One-Year Changes in Bioelectrical Impedance Data in Adolescent Athletes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Anthropometric Measurements
2.4. Bioelectrical Impedance Analysis
2.5. Statistical Analyses
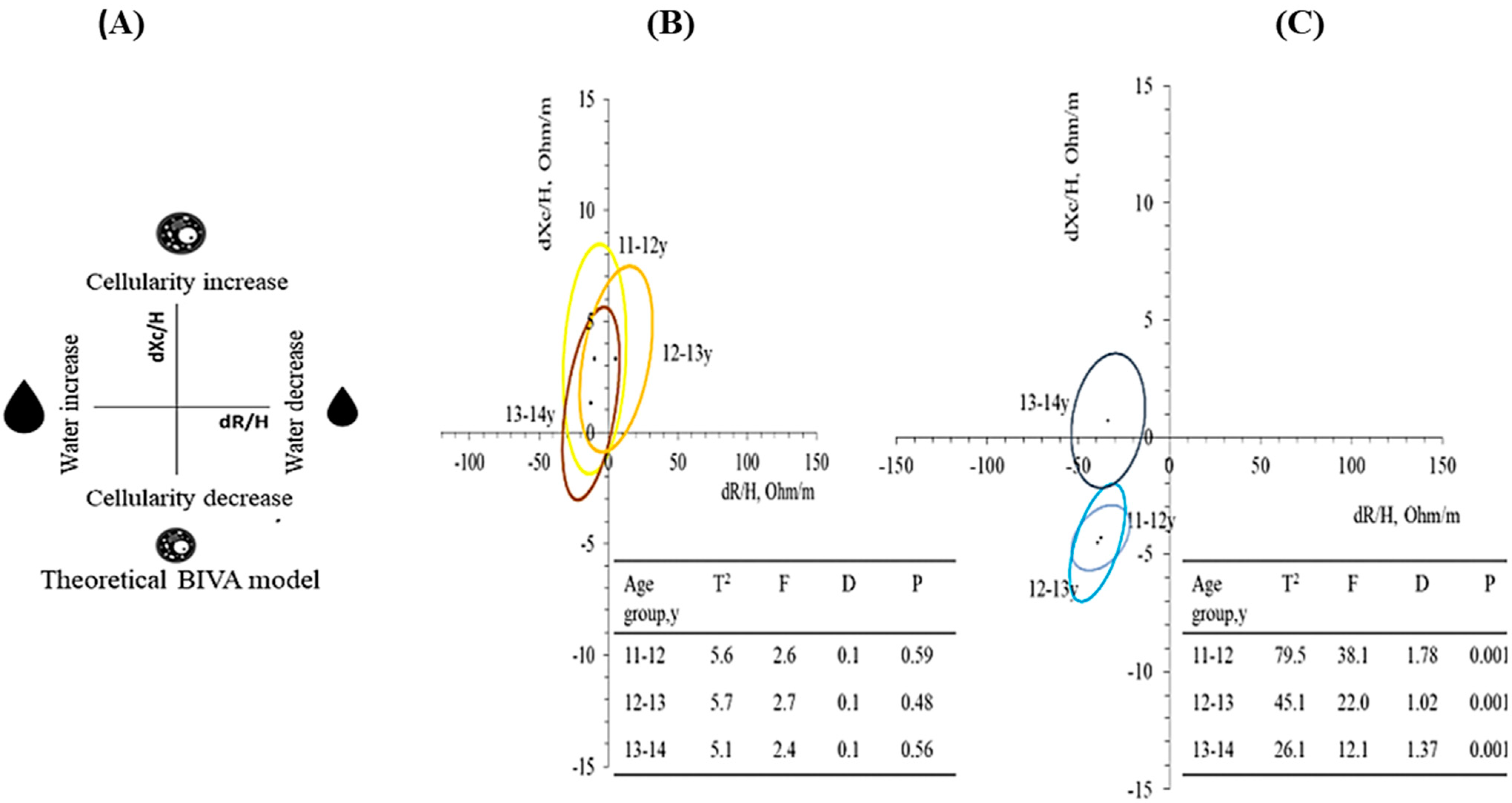
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malina, R.M.; Geithner, C.A. Body Composition of Young Athletes. Am. J. Lifestyle Med. 2011, 5, 262–278. [Google Scholar] [CrossRef]
- Ortega, F.B.; Ruiz, J.R.; Castillo, M.J.; Sjöström, M. Physical fitness in childhood and adolescence: A powerful marker of health. Int. J. Obes. 2008, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rogol, A.D.; Clark, P.A.; Roemmich, J.N. Growth and pubertal development in children and adolescents: Effects of diet and physical activity. Am. J. Clin. Nutr. 2000, 72, 521S–528S. [Google Scholar] [CrossRef] [PubMed]
- Bielemann, R.M.; Domingues, M.R.; Horta, B.L.; Menezes, A.M.B.; Gonçalves, H.; Assunção, M.C.F.; Hallal, P.C. Physical activity throughout adolescence and bone mineral density in early adulthood: The 1993 Pelotas (Brazil) Birth Cohort Study. Osteoporos. Int. 2014, 25, 2007–2015. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Desbrow, B.; Cox, G.; Desbrow, B.; Burke, L.M.; Cox, G.R.; Sawyer, S.M. Sports Dietitians Australia Position Statement: Sports Nutrition for the Adolescent Athlete Sports Dietitians Australia Position Statement: Sports Nutrition for the Adolescent Athlete. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Pierson, R.N., Jr.; Heymsfield, S.B. The five-level model: A new approach to organizing body-composition research. Am. J. Clin. Nutr. 1992, 56, 19–28. [Google Scholar] [CrossRef]
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R.; Charlier, R.; Caspers, M.; Knaeps, S.; Mertens, E.; Lambrechts, D.; Lefevre, J.; et al. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Koury, J.C.; Ribeiro, M.A.; Massarani, F.A.; Vieira, F.; Marini, E. Fat-free mass in adolescent athletes: Accuracy of bioimpedance equations and identification of new predictive equations. Nutrition 2019, 60, 59–65. [Google Scholar] [CrossRef]
- Horlick, M.; Arpadi, S.M.; Bethel, J.; Wang, J.; Moye, J.; Cuff, P.; Pierson, R.N.; Kotler, D. Bioelectrical impedance analysis models for prediction of total body water and fat-free mass in healthy and HIV-infected children and adolescents. Am. J. Clin. Nutr. 2002, 76, 991–999. [Google Scholar] [CrossRef]
- Pietrobelli, A.; Andreoli, A.; Cervelli, V.; Carbonelli, M.G.; Peroni, D.G.; De Lorenzo, A. Predicting fat-free mass in children using bioimpedance analysis. Acta Diabetol. 2003, 40, s212–s215. [Google Scholar] [CrossRef]
- Deurenberg, P.; Van Der Kooy, K.; Leenen, R.; Weststrate, J.A.; Seidell, J. Sex and age specific prediction formulas for estimating body composition from bioelectrical impedance: A cross-validation study. Int. J. Obes. 1991, 15, 17–25. [Google Scholar]
- da Costa, R.F.; Silva, A.M.; Masset, K.V.d.S.B.; Cesário, T.d.M.; Cabral, B.G.d.A.T.; Ferrari, G.; Dantas, P.M.S. Development and Cross-Validation of a Predictive Equation for Fat-Free Mass in Brazilian Adolescents by Bioelectrical Impedance. Front. Nutr. 2022, 9, 820736. [Google Scholar] [CrossRef]
- Koury, J.C.; Trugo, N.M.; Torres, A.G. Phase Angle and Bioelectrical Impedance Vectors in Adolescent and Adult Male Athletes. Int. J. Sports Physiol. Perform. 2014, 9, 798–804. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gomez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis? Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gómez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Silva, A.M.; Matias, C.N.; Nunes, C.L.; Santos, D.A.; Marini, E.; Lukaski, H.C.; Sardinha, L.B. Lack of agreement of in vivo raw bioimpedance measurements obtained from two single and multi-frequency bioelectrical impedance devices. Eur. J. Clin. Nutr. 2018, 73, 1077–1083. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Barbosa-Silva, T.G.; Heymsfield, S.B. Bioelectrical impedance analysis in the assessment of sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 366–374. [Google Scholar] [CrossRef]
- Domingos, C.; Matias, C.N.; Cyrino, E.S.; Sardinha, L.B.; Silva, A.M. The usefulness of Tanita TBF-310 for body composition assessment in Judo athletes using a four-compartment molecular model as the reference method. Rev. Assoc. Med. Bras. 2019, 65, 1283–1289. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N.; Chumlea, W.C.; Roche, A.F. Bioelectric impedance phase angle and body composition. Am. J. Clin. Nutr. 1988, 48, 16–23. [Google Scholar] [CrossRef]
- Marini, E.; Campa, F.; Buffa, R.; Stagi, S.; Matias, C.N.; Toselli, S.; Sardinha, L.B.; Silva, A.M. Phase angle and bioelectrical impedance vector analysis in the evaluation of body composition in athletes. Clin. Nutr. 2020, 39, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Buffa, R.; Floris, G.; Marini, E. Bioelectrical impedance vector in pre- and postmenarcheal females. Nutrition 2002, 18, 474–478. [Google Scholar] [CrossRef]
- Di Vincenzo, O.; Marra, M.; Scalfi, L. Bioelectrical impedance phase angle in sport: A systematic review. J. Int. Soc. Sports Nutr. 2019, 16, 49. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Barbosa-Silva, T.G.; Bielemann, R.M.; Gallagher, D.; Heymsfield, S.B. Phase angle and its determinants in healthy subjects: Influence of body composition. Am. J. Clin. Nutr. 2016, 103, 712–716. [Google Scholar] [CrossRef]
- Norman, K.; Stobäus, N.; Pirlich, M.; Bosy-Westphal, A. Bioelectrical phase angle and impedance vector analysis—Clinical relevance and applicability of impedance parameters. Clin. Nutr. 2012, 31, 854–861. [Google Scholar] [CrossRef]
- Cattem, M.V.d.O.; Sinforoso, B.T.; Campa, F.; Koury, J.C. Bioimpedance Vector Patterns according to Age and Handgrip Strength in Adolescent Male and Female Athletes. Int. J. Environ. Res. Public Health 2021, 18, 6069. [Google Scholar] [CrossRef]
- Ballarin, G.M.; Valerio, G.; Alicante, P.M.; Di Vincenzo, O.M.; Scalfi, L. Bioelectrical Impedance Analysis (BIA)- Derived Phase Angle in Children and Adolescents: A Systematic Review. J. Pediatr. Gastroenterol. Nutr. 2022, 75, 120–130. [Google Scholar] [CrossRef]
- Ackland, T.; Lohman, T.G.; Sundgot-Borgen, J.; Maughan, R.J.; Meyer, N.L.; Stewart, A.; Müller, W.; Ackland, W.P.T.R. Current Status of Body Composition Assessment in Sport: Review and Position Statement on Behalf of the Ad Hoc Research Working Group on Body Composition Health and Performance, under the Auspices of the I.O.C. Medical Commission. Sports Med. 2012, 42, 227–249. [Google Scholar] [CrossRef]
- Castizo-Olier, J.; Irurtia, A.; Jemni, M.; Carrasco-Marginet, M.; Fernández-García, R.; Rodríguez, F.A. Bioelectrical impedance vector analysis (BIVA) in sport and exercise: Systematic review and future perspectives. PLoS ONE 2018, 13, e0197957. [Google Scholar] [CrossRef] [PubMed]
- Campa, F.; Toselli, S.; Mazzilli, M.; Gobbo, L.A.; Coratella, G. Assessment of Body Composition in Athletes: A Narrative Review of Available Methods with Special Reference to Quantitative and Qualitative Bioimpedance Analysis. Nutrients 2021, 13, 1620. [Google Scholar] [CrossRef] [PubMed]
- Stagi, S.; Silva, A.M.; Jesus, F.; Campa, F.; Cabras, S.; Earthman, C.P.; Marini, E. Usability of classic and specific bioelectrical impedance vector analysis in measuring body composition of children. Clin. Nutr. 2022, 41, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Buffa, R.; Mereu, E.; Comandini, O.; Ibanez, M.E.; Marini, E. Bioelectrical impedance vector analysis (BIVA) for the assessment of two-compartment body composition. Eur. J. Clin. Nutr. 2014, 68, 1234–1240. [Google Scholar] [CrossRef]
- Mereu, E.; Buffa, R.; Lussu, P.; Marini, E. Phase angle, vector length, and body composition. Am. J. Clin. Nutr. 2016, 104, 845–847. [Google Scholar] [CrossRef]
- Piccoli, A.; Pillon, L.; Dumler, F. Impedance vector distribution by sex, race, body mass index, and age in the United States: Standard reference intervals as bivariate Z scores. Nutrition 2002, 18, 153–167. [Google Scholar] [CrossRef]
- Piccoli, A.; Rossi, B.; Pillon, L.; Bucciante, G. A new method for monitoring body fluid variation by bioimpedance analysis: The RXc graph. Kidney Int. 1994, 46, 534–539. [Google Scholar] [CrossRef]
- Campa, F.; Matias, C.; Gatterer, H.; Toselli, S.; Koury, J.C.; Andreoli, A.; Melchiorri, G.; Sardinha, L.B.; Silva, A.M. Classic Bioelectrical Impedance Vector Reference Values for Assessing Body Composition in Male and Female Athletes. Int. J. Environ. Res. Public Health 2019, 16, 5066. [Google Scholar] [CrossRef]
- Koury, J.C.; de Oliveira-Junior, A.V.; Portugal, M.R.C.; de Oliveira, K.d.J.F.; Donangelo, C.M. Bioimpedance parameters in adolescent athletes in relation to bone maturity and biochemical zinc indices. J. Trace Elem. Med. Biol. 2018, 46, 26–31. [Google Scholar] [CrossRef]
- Ferreira, A.; Ara, D.; Batalha, N.; Collado-Mateo, D. Phase Angle from Bioelectric Impedance and Maturity-Related Factors in Adolescent Athletes: A Systematic Review. Sustainability 2020, 12, 4806. [Google Scholar]
- Campa, F.; Silva, A.M.; Iannuzzi, V.; Mascherini, G.; Benedetti, L.; Toselli, S. The Role of Somatic Maturation on Bioimpedance Patterns and Body Composition in Male Elite Youth Soccer Players. Int. J. Environ. Res. Public Health 2019, 16, 4711. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.A.; Hitchen, P.J.; Stratton, G. The importance of considering biological maturity when assessing physical fitness measures in girls and boys aged 10 to 16 years. Ann. Hum. Biol. 2000, 27, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Toselli, S.; Marini, E.; Latessa, P.M.; Benedetti, L.; Campa, F. Maturity Related Differences in Body Composition Assessed by Classic and Specific Bioimpedance Vector Analysis among Male Elite Youth Soccer Players. Int. J. Environ. Res. Public Health 2020, 17, 729. [Google Scholar] [CrossRef] [PubMed]
- De Palo, T.; Messina, G.; Edefonti, A.; Perfumo, F.; Pisanello, L.; Peruzzi, L.; Di Iorio, B.; Mignozzi, M.; Vienna, A.; Conti, G.; et al. Normal values of the bioelectrical impedance vector in childhood and puberty. Nutrition 2000, 16, 417–424. [Google Scholar] [CrossRef]
- Mathias-Genovez, M.G.; Oliveira, C.C.; Camelo, J.S.; Del Ciampo, L.A.; Monteiro, J.P. Bioelectrical Impedance of Vectorial Analysis and Phase Angle in Adolescents. J. Am. Coll. Nutr. 2016, 35, 262–270. [Google Scholar] [CrossRef]
- Malina, R.M.; Rogol, A.D.; Cumming, S.P.; Coelho e Silva, M.J.; Figueiredo, A.J. Biological maturation of youth athletes: Assessment and implications. Br. J. Sports Med. 2015, 49, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Pearson, D.; Naughton, G.; Torode, M. Predictability of physiological testing and the role of maturation in talent identification for adolescent team sports. J. Sci. Med. Sport 2006, 9, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Orsso, C.E.; Gonzalez, M.C.; Maisch, M.J.; Haqq, A.M.; Prado, C.M. Using bioelectrical impedance analysis in children and adolescents: Pressing issues. Eur. J. Clin. Nutr. 2021, 76, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.F.; Matias, C.N.; Campa, F.; Morgado, J.P.; Franco, P.; Quaresma, P.; Almeida, N.; Curto, D.; Toselli, S.; Monteiro, C.P. Bioimpedance Vector Patterns Changes in Response to Swimming Training: An Ecological Approach. Int. J. Environ. Res. Public Health 2020, 17, 4851. [Google Scholar] [CrossRef] [PubMed]
- De Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- VanItallie, T.B.; Yang, M.U.; Heymsfield, S.B.; Funk, R.C.; Boileau, R.A. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 52, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Pastori, G. BIVA Software 2002; Department of Medical and Surgical Sciences University of Padova: Padua, Italy, 2002. [Google Scholar]
- Roemmich, J.N.; Clark, P.A.; Weltman, A.; Rogol, A.D.; Welt-man, A.; Rogol Alterations, A.D. Alterations in growth and body composition during puberty. I. Comparing multicompartment body composition models. J. Appl. Physiol. 1997, 83, 927–935. [Google Scholar] [CrossRef]
- Coratella, G.; Campa, F.; Matias, C.N.; Toselli, S.; Koury, J.C.; Andreoli, A.; Sardinha, L.B.; Silva, A.M. Generalized bioelectric impedance-based equations underestimate body fluids in athletes. Scand. J. Med. Sci. Sports 2021, 31, 2123–2132. [Google Scholar] [CrossRef]
- Marini, E.; Buffa, R.; Gobbo, L.A.; Salinas-Escudero, G.; Stagi, S.; García-Peña, C.; Sánchez-García, S.; Carrillo-Vega, M.F. Interpopulation Similarity of Sex and Age-Related Body Composition Variations among Older Adults. Int. J. Environ. Res. Public Health 2020, 17, 6047. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Sergi, G.; Succa, V.; Saragat, B.; Sarti, S.; Coin, A.; Manzato, E.; Buffa, R. Efficacy of specific bioelectrical impedance vector analysis (BIVA) for assessing body composition in the elderly. J. Nutr. Health Aging 2012, 17, 515–521. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.; Young, A.J.; Sawka, M.N. Bioelectrical Impedance to Estimate Changes in Hydration Status. Int. J. Sports Med. 2002, 23, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ballarin, G.; Valerio, G.; Alicante, P.; Di Vincenzo, O.; Monfrecola, F.; Scalfi, L. Could BIA-derived phase angle predict health-related musculoskeletal fitness? A cross-sectional study in young adults. Nutrition 2024, 112388. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
| Female n [%] | Male n [%] | |
|---|---|---|
| Athletics | 12 [23] | 20 [24] |
| Soccer | 8 [16] | 21 [26] |
| Volleyball | 11 [21] | 20 [24] |
| Swimming | 6 [12] | 7 [8.5] |
| Table tennis | 8 [16] | 8 [10] |
| Handball | 6 [12] | 6 [7.5] |
| Female | Male | ||||||
|---|---|---|---|---|---|---|---|
| 11 to 12y | 12 to 13y | 13 to 14y | 11 to 12y | 12 to 13y | 13 to 14y | ||
| n = 16 | n = 23 | n = 16 | n = 25 | n = 43 | n = 14 | ||
| T0 | 11.18 ± 0.26 | 12.32 ± 0.34 | 13.22 ± 0.32 | 11.27 ± 0.24 | 12.36 ± 0.29 | 13.41 ± 0.31 | |
| Age (years) | T1 | 12.25 ± 0.21 | 13.01 ± 0.22 | 14.19 ± 0.29 | 12.35 ± 0.27 | 13.03 ± 0.35 | 14.20 ± 0.34 |
| p-value | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Weight (kg) | T0 | 48.3 ± 12.7 | 48.0 ± 10.9 | 52.9 ± 6.5 | 45.0 ± 12.5 | 47.9 ± 10.1 | 53.3 ± 9.6 |
| T1 | 53.5 ± 11.9 | 53.7 ± 12.4 | 55.4 ± 6.1 | 51.7 ± 13.4 | 54.8 ± 10.8 | 58.7 ± 10.1 | |
| p-value | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Change | 5.2 ± 2.7 a | 5.7 ± 3.2 a | 2.4 ± 2.3 b | 6.7 ± 4.1 | 6.9 ± 2.8 | 5.4 ± 3.4 | |
| % | 11.9 | 12.1 | 4.8 | 15.6 | 14.8 | 10.5 | |
| Height (cm) | T0 | 152.4 ± 7.0 a | 156.5 ± 6.7 a,b | 160.4 ± 6.7 b | 149.6 ± 8.3 a | 154.7 ± 7.4 b | 161.3 ± 7.9 c |
| T1 | 157.3 ± 6.5 | 159.8 ± 6.2 | 162.3 ± 6.7 | 157.8 ± 9.6 a | 163.2 ± 7.2 b | 168.4 ± 7.6 b | |
| p-value | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Change | 4.9 ± 2.1 a | 3.3 ± 2.7 a,b | 1.9 ± 1.2 b | 8.2 ± 2.7 | 8.5 ± 3.0 | 7.1 ± 2.7 | |
| % | 3.3 | 2.2 | 1.2 | 5.5 | 5.5 | 4.5 | |
| T0 | 20.6 ± 4.2 | 19.4 ± 3.4 | 20.6 ± 2.7 | 19.8 ± 3.7 | 19.9 ± 3.2 | 20.2 ± 2.7 | |
| BMI (kg/m2) | T1 | 21.5 ± 3.8 | 20.9 ± 3.8 | 21.1 ± 2.4 | 20.5 ± 3.6 | 20.4 ± 3.2 | 20.6 ± 2.9 |
| p-value | 0.005 | 0.001 | 0.102 | 0.061 | 0.002 | 0.288 | |
| Change | 0.9 ± 1.1 a,b | 1.4 ± 0.9 a | 0.4 ± 1.0 b | 0.7 ± 1.8 | 0.6 ± 1.1 | 0.4 ± 1.3 | |
| % | 4.9 | 7.4 | 2.5 | 4.0 | 3.1 | 2.0 | |
| R/H (Ω/m) | T0 | 414.1 ± 67.1 | 405.7 ± 47.1 | 386.5 ± 26.5 | 414.6 ± 63.7 a | 383.8 ± 58.2 b | 368.4 ± 49.5 b |
| T1 | 404.3 ± 74.3 | 393.2 ± 46.2 | 392.1 ± 54.0 | 376.8 ± 67.1 a | 344.3 ± 52.2 b | 334.8 ± 46.3 c | |
| p-value | 0.239 | 0.133 | 0.556 | 0.001 | 0.001 | 0.001 | |
| Change | −9.8 ± 32.1 | −12.5 ± 38.4 | 5.6 ± 37.1 | −37.8 ± 30.6 | −39.5 ± 39.0 | −33.6 ± 26.1 | |
| % | −2.4 | −2.7 | 1.2 | −9.2 | −9.8 | −8.9 | |
| Xc/H (Ω/m) | T0 | 41.1 ± 4.2 | 42.3 ± 5.7 | 41.9 ± 3.7 | 45.3 ± 7.0 a | 42.1 ± 7.0 a,b | 38.9 ± 5.2 b |
| T1 | 44.4 ± 7.5 | 43.7 ± 6.8 | 45.2 ± 6.2 | 41.0 ± 7.3 | 37.6 ± 5.3 | 39.6 ± 5.9 | |
| p-value | 0.092 | 0.448 | 0.040 | 0.001 | 0.001 | 0.517 | |
| Change | 3.3 ± 7.3 | 1.3 ± 8.1 | 3.3 ± 5.9 | −4.3 ± 2.6 a | −4.5 ± 6.4 a | 0.7 ± 3.7 b | |
| % | 8.4 | 4.5 | 8.2 | −9.7 | −9.5 | 1.9 | |
| Z/H (Ω/m) | T0 | 416.2 ± 67.1 | 407.9 ± 47.2 | 388.7 ± 26.6 | 417.1 ± 64.0 | 386.2 ± 58.4 | 370.5 ± 49.5 |
| T1 | 406.8 ± 74.1 | 395.7 ± 46.4 | 394.7 ± 54.0 | 379.1 ± 67.3 | 346.4 ± 52.3 | 337.2 ± 46.2 | |
| p-value | 0.260 | 0.142 | 0.530 | 0.001 | 0.001 | 0.001 | |
| Change | −9.4 ± 32.0 | −12.2 ± 38.6 | 6.0 ± 37.1 | −38.0 ± 30.6 | −39.8 ± 39.1 | −33.3 ± 25.9 | |
| % | −2.3 | −2.6 | 1.3 | −9.2 | −9.8 | −8.8 | |
| PhA (°) | T0 | 5.74 ± 0.57 a | 5.98 ± 0.64 a,b | 6.20 ± 0.46 b | 6.3 ± 0.6 | 6.3 ± 0.7 | 6.1 ± 0.7 |
| T1 | 6.40 ± 1.37 | 6.35 ± 0.85 | 6.64 ± 0.93 | 6.2 ± 0.6 a | 6.3 ± 0.7 a | 6.8 ± 1.1 b | |
| p-value | 0.048 | 0.120 | 0.084 | 0.833 | 0.926 | 0.007 | |
| Change | 0.7 ± 1.2 a | 0.4 ± 1.1 b | 0.4 ± 0.9 b | 0.0 ± 0.5 a | 0.0 ± 0.8 a | 0.7 ± 0.9 b | |
| % | 11.5 | 7.4 | 7.3 | −0.1 | 0.4 | 12.4 | |
| FFM (kg) | T0 | 36.2 ± 6.3 | 37.3 ± 5.1 | 40.1 ± 2.6 | 36.1 ± 6.3 a | 39.9 ± 6.6 a,b | 43.3 ± 6.4 b |
| T1 | 39.0 ± 6.8 | 40.0 ± 5.4 | 41.2 ± 4.3 | 42.1 ± 8.2 a | 46.5 ± 7.1 a,b | 49.5 ± 6.8 b | |
| p-value | 0.001 | 0.001 | 0.139 | 0.001 | 0.001 | 0.001 | |
| Change | 2.8 ± 2.7 a | 2.7 ± 3.3 a | 1.1 ± 2.9 b | 5.9 ± 3.2 | 6.6 ± 3.8 | 6.1 ± 3.0 | |
| % | 8.0 | 7.6 | 2.7 | 16.3 | 17.3 | 14.5 | |
| FFMI (kg/m2) | T0 | 15.5 ± 2.1 | 15.2 ± 1.3 | 15.6 ± 1.1 | 16.0 ± 1.4 | 16.6 ± 1.7 | 16.6 ± 1.5 |
| T1 | 15.7 ± 2.2 | 15.6 ± 1.4 | 15.7 ± 1.7 | 16.7 ± 1.7 | 17.4 ± 1.8 | 17.4 ± 1.7 | |
| p-value | 0.413 | 0.058 | 0.804 | 0.001 | 0.001 | 0.009 | |
| Change | 0.2 ± 0.9 | 0.4 ± 1.0 | 0.1 ± 1.1 | 0.7 ± 0.9 | 0.8 ± 1.2 | 0.8 ± 1.0 | |
| % | 1.2 | 3.0 | 0.3 | 4.5 | 5.1 | 4.9 | |
| Maturity (%) * | T0 | 56.3 | 91.3 | 100 | 0 | 0 | 100 |
| T1 | 100 | 100 | 100 | 0 | 100 | 100 | |
| Female | Initial Age (T0) | Time Interval (After One Year) | Initial Age*Time Interval | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| PhA (°) | 1.67 | 0.192 | 10.40 | 0.002 | 0.535 | 0.716 |
| R/H (Ω/m) | 1.42 | 0.244 | 0.37 | 0.544 | 0.370 | 0.689 |
| Xc/H (Ω/m) | 0.198 | 0.821 | 6.49 | 0.012 | 0.464 | 0.629 |
| Z/H (Ω/m) | 1.39 | 0.250 | 0.32 | 0.570 | 0.376 | 0.687 |
| Male | Initial Age (T0) | Time Interval (After One Year) | Initial Age*Time Interval | |||
| F | p | F | p | F | p | |
| PhA (°) | 0.70 | 0.498 | 3.59 | 0.060 | 3.440 | 0.035 |
| R/H (Ω/m) | 6.63 | 0.002 | 12.96 | <0.001 | 0.164 | 0.853 |
| Xc/H (Ω/m) | 4.64 | 0.011 | 5.10 | 0.025 | 2.840 | 0.063 |
| Z/H (Ω/m) | 6.64 | 0.002 | 12.93 | <0.001 | 0.174 | 0.841 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cattem, M.V.d.O.; Orsso, C.E.; Gonzalez, M.C.; Koury, J.C. One-Year Changes in Bioelectrical Impedance Data in Adolescent Athletes. Nutrients 2024, 16, 701. https://doi.org/10.3390/nu16050701
Cattem MVdO, Orsso CE, Gonzalez MC, Koury JC. One-Year Changes in Bioelectrical Impedance Data in Adolescent Athletes. Nutrients. 2024; 16(5):701. https://doi.org/10.3390/nu16050701
Chicago/Turabian StyleCattem, Marcus Vinícius de Oliveira, Camila E. Orsso, Maria Cristina Gonzalez, and Josely Correa Koury. 2024. "One-Year Changes in Bioelectrical Impedance Data in Adolescent Athletes" Nutrients 16, no. 5: 701. https://doi.org/10.3390/nu16050701
APA StyleCattem, M. V. d. O., Orsso, C. E., Gonzalez, M. C., & Koury, J. C. (2024). One-Year Changes in Bioelectrical Impedance Data in Adolescent Athletes. Nutrients, 16(5), 701. https://doi.org/10.3390/nu16050701






