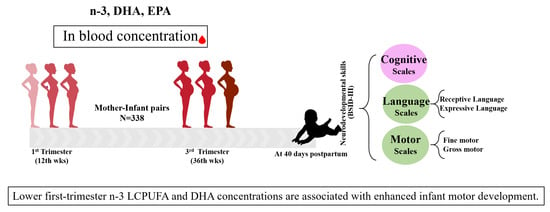
The Maternal Omega-3 Long-Chain Polyunsaturated Fatty Acid Concentration in Early Pregnancy and Infant Neurodevelopment: The ECLIPSES Study
Abstract
1. Introduction
2. Materials and Methods
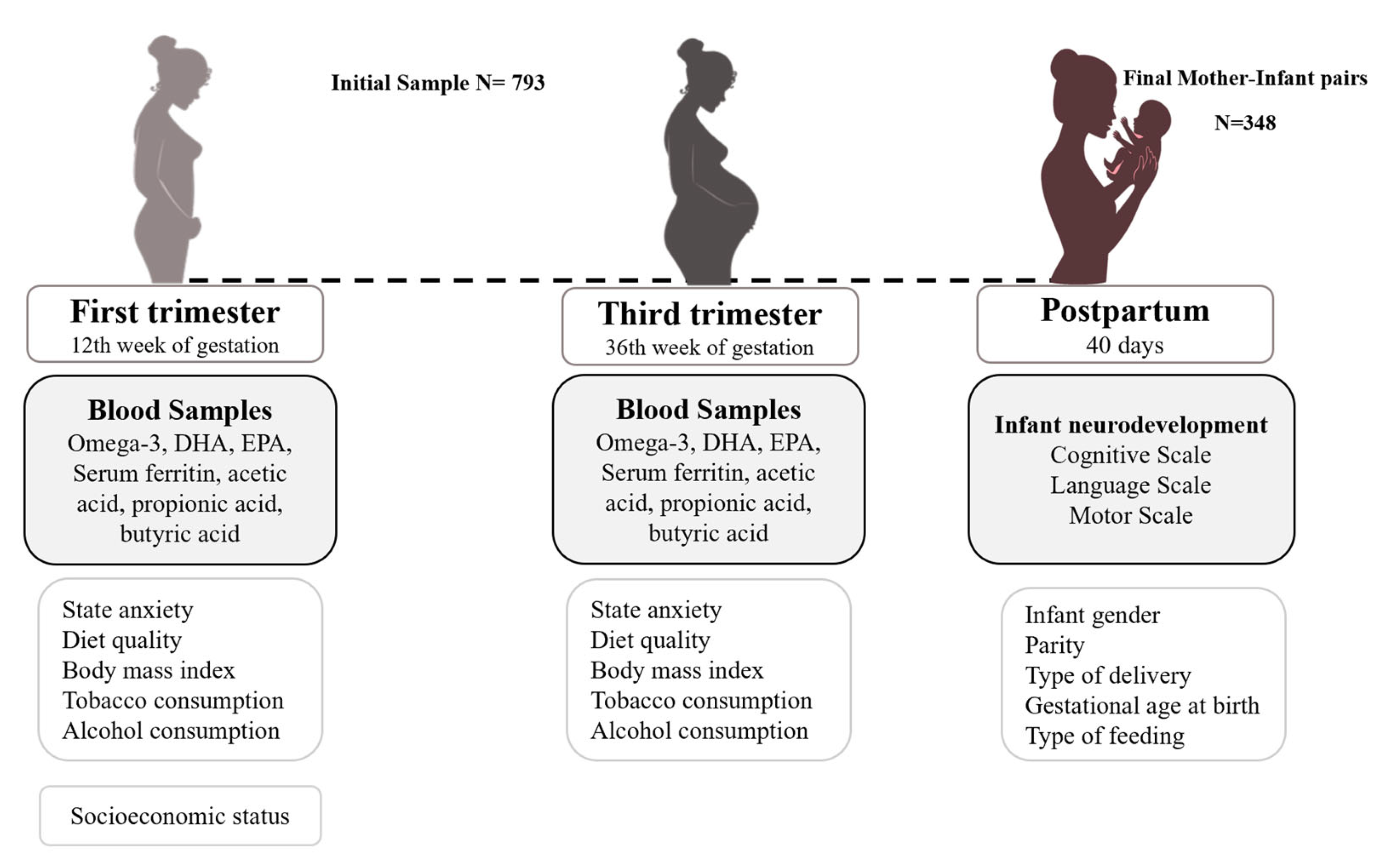
2.1. Study Design and Procedure
2.2. Instruments and Data Collection
2.2.1. Main Measurements
2.2.2. Adjustment Measurements
Prenatal Psychological Distress
Sociodemographic Data
Lifestyle Habits
Clinical Data
Obstetrical and Birth Data
2.3. Statistical Analysis
3. Results
3.1. General Characteristics of the Sample
3.2. Infant Cognitive Development According to Mothers’ n-3 LCPUFA, DHA, and EPA Levels in First and Third Trimesters
3.3. Predictive Relationship between n-3 LCPUFA and DHA Serum Levels at First Trimester and Infant Motor and Gross Motor Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colombo, J.; Gustafson, K.M.; Carlson, S.E. Critical and Sensitive Periods in Development and Nutrition. Ann. Nutr. Metab. 2019, 75, 34–42. [Google Scholar] [CrossRef]
- Cusick, S.E.; Georgieff, M.K. The Role of Nutrition in Brain Development: The Golden Opportunity of the “First 1000 Days” Brain Development in Late Fetal and Early Postnatal Life. J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef]
- Büyükuslu, N.; Ovalı, S.; Altuntaş, Ş.L.; Batırel, S.; Yiğit, P.; Garipağaoğlu, M. Supplementation of Docosahexaenoic Acid (DHA) / Eicosapentaenoic Acid (EPA) in a Ratio of 1/1.3 during the Last Trimester of Pregnancy Results in EPA Accumulation in Cord Blood. Prostaglandins Leukot Essent Fat. Acids 2017, 125, 32–36. [Google Scholar] [CrossRef]
- Martinat, M.; Rossitto, M.; Di Miceli, M.; Layé, S. Perinatal Dietary Polyunsaturated Fatty Acids in Brain Development, Role in Neurodevelopmental Disorders. Nutrients 2021, 13, 1185. [Google Scholar] [CrossRef]
- Dinicolantonio, J.J.; O’keefe, J.H. The Importance of Marine OMEGA-3S for Brain Development and the Prevention and Treatment of Behavior, Mood, and Other Brain Disorders. Nutrients 2020, 12, 2333. [Google Scholar] [CrossRef]
- Clandinin, M.T.; Chappell, J.E.; Leong, S.; Heim, T.; Swyer, P.R.; Chance, G.W. Intrauterine Fatty Acid Accretion in Infant Brain: Implications for Fatty Acid Requirements. Early Hum. Dev. 1980, 4, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Clandinin, M.T.; Chappell, J.E.; Leong, S.; Heim, T.; Swyer, P.R.; Chance, G.W. Extrauterine Fatty Acid Accretion in Infant Brain: Implications for Fatty Acid Requirements. Early Hum. Dev. 1980, 4, 131–138. [Google Scholar] [CrossRef]
- Martinez, M. Tissue Levels of Polyunsaturated Fatty Acids during Early Human Development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef] [PubMed]
- Georgieff, M.K.; Ramel, S.E.; Cusick, S.E. Nutritional Influences on Brain Development. Acta Paediatr. Int. J. Paediatr. 2018, 107, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.F.; Roberts, R.M.; Makrides, M. The Influence of Omega-3 Long-Chain Polyunsaturated Fatty Acid, Docosahexaenoic Acid, on Child Behavioral Functioning: A Review of Randomized Controlled Trials of Dha Supplementation in Pregnancy, the Neonatal Period and Infancy. Nutrients 2021, 13, 415. [Google Scholar] [CrossRef] [PubMed]
- Judge, M.P.; Harel, O.; Lammi-Keefe, C.J. Maternal Consumption of a Docosahexaenoic Acid-Containing Functional Food during Pregnancy: Benefit for Infant Performance on Problem-Solving but Not on Recognition Memory Tasks at Age 9 Mo. Am. J. Clin. Nutr. 2007, 85, 1572–1577. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Yelland, L.; Quinlivan, J.; Ryan, P.; Doyle, L.W.; Anderson, P.; Else, P.L.; Meyer, B.J.; et al. Effect of DHA Supplementation during Pregnancy on Maternal Depression and Neurodevelopment of Young Children: A Randomized Controlled Trial. JAMA 2010, 304, 1675–1683. [Google Scholar] [CrossRef]
- Colombo, J.; Jill Shaddy, D.; Gustafson, K.; Gajewski, B.J.; Thodosoff, J.M.; Kerling, E.; Carlson, S.E. The Kansas University DHA Outcomes Study (KUDOS) Clinical Trial: Long-Term Behavioral Follow-up of the Effects of Prenatal DHA Supplementation. Am. J. Clin. Nutr. 2019, 109, 1380–1392. [Google Scholar] [CrossRef]
- Mulder, K.A.; King, D.J.; Innis, S.M. Omega-3 Fatty Acid Deficiency in Infants before Birth Identified Using a Randomized Trial of Maternal DHA Supplementation in Pregnancy. PLoS ONE 2014, 9, 19–22. [Google Scholar] [CrossRef]
- Gould, J.F.; Smithers, L.G.; Makrides, M. The Effect of Maternal Omega-3 (N23) LCPUFA Supplementation during Pregnancy on Early Childhood Cognitive and Visual Development: A Systematic Review and Meta-Analysis of Randomized Controlled Trials1-3. Am. J. Clin. Nutr. 2013, 97, 531–544. [Google Scholar] [CrossRef]
- Middleton, P.; Gomersall, J.C.; Gould, J.F.; Shepherd, E.; Olsen, S.F.; Makrides, M. Omega-3 Fatty Acid Addition during Pregnancy. Cochrane Database Syst. Rev. 2018, 2018, CD003402. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.; Rao, S.C.; Schulzke, S.M.; Patole, S.K.; Simmer, K. Longchain Polyunsaturated Fatty Acid Supplementation in Preterm Infants. Cochrane Database Syst. Rev. 2016, 2016, CD000375. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Noguera, M.F.; Calvache, J.A.; Bonfill Cosp, X.; Kotanidou, E.P.; Galli-Tsinopoulou, A. Supplementation with Long Chain Polyunsaturated Fatty Acids (LCPUFA) to Breastfeeding Mothers for Improving Child Growth and Development. Cochrane Database Syst. Rev. 2015, 2015, CD007901. [Google Scholar] [CrossRef] [PubMed]
- Jasani, B.; Simmer, K.; Patole, S.K.; Rao, S.C. Long Chain Polyunsaturated Fatty Acid Supplementation in Infants Born at Term. Cochrane Database Syst. Rev. 2017, 2017, CD000376. [Google Scholar] [CrossRef] [PubMed]
- Spiller, P.; Hibbeln, J.R.; Myers, G.; Vannice, G.; Golding, J.; Crawford, M.A.; Strain, J.J.; Connor, S.L.; Brenna, J.T.; Kris-Etherton, P.; et al. An Abundance of Seafood Consumption Studies Presents New Opportunities to Evaluate Effects on Neurocognitive Development. Prostaglandins Leukot Essent Fat. Acids 2019, 151, 8–13. [Google Scholar] [CrossRef]
- Hibbeln, C.J.R.; Spiller, P.; Brenna, J.T.; Golding, J.; Holub, B.J.; Harris, W.S.; Kris-Etherton, P.; Lands, B.; Connor, S.L.; Myers, G.; et al. Relationships between Seafood Consumption during Pregnancy and Childhood and Neurocognitive Development: Two Systematic Reviews. Prostaglandins Leukot Essent Fat. Acids 2019, 151, 14–36. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.A.; Torrent, M.; Julvez, J.; Ribas-Fitó, N.; Kogevinas, M.; Sunyer, J. Maternal Fish and Other Seafood Intakes during Pregnancy and Child Neurodevelopment at Age 4 Years. Public Health Nutr. 2009, 12, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Daniels, J.L.; Longnecker, M.P.; Rowland, A.S.; Golding, J. Fish Intake during Pregnancy and Early Cognitive Development of Offspring. Epidemiology 2004, 15, 394–402. [Google Scholar] [CrossRef]
- Oken, E.; Radesky, J.S.; Wright, R.O.; Bellinger, D.C.; Amarasiriwardena, C.J.; Kleinman, K.P.; Hu, H.; Gillman, M.W. Maternal Fish Intake during Pregnancy, Blood Mercury Levels, and Child Cognition at Age 3 Years in a US Cohort. Am. J. Epidemiol. 2008, 167, 1171–1181. [Google Scholar] [CrossRef]
- Gale, C.R.; Robinson, S.M.; Godfrey, K.M.; Law, C.M.; Schlotz, W.; O’Callaghan, F.J. Oily Fish Intake during Pregnancy—Association with Lower Hyperactivity but Not with Higher Full-Scale IQ in Offspring. J. Child Psychol. Psychiatry 2008, 49, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Hibbeln, J.R.; Davis, J.M.; Steer, C.; Emmett, P.; Rogers, I.; Williams, C.; Golding, J. Maternal Seafood Consumption in Pregnancy and Neurodevelopmental Outcomes in Childhood (ALSPAC Study): An Observational Cohort Study. Lancet 2007, 369, 578–585. [Google Scholar] [CrossRef]
- Oken, E.; Østerdal, M.L.; Gillman, M.W.; Knudsen, V.K.; Halldorsson, T.I.; Strøm, M.; Bellinger, D.C.; Hadders-Algra, M.; Michaelsen, K.F.; Olsen, S.F. Associations of Maternal Fish Intake during Pregnancy and Breastfeeding Duration with Attainment of Developmental Milestones in Early Childhood: A Study from the Danish National Birth Cohort. Am. J. Clin. Nutr. 2008, 88, 789–796. [Google Scholar] [CrossRef]
- Rioux, F.M.; Bélanger-Plourde, J.; Leblanc, C.P.; Vigneau, F. Relationship Between Maternal DHA and Iron Status: And Infants’ Cognitive Performance. Can. J. Diet. Pract. Res. 2011, 72, e140–e146. [Google Scholar] [CrossRef]
- Brouwer-Brolsma, E.M.; van de Rest, O.; Godschalk, R.; Zeegers, M.P.A.; Gielen, M.; de Groot, R.H.M. Associations between Maternal Long-Chain Polyunsaturated Fatty Acid Concentrations and Child Cognition at 7 Years of Age: The MEFAB Birth Cohort. Prostaglandins Leukot Essent Fat. Acids 2017, 126, 92–97. [Google Scholar] [CrossRef]
- Strain, J.J.; Davidson, P.W.; Bonham, M.P.; Duffy, E.M.; Stokes-Riner, A.; Thurston, S.W.; Wallace, J.M.W.; Robson, P.J.; Shamlaye, C.F.; Georger, L.A.; et al. Associations of Maternal Long-Chain Polyunsaturated Fatty Acids, Methyl Mercury, and Infant Development in the Seychelles Child Development Nutrition Study. Neurotoxicology 2008, 29, 776–782. [Google Scholar] [CrossRef]
- Braarud, H.C.; Markhus, M.W.; Skotheim, S.; Stormark, K.M.; Frøyland, L.; Graff, I.E.; Kjellevold, M. Maternal DHA Status during Pregnancy Has a Positive Impact on Infant Problem Solving: A Norwegian Prospective Observation Study. Nutrients 2018, 10, 529. [Google Scholar] [CrossRef] [PubMed]
- Arija, V.; Fargas, F.; March, G.; Abajo, S.; Basora, J.; Canals, J.; Ribot, B.; Aparicio, E.; Serrat, N.; Hernández-Martínez, C.; et al. Adapting Iron Dose Supplementation in Pregnancy for Greater Effectiveness on Mother and Child Health: Protocol of the ECLIPSES Randomized Clinical Trial. BMC Pregnancy Childbirth 2014, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, L.I.; Arija, V.; Aranda, N.; Aparicio, E.; Serrat, N.; Fargas, F.; Ruiz, F.; Pallejà, M.; Coronel, P.; Gimeno, M.; et al. The Effectiveness of Different Doses of Iron Supplementation and the Prenatal Determinants of Maternal Iron Status in Pregnant Spanish Women: ECLIPSES Study. Nutrients 2019, 11, 2418. [Google Scholar] [CrossRef] [PubMed]
- Baylay, N. Bayley Scales for Infant and Toddler Development, 3rd ed.; Psychological Corporation: San Antonio, TX, USA, 2006. [Google Scholar]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. STAI Cuestionario de Ansiedad Estado Rasgo; TEA Ediciones: Madrid, Spain, 1997. [Google Scholar]
- Hollingshead, A.B. Four Factor Index of Social Status. Yale J. Sociol. 2011, 8, 21–52. [Google Scholar]
- Institut d’Estadística de Catalunya. Catalan Classification of Occupations; Institut d’Estadística de Catalunya; Catalonia, Spain: 2011. Available online: https://www.idescat.cat/metodes/classificacions/cco-2011-ca?lang=es/ (accessed on 27 November 2023).
- Validación de Un Cuestionario de Frecuencia de Consumo Alimentario Corto: Reproducibilidad y Validez. Available online: https://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S0212-16112008000300011 (accessed on 25 October 2022).
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Fagerström, K.O. Measuring Degree of Physical Dependence to Tobacco Smoking with Reference to Individualization of Treatment. Addict. Behav. 1978, 3, 235–241. [Google Scholar] [CrossRef]
- Otto, S.J.; Houwelingen, A.C.; Antal, M.; Manninen, A.; Godfrey, K.; López-Jaramillo, P.; Hornstra, G. Maternal and Neonatal Essential Fatty Acid Status in Phospholipids: An International Comparative Study. Eur. J. Clin. Nutr. 1997, 51, 232–242. [Google Scholar] [CrossRef]
- Human Metabolome Database. Available online: https://hmdb.ca/ (accessed on 27 November 2023).
- van der Wurff, I.S.M.; Bakker, E.C.; Hornstra, G.; Kirschner, P.A.; Gielen, M.; Godschalk, R.W.L.; Kremers, S.; Zeegers, M.P.; de Groot, R.H.M. Association between Prenatal and Current Exposure to Selected LCPUFAs and School Performance at Age 7. Prostaglandins Leukot Essent Fat. Acids 2016, 108, 22–29. [Google Scholar] [CrossRef]
- Strain, J.J.; Davidson, P.W.; Thurston, S.W.; Harrington, D.; Mulhern, M.S.; McAfee, A.J.; van Wijngaarden, E.; Shamlaye, C.F.; Henderson, J.; Watson, G.E.; et al. Maternal PUFA Status but Not Prenatal Methylmercury Exposure Is Associated with Children’s Language Functions at Age Five Years in the Seychelles. J. Nutr. 2012, 142, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Thomason, M.E.; Hect, J.; Waller, R.; Manning, J.H.; Stacks, A.M.; Beeghly, M.; Boeve, J.L.; Wong, K.; Van Den Heuvel, M.I.; Hernandez-Andrade, E.; et al. Prenatal Neural Origins of Infant Motor Development: Associations between Fetal Brain and Infant Motor Development. Dev. Psychopathol. 2018, 30, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Borsani, E.; Della Vedova, A.M.; Rezzani, R.; Rodella, L.F.; Cristini, C. Correlation between Human Nervous System Development and Acquisition of Fetal Skills: An Overview. Brain Dev. 2019, 41, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Shulkin, M.; Pimpin, L.; Bellinger, D.; Kranz, S.; Fawzi, W.; Duggan, C.; Mozaffarian, D. N-3 Fatty Acid Supplementation in Mothers, Preterm Infants, and Term Infants and Childhood Psychomotor and Visual Development: A Systematic Review and Meta-Analysis. J. Nutr. 2018, 148, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Gawlik, N.R.; Anderson, A.J.; Makrides, M.; Kettler, L.; Gould, J.F. The Influence of DHA on Language Development: A Review of Randomized Controlled Trials of DHA Supplementation in Pregnancy, the Neonatal Period, and Infancy. Nutrients 2020, 12, 3106. [Google Scholar] [CrossRef] [PubMed]
- Nevins, J.E.H.; Donovan, S.M.; Snetselaar, L.; Dewey, K.G.; Novotny, R.; Stang, J.; Taveras, E.M.; Kleinman, R.E.; Bailey, R.L.; Raghavan, R.; et al. Omega-3 Fatty Acid Dietary Supplements Consumed during Pregnancy and Lactation and Child Neurodevelopment: A Systematic Review. J. Nutr. 2021, 151, 3483–3494. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Londono Tobon, A.; Diaz Stransky, A.; Ross, D.A.; Stevens, H.E. Effects of Maternal Prenatal Stress: Mechanisms, Implications, and Novel Therapeutic Interventions. Biol. Psychiatry 2016, 80, e85–e87. [Google Scholar] [CrossRef]
- Cattane, N.; Räikkönen, K.; Anniverno, R.; Mencacci, C.; Riva, M.A.; Pariante, C.M.; Cattaneo, A. Depression, Obesity and Their Comorbidity during Pregnancy: Effects on the Offspring’s Mental and Physical Health. Mol. Psychiatry 2021, 26, 462–481. [Google Scholar] [CrossRef]
- Tsuduki, T.; Honma, T.; Nakagawa, K.; Ikeda, I.; Miyazawa, T. Long-Term Intake of Fish Oil Increases Oxidative Stress and Decreases Lifespan in Senescence-Accelerated Mice. Nutrition 2011, 27, 334–337. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M. The Impact of Maternal Gut Microbiota during Pregnancy on Fetal Gut–Brain Axis Development and Life-Long Health Outcomes. Microorganisms 2023, 11, 2199. [Google Scholar] [CrossRef]
- Del Rosario, C.; Slevin, M.; Molloy, E.J.; Quigley, J.; Nixon, E. How to Use the Bayley Scales of Infant and Toddler Development. Arch. Dis. Child. Educ. Pract. 2021, 106, 108–112. [Google Scholar] [CrossRef]
- Kvestad, I.; Hysing, M.; Ranjitkar, S.; Shrestha, M.; Ulak, M.; Chandyo, R.K.; Strand, T.A. The Stability of the Bayley Scales in Early Childhood and Its Relationship with Future Intellectual Abilities in a Low to Middle Income Country. Early Hum. Dev. 2022, 170, 105610. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Rodríguez, J.; Díaz-López, A.; Canals-Sans, J.; Arija, V. Maternal Vitamin B12 Status during Pregnancy and Early Infant Neurodevelopment: The ECLIPSES Study. Nutrients 2023, 15, 1529. [Google Scholar] [CrossRef] [PubMed]
- Voltas, N.; Canals, J.; Hernández-Martínez, C.; Serrat, N.; Basora, J.; Arija, V. Effect of Vitamin d Status during Pregnancy on Infant Neurodevelopment: The Eclipses Study. Nutrients 2020, 12, 3196. [Google Scholar] [CrossRef] [PubMed]
| Maternal Characteristics during Pregnancy | |
|---|---|
| Age (years), mean ± SD | 38.8 ± 5.1 |
| Family socioeconomic level, n (%) | |
| Low | 144 (41.4) |
| Mid | 152 (43.7) |
| High | 52 (14.9) |
| Mother’s anxiety (total score) during pregnancy, mean ± SD | 17.5 ± 8.8 |
| Quality of diet in the first/third trimesters (total score), mean ± SD | 9.9 ± 2.1 |
| Tobacco consumption, n (%) | |
| No | 296 (85.1) |
| Yes | 52 (14.9) |
| Alcohol consumption, n (%) | |
| No | 298 (85.5) |
| Yes | 50 (14.5) |
| BMI change in the first and third trimesters (kg/m2), mean ± SD | 4.0 ± 1.4 |
| Serum ferritin (µg/L), mean ± SD | |
| First trimester | 40.9 ± 28.3 |
| Third trimester | 16.2 ± 9.1 |
| Acetic acid (μmol/L), mean ± SD | |
| First trimester | 49.4 ± 21.6 |
| Third trimester | 48.6 ± 18.5 |
| Propionic acid (μmol/L), mean ± SD | |
| First trimester | 3.5 ± 0.9 |
| Third trimester | 3.5 ± 1.0 |
| Butyric acid (μmol/L), mean ± SD | |
| First trimester | 0.7 ± 0.3 |
| Third trimester | 0.8 ± 0.4 |
| n-3 LCPUFA (μmol/L), mean ± SD | |
| First trimester | 281.2 ± 96.9 |
| Tertile 1 | 180.7 ± 28.3 |
| Tertile 2 | 264.8 ± 23.7 |
| Tertile 3 | 390.8 ± 65.6 |
| Third trimester | 261.5 ± 83.1 |
| Tertile 1 | 175.3 ± 23.8 |
| Tertile 2 | 247.4 ± 22.5 |
| Tertile 3 | 359.4 ± 52.6 |
| DHA (μmol/L), mean ± SD | |
| First trimester | 242.5 ± 75.0 |
| Tertile 1 | 162.4 ± 24.5 |
| Tertile 2 | 233.6 ± 21.1 |
| Tertile 3 | 326.3 ± 46.2 |
| Third trimester | 237.0 ± 70.5 |
| Tertile 1 | 164.1 ± 21.8 |
| Tertile 2 | 224.6 ± 18.6 |
| Tertile 3 | 319.0 ± 43.6 |
| EPA (μmol/L), mean ± SD | |
| First trimester | 37.5 ± 25.6 |
| Tertile 1 | 14.0 ± 4.0 |
| Tertile 2 | 29.8 ± 5.1 |
| Tertile 3 | 65.3 ± 23.2 |
| Third trimester | 24.0 ± 16.7 |
| Tertile 1 | 8.5 ± 3.4 |
| Tertile 2 | 19.1 ± 3.6 |
| Tertile 3 | 42.9 ± 13.9 |
| Obstetrical outcomes | |
| Previous parity, n (%) | |
| Nulliparous | 191 (55.0) |
| Multiparous | 157 (45.0) |
| Gestational age at birth (weeks), mean ± SD | 39.8 ± 1.2 |
| Type of delivery, n (%) | |
| Eutocic | 228(65.6) |
| Dystocic | 120 (34.4) |
| Baby characteristics | |
| Infant’s gender, n (%) | |
| Boy | 185 (53.2) |
| Girl | 163 (46.8) |
| Type of feeding, n (%) | |
| Formula | 64 (18.4) |
| Breastfeeding | 284 (81.6) |
| Infant cognitive development, mean ± SD | |
| Cognitive scale | 102.10 ± 8.0 |
| Language scale | 96.5 ± 8.2 |
| Receptive | 10.6 ± 2.0 |
| Expressive | 8.1 ± 1.5 |
| Motor scale | 107.7 ± 11.5 |
| Fine | 11.5 ± 1.9 |
| Gross | 11.0 ± 2.2 |
| n-3 LCPUFA | First Trimester | Third Trimester | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 a < 224.3653 μmol/L | Tertile 2 b 224.3653 μmol/L–312.6929 μmol/L | Tertile 3 c > 312.692 μmol/L | p | Post Hoc | Tertile 1 a < 211.6078 μmol/L | Tertile 2 b 20,211.6078 μmol/L–287.8421 μmol/L | Tertile 3 c > 287.8421 μmol/L | p | Post Hoc | ||
| Cognitive Index | Unadjusted mean (SD) | 102.87 (8.0) | 102.30 (8.8) | 100.95 (7.4) | 0.190 | 102.64 (7.6) | 101.10 (9.3) | 102.59 (7.3) | 0.267 | ||
| Adjusted mean | 102.572 | 102.523 | 100.952 | 0.255 | 102.888 | 101.059 | 102.133 | 0.265 | |||
| Language Index | Unadjusted mean (SD) | 96.69 (7.4) | 96.91 (8.9) | 96.12 (8.4) | 0.758 | 97.09 (7.7) | 95.48 (9.0) | 97.13 (8.1) | 0.234 | ||
| Adjusted mean | 96.476 | 97.013 | 96.103 | 0.725 | 97.369 | 95.114 | 96.590 | 0.149 | |||
| Receptive | Unadjusted mean (SD) | 10.77 (1.9) | 10.72 (2.2) | 10.48 (2.2) | 0.553 | 10.81 (1.8) | 10.33 (2.3) | 10.94 (2.0) | 0.070 | ||
| Adjusted mean | 10.616 | 10.739 | 10.520 | 0.755 | 10.833 | 10.282 | 10.846 | 0.101 | |||
| Expressive | Unadjusted mean (SD) | 8.07 (1.5) | 8.19 (1.6) | 8.17 (1.6) | 0.843 | 8.18 (1.5) | 8.09 (1.7) | 8.05 (1.6) | 0.847 | ||
| Adjusted mean | 8.150 | 8.196 | 8.134 | 0.956 | 8.240 | 8.015 | 7.966 | 0.437 | |||
| Motor Index | Unadjusted mean (SD) | 110.23 (10.3) | 107.35 (10.9) | 105.58 (13.2) | 0.011 | a–c 0.009 | 108.23 (9.8) | 107.20 (11.0) | 107.64 (13.8) | 0.804 | |
| Adjusted mean | 110.165 | 106.603 | 105.736 | 0.025 | a–c 0.028 | 108.534 | 106.630 | 107.075 | 0.509 | ||
| Fine | Unadjusted mean (SD) | 11.67 (1.9) | 11.44 (1.9) | 11.38 (1.9) | 0.508 | 11.55 (2.0) | 11.46 (2.0) | 11.58 (1.8) | 0.883 | ||
| Adjusted mean | 11.669 | 11.321 | 11.391 | 0.438 | 11.613 | 11.321 | 11.530 | 0.554 | |||
| Gross | Unadjusted mean (SD) | 11.72 (2.3) | 10.95 (2.3) | 10.71 (2.2) | 0.003 | a–b 0.035 a–c 0.003 | 11.10 (2.0) | 10.97 (2.4) | 11.18 (2.4) | 0.794 | |
| Adjusted mean | 11.679 | 10.863 | 10.744 | 0.010 | a–b 0.038 a–c 0.014 | 11.112 | 10.929 | 11.079 | 0.840 | ||
| Docosahexaenoic Acid | First Trimester | Third Trimester | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 a < 199.0497 μmol/L | Tertile 2 b 199.0497 μmol/L –268.8105 μmol/L | Tertile 3 c > 268.8105 μmol/L | p | Post Hoc | Tertile 1 a < 195.1726 μmol/L | Tertile 2 b 195.1726 μmol/L –264.4524 μmol/L | Tertile 3 c > 264.4524 μmol/L | p | Post Hoc | ||
| Cognitive Index | Unadjusted mean (SD) | 102.73 (8.0) | 102.48 (8.8) | 100.90 (7.5) | 0.182 | 102.05 (8.1) | 101.40 (9.1) | 102.83 (7.0) | 0.417 | ||
| Adjusted mean | 102.444 | 102.667 | 100.907 | 0.228 | 102.358 | 101.233 | 102.442 | 0.477 | |||
| Language Index | Unadjusted mean (SD) | 96.75 (7.6) | 96.55 (9.1) | 96.72 (7.8) | 0.982 | 96.58 (7.7) | 95.70 (9.2) | 97.31 (8.0) | 0.344 | ||
| Adjusted mean | 96.346 | 96.744 | 96.796 | 0.919 | 96.954 | 95.107 | 96.896 | 0.193 | |||
| Receptive | Unadjusted mean (SD) | 10.68 (1.9) | 10.67 (2.2) | 10.68 (2.1) | 0.999 | 10.71 (1.9) | 10.30 (2.3) | 11.03 (2.0) | 0.029 | b–c 0.024 | |
| Adjusted mean | 10.495 | 10.705 | 10.727 | 0.710 | 10.787 | 10.174 | 10.963 | 0.057 | |||
| Expressive | Unadjusted mean (SD) | 8.17 (1.5) | 8.12 (1.6) | 8.19 (1.5) | 0.937 | 8.10 (1.4) | 8.19 (1.8) | 8.03 (1.6) | 0.732 | ||
| Adjusted mean | 8.215 | 8.144 | 8.169 | 0.948 | 8.149 | 8.110 | 7.959 | 0.657 | |||
| Motor Index | Unadjusted mean (SD) | 110.08 (10.7) | 107.87 (10.5) | 105.10 (13.1) | 0.005 | a–c 0.004 | 108.49 (9.9) | 106.47 (11.0) | 108.11 (13.6) | 0.386 | |
| Adjusted mean | 109.710 | 107.448 | 105.208 | 0.029 | a–c 0.024 | 108.688 | 105.907 | 107.596 | 0.252 | ||
| Fine | Unadjusted mean (SD) | 11.59 (2.0) | 11.55 (1.9) | 11.38 (1.9) | 0.671 | 11.50 (2.0) | 11.41 (2.0) | 11.66 (1.8) | 0.612 | ||
| Adjusted mean | 11.548 | 11.493 | 11.365 | 0.800 | 11.557 | 11.272 | 11.616 | 0.399 | |||
| Gross | Unadjusted mean (SD) | 11.75 (2.3) | 11.01 (2.2) | 10.55 (2.2) | <0.001 | a–b 0.044 a–c >0.001 | 11.24 (2.0) | 10.78 (2.4) | 11.24 (2.4) | 0.219 | |
| Adjusted mean | 11.669 | 10.949 | 10.595 | 0.004 | a–c 0.003 | 11.222 | 10.759 | 11.138 | 0.321 | ||
| Eicosapentaenoic Acid | First Trimester | Third Trimester | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tertile 1 a < 20.9746 μmol/L | Tertile 2 b 20.9746 μmol/L –38.6014 μmol/L | Tertile 3 c > 38.6014 μmol/L | p | Tertile 1 a < 14.2267 μmol/L | Tertile 2 b 14.2267 μmol/L –27.1558 μmol/L | Tertile 3 c > 27.1558 μmol/L | p | ||
| Cognitive Index | Unadjusted mean (SD) | 102.78 (7.8) | 102.03 (8.5) | 101.59 (7.5) | 0.556 | 102.36 (8.2) | 102.41 (8.2) | 101.52 (8.0) | 0.655 |
| Adjusted mean | 102.925 | 102.166 | 101.287 | 0.373 | 102.590 | 102.190 | 101.300 | 0.500 | |
| Language Index | Unadjusted mean (SD) | 96.38 (7.6) | 97.29 (8.5) | 96.22 (8.4) | 0.572 | 96.72 (8.2) | 96.14 (8.3) | 96.97 (8.7) | 0.741 |
| Adjusted mean | 96.266 | 97.479 | 96.049 | 0.400 | 96.810 | 95.769 | 96.611 | 0.645 | |
| Receptive | Unadjusted mean (SD) | 10.77 (2.0) | 10.81 (2.2) | 10.47 (2.1) | 0.395 | 10.73 (2.0) | 10.62 (2.2) | 10.76 (2.1) | 0.873 |
| Adjusted mean | 10.639 | 10.866 | 10.451 | 0.366 | 10.751 | 10.515 | 10.737 | 0.687 | |
| Expressive | Unadjusted mean (SD) | 7.97 (1.5) | 8.23 (1.6) | 8.22 (1.5) | 0.398 | 8.13 (1.5) | 8.03 (1.6) | 8.18 (1.6) | 0.755 |
| Adjusted mean | 8.055 | 8.241 | 8.171 | 0.714 | 8.135 | 8.004 | 8.079 | 0.850 | |
| Motor Index | Unadjusted mean (SD) | 108.49 (10.4) | 108.09 (11.2) | 106.72 (13.1) | 0.499 | 108.07 (10.4) | 107.50 (10.3) | 107.42 (14.0) | 0.906 |
| Adjusted mean | 108.463 | 107.844 | 106.318 | 0.444 | 108.106 | 107.345 | 106.736 | 0.726 | |
| Fine | Unadjusted mean (SD) | 11.37 (1.8) | 11.48 (2.0) | 11.64 (1.9) | 0.599 | 11.42 (2.0) | 11.53 (1.9) | 11.58 (1.9) | 0.828 |
| Adjusted mean | 11.380 | 11.433 | 11.568 | 0.799 | 11.446 | 11.469 | 11.490 | 0.988 | |
| Gross | Unadjusted mean (SD) | 11.41 (2.4) | 11.18 (2.3) | 10.83 (2.2) | 0.168 | 11.17 (2.2) | 10.96 (2.3) | 11.14 (2.3) | 0.749 |
| Adjusted mean | 11.356 | 11.160 | 10.799 | 0.249 | 11.143 | 10.954 | 11.054 | 0.853 | |
| Motor Index | Gross Motor | Motor Index | Gross Motor | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | ||
| n-3 LCPUFA (μmol/L) | −0.015 | 0.034 | −0.004 | 0.012 | DHA (μmol/L) | −0.021 | 0.029 | −0.005 | 0.003 |
| SES (low/mid/high) | −0.010 | 0.854 | 0.013 | 0.232 | SES (low/mid/high) | −0.011 | 0.847 | 0.012 | 0.260 |
| Mother’s anxiety (total score) | −0.011 | 0.886 | 0.006 | 0.710 | Mother’s anxiety (total score) | −0.012 | 0.873 | 0.005 | 0.728 |
| Tobacco consumption (Yes/No) | 4.453 | 0.022 | 0.849 | 0.024 | Tobacco consumption (Yes/No) | 4.349 | 0.025 | 0.817 | 0.031 |
| Alcohol consumption (Yes/No) | 1.845 | 0.324 | 0.316 | 0.384 | Alcohol consumption (Yes/No) | 1.771 | 0.342 | 0.318 | 0.379 |
| BMI change (kg/m2) | 0.098 | 0.839 | −0.032 | 0.730 | BMI change (kg/m2) | 0.100 | 0.834 | −0.037 | 0.695 |
| Quality of diet (total score) | 0.335 | 0.275 | 0.040 | 0.507 | Quality of diet (total score) | 0.326 | 0.282 | 0.031 | 0.593 |
| Serum ferritin (μg/L) | 0.008 | 0.742 | 0.005 | 0.277 | Serum ferritin (μg/L) | 0.010 | 0.691 | 0.005 | 0.248 |
| Acetic acid (μmol/L) | 0.080 | 0.032 | 0.011 | 0.140 | Acetic acid (μmol/L) | 0.080 | 0.033 | 0.010 | 0.169 |
| Propionic acid (μmol/L) | −1.703 | 0.077 | −0.344 | 0.067 | Propionic acid (μmol/L) | −1.651 | 0.087 | −0.325 | 0.082 |
| Butyric acid (μmol/L) | −1.441 | 0.559 | 0.183 | 0.703 | Butyric acid (μmol/L) | −1.457 | 0.553 | 0.221 | 0.643 |
| Parity (nulliparous/multiparous) | 2.540 | 0.077 | 0.388 | 0.164 | Parity (nulliparous/multiparous) | 2.528 | 0.077 | 0.410 | 0.140 |
| Gestational age at birth (weeks) | 1.403 | 0.011 | 0.225 | 0.036 | Gestational age at birth (weeks) | 1.368 | 0.012 | 0.234 | 0.027 |
| Infant’s gender (boy/girl) | 0.459 | 0.734 | −0.032 | 0.902 | Infant’s gender (boy/girl) | 0.430 | 0.749 | −0.061 | 0.817 |
| Type of delivery (eutocic/dystocic) | 0.607 | 0.680 | 0.079 | 0.783 | Type of delivery (eutocic/dystocic) | 0.619 | 0.672 | 0.072 | 0.799 |
| Type of infant feeding (formula/breastfeeding) | 0.961 | 0.584 | 0.367 | 0.283 | Type of infant feeding (formula/breastfeeding) | 0.944 | 0.590 | 0.352 | 0.301 |
| Adjusted global model | R2 × 100 = 4.4 F16/294 = 1.895 p = 0.021 | R2 × 100 = 4.7 F16/294 = 1.961 p = 0.015 | R2 × 100 = 4.5 F16/295 = 1.923 p = 0.018 | R2 × 100 = 5.8 F16/295 = 2.195 p = 0.005 | |||||
| Adjusted model with significant variables | R2 × 100 = 4.7 F4/329 = 5.138 p = 0.001 | R2 × 100 = 4.5 F3/333 = 6.221 p = 0.000 | R2 × 100 = 4.9 F4/330 = 5.328 p = 0.000 | R2 × 100 = 5.5 F3/334 = 7.598 p = 0.000 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahabi, B.; Hernández-Martínez, C.; Voltas, N.; Canals, J.; Arija, V. The Maternal Omega-3 Long-Chain Polyunsaturated Fatty Acid Concentration in Early Pregnancy and Infant Neurodevelopment: The ECLIPSES Study. Nutrients 2024, 16, 687. https://doi.org/10.3390/nu16050687
Shahabi B, Hernández-Martínez C, Voltas N, Canals J, Arija V. The Maternal Omega-3 Long-Chain Polyunsaturated Fatty Acid Concentration in Early Pregnancy and Infant Neurodevelopment: The ECLIPSES Study. Nutrients. 2024; 16(5):687. https://doi.org/10.3390/nu16050687
Chicago/Turabian StyleShahabi, Behnaz, Carmen Hernández-Martínez, Núria Voltas, Josefa Canals, and Victoria Arija. 2024. "The Maternal Omega-3 Long-Chain Polyunsaturated Fatty Acid Concentration in Early Pregnancy and Infant Neurodevelopment: The ECLIPSES Study" Nutrients 16, no. 5: 687. https://doi.org/10.3390/nu16050687
APA StyleShahabi, B., Hernández-Martínez, C., Voltas, N., Canals, J., & Arija, V. (2024). The Maternal Omega-3 Long-Chain Polyunsaturated Fatty Acid Concentration in Early Pregnancy and Infant Neurodevelopment: The ECLIPSES Study. Nutrients, 16(5), 687. https://doi.org/10.3390/nu16050687