor for Multiple Sclerosis: An Update. Acta Neurol. Scand. 2012, 126, 58–62. [Google Scholar] [CrossRef]
- Gerasimidi Vazeou, A.; Kordonouri, O.; Witsch, M.; Hermann, J.M.; Forsander, G.; de Beaufort, C.; Veeze, H.J.; Maffeis, C.; Cherubini, V.; Cinek, O.; et al. Seasonality at the Clinical Onset of Type 1 Diabetes-Lessons from the SWEET Database. Pediatr. Diabetes 2016, 17, 32–37. [Google Scholar] [CrossRef]
- Zipitis, C.S.; Akobeng, A.K. Vitamin D Supplementation in Early Childhood and Risk of Type 1 Diabetes: A Systematic Review and Meta-Analysis. Arch. Dis. Child 2008, 93, 512–517. [Google Scholar] [CrossRef]
- Dong, J.Y.; Zhang, W.G.; Chen, J.J.; Zhang, Z.L.; Han, S.F.; Qin, L.Q. Vitamin D Intake and Risk of Type 1 Diabetes: A Meta-Analysis of Observational Studies. Nutrients 2013, 5, 3551–3562. [Google Scholar] [CrossRef]
- Setty-Shah, N.; Maranda, L.; Nwosu, B.U. Increased Risk for Vitamin D Deficiency in Obese Children with Both Celiac Disease and Type 1 Diabetes. Gastroenterol. Res. Pract. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Patterson, C.C.; Harjutsalo, V.; Rosenbauer, J.; Neu, A.; Cinek, O.; Skrivarhaug, T.; Rami-Merhar, B.; Soltesz, G.; Svensson, J.; Parslow, R.C.; et al. Trends and Cyclical Variation in the Incidence of Childhood Type 1 Diabetes in 26 European Centres in the 25 Year Period 1989–2013: A Multicentre Prospective Registration Study. Diabetologia 2019, 62, 408–417. [Google Scholar] [CrossRef]
- Gesuita, R.; Rabbone, I.; Marconi, V.; De Sanctis, L.; Marino, M.; Tiberi, V.; Iannilli, A.; Tinti, D.; Favella, L.; Giorda, C.; et al. Trends and Cyclic Variation in the Incidence of Childhood Type 1 Diabetes in Two Italian Regions over 33 Years and during the COVID-19 Pandemic. Diabetes Obes. Metab. 2023, 25, 1698–1703. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, Epidemiology, and Classification of Diabetes in Children and Adolescents. Pediatr. Diabetes 2018, 19, 7–19. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the Diagnosis of Coeliac Disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; de’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in Pediatric Age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, Jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef]
- Onis, M. WHO Child Growth Standards Based on Length/Height, Weight and Age. Acta Paediatr. 2007, 95, 76–85. [Google Scholar] [CrossRef]
- Oilinki, T.; Otonkoski, T.; Ilonen, J.; Knip, M.; Miettinen, P. Prevalence and Characteristics of Diabetes among Somali Children and Adolescents Living in Helsinki, Finland. Pediatr. Diabetes 2012, 13, 176–180. [Google Scholar] [CrossRef]
- Zanchi, C.; di Leo, G.; Ronfani, L.; Martelossi, S.; Not, T.; Ventura, A. Bone Metabolism in Celiac Disease. J. Pediatr. 2008, 153, 262–265. [Google Scholar] [CrossRef]
- Margoni, D.; Chouliaras, G.; Duscas, G.; Voskaki, I.; Voutsas, N.; Papadopoulou, A.; Panayiotou, J.; Roma, E. Bone Health in Children With Celiac Disease Assessed By Dual X-Ray Absorptiometry. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 680–684. [Google Scholar] [CrossRef]
- Stenson, W.F.; Newberry, R.; Lorenz, R.; Baldus, C.; Civitelli, R. Increased Prevalence of Celiac Disease and Need for Routine Screening Among Patients With Osteoporosis. Arch. Intern. Med. 2005, 165, 393. [Google Scholar] [CrossRef]
- Zingone, F.; Ciacci, C. The Value and Significance of 25(OH) and 1,25(OH) Vitamin D Serum Levels in Adult Coeliac Patients: A Review of the Literature. Dig. Liver Dis. 2018, 50, 757–760. [Google Scholar] [CrossRef]
- Tanpowpong, P.; Camargo, C.A. Early-Life Vitamin D Deficiency and Childhood-Onset Coeliac Disease. Public Health Nutr. 2014, 17, 823–826. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the Immune System. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Rosen, Y.; Daich, J.; Soliman, I.; Brathwaite, E.; Shoenfeld, Y. Vitamin D and Autoimmunity. Scand. J. Rheumatol. 2016, 45, 439–447. [Google Scholar] [CrossRef]
- Bikle, D. Nonclassic Actions of Vitamin D. J. Clin. Endocrinol. Metab. 2009, 94, 26–34. [Google Scholar] [CrossRef]
- Galușca, D.; Popoviciu, M.S.; Babeș, E.E.; Vidican, M.; Zaha, A.A.; Babeș, V.V.; Jurca, A.D.; Zaha, D.C.; Bodog, F. Vitamin D Implications and Effect of Supplementation in Endocrine Disorders: Autoimmune Thyroid Disorders (Hashimoto’s Disease and Grave’s Disease), Diabetes Mellitus and Obesity. Medicina 2022, 58, 194. [Google Scholar] [CrossRef]
- Bhalla, A.K.; Amento, E.P.; Clemens, T.L.; Holick, M.F.; Krane, S.M. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: Presence in monocytes and induction in t lymphocytes following activation. J. Clin. Endocrinol. Metab. 1983, 57, 1308–1310. [Google Scholar] [CrossRef]
- Provvedini, D.M.; Tsoukas, C.D.; Deftos, L.J.; Manolagas, S.C. 1,25-Dihydroxyvitamin D3 Receptors in Human Leukocytes. Science (1979) 1983, 221, 1181–1183. [Google Scholar] [CrossRef]
- Borges, M.C.; Martini, L.A.; Rogero, M.M. Current Perspectives on Vitamin D, Immune System, and Chronic Diseases. Nutrition 2011, 27, 399–404. [Google Scholar] [CrossRef]
- Pozzilli, P.; Manfrini, S.; Crinò, A.; Picardi, A.; Leomanni, C.; Cherubini, V.; Valente, L.; Khazrai, M.; Visalli, N. Low Levels of 25-Hydroxyvitamin D3 and 1,25-Dihydroxyvitamin D3 in Patients with Newly Diagnosed Type 1 Diabetes. Horm. Metab. Res. 2005, 37, 680–683. [Google Scholar] [CrossRef]
- Janner, M.; Ballinari, P.; Mullis, P.; Flück, C. High Prevalence of Vitamin D Deficiency in Children and Adolescents with Type 1 Diabetes. Swiss Med. Wkly. 2010, 140, w13091. [Google Scholar] [CrossRef][Green Version]
- Yasemin, A.; Ralph, S.L. A Review of the Principles of Radiological Assessment of Skeletal Dysplasias. J. Clin. Res. Pediatr. Endocrinol. 2011, 3, 163–178. [Google Scholar] [CrossRef]
- Adorini, L. Intervention in Autoimmunity: The Potential of Vitamin D Receptor Agonists. Cell Immunol. 2005, 233, 115–124. [Google Scholar] [CrossRef]
- Harvey, J.N.; Hibbs, R.; Maguire, M.J.; O’Connell, H.; Gregory, J.W. The Changing Incidence of Childhood-Onset Type 1 Diabetes in Wales: Effect of Gender and Season at Diagnosis and Birth. Diabetes Res. Clin. Pract. 2021, 175, 108739. [Google Scholar] [CrossRef]
- Weng, J.; Zhou, Z.; Guo, L.; Zhu, D.; Ji, L.; Luo, X.; Mu, Y.; Jia, W. Incidence of Type 1 Diabetes in China, 2010–2013: Population Based Study. BMJ 2018, 360, j5295. [Google Scholar] [CrossRef]
- Weets, I.; Kaufman, L.; van der Auwera, B.; Crenier, L.; Rooman, R.P.A.; de Block, C.; Casteels, K.; Weber, E.; Coeckelberghs, M.; Laron, Z.; et al. Seasonality in Clinical Onset of Type 1 Diabetes in Belgian Patients above the Age of 10 Is Restricted to HLA-DQ2/DQ8-Negative Males, Which Explains the Male to Female Excess in Incidence. Diabetologia 2004, 47, 614–621. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gregoriou, E.; Mamais, I.; Tzanetakou, I.; Lavranos, G.; Chrysostomou, S. The Effects of Vitamin D Supplementation in Newly Diagnosed Type 1 Diabetes Patients: Systematic Review of Randomized Controlled Trials. Rev. Diabet. Stud. 2017, 14, 260–268. [Google Scholar] [CrossRef]
- Treiber, G.; Prietl, B.; Fröhlich-Reiterer, E.; Lechner, E.; Ribitsch, A.; Fritsch, M.; Rami-Merhar, B.; Steigleder-Schweiger, C.; Graninger, W.; Borkenstein, M.; et al. Cholecalciferol Supplementation Improves Suppressive Capacity of Regulatory T-Cells in Young Patients with New-Onset Type 1 Diabetes Mellitus—A Randomized Clinical Trial. Clin. Immunol. 2015, 161, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, M.A.L.; Sato, M.N.; Finazzo, C.; Duarte, A.J.S.; Dib, S.A. Effect of Cholecalciferol as Adjunctive Therapy With Insulin on Protective Immunologic Profile and Decline of Residual β-Cell Function in New-Onset Type 1 Diabetes Mellitus. Arch. Pediatr. Adolesc. Med. 2012, 166, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ataie-Jafari, A.; Loke, S.-C.; Rahmat, A.B.; Larijani, B.; Abbasi, F.; Leow, M.K.S.; Yassin, Z. A Randomized Placebo-Controlled Trial of Alphacalcidol on the Preservation of Beta Cell Function in Children with Recent Onset Type 1 Diabetes. Clin. Nutr. 2013, 32, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Raab, J.; Giannopoulou, E.Z.; Schneider, S.; Warncke, K.; Krasmann, M.; Winkler, C.; Ziegler, A.-G. Prevalence of Vitamin D Deficiency in Pre-Type 1 Diabetes and Its Association with Disease Progression. Diabetologia 2014, 57, 902–908. [Google Scholar] [CrossRef]
- Mosca, C.; Thorsteinsdottir, F.; Abrahamsen, B.; Rumessen, J.J.; Händel, M.N. Newly Diagnosed Celiac Disease and Bone Health in Young Adults: A Systematic Literature Review. Calcif. Tissue Int. 2022, 110, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Gerenli, N.; Dursun, F.; Çeltik, C.; Kırmızıbekmez, H. Significant Improvement in Bone Mineral Density in Pediatric Celiac Disease: Even at Six Months with Gluten-Free Diet. J. Pediatr. Endocrinol. Metab. 2021, 34, 341–348. [Google Scholar] [CrossRef]
- Carlberg, C.; Haq, A. The Concept of the Personal Vitamin D Response Index. J. Steroid. Biochem. Mol. Biol. 2018, 175, 12–17. [Google Scholar] [CrossRef]
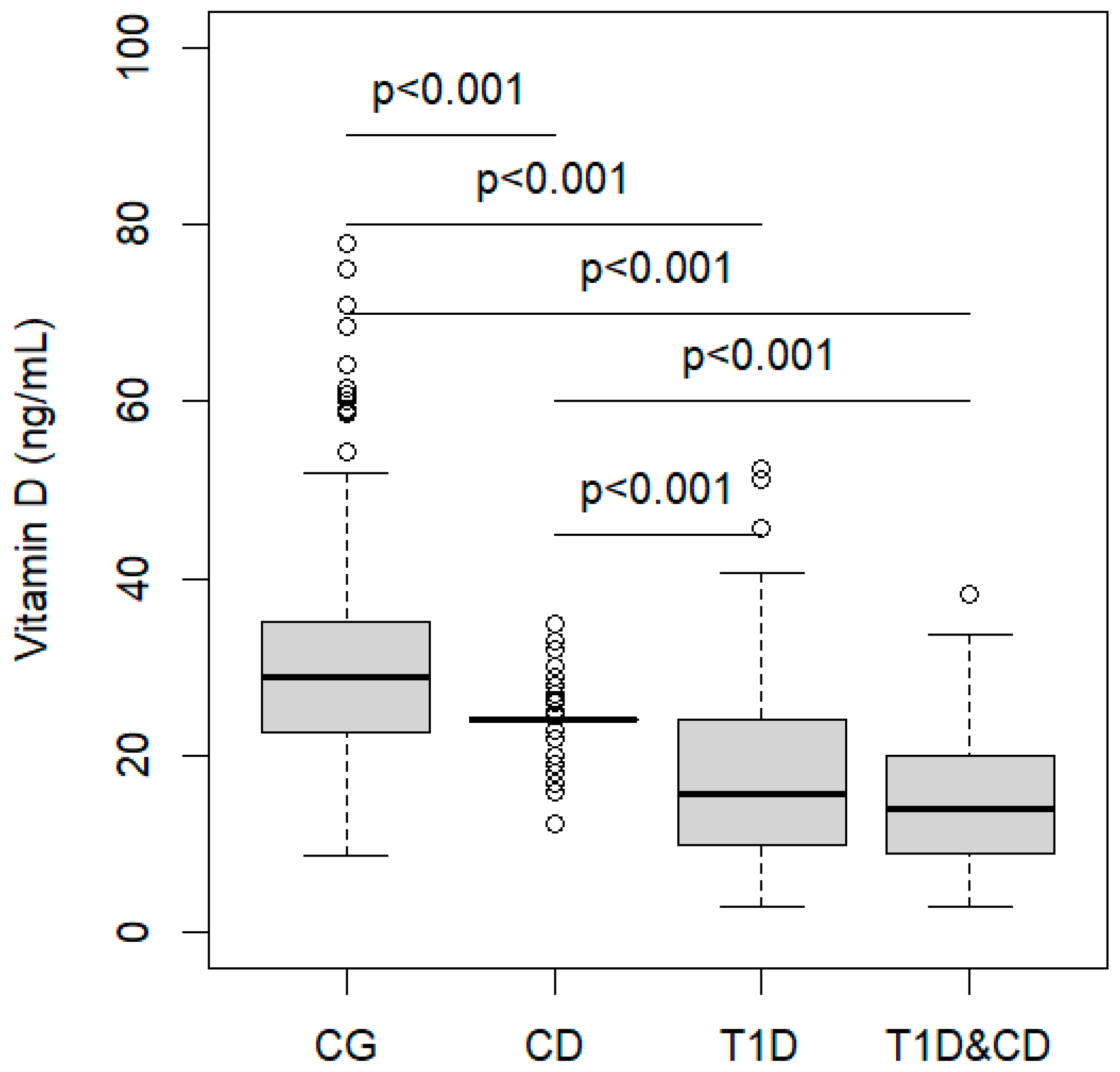

| CG (n = 131) | CD (n = 131) | T1D (n = 109) | T1D&CD (n = 22) | p | |
|---|---|---|---|---|---|
| Gender, male, n (%) | 50 (38.2) | 50 (38.2) | 64 (58.7) | 12 (54.5) | 0.003 |
| Age (years), median (IQR) | 8 (7–9) | 8 (6–11) | 10 (7–13) | 8 (4–12) | <0.001 # |
| Vitamin D classes, n (%) | |||||
| Severe deficiency | 2 (1.5) | 0 (0) | 27 (24.8) | 7 (31.8) | <0.001 |
| Deficiency | 16 (12.2) | 5 (3.8) | 41 (37.6) | 10 (45.5) | |
| Insufficiency | 58 (44.3) | 121 (92.4) | 29 (26.6) | 2 (9.1) | |
| Sufficiency | 55 (42) | 5 (3.8) | 12 (11) | 3 (13.6) | |
| BMI in classes | |||||
| Underweight (BMI-SDS < 0) | 100 (76.3) | 33 (68.8) | 72 (66.1) | 15 (68.2) | 0.135 |
| Normal weight (0 ≤ BMI-SDS ≤ 1.28) | 29 (22.1) | 15 (31.2) | 29 (26.6) | 6 (27.3) | |
| Overweight (BMI-SDS > 1.28) | 2 (1.5) | 0 (0) | 8 (7.3) | 1 (4.5) | |
| BMI (Kg/m2), median (IQR) | 17 (15–18) | 17 (15–19) | 17 (15–20) | 6 (14–18) | 0.305 |
| Estimate | 95% CI | p | |
|---|---|---|---|
| Intercept | 22.1 | 15.1; 29.1 | <0.001 |
| CD | −0.8 | −6.9; 5.3 | 0.793 |
| T1D | −10.0 | −15.1; −4.9 | <0.001 |
| CD and T1D | −14.2 | −23.5; −4.9 | 0.003 |
| Spring | 0.8 | −3.8; 5.4 | 0.731 |
| Summer | 14.6 | 9.3; 19.8 | <0.001 |
| Autumn | 16.6 | 12.1; 21.1 | <0.001 |
| BMI (Kg/m2) | 0.1 | −0.2; 0.5 | 0.541 |
| CD in Spring | −0.1 | −8.6; 8.4 | 0.984 |
| T1D in Spring | 2.1 | −5; 9.2 | 0.565 |
| T1D&CD in Spring | −7.3 | −24.2; 9.7 | 0.398 |
| CD in Summer | −14.2 | −24; −4.4 | 0.005 |
| T1D in Summer | −11.2 | −18.9; −3.4 | 0.005 |
| T1D&CD in Summer | −5.7 | −19.1; 7.7 | 0.403 |
| CD in Autumn | −15.7 | −25.1; −6.2 | 0.001 |
| T1D in Autumn | −9.8 | −16.7; −3 | 0.005 |
| T1D&CD in Autumn | −6.3 | −17.8; 5.3 | 0.284 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, M.; Galeazzi, T.; Gesuita, R.; Ricci, S.; Catassi, C.; Cherubini, V.; Lionetti, E. Differences in Plasma 25-Hydroxyvitamin D Levels at Diagnosis of Celiac Disease and Type 1 Diabetes. Nutrients 2024, 16, 743. https://doi.org/10.3390/nu16050743
Marino M, Galeazzi T, Gesuita R, Ricci S, Catassi C, Cherubini V, Lionetti E. Differences in Plasma 25-Hydroxyvitamin D Levels at Diagnosis of Celiac Disease and Type 1 Diabetes. Nutrients. 2024; 16(5):743. https://doi.org/10.3390/nu16050743
Chicago/Turabian StyleMarino, Monica, Tiziana Galeazzi, Rosaria Gesuita, Salima Ricci, Carlo Catassi, Valentino Cherubini, and Elena Lionetti. 2024. "Differences in Plasma 25-Hydroxyvitamin D Levels at Diagnosis of Celiac Disease and Type 1 Diabetes" Nutrients 16, no. 5: 743. https://doi.org/10.3390/nu16050743
APA StyleMarino, M., Galeazzi, T., Gesuita, R., Ricci, S., Catassi, C., Cherubini, V., & Lionetti, E. (2024). Differences in Plasma 25-Hydroxyvitamin D Levels at Diagnosis of Celiac Disease and Type 1 Diabetes. Nutrients, 16(5), 743. https://doi.org/10.3390/nu16050743








