Complementary Feeding: Tradition, Innovation and Pitfalls
Abstract
1. Introduction
2. Tradition
2.1. Human Milk and Infant Formula
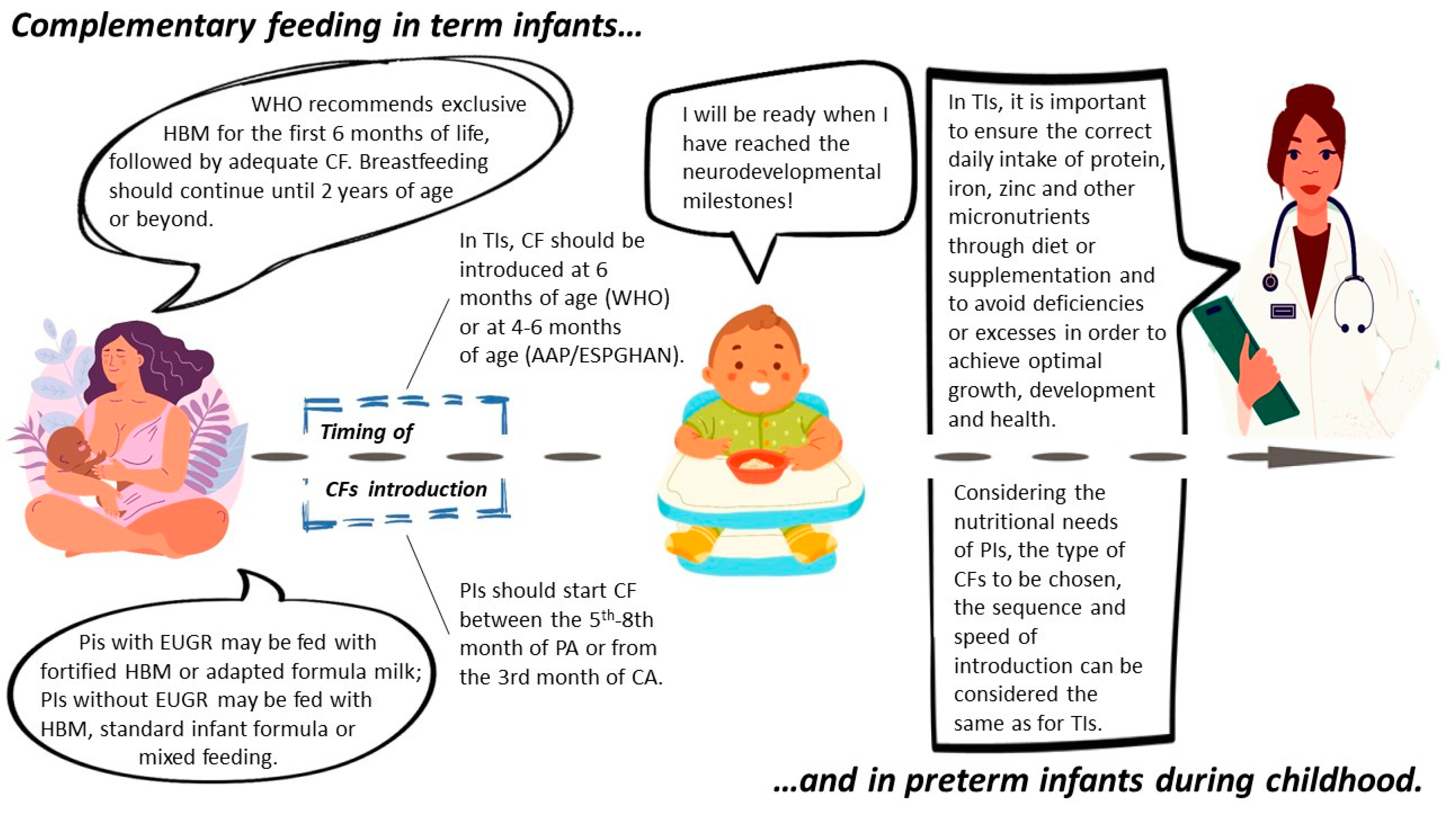
2.2. Traditional Complementary Feeding: Definition and Characteristics
2.3. Complementary Feeding Patterns Worldwide
2.4. Complementary Feeding in Preterm Infants
3. Baby-Led Weaning and On-Demand Complementary Feeding
4. Plant-Based Complementary Feeding
5. Complementary Feeding Practices and Risk for Non-Communicable Diseases
5.1. Overweight and Obesity
5.2. Type 1 Diabetes
5.3. Celiac Disease
6. Complementary Feeding and Food Allergy
Timing of Solid Food Introduction and Risk of Food Allergy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AAP | American Academy of Pediatrics |
| AD | Atopic Dermatitis |
| ALA | Alfa-Linolenic Acid |
| BB | Breastfed |
| BLCF | Baby-Led Complementary Feeding |
| BLISS | Baby-Led Introduction to SolidS |
| BLW | Baby-Led Weaning |
| CD | Celiac Disease |
| CF | Complementary feeding |
| CFs | Complementary foods |
| CM | Cow Milk |
| DHA | Docosahexaenoic Acid |
| EA | Egg Allergy |
| EAT | Enquiring About Tolerance |
| EFSA | European Food Safety Authority |
| ESPGHAN | European Society for Paediatric Gastroenterology Hepatology and Nutrition |
| EUGR | Extra Uterine Growth Restriction |
| FA | Food Allergy |
| FIMP | Italian Federation of Pediatricians |
| FF | Formula-fed |
| HEAP | Hens Egg Allergy Prevention |
| HM | Human Breast Milk |
| ID | Iron Deficiency |
| IDA | Iron-Deficiency Anemia |
| IGF 1 | Insulin-Like Growth Factor 1 |
| LA | Linoleic Acid |
| LCPUFAs | Long-Chain Polyunsaturated Fatty Acids |
| LEAP | Learning Early About Peanut Allergy Study |
| NCDs | Non-Communicable Diseases |
| NRCF | Non-Responsive Complementary Feeding |
| PETIT | Prevention of Egg Allergy with Tiny Amount Intake |
| PLW | Parent-Led Weaning |
| PCPs | Primary Care Pediatricians |
| PIs | Preterm Infants |
| RCF | Responsive Complementary Feeding |
| SACN | UK Scientific Advisory Committee on Nutrition |
| SIDOAhD | Italian Society for Developmental Origins of Health and Disease |
| SIGENP | Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition |
| SIMP | Italian Society of Perinatal Medicine |
| SIN | Italian Society of Neonatology |
| SIP | Italian Pediatric Society |
| SPT | Skin Prick Tests |
| STEP | Australian Study Starting Time of Egg Protein |
| SW | Standard Weaning |
| TEDDY | The Environmental Determinants of Diabetes in the Young |
| T1DM | Type 1 Diabetes Mellitus |
| T2DM | Type 2 Diabetes Mellitus |
| TRIGR | Trial to Reduce IDDM in the Genetically at-Risk |
| Tis | Term Infants |
| WHO | World Health Organization |
References
- World Health Organization; UNICEF. Strengthening Action to Improve Feeding of Infants and Young Children 6–23 Months of Age in Nutrition and Child Health Programmes: Geneva, 6–9 October 2008. Report of Proceedings. Available online: https://iris.who.int/bitstream/handle/10665/44034/9789241597890%20eng.pdf;jsessionid=87A00C0BA248EC40EE35A22A2E9984DB?sequence=1 (accessed on 4 September 2023).
- Barker, D.J.P. Editorial: The developmental origins of adult disease. Eur. J. Epidemiol. 2002, 18, 733–736. [Google Scholar] [CrossRef]
- Brambilla, P.; Giussani, M.; Picca, M.; Bottaro, G.; Buzzetti, R.; Milani, G.P.; Agostoni, C.; Becherucci, P. Do the opinions of pediatricians influence their recommendations on complementary feeding? Preliminary results. Eur. J. Pediatr. 2020, 179, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Hamer, D.H.; Solomon, H.; Das, G.; Knabe, T.; Beard, J.; Simon, J.; Nisar, Y.B.; MacLeod, W.B. Importance of breastfeeding and complementary feeding for management and prevention of childhood diarrhoea in low- and middle-income countries. J. Glob. Health 2022, 12, 10011. [Google Scholar] [CrossRef] [PubMed]
- Gatica-Domínguez, G.; Neves, P.A.; Barros, A.J.; Victora, C.G. Complementary Feeding Practices in 80 Low- and Middle-Income Countries: Prevalence of and Socioeconomic Inequalities in Dietary Diversity, Meal Frequency, and Dietary Adequacy. J. Nutr. 2021, 151, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Βasdeki, A.M.; Fatouros, D.G.; Βiliaderis, C.G.; Moschakis, T. Physicochemical properties of human breast milk during the second year of lactation. Curr. Res. Food Sci. 2021, 4, 565–576. [Google Scholar] [CrossRef]
- Skórka, A.; Pieścik-Lech, M.; Kołodziej, M.; Szajewska, H. Infant formulae supplemented with prebiotics: Are they better than unsupplemented formulae? An updated systematic review. Br. J. Nutr. 2018, 119, 810–825. [Google Scholar] [CrossRef]
- Riva, E.; Verduci, E.; Agostoni, C.; Giovannini, M. Closer to the gold standard: An appraisal of formulae available in Italy for use in formula-fed infants. J. Int. Med. Res. 2005, 33, 595–611. [Google Scholar] [CrossRef]
- Capra, M.E.; Stanyevic, B.; Giudice, A.; Monopoli, D.; Decarolis, N.M.; Esposito, S.; Biasucci, G. Long-Chain Polyunsaturated Fatty Acids Effects on Cardiovascular Risk in Childhood: A Narrative Review. Nutrients 2023, 15, 1661. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Martin, C.R.; Ling, P.R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, 279. [Google Scholar] [CrossRef]
- Underwood, M.A. Human milk for the premature infant. Pediatr. Clin. N. Am. 2013, 60, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Baker, S.; Cleghorn, G.; Neto, U.F.; Gopalan, S.; Hernell, O.; Hock, Q.S.; Jirapinyo, P.; Lonnerdal, B.; Pencharz, P.; et al. Global standard for the composition of infant formula: Recommendations of an ESPGHAN coordinated international expert group. J. Pediatr. Gastroenterol. Nutr. 2005, 41, 584–599. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Exclusive Breastfeeding for Optimal Growth, Development and Health of Infants. Available online: https://www.who.int/tools/elena/interventions/exclusive-breastfeeding (accessed on 2 September 2023).
- Pan American Health Organization, World Health Organization. Guiding principles for Complementary Feeding of the Breastfed Children. Available online: https://www.who.int/publications/i/item/9275124604 (accessed on 4 September 2023).
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens); Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.-I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Scientific Opinion on the appropriate age range for introduction of complementary feeding into an infant’s diet. EFSA J. 2019, 17, 5780. [Google Scholar] [CrossRef]
- Dewey, K. Guiding Principles for Feeding Non-Breastfed Children 6–24 Months of Age; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Gartner, L.M.; Morton, J.; Lawrence, R.A.; Naylor, A.J.; O’Hare, D.; Schanler, R.J.; Eidelman, A.I. Breastfeeding and the use of human milk. Pediatrics 2005, 115, 496–506. [Google Scholar] [PubMed]
- Section on Breastfeeding; Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the use of human milk. Pediatrics 2012, 129, e827–e841. [Google Scholar]
- Fernandes, C.; Martins, F.; Santos, A.F.; Fernandes, M.; Veríssimo, M. Complementary Feeding Methods: Associations with Feeding and Emotional Responsiveness. Children 2023, 10, 464. [Google Scholar] [CrossRef]
- Naylor, A.J.; Morrow, A.L. Developmental Readiness of Normal Full Term Infants to Progress from Exclusive Breastfeeding to the Introduction of Complementary Foods: Reviews of the Relevant Literature Concerning Infant Immunologic, Gastrointestinal, Oral Motor and Maternal Reproductive and Lactational Development; The Linkages Project and Wellstart International: Washington, DC, USA, 2001. [Google Scholar]
- Caroli, M.; Vania, A.; Verga, M.C.; Di Mauro, G.; Bergamini, M.; Cuomo, B.; D’Anna, R.; D’Antonio, G.; Dello Iacono, I.; Dessì, A.; et al. Recommendations on Complementary Feeding as a Tool for Prevention of Non-Communicable Diseases (NCDs)-Paper Co-Drafted by the SIPPS, FIMP, SIDOHaD, and SINUPE Joint Working Group. Nutrients 2022, 14, 257. [Google Scholar] [CrossRef]
- Acevedo, N.; Alhamwe, B.A.; Caraballo, L.; Ding, M.; Ferrante, A.; Garn, H.; Garssen, J.; Hii, C.S.; Irvine, J.; Llinás-Caballero, K.; et al. Perinatal and early-life nutrition, epigenetics, and allergy. Nutrients 2021, 13, 724. [Google Scholar] [CrossRef]
- Domellöf, M.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Iron requirements of infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 119–129. [Google Scholar] [CrossRef]
- Obbagy, J.E.; English, L.K.; Psota, T.L.; Wong, Y.P.; Butte, N.F.; Dewey, K.G.; Fox, M.K.; Greer, F.R.; Krebs, N.F.; Scanlon, K.S.; et al. Complementary feeding and micronutrient status: A systematic review. Am. J. Clin. Nutr. 2019, 109 (Suppl. S7), 852S–871S. [Google Scholar] [CrossRef] [PubMed]
- Miniello, V.L.; Verga, M.C.; Miniello, A.; Di Mauro, C.; Diaferio, L.; Francavilla, R. Complementary feeding and iron status: “the unbearable lightness of being” infants. Nutrients 2021, 13, 4201. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.; Heath, A.L.; Williams, S.M.; Cameron, S.L.; Fleming, E.A.; Taylor, B.J.; Wheeler, B.J.; Gibson, R.S.; Taylor, R.W. Baby-Led Introduction to SolidS (BLISS) study: A randomised controlled trial of a baby-led approach to complementary feeding. BMC Pediatr. 2015, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on the essential composition of infant and follow-on formulae. EFSA J. 2014, 12, 3760. [Google Scholar] [CrossRef]
- Sazawal, S.; Black, R.E.; Menon, V.P.; Dinghra, P.; Caulfield, L.E.; Dhingra, U.; Bagati, A. Zinc supplementation in infants born small for gestational age reduces mortality: A prospective, randomized, controlled trial. Pediatrics 2001, 108, 1280–1286. [Google Scholar] [CrossRef]
- Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Domellof, M.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R.; et al. Vitamin D in the healthy European paediatric population. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 692–701. [Google Scholar] [CrossRef]
- Corsello, A.; Milani, G.P.; Giannì, M.L.; Dipasquale, V.; Romano, C.; Agostoni, C. Different Vitamin D Supplementation Strategies in the First Years of Life: A Systematic Review. Healthcare 2022, 10, 1023. [Google Scholar] [CrossRef]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef]
- Taylor, S.N.; Fenton, T.R.; Groh-Wargo, S.; Gura, K.; Martin, C.R.; Griffin, I.J.; Rozga, M.; Moloney, L. Exclusive Maternal Milk Compared with Exclusive Formula on Growth and Health Outcomes in Very-Low-Birthweight Preterm Infants: Phase II of the Pre-B Project and an Evidence Analysis Center Systematic Review. Front. Pediatr. 2022, 9, 793311. [Google Scholar] [CrossRef]
- Koletzko, B.; Dodds, P.F.; Akerblom, H.; Ashwell, M. Early Nutrition and Its Later Consequences: New Opportunities; Springer Publishers: New York, NY, USA, 2005. [Google Scholar]
- Victor, R.; Baines, S.K.; Agho, K.E.; Dibley, M.J. Factors associated with inappropriate complementary feeding practices among children aged 6-23 months in Tanzania. Matern. Child Nutr. 2014, 10, 545–561. [Google Scholar] [CrossRef] [PubMed]
- Kabir, I.; Khanam, M.; Agho, K.E.; Mihrshahi, S.; Dibley, M.J.; Roy, S.K. Determinants of inappropriate complementary feeding practices in infant and young children in Bangladesh: Secondary data analysis of Demographic Health Survey 2007. Matern. Child Nutr. 2012, 8 (Suppl. S1), 11–27. [Google Scholar] [CrossRef] [PubMed]
- Arikpo, D.; Edet, E.S.; Chibuzor, M.T.; Odey, F.; Caldwell, D.M. Educational interventions for improving primary caregiver complementary feeding practices for children aged 24 months and under. Cochrane Database Syst. Rev. 2018, 5, CD011768. [Google Scholar] [CrossRef] [PubMed]
- Fein, S.B.; Labiner-Wolfe, J.; Scanlon, K.S.; Grummer-Strawn, L.M. Selected complementary feeding practices and their association with maternal education. Pediatrics 2008, 122 (Suppl. S2), S91–S97. [Google Scholar] [CrossRef] [PubMed]
- Caroli, M.; Mele, R.M.; Tomaselli, M.A.; Cammisa, M.; Longo, F.; Attolini, E. Complementary feeding patterns in Europe with a special focus on Italy. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Sirkka, O.; Abrahamse-Berkeveld, M.; van der Beek, E.M. Complementary Feeding Practices among Young Children in China, India, and Indonesia: A Narrative Review. Curr. Dev. Nutr. 2022, 6, nzac092. [Google Scholar] [CrossRef]
- Schiess, S.; Grote, V.; Scaglioni, S.; Luque, V.; Martin, F.; Stolarczyk, A.; Vecchi, F.; Koletzko, B.; European Childhood Obesity Project. Introduction of complementary feeding in 5 European countries. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 92–98. [Google Scholar] [CrossRef]
- Giovannini, M.; Riva, E.; Banderali, G.; Scaglioni, S.; Veehof, S.H.; Sala, M.; Radaelli, G.; Agostoni, C. Feeding practices of infants through the first year of life in Italy. Acta Paediatr. 2004, 93, 492–497. [Google Scholar] [CrossRef]
- Wright, C.M.; Parkinson, K.N.; Drewett, R.F. Why are babies weaned early? Data from a prospective population based cohort study. Arch. Dis. Child. 2004, 89, 813–816. [Google Scholar] [CrossRef]
- Brekke, H.K.; Ludvigsson, J.F.; van Odijk, J.; Ludvigsson, J. Breastfeeding and introduction of solid foods in Swedish infants: The All Babies in Southeast Sweden study. Br. J. Nutr. 2005, 94, 377–382. [Google Scholar] [CrossRef]
- Riskin, A. Meeting the nutritional needs of premature babies: Their future is in our hands. Br. J. Hosp. Med. 2017, 78, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, M.E.; Panza, R.; Cresi, F.; Salvatori, G.; Corvaglia, L.; Aceti, A.; Giannì, M.L.; Liotto, N.; Ilardi, L.; Laforgia, N.; et al. Complementary feeding in preterm infants: A position paper by Italian neonatal, paediatric and paediatric gastroenterology joint societies. Ital. J. Pediatr. 2022, 48, 143. [Google Scholar] [CrossRef] [PubMed]
- Liotto, N.; Cresi, F.; Beghetti, I.; Roggero, P.; Menis, C.; Corvaglia, L.; Mosca, F.; Aceti, A.; on Behalf of the Study Group on Neonatal Nutrition and Gastroenterology-Italian Society of Neonatology. Complementary Feeding in Preterm Infants: A Systematic Review. Nutrients 2020, 12, 1843. [Google Scholar] [CrossRef] [PubMed]
- DHSS (Department of Health and Social Security). Present day practice in infant feeding. Lancet 1988, 331, 975–976. [Google Scholar] [CrossRef]
- King, C. An evidence based guide to weaning preterm infants. Paediatr. Child Health 2009, 19, 405–414. [Google Scholar] [CrossRef]
- Palmer, D.J.; Makrides, M. Introducing solid foods to preterm infants in developed countries. Ann. Nutr. Metab. 2012, 60 (Suppl. S2), 31–38. [Google Scholar] [CrossRef] [PubMed]
- Fanaro, S.; Borsari, G.; Vigi, V. Complementary feeding practices in preterm infants: An observational study in a cohort of Italian infants. J. Pediatr. Gastroenterol. Nutr. 2007, 45 (Suppl. S3), S210–S214. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, M.A.; Rust, P.; Masztalerz-Kozubek, D.; Bichler, J.; Hamułka, J. Factors Influencing the Age of Complementary Feeding-A Cross-Sectional Study from Two European Countries. Int. J. Environ. Res. Public Health 2019, 16, 3799. [Google Scholar] [CrossRef]
- Cleary, J.; Dalton, S.M.; Harman, A.; Wright, I.M. Current practice in the introduction of solid foods for preterm infants. Public Health Nutr. 2020, 23, 94–101. [Google Scholar] [CrossRef]
- Giannì, M.L.; Bezze, E.; Colombo, L.; Rossetti, C.; Pesenti, N.; Roggero, P.; Sannino, P.; Muscolo, S.; Plevani, L.; Mosca, F. Complementary Feeding Practices in a Cohort of Italian Late Preterm Infants. Nutrients 2018, 10, 1861. [Google Scholar] [CrossRef]
- Braid, S.; Harvey, E.M.; Bernstein, J.; Matoba, N. Early introduction of complementary foods in preterm infants. J. Pediatr. Gastroenterol. Nutr. 2015, 60, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Menezes, L.V.P.; Steinberg, C.; Nóbrega, A.C. Complementary feeding in infants born prematurely. Codas 2018, 30, e20170157. [Google Scholar] [CrossRef] [PubMed]
- Crapnell, T.L.; Rogers, C.E.; Neil, J.J.; Inder, T.E.; Woodward, L.J.; Pineda, R.G. Factors associated with feeding difficulties in the very preterm infant. Acta Paediatr. 2013, 102, e539–e545. [Google Scholar] [CrossRef] [PubMed]
- Lau, C. Development of infant oral feeding skills: What do we know? Am. J. Clin. Nutr. 2016, 103, 616S–621S. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, K.; Spittle, A.J.; Slattery, J.M.; Morgan, A.T. Oromotor Feeding in Children Born Before 30 Weeks’ Gestation and Term-Born Peers at 12 Months’ Corrected Age. J. Pediatr. 2016, 178, 113–118.e1. [Google Scholar] [CrossRef] [PubMed]
- Lipner, H.S.; Huron, R.F. Developmental and Interprofessional Care of the Preterm Infant: Neonatal Intensive Care Unit through High-Risk Infant Follow-up. Pediatr. Clin. N. Am. 2018, 65, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Elfzzani, Z.; Kwok, T.C.; Dorling, J. Education of family members to support weaning to solids and nutrition in later infancy in term-born infants. Cochrane Database Syst. Rev. 2020, 7, CD012241. [Google Scholar] [CrossRef] [PubMed]
- Chiale, F.; Maggiora, E.; Aceti, A.; Liotto, N.; Coscia, A.; Peila, C.; Baldassarre, M.E.; Bertino, E.; Cresi, F. Complementary Feeding: Recommendations for the Introduction of Allergenic Foods and Gluten in the Preterm Infant. Nutrients 2021, 13, 2477. [Google Scholar] [CrossRef]
- Capra, M.E.; Monopoli, D.; Decarolis, N.M.; Giudice, A.; Stanyevic, B.; Esposito, S.; Biasucci, G. Dietary Models and Cardiovascular Risk Prevention in Pediatric Patients. Nutrients 2023, 15, 3664. [Google Scholar] [CrossRef]
- Bivi, D.; Di Chio, T.; Geri, F.; Morganti, R.; Goggi, S.; Baroni, L.; Mumolo, M.G.; de Bortoli, N.; Peroni, D.G.; Marchi, S.; et al. Raising Children on a Vegan Diet: Parents’ Opinion on Problems in Everyday Life. Nutrients 2021, 13, 1796. [Google Scholar] [CrossRef]
- Baldassarre, M.E.; Panza, R.; Farella, I.; Posa, D.; Capozza, M.; Mauro, A.D.; Laforgia, N. Vegetarian and Vegan Weaning of the Infant: How Common and How Evidence-Based? A Population-Based Survey and Narrative Review. Int. J. Environ. Res. Public Health 2020, 17, 4835. [Google Scholar] [CrossRef] [PubMed]
- ESPGHAN Committee on Nutrition; Aggett, P.J.; Agostoni, C.; Axelsson, I.; De Curtis, M.; Goulet, O.; Hernell, O.; Koletzko, B.; Lafeber, H.N.; Michaelsen, K.F.; et al. Feeding preterm infants after hospital discharge: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Mazzantini, S.; Zuccotti, G.V. Nutrition in the First 1000 Days: The Origin of Childhood Obesity. Int. J. Environ. Res. Public Health 2016, 13, 838. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Moreno, L.; Puntis, J.; Rigo, J.; et al. Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Rapley, G.; Forste, R.; Cameron, S.; Brown, A.; Wright, C. Baby-Led Weaning: A New Frontier? ICAN Infant Child Adolesc. Nutr. 2015, 7, 77–85. [Google Scholar] [CrossRef]
- Brown, A.; Jones, S.W.; Rowan, H. Baby-Led Weaning: The Evidence to Date. Curr. Nutr. Rep. 2017, 6, 148–156. [Google Scholar] [CrossRef]
- Taylor, R.W.; Williams, S.M.; Fangupo, L.J.; Wheeler, B.J.; Taylor, B.J.; Daniels, L.; Fleming, E.A.; McArthur, J.; Morison, B.; Erickson, L.W.; et al. Effect of a Baby-Led Approach to Complementary Feeding on Infant Growth and Overweight: A Randomized Clinical Trial. JAMA Pediatr. 2017, 171, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Conlon, C.A.; Haszard, J.J.; Beck, K.L.; von Hurst, P.R.; Taylor, R.W.; Heath, A.M. Food fussiness and early feeding characteristics of infants following Baby-Led Weaning and traditional spoon-feeding in New Zealand: An internet survey. Appetite 2018, 130, 110–116. [Google Scholar] [CrossRef]
- Komninou, S.; Halford, J.C.G.; Harrold, J.A. Differences in parental feeding styles and practices and toddler eating behaviour across complementary feeding methods: Managing expectations through consideration of effect size. Appetite 2019, 137, 198–206. [Google Scholar] [CrossRef]
- Cameron, S.L.; Heath, A.L.; Taylor, R.W. How feasible is Baby-led Weaning as an approach to infant feeding? A review of the evidence. Nutrients 2012, 4, 1575–1609. [Google Scholar] [CrossRef]
- Alpers, B.; Blackwell, V.; Clegg, M.E. Standard v. baby-led complementary feeding: A comparison of food and nutrient intakes in 6-12-month-old infants in the UK. Public Health Nutr. 2019, 22, 2813–2822. [Google Scholar] [CrossRef] [PubMed]
- Rowan, H.; Lee, M.; Brown, A. Differences in dietary composition between infants introduced to complementary foods using Baby-led weaning and traditional spoon feeding. J. Hum. Nutr. Diet. 2019, 32, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.; Taylor, R.W.; Williams, S.M.; Gibson, R.S.; Fleming, E.A.; Wheeler, B.J.; Taylor, B.J.; Haszard, J.J.; Heath, A.M. Impact of a modified version of baby-led weaning on iron intake and status: A randomised controlled trial. BMJ Open 2018, 8, e019036. [Google Scholar] [CrossRef] [PubMed]
- Carruth, B.R.; Skinner, J.D. Feeding behaviors and other motor development in healthy children (2–24 months). J. Am. Coll. Nutr. 2002, 21, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Özyüksel, G.; Soyer, T.; Üzümcügil, F.; Yalçın, Ş.; Ekinci, S.; Karnak, İ.; Çiftçi, A.Ö.; Tanyel, F.C. Foreign Body Aspiration in Infants: Role of Self-Feeding. Pediatr. Allergy Immunol. Pulmonol. 2019, 32, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Fangupo, L.J.; Heath, A.M.; Williams, S.M.; Erickson Williams, L.W.; Morison, B.J.; Fleming, E.A.; Taylor, B.J.; Wheeler, B.J.; Taylor, R.W. A Baby-Led Approach to Eating Solids and Risk of Choking. Pediatrics 2016, 138, e20160772. [Google Scholar] [CrossRef] [PubMed]
- Boswell, N. Complementary Feeding Methods-A Review of the Benefits and Risks. Int. J. Environ. Res. Public Health 2021, 18, 7165. [Google Scholar] [CrossRef]
- Alvisi, P.; Congiu, M.; Ficara, M.; De Gregorio, P.; Ghio, R.; Spisni, E.; Di Saverio, P.; Labriola, F.; Lacorte, D.; Lionetti, P. Complementary Feeding in Italy: From Tradition to Innovation. Children 2021, 8, 638. [Google Scholar] [CrossRef]
- Addessi, E.; Galloway, A.T.; Wingrove, T.; Brochu, H.; Pierantozzi, A.; Bellagamba, F.; Farrow, C.V. Baby-led weaning in Italy and potential implications for infant development. Appetite 2021, 164, 105286. [Google Scholar] [CrossRef]
- Bergamini, M.; Simeone, G.; Verga, M.C.; Doria, M.; Cuomo, B.; D’Antonio, G.; Dello Iacono, I.; Di Mauro, G.; Leonardi, L.; Miniello, V.L.; et al. Complementary Feeding Caregivers’ Practices and Growth, Risk of Overweight/Obesity, and Other Non-Communicable Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2646. [Google Scholar] [CrossRef]
- Ferrara, P.; Corsello, G.; Quattrocchi, E.; Dell’Aquila, L.; Ehrich, J.; Giardino, I.; Pettoello-Mantovani, M. Caring for Infants and Children Following Alternative Dietary Patterns. J. Pediatr. 2017, 187, 339–340.e1. [Google Scholar] [CrossRef] [PubMed]
- Eurispes. Rapporto Italia 2023. Available online: https://eurispes.eu/news/risultati-del-rapporto-italia-2023/ (accessed on 1 October 2023).
- Simeone, G.; Bergamini, M.; Verga, M.C.; Cuomo, B.; D’Antonio, G.; Iacono, I.D.; Mauro, D.D.; Mauro, F.D.; Mau-ro, G.D.; Leonardi, L.; et al. Do Vegetarian Diets Provide Adequate Nutrient Intake during Complementary Feeding? A Systematic Review. Nutrients 2022, 14, 3591. [Google Scholar] [CrossRef] [PubMed]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council (Hg). Eat for Health. Australian Dietary Guidelines; National Health and Medical Research Council: Canberra, Australia, 2013.
- Direção-Geral da Saúde (Hg). National Programme for the Promotion of a Healthy Diet, Guidelines for a Healthy Vegetarian Diet; Direção-Geral da Saúde: Lissabon, Portugal, 2015. [Google Scholar]
- Amit, M. Vegetarian diets in children and adolescents. Paediatr. Child Health 2010, 15, 303–314. [Google Scholar]
- Richter, M.; Boeing, H.; Grünewald-Funk, D.; Heseker, H.; Kroke, A.; Leschik-Bonnet, E.; Oberritter, H.; Strohm, D.; Watzl, B. Vegan Diet Position of the German Nutrition Society (DGE). Ernaehrungs Umsch. Int. 2016, 63, 92–102. [Google Scholar]
- SIPPS; FIMP; SIMA; SIMP. Position Paper—Diete vegetariane in gravidanza ed età evolutiva. Riv. Ital. Pediatr. Prev. Soc. 2017, 12 (Suppl. S3), 119–193. [Google Scholar]
- Mangels, A.R.; Messina, V. Considerations in planning vegan diets: Infants. J. Am. Diet. Assoc. 2001, 101, 670–677. [Google Scholar] [CrossRef]
- Farella, I.; Panza, R.; Baldassarre, M.E. The Difficult Alliance between Vegan Parents and Pediatrician: A Case Report. Int. J. Environ. Res. Public Health 2020, 17, 6380. [Google Scholar] [CrossRef]
- Budreviciute, A.; Damiati, S.; Sabir, D.K.; Onder, K.; Schuller-Goetzburg, P.; Plakys, G.; Katileviciute, A.; Khoja, S.; Kodzius, R. Management and Prevention Strategies for Non-communicable Diseases (NCDs) and Their Risk Factors. Front. Public Health 2020, 8, 788. [Google Scholar] [CrossRef]
- Mallisetty, Y.; Mukherjee, N.; Jiang, Y.; Chen, S.; Ewart, S.; Arshad, S.H.; Holloway, J.W.; Zhang, H.; Karmaus, W. Epigenome-Wide Association of Infant Feeding and Changes in DNA Methylation from Birth to 10 Years. Nutrients 2021, 13, 99. [Google Scholar] [CrossRef]
- Latorre-Millán, M.; Rupérez, A.I.; González-Gil, E.M.; Santaliestra-Pasías, A.; Vázquez-Cobela, R.; Gil-Campos, M.; Aguilera, C.M.; Gil, Á.; Moreno, L.A.; Leis, R.; et al. Dietary patterns and their association with body composition and cardiometabolic markers in children and adolescents: Genobox cohort. Nutrients 2020, 12, 3424. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Foskey, R.J.; Allen, K.J.; Dharmage, S.C.; Koplin, J.J.; Ponsonby, A.L.; Lowe, A.J.; Matheson, M.C.; Tang, M.L.; Gurrin, L.; et al. The Impact of Timing of Introduction of Solids on Infant Body Mass Index. J. Pediatr. 2016, 179, 104–110.e1. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.W.; Lee, M.; Brown, A. Spoonfeeding is associated with increased infant weight but only amongst formula-fed infants. Matern. Child Nutr. 2020, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Martinón-Torres, N.; Carreira, N.; Picáns-Leis, R.; Pérez-Ferreirós, A.; Kalén, A.; Leis, R. Baby-led weaning: What role does it play in obesity risk during the first years? A systematic review. Nutrients 2021, 13, 1009. [Google Scholar] [CrossRef] [PubMed]
- Dogan, E.; Yilmaz, G.; Caylan, N.; Turgut, M.; Gokcay, G.; Oguz, M.M. Baby-led complementary feeding: Randomized controlled study. Pediatr. Int. 2018, 60, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, M.; Makki, K.; Storelli, G.; Machuca-Gayet, I.; Srutkova, D.; Hermanova, P.; Martino, M.E.; Balmand, S.; Hudcovic, T.; Heddi, A.; et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 2016, 351, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensen, J.M.; et al. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016, 351, aad3311. [Google Scholar] [CrossRef]
- Kamng’ona, A.W.; Young, R.; Arnold, C.D.; Kortekangas, E.; Patson, N.; Jorgensen, J.M.; Prado, E.L.; Chaima, D.; Malamba, C.; Ashorn, U.; et al. The association of gut microbiota characteristics in Malawian infants with growth and inflammation. Sci. Rep. 2019, 9, 12893. [Google Scholar] [CrossRef]
- Forbes, J.D.; Azad, M.B.; Vehling, L.; Tun, H.M.; Konya, T.B.; Guttman, D.S.; Field, C.J.; Lefebvre, D.; Sears, M.R.; Becker, A.B.; et al. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. 2018, 172, e181161. [Google Scholar] [CrossRef]
- Townsend, E.; Pitchford, N.J. Baby knows best? The impact of weaning style on food preferences and body mass index in early childhood in a case-controlled sample. BMJ Open 2012, 2, e000298. [Google Scholar] [CrossRef]
- Tang, M.; Matz, K.L.; Berman, L.M.; Davis, K.N.; Melanson, E.L.; Frank, D.N.; Hendricks, A.E.; Krebs, N.F. Effects of Complementary Feeding with Different Protein-Rich Foods on Infant Growth and Gut Health: Study Protocol. Front. Pediatr. 2022, 9, 793215. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Ma, C.; Weinheimer-Haus, E.M.; Robertson, C.E.; Kofonow, J.M.; Berman, L.M.; Waljee, A.; Zhu, J.; Frank, D.N.; Krebs, N.F. Different gut microbiota in U.S. formula-fed infants consuming a meat vs. dairy-based complementary foods: A randomized controlled trial. Front. Nutr. 2023, 9, 1063518. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Hachelaf, W.; Suau, A.; Boudraa, G.; Mangin, I.; Touhami, M.; Bouziane-Nedjadi, K.; Pochart, P. A longitudinal study of infant faecal microbiota during weaning. FEMS Microbiol. Ecol. 2006, 58, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Broekaert, I.; Demmelmair, H.; Franke, J.; Hannibal, I.; Oberle, D.; Schiess, S.; Baumann, B.T.; Verwied-Jorky, S. Protein intake in the first year of life: A risk factor for later obesity? The E.U. childhood obesity project. Adv. Exp. Med. Biol. 2005, 569, 69–79. [Google Scholar] [CrossRef]
- Grote, V.; Theurich, M. Complementary feeding and obesity risk. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Lund-Blix, N.A.; Stene, L.C.; Rasmussen, T.; Torjesen, P.A.; Andersen, L.F.; Rønningen, K.S. Infant feeding in relation to islet autoimmunity and type 1 diabetes in genetically susceptible children: The MIDIA study. Diabetes Care 2015, 38, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Knip, M.; Virtanen, S.M.; Seppä, K.; Ilonen, J.; Savilahti, E.; Vaarala, O.; Reunanen, A.; Teramo, K.; Hämäläinen, A.M.; Paronen, J.; et al. Dietary Intervention in Infancy and Later Signs of Beta-Cell Autoimmunity. N. Engl. J. Med. 2010, 363, 1900–1908. [Google Scholar] [CrossRef]
- Hyytinen, M.; Savilahti, E.; Virtanen, S.M.; Härkönen, T.; Ilonen, J.; Luopajärvi, K.; Uibo, R.; Vaarala, O.; Åkerblom, H.K.; Knip, M.; et al. Avoidance of Cow’s Milk–Based Formula for At-Risk Infants Does Not Reduce Development of Celiac Disease: A Randomized Controlled Trial. Gastroenterology 2017, 153, 961–970.e3. [Google Scholar] [CrossRef]
- Rewers, M.; Hyöty, H.; Lernmark, Å.; Hagopian, W.; She, J.X.; Schatz, D.; Ziegler, A.G.; Toppari, J.; Akolkar, B.; Krischer, J. The environmental determinants of diabetes in the young (TEDDY) study. Ann. N. Y. Acad. Sci. 2008, 1150, 1–13. [Google Scholar] [CrossRef]
- Hummel, S.; Weiß, A.; Bonifacio, E.; Agardh, D.; Akolkar, B.; Aronsson, C.A.; Hagopian, W.A.; Koletzko, S.; Krischer, J.P.; Lernmark, Å.; et al. Associations of breastfeeding with childhood autoimmunity, allergies, and overweight: The Environmental Determinants of Diabetes in the Young (TEDDY) study. Am. J. Clin. Nutr. 2021, 114, 134–142. [Google Scholar] [CrossRef]
- Uusitalo, U.; Mramba, L.K.; Aronsson, C.A.; Vehik, K.; Yang, J.; Hummel, S.; Lernmark, Å.; Rewers, M.; Hagopian, W.; McIndoe, R.; et al. HLA Genotype and Probiotics Modify the Association Between Timing of Solid Food Introduction and Islet Autoimmunity in the TEDDY Study. Diabetes Care 2023, 46, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Pieścik-Lech, M.; Chmielewska, A.; Shamir, R.; Szajewska, H. Systematic review: Early infant feeding and the risk of type 1 Diabetes. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Beyerlein, A.; Liu, X.; Uusitalo, U.M.; Harsunen, M.; Norris, J.M.; Foterek, K.; Virtanen, S.M.; Rewers, M.J.; She, J.X.; Simell, O.; et al. Dietary intake of soluble fiber and risk of islet autoimmunity by 5 y of age: Results from the TEDDY study. Am. J. Clin. Nutr. 2015, 102, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Niinistö, S.; Erlund, I.; Lee, H.S.; Uusitalo, U.; Salminen, I.; Aronsson, C.A.; Parikh, H.M.; Liu, X.; Hummel, S.; Toppari, J.; et al. Children’s erythrocyte fatty acids are associated with the risk of islet autoimmunity. Sci. Rep. 2021, 11, 3627. [Google Scholar] [CrossRef]
- Szajewska, H.; Shamir, R.; Mearin, L.; Ribes-Koninckx, C.; Catassi, C.; Domellöf, M.; Fewtrell, M.S.; Husby, S.; Papadopoulou, A.; Vandenplas, Y.; et al. Gluten introduction and the risk of coeliac disease: A position paper by the european society for pediatric gastroenterology, hepatology, and nutrition. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Knip, M.; Åkerblom, H.K.; Becker, D.; Dosch, H.M.; Dupre, J.; Fraser, W.; Howard, N.; Ilonen, J.; Krischer, J.P.; Kordonouri, O.; et al. Hydrolyzed infant formula and early β-cell autoimmunity: A randomized clinical trial. JAMA 2014, 311, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- du Toit, G.; Tsakok, T.; Lack, S.; Lack, G. Prevention of food allergy. J. Allergy Clin. Immunol. 2016, 137, 998–1010. [Google Scholar] [CrossRef]
- Hong, X.; Ladd-Acosta, C.; Hao, K.; Sherwood, B.; Ji, H.; Keet, C.A.; Kumar, R.; Caruso, D.; Liu, X.; Wang, G.; et al. Epigenome-wide association study links site-specific DNA methylation changes with cow’s milk allergy. J. Allergy Clin. Immunol. 2016, 138, 908–911.e9. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics 2000, 106 Pt 1, 346–349. [Google Scholar] [CrossRef]
- Devonshire, A.L.; Robison, R.G. Prevention of food allergy. Allergy Asthma Proc. 2019, 40, 450–452. [Google Scholar] [CrossRef]
- Prescott, S.L.; Pawankar, R.; Allen, K.J.; Campbell, D.E.; Sinn, J.K.; Fiocchi, A.; Ebisawa, M.; Sampson, H.A.; Beyer, K.; Lee, B.W. A global survey of changing patterns of food allergy burden in children. World Allergy Organ. J. 2013, 6, 21. [Google Scholar] [CrossRef]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; American Academy of Pediatrics Committee on Nutrition; American Academy of Pediatrics Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 2008, 121, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.L.; Smith, P.; Tang, M.; Palmer, D.J.; Sinn, J.; Huntley, S.J.; Cormack, B.; Heine, R.G.; Gibson, R.A.; Makrides, M. The importance of early complementary feeding in the development of oral tolerance: Concerns and controversies. Pediatr. Allergy Immunol. 2008, 19, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, J.S.; Ogston, S.A.; Clark, A.; Florey, C.D.; Howie, P.W. Relation between early intro-duction of solid food to infants and their weight and illnesses during the first two years of life. BMJ 1993, 306, 1572–1576. [Google Scholar] [CrossRef] [PubMed]
- Zutavern, A.; Brockow, I.; Schaaf, B.; Bolte, G.; von Berg, A.; Diez, U.; Borte, M.; Herbarth, O.; Wichmann, H.E.; Heinrich, J.; et al. Timing of solid food introduction in relation to atopic dermatitis and atopic sensitization: Results from a prospective birth cohort study. Pediatrics 2006, 117, 401–411. [Google Scholar] [CrossRef] [PubMed]
- West, C.E.; D’Vaz, N.; Prescott, S.L. Dietary immunomodulatory factors in the development of immune tolerance. Curr. Allergy Asthma Rep. 2011, 11, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Zutavern, A.; Brockow, I.; Schaaf, B.; von Berg, A.; Diez, U.; Borte, M.; Kraemer, U.; Herbarth, O.; Behrendt, H.; Wichmann, H.E.; et al. Timing of solid food intro-duction in relation to eczema, asthma, allergic rhinitis, and food and inhalant sensiti-zation at the age of 6 years: Results from the prospective birth cohort study LISA. Pediatrics 2008, 121, e44–e52. [Google Scholar] [CrossRef]
- Du Toit, G.; Katz, Y.; Sasieni, P.; Mesher, D.; Maleki, S.J.; Fisher, H.R.; Fox, A.T.; Turcanu, V.; Amir, T.; Zadik-Mnuhin, G.; et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J. Allergy Clin. Immunol. 2008, 122, 984–991. [Google Scholar] [CrossRef]
- Høst, A.; Halken, S.; Muraro, A.; Dreborg, S.; Niggemann, B.; Aalberse, R.; Arshad, S.H.; von Berg, A.; Carlsen, K.H.; Duschén, K.; et al. Dietary prevention of allergic diseases in infants and small children. Pediatr. Allergy Immunol. 2008, 19, 1–4. [Google Scholar] [CrossRef]
- Australasian Society of Clinical Immunology and Allergy. Infant Feeding and Allergy Prevention. Available online: http://www.allergy.org.au/images/pcc/ASCIA_guidelines_infant_feeding_and_allergy_prevention.pdf (accessed on 28 April 2019).
- Tham, E.H.; Shek, L.P.; Van Bever, H.P.; Vichyanond, P.; Ebisawa, M.; Wong, G.W.; Lee, B.W.; Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI). Early introduction of allergenic foods for the prevention of food allergy from an Asian perspective-An Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI) consensus statement. Pediatr. Allergy Immunol. 2018, 29, 18–27. [Google Scholar] [CrossRef]
- Fifty-Fourth World Health Assembly. Provisional Agenda Item 13.1.1. Global Strategy for Infant and Young Child Feeding: The Optimal Duration of Exclusive Breastfeeding; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized trial of peanut consumption in infants at risk for peanut allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Du Toit, G.; Sayre, P.H.; Roberts, G.; Sever, M.L.; Lawson, K.; Bahnson, H.T.; Brough, H.A.; Santos, A.F.; Harris, K.M.; Radulovic, S.; et al. Effect of Avoidance on Peanut Allergy after Early Peanut Consumption. N. Engl. J. Med. 2016, 374, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- du Toit, G.; Sayre, P.H.; Roberts, G.; Lawson, K.; Sever, M.L.; Bahnson, H.T.; Fisher, H.R.; Feeney, M.; Radulovic, S.; Basting, M.; et al. Allergen specificity of early peanut consumption and effect on development of allergic disease in the Learning Early About Peanut Allergy study cohort. J. Allergy Clin. Immunol. 2018, 141, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, D.M.; Sicherer, S.; Greenhawt, M.; Campbell, D.; Chan, E.S.; Muraro, A.; Halken, S.; Katz, Y.; Ebisawa, M.; Eichenfield, L.; et al. Consensus communication on early peanut introduction and the prevention of peanut allergy in high-risk infants. Allergy Asthma Clin. Immunol. 2015, 11, 23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Togias, A.; Cooper, S.F.; Acebal, M.L.; Assa’ad, A.; Baker, J.R., Jr.; Beck, L.A.; Block, J.; Byrd-Bredbenner, C.; Chan, E.S.; Eichenfield, L.F.; et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases-sponsored expert panel. J. Allergy Clin. Immunol. 2017, 139, 29–44. [Google Scholar] [CrossRef]
- Gupta, R.S.; Bilaver, L.A.; Johnson, J.L.; Hu, J.W.; Jiang, J.; Bozen, A.; Martin, J.; Reese, J.; Cooper, S.F.; Davis, M.M.; et al. Assessment of Pediatrician Awareness and Implementation of the Addendum Guidelines for the Prevention of Peanut Allergy in the United States. JAMA Netw. Open 2020, 3, e2010511. [Google Scholar] [CrossRef] [PubMed]
- Bellach, J.; Schwarz, V.; Ahrens, B.; Trendelenburg, V.; Aksünger, Ö.; Kalb, B.; Niggemann, B.; Keil, T.; Beyer, K. Randomized placebo-controlled trial of hen’s egg consumption for primary prevention in infants. J. Allergy Clin. Immunol. 2017, 139, 1591–1599.e2. [Google Scholar] [CrossRef]
- Palmer, D.J.; Sullivan, T.R.; Gold, M.S.; Prescott, S.L.; Makrides, M. Randomized controlled trial of early regular egg intake to prevent egg allergy. J. Allergy Clin. Immunol. 2017, 139, 1600–1607.e2. [Google Scholar] [CrossRef]
- Natsume, O.; Kabashima, S.; Nakazato, J.; Yamamoto-Hanada, K.; Narita, M.; Kondo, M.; Saito, M.; Kishino, A.; Takimoto, T.; Inoue, E.; et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 276–286. [Google Scholar] [CrossRef]
- Tham, E.H.; Lee, B.W.; Chan, Y.H.; Loo, E.X.L.; Toh, J.Y.; Goh, A.; Teoh, O.H.; Yap, F.; Tan, K.H.; Godfrey, K.M.; et al. Low Food Allergy Prevalence Despite Delayed Introduction of Allergenic Foods-Data from the GUSTO Cohort. J. Allergy Clin. Immunol. Pract. 2018, 6, 466–475.e1. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on the Evaluation of the Addition of Ingredients New to Infant Formula. Comparing Infant Formulas with Human Milk. National Academies Press (US): Washington, DC, USA, 2004. Available online: https://www.ncbi.nlm.nih.gov/books/NBK215837/ (accessed on 6 February 2019).
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; Committee on Nutrition; Section on Allergy and Immunology. The Effects of Early Nutritional Interventions on the Development of Atopic Disease in Infants and Children: The Role of Maternal Dietary Restriction, Breastfeeding, Hydrolyzed Formulas, and Timing of Introduction of Allergenic Complementary Foods. Pediatrics 2019, 143, e20190281. [Google Scholar] [CrossRef]
- Alvisi, P.; Brusa, S.; Alboresi, S.; Amarri, S.; Bottau, P.; Cavagni, G.; Corradini, B.; Landi, L.; Loroni, L.; Marani, M.; et al. Recommendations on complementary feeding for healthy, full-term infants. Ital. J. Pediatr. 2015, 41, 36. [Google Scholar] [CrossRef]
- Perkin, M.R.; Logan, K.; Marrs, T.; Radulovic, S.; Craven, J.; Flohr, C.; Lack, G.; EAT Study Team. Enquiring About Tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen. J. Allergy Clin. Immunol. 2016, 137, 1477–1486.e8. [Google Scholar] [CrossRef]
| Variable | Colostrum (1–5 Days) | Mature Milk (>14 Days) | Cows’ Milk–Based Starting Infant Formula (Min–Max) | Cows’Milk-Based Starting Infant Formula (Min–Max) |
|---|---|---|---|---|
| Energy | 50–60 kcal/100 mL | 65–70 kcal/100 mL | 60–70 kcal/100 mL | 60–70 kcal/100 mL |
| Carbohydrate | 50–62 g/L | 60–70 g/L | 9.0–14.0 g/100 kcal | 54–98 g/L |
| Total protein | 14–16 g/L | 8–10 g/L | 1.8–3 g/100 kcal | 10.8–21 g/L |
| Total fat | 15–20 g/L | 35–40 g/L | 4.4–6.0 g/100 kcal | 26–42 g/L |
| Iron | 0.5–1.0 mg/L | 0.3–0.7 mg/L | 0.3–1.3 mg/100 kcal | 2–9 mg/L |
| Calcium | 250 mg/L | 200–250 mg/L | 50–140 mg/100 kcal | 300–980 mg/L |
| Phosphorus | 120–160 mg/L | 120–140 mg/L | 25–90 mg/100 kcal | 150–630 mg/L |
| Magnesium | 30–35 mg/L | 30–35 mg/L | 5–15 mg/100 kcal | 30–105 mg/L |
| Sodium | 300–400 mg/L | 150–250 mg/L | 20–60 mg/100 kcal | 120–420 mg/L |
| Chloride | 600–800 mg/L | 400–450 mg/L | 50–160 mg/100 kcal | 300–1120 mg/L |
| Potassium | 600–700 mg/L | 400–550 mg/L | 60–160 mg/100 kcal | 360–1120 mg/L |
| Manganese | 5–12 μg/L | 3–4 μg/L | 1–50 μg/100 kcal | 6–350 ug/L |
| Iodine | 40–50 μg/L | 140–150 μg/L | 10–50 μg/100 kcal | 60–350 μg/L |
| Selenium | 25–32 μg/L | 10–25 μg/L | 1–9 μg/100 kcal | 9–63 μg/L |
| 6–12 Months | 12–24 Months | 24–36 Months | |
|---|---|---|---|
| Proteins | 14% | 14% | 14% |
| Carbohydrates | 45–55% | 45–60% | 45–60% |
| Fats | 40% | 35–40% | 35–40% |
| Fibers | 680–940 kcal/day | 10 g/day |
| FOOD | AGE | |||
|---|---|---|---|---|
| 6–9 Months | 9–12 Months | 12–18 Months | 18–24 Months | |
| Cereals creams (rice, corn, and tapioca) | 25–30 g | |||
| Baby pasta and rice | 25–30 g | |||
| Bread | 5–10 g | |||
| Vegetable broth | 30–40 g | |||
| Fruits | 40 g (fresh fruit) 40 g (fruit puree) | 50 g (twice a day) 40 g (fruit puree) | 50 g three times a day | |
| Vegetables | 20 g | 30 g | ||
| Fish | 20 g (fresh fish), 40 g (fish puree) | 25 g (fresh fish) | ||
| Meat | 10 g (fresh meat), 40 g (meat puree) | 15–20 g (fresh meat) | ||
| Eggs | ¼ well cooked | ½ well cooked | ||
| Legumes | 25 g (Fresh peas) 10 g (Dried legumes) 40 g (legumes puree) | 30 g (Fresh peas) 30 g (Fresh green beans) 15–20 g (Dried legumes) | ||
| Extra virgin olive oil | 10 g | |||
| Dietary Recommendations for Term Infants (TIs) 0–6 Months | ||
|---|---|---|
| American Academy of Pediatrics (AAP) | ESPGHAN | Italian Intersociety Document |
|
|
|
| Dietary recommendations for TIs 7–12 months | ||
| AAP | ESPGHAN | Italian Intersociety Document |
|
|
|
WHO
| ||
| Food | Iron Content (mg/100 g of Food) |
|---|---|
| Dried borlotti beans | 9 |
| Whole chicken egg | 6.3 |
| Oatmeal | 5.2 |
| Dried peas | 4.5 |
| Seabass | 4.1 |
| Pork meat | 4 |
| Horse meat | 3.9 |
| Lamb meat | 3.2 |
| Anchovies | 3.2 |
| Guinea fowl | 2.8 |
| Beef meat | 2.3 |
| Veal | 2.3 |
| Mackerel | 2.1 |
| Trout | 2 |
| Chicken Meat | 0.23 |
| Cow’s milk | 0.1–0.2 |
| Food | Zinc Content (mg/100 g of Food) |
|---|---|
| Grana Padano (cheese) | 11 |
| Lamb meat | 5.8 |
| Sardines | 5.7 |
| Turkey meat | 5.1 |
| Beef | 5 |
| Anchovies | 4.2 |
| Parmigiano Reggiano (Parmesan cheese) | 4 |
| Rabbit meat | 3.9 |
| Sardines | 3.9 |
| Dried cannellini beans | 3.6 |
| Pork meat | 3.5 |
| Guinea fowl | 3.8 |
| Dried chickpeas | 3.2 |
| Died borlotti beans/dried lentils | 2.9 |
| Chicken meat | 2.8 |
| Hen eggs, yolk | 2.14 |
| Item | Recommendation for Preterm Infants (PIs) | Evidence |
|---|---|---|
| Recommended time for initiation of complementary feeding | PIs should start complementary feeding (CF) between 5 and 8 months of postnatal age (PA) or from 3 months of correct age (CA), so that the neurodevelopmental milestones are attained. | Certainty of evidence: moderate; grade of recommendation: strong. |
| Management of PIs with comorbidities and/or oral dysfunction | Preterm infants with comorbidities or oral dysfunctions may require a multidisciplinary assessment to evaluate when and how CF should be started. | Certainty of evidence: low; grade of recommendation: weak. |
| Complementary Foods (CFs) recommended during CF | Recommendations for PIs regarding type of foods to choose, sequence and speed of introduction may be considered the same as for term infants, currently. Consider starting CF encompassing sources of carbohydrates, proteins and vegetable fats (extra-virgin olive oil) and paying special attention to the intake of micronutrients (e.g., iron and vitamins). | Certainty of evidence: low; grade of recommendation: weak. |
| Risk of developing overweight/obesity in relation to an early onset of CF | Timing of CF start in PIs is unlikely to influence the incidence of overweight and obesity in childhood and adulthood, so the onset of CF should not be delayed for this purpose. | Certainty of evidence: moderate; grade of recommendation: strong. |
| Risk of developing allergy in relation to an early onset of CF | The introduction of allergenic foods (e.g., eggs, fish, tomato, peanuts) may not be delayed in PIs. | Certainty of evidence: very low; grade of recommendation: weak. |
| Vegetarian and Vegan CF regimens in PIs | Vegetarian and vegan weaning may be carefully planned in PIs. | Certainty of evidence: very low; grade of recommendation: weak. |
| Recommended type of lactation during CF | Infants without Extra Uterine Growth Restriction (EUGR) may be fed with exclusive human breast milk (HM), standard infant formula enriched with long-chain polyunsaturated fatty acids (LCPUFAs), or mixed feeding (in case of inadequate amounts of HM). Infants with EUGR or at high risk of long-term growth failure may be fed with fortified HM or formula milk adapted for PIs as long as necessary to gain an optimal weight for CA. | Certainty of evidence: low; grade of recommendation: weak. |
| Traditional Weaning (TW) | Baby-Led Weaning (BLW) | BLISS (Baby-Led Introduction to SolidS) | On-Demand Complementary Feeding | |
|---|---|---|---|---|
| Parents involvement | Yes (spoon feeding) | No (use of hands) | No (use of hands), parents are instructed about relatives’ concerns | Yes (possibly use of spoon) |
| Food texture | Purees, semisolid, finger foods, solid | Finger foods | Finger foods | Based on the level of psycho-neuro-motor and physical development |
| Benefits |
|
|
|
|
| Risks |
|
|
|
| Food | 4–6 Months | 6–8 Months | 9–10 Months | 11–12 Months |
|---|---|---|---|---|
| Milk | Human milk Soy formula | Human milk Soy formula | Human milk Soy formula | Human milk Soy formula |
| Cereal and cereal-derived food | Iron fortified infant cereal (usually rice is the first introduced) | Infant cereal, crackers, unsweetened dry cereal for breakfast | Infant cereal, crackers, toast, unsweetened dry cereal for breakfast, soft bread | Infant cereal, crackers, toast, unsweetened dry cereal for breakfast, soft bread, rice pasta |
| Fruits and vegetables | - | Strained fruit or vegetables, fruit or vegetable juice | Soft or cooked fruit, fruit juice, cooked mashed vegetable, vegetable juice | Soft, canned or cooked fruit, peeled raw fruit, fruit juice, cooked pieces of vegetable, vegetables juice |
| Pulses | - | Pureed legumes | Pureed legumes, | Mashed legumes |
| Other food items with high protein content | Tofu, soy yogurt (after 8 months) | Soy cheese, soy yogurt (after 8 months) | Tofu, soy cheese or yogurt, tempeh | |
| Other food items with high fat content | Olive oil | Olive oil | Olive oil | Olive oil Small amount of light margarine |
| Daily Requirement 0–12 Months | Plant-Based Food That Contains | Supplementation | |
|---|---|---|---|
| Vitamin B12 | 0.5–0.8 µg/day | Algae, fungi, tempeh | Necessary |
| Calcium | 500 mg/day | Dairy products, broccoli, kale, cabbage, soy drinks, tofu. Nuts, dried beans, spinach (low bioavailability) | Not necessary |
| Vitamin D | 10 µg/day (400 IU/day) | Dairy products or cereals, fortified soy drink | Suggested |
| Iron | 6–8 mg/day | Soaking pulses, iron-rich vegetables, iron-fortified food (cereals) | Not necessary |
| Zinc | 5 mg/day | Zinc-fortified cereal, whole seeds, nuts, legumes, dairy products | Not necessary |
| Iodine | 50–80 µg/day | Dairy products, breast milk, infant formula | Not necessary |
| Guidelines | |
|---|---|
| American Academy of Pediatrics (AAP) 2019 |
|
| Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI); 2017 |
|
| National Institute of Allergy and Infectious Diseases (NIAID), 2017 |
|
| European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN), 2017 |
|
| Asian Pacific Association of Pediatric Allergy, Respirology and Immunology (APAPARI), 2018 |
|
| German Society for Allergology and Clinical Immunology (DGAKI) |
|
| European Academy of Allergy and Immunology (EAACI) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capra, M.E.; Decarolis, N.M.; Monopoli, D.; Laudisio, S.R.; Giudice, A.; Stanyevic, B.; Esposito, S.; Biasucci, G. Complementary Feeding: Tradition, Innovation and Pitfalls. Nutrients 2024, 16, 737. https://doi.org/10.3390/nu16050737
Capra ME, Decarolis NM, Monopoli D, Laudisio SR, Giudice A, Stanyevic B, Esposito S, Biasucci G. Complementary Feeding: Tradition, Innovation and Pitfalls. Nutrients. 2024; 16(5):737. https://doi.org/10.3390/nu16050737
Chicago/Turabian StyleCapra, Maria Elena, Nicola Mattia Decarolis, Delia Monopoli, Serena Rosa Laudisio, Antonella Giudice, Brigida Stanyevic, Susanna Esposito, and Giacomo Biasucci. 2024. "Complementary Feeding: Tradition, Innovation and Pitfalls" Nutrients 16, no. 5: 737. https://doi.org/10.3390/nu16050737
APA StyleCapra, M. E., Decarolis, N. M., Monopoli, D., Laudisio, S. R., Giudice, A., Stanyevic, B., Esposito, S., & Biasucci, G. (2024). Complementary Feeding: Tradition, Innovation and Pitfalls. Nutrients, 16(5), 737. https://doi.org/10.3390/nu16050737









