Abstract
Metabolic syndrome (MetS) denotes a constellation of risk factors associated with the development of cardiovascular disease, with its roots potentially traced back to early life. Given the pivotal role of oxidative stress and dysbiotic gut microbiota in MetS pathogenesis, comprehending their influence on MetS programming is crucial. Targeting these mechanisms during the early stages of life presents a promising avenue for preventing MetS later in life. This article begins by examining detrimental insults during early life that impact fetal programming, ultimately contributing to MetS in adulthood. Following that, we explore the role of oxidative stress and the dysregulation of gut microbiota in the initiation of MetS programming. The review also consolidates existing evidence on how gut-microbiota-targeted interventions can thwart oxidative-stress-associated MetS programming, encompassing approaches such as probiotics, prebiotics, postbiotics, and the modulation of bacterial metabolites. While animal studies demonstrate the favorable effects of gut-microbiota-targeted therapy in mitigating MetS programming, further clinical investigations are imperative to enhance our understanding of manipulating gut microbiota and oxidative stress for the prevention of MetS.
1. Introduction
Metabolic syndrome (MetS), characterized by a combination of conditions such as obesity, insulin resistance, hypertension, and dyslipidemia, stands as a paramount threat to global health. It not only contributes to two-thirds of non-communicable disease (NCD) deaths worldwide, but also escalates the risk of cardiovascular disease (CVD) [1]. Despite varied definitions of MetS [2], its global prevalence is estimated to impact approximately one-quarter of the global population [1]. While several treatments have been applied clinically for diverse MetS phenotypes, a notable absence of specific therapeutic regimens persists, emphasizing the need for preventive interventions to curb the rising prevalence of MetS alongside treatment-focused approaches [3].
Emerging evidence suggests that the early-life environment can exert enduring effects on lifelong human well-being. Vulnerability to MetS may initiate with events occurring in the prenatal and infancy phases, leaving lasting impacts in relation to MetS and its associated complications in adulthood [4,5,6]. Referred to as the developmental origin of health and disease (DOHaD) [7], this theory underscores how early-life environmental exposures shape later health and disease risk. Consequently, efforts have been directed towards identifying early-life risk factors, preventing their exposures, particularly during pregnancy and lactation, and exploring interventions to enhance long-term outcomes, known as reprogramming.
Several early-life risk factors, often associated with adverse in utero environments, may contribute to susceptibility to adult MetS [8,9]. However, accumulating evidence suggests that the interplay between oxidative stress and dysbiosis in the gut microbiota is pivotal in the developmental programming of MetS.
Compelling evidence indicates that the mechanisms underlying MetS risk factors may originate in the gut microbiome [10]. Interactions within the gut microbiota community can process external cues contributing to major MetS components, such as obesity, hyperglycemia, dyslipidemia, and hypertension [10]. Furthermore, recent findings implicate gut-microbial-derived metabolites like tryptophan-derived uremic toxins and trimethylamine N-oxide (TMAO) in elevating CVD risk [11,12]. The intricate connection between diet, gut microbiota, and metabolic outcomes is well established [13], linking maternal imbalanced diets to developmental origins of MetS through disruptions in gut microbiota composition or function.
Oxidative stress arises when an excess of reactive oxygen species (ROS) overwhelms the antioxidant defense system, and its involvement in the etiology of various disorders has been uncovered [14]. The involvement of oxidative stress in MetS is evident as both a contributing factor and a resultant condition. Although the acknowledged role of oxidative stress in the pathogenesis of MetS is widespread [15], additional clarification is required to fully understand its causative effects on the programming of MetS.
A balanced gut microbiome depends on a stable redox balance, and gut microbiota dysbiosis disrupts this equilibrium. There is growing interest in targeting the gut microbiota through diet and nutritional approaches. This review aims to delve deeper into the interplay between oxidative stress and gut microbiota and their impact on the developmental origins of MetS. We discuss potential mechanisms involved in MetS development, focusing on the role of oxidative stress and gut microbiota dysbiosis. Subsequently, we summarize nutritional approaches targeting gut microbiota in animal models to prevent oxidative-stress-associated MetS programming. Gut-microbiota-targeted therapies have been employed clinically to address obesity and associated disorders. However, there is a notable lack of information regarding their potential application in the context of MetS programming in humans within the field of DOHaD. Figure 1 illustrates the interrelationships between early-life adverse environments, oxidative stress, gut microbiota, and the developmental programming of MetS.
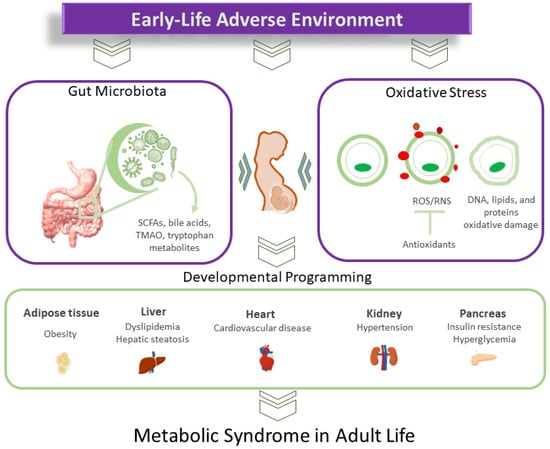
Figure 1.
Diagram illustrating the intricate connections between oxidative stress and gut microbiota in the developmental programming of metabolic syndrome.
2. MetS of Developmental Origins
Several lines of epidemiological evidence have substantiated the link between adverse early-life conditions and the heightened risk of offspring developing MetS. First, research indicates that offspring exposed to famines exhibit an increased susceptibility to developing MetS [16,17,18]. The Dutch Famine Birth Cohort Study demonstrated that maternal famine exposure programmed offspring towards conditions such as obesity, dyslipidemia, hypertension, insulin resistance, and CVD in adulthood [18]. Another strand of evidence stems from twin pregnancy studies, revealing an association between low birth weight (LBW) and specific MetS characteristics as twins mature [19,20]. Additionally, numerous studies have established that LBW correlates with various aspects of adult MetS, including hypertension [21], obesity [22], insulin resistance [23], non-alcoholic fatty liver disease (NAFLD), and dyslipidemia [24]. Notably, given that CVD is a significant complication of MetS, there is a notable surge in CVD risk associated with LBW [25]. Lastly, various early-life risk factors linked to subsequent MetS have been identified in observational studies. These factors during pregnancy encompass environmental chemical exposure [26], maternal obesity [27], gestational diabetes [28], cigarette smoke [29], and maternal stress [30]. In addition to maternal factors, adverse cardiometabolic outcomes in offspring are associated with paternal obesity, advanced paternal age at conception, paternal diabetes mellitus, and paternal smoking [31,32].
While human studies establish a connection between adverse in utero environments and later-life MetS, the underlying molecular mechanisms of MetS programming necessitate investigation to devise successful reprogramming strategies. The exploration of innovative interventions has been significantly aided by the pivotal role played by animal models in establishing the biological plausibility of these concepts [6,9].
To emulate facets of MetS observed in humans, various adverse early-life environments, such as maternal nutritional deficiencies and imbalances [9,33], maternal medical issues [34,35], exposure to environmental chemicals [36,37], and medication use [38,39], have been examined to create animal models of offspring MetS [6]. It is noteworthy that, currently, no single animal model fully replicates all features of human MetS. Most research on MetS programming employs models displaying specific components of MetS [6]. Although a broad range of maternal insults are linked to certain MetS features, only some animal models present two or more components of MetS in adult offspring [6]. In addition to commonly used rats, other animals, including mice [40], sheep [41], swine [42], rabbits [43], and non-human primates [44], have been utilized as animal models for studying MetS programming.
To date, animal studies have shed light on several mechanistic frameworks proposed to elucidate the biological basis of MetS programming, including oxidative stress [45,46], dysbiotic gut microbiota [47], impaired nutrient sensing [48], epigenetic regulation [49], aberrant activation of the renin–angiotensin system (RAS) [50], deficient nitric oxide (NO) [51], and glucocorticoid programming [52].
While the core mechanisms involved in MetS programming still require further exploration, early-life adverse insults may mediate these interconnected mechanisms and impact multiple interacting organ systems, ultimately leading to adult MetS. The primary emphasis of this review centers on rat models concerning MetS programming associated with oxidative stress and gut microbiota dysbiosis. A more in-depth discussion of these models will follow in the subsequent section.
3. Oxidative Stress and MetS of Developmental Origins
3.1. Oxidative Stress during Pregnancy
Throughout pregnancy, maintaining a delicate balance between reactive oxygen species (ROS) and antioxidants is crucial to create an optimal environment for the developing fetus [53]. ROS, while playing a pivotal role in the regulation of transcription factors and signaling pathways essential for fetal growth and development, can become a double-edged sword. Normal ROS levels are necessary for these processes, but an excess of ROS can lead to oxidative stress, causing cellular damage. Factors contributing to increased ROS production include elevated oxygen consumption, heightened metabolism, and an increased utilization of fatty acids. The excessive production of ROS disrupts these vital processes, leading to complications in maternal health during pregnancy [54].
Oxidative stress plays a significant role in the inactivation of nitric oxide (NO) as a result of excessive ROS. NO, a well-known vasodilator, governs much of the feto-placental circulation [55]. The production of NO can be inhibited by its endogenous inhibitor, asymmetric dimethylarginine (ADMA). ADMA levels decrease in the first trimester and then rise as gestational age increases [56,57]. This physiological reduction in ADMA, accompanied by high NO levels, represents a hemodynamic adaptation in early pregnancy to meet the heightened need for organ perfusion and maintain uterine relaxation. Conversely, an increase in ADMA in later gestation supports higher uterine muscle contractile activity, which is crucial for successful delivery [58].
Several maternal medical conditions and complications of pregnancy, now recognized to induce oxidative stress, encompass maternal undernutrition, obesity, preeclampsia, gestational diabetes, intrauterine growth retardation (IUGR), and maternal smoking [45,59,60,61]. Notably, gestational diabetes, preeclampsia, and maternal undernutrition are associated with elevated ADMA levels. In summary, these observations highlight that an imbalance between ROS and NO leads to oxidative stress, a fundamental mechanism participating in fetal programming during compromised pregnancies.
3.2. Oxidative Stress in Animal Models of MetS of Developmental Origins
While the notion of oxidative stress playing a pathogenic role in MetS has been suggested [15], there is a limited number of studies delving into the consequences of early-life oxidative stress on the emergence of MetS in offspring. Table 1 offers a comprehensive summary of the current literature, spotlighting investigations that examine oxidative stress in animal models displaying at least two features of MetS in their offspring [61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107]. Given their relatively shorter life span, these investigations have predominantly utilized rat models. The table includes the age at which offspring were assessed, with the age range for rats spanning from 12 to 52 weeks, equivalent to young to middle adulthood in humans [108].

Table 1.
Offspring MetS in animal models related to oxidative stress.
Various adverse maternal conditions have been utilized to induce rodent models of MetS programming. These conditions encompass maternal undernutrition, maternal overnutrition, maternal illness, pregnancy complications, maternal smoking, and chemical exposure. Protocols involving caloric or protein restriction have been employed to simulate human famine research. Maternal caloric restriction has been shown to result in hypertension and insulin resistance in adult progeny [61,62,63]. Similarly, offspring from dams exposed to protein restriction exhibited elevated blood pressure (BP) and insulin resistance [64,65]. Conversely, MetS programming can also be initiated by maternal overnutrition. Offspring exposed to maternal high fructose intake demonstrated hypertension, insulin resistance, dyslipidemia, and obesity [66,67,68]. Similarly, a maternal diet rich in saturated fat led to programmed hypertension, obesity, dyslipidemia, and hyperinsulinemia [72,73,74,75,76,77].
Maternal diseases and complications during pregnancy have been linked to offspring MetS. For instance, the offspring of diabetic rat dams exhibited hypertension, obesity, insulin resistance, and dyslipidemia [78,79,80]. Although various animal models of maternal diabetes have been developed, only streptozotocin (STZ)-induced diabetes has been explored in the context of MetS of developmental origins [78,79,80]. Maternal inflammation induced by lipopolysaccharide (LPS) and maternal uteroplacental insufficiency have been associated with hypertension and insulin resistance in adult progeny [83,84,85,86,87]. Additionally, maternal stress induced by excessive glucocorticoids has been studied in relation to offspring MetS, revealing associations with hypertension, obesity, and insulin resistance following maternal dexamethasone exposure [89,90,91].
Exposure to environmental toxins/chemicals during pregnancy has also been implicated in the developmental programming of MetS. Perinatal nicotine exposure has been linked to hypertension, hyperlipidemia, and steatosis in adult offspring [97,98,99]. Pregnant rats intubated with 1 g of ethanol/kg at days 13 and 14 of gestation exhibited hypertension and insulin resistance in offspring at 6 months of age [100,101]. Furthermore, maternal exposure to bisphenol A (BPA) [103,104] or di-n-butyl phthalate (DEHP) [105,106] resulted in an increase in BP and insulin resistance in adult rat offspring.
3.3. Mechanisms of Oxidative Stress
Mechanisms underlying oxidative stress encompass a range of processes that culminate in an overproduction of reactive oxygen species (ROS), potentially causing harm to cellular components. Key mechanisms include mitochondrial dysfunction, inflammation, metal ion imbalance, oxidative enzymatic reactions (such as NADPH oxidase and xanthine oxidase), ischemia–reperfusion injury, endoplasmic reticulum stress, lipid peroxidation, DNA damage, and compromised antioxidant defenses [14,15,53]. These factors collectively contribute to the intricate web of events associated with oxidative stress, impacting cellular health and function. The mechanisms underlying increased oxidative stress in MetS programming are not fully elucidated, but they involve the heightened expression of NADPH oxidase [92,97,102], elevated levels of ROS [71,82,88,96,107], diminished activity of antioxidant enzymes [64,76,82,92,99,102], increased peroxynitrite [62,81], elevated ADMA [61,93,103], and reduced NO [61,62,69,78,89,103]. In MetS of developmental origins, oxidative stress can manifest in various organs, including the brain [70,96], kidneys [61,85], vessels [62], and liver [99].
The impaired removal of ROS due to reduced antioxidant capacities is a contributing factor to oxidative-stress-associated MetS programming. Models of MetS programming have demonstrated a decrease in glutathione [64] and a reduction in the activities of antioxidant enzymes such as SOD [76,82,102], glutathione peroxidase 1 [92,99], and catalase [102]. Numerous specific markers of lipid, protein, and DNA oxidative damage have been investigated in various programmed MetS models, including malondialdehyde (MDA) [76,85,97], F2-isoprostanes [64,88], thiobarbituric acid reactive substances (TBARS) [81], 4-hydroxynonenal (4-NHE) [99], and 8-hydroxydeoxyguanosine (8-OHdG) [61,69,77,103].
The disruption of the ADMA/NO pathway is another oxidative-stress-associated mechanism involved in MetS programming. Table 1 highlights the crucial role of ADMA in several models of oxidative-stress-associated MetS programming, such as caloric restriction [61], maternal diabetes [78], maternal stress [93], and BPA exposure [103]. Likewise, deficient NO levels were observed in these models.
4. Gut Microbiota and MetS of Developmental Origins
4.1. Gut Microbiota
The gut microbiota constitutes a symbiotic community comprising trillions of microbes belonging to over 1000 species actively involved in modulating the host’s physiological functions. These functions include regulating gut homeostasis, influencing the absorption and metabolism of dietary nutrients, impacting the immune system, controlling BP, and contributing to drug metabolism [109]. The composition of the gut microbiota is highly individualized and undergoes transformations throughout the human lifespan, influenced by factors such as diet, lifestyle, medications, environment, and various diseases [109]. Despite the presence of some evidence that supports the transmission of microbes from mothers to fetuses during pregnancy, the existence of the prenatal microbiome has been a topic of considerable debate in recent years [110]. Microbial colonization of the neonatal gut begins shortly after birth, with the infant microbiota achieving an adult-like composition by the age of 2–3 years [111]. The mother’s microbiome significantly influences the composition of the offspring’s gut microbiome [112]. Notably, multiple factors, including maternal conditions, feeding type, delivery method, antibiotic exposure, ecological factors, and gestational age, are associated with the composition of the offspring’s gut microbiome [112].
Several of the aforementioned factors implicated in the developmental origins of MetS have been linked to disturbances in the gut microbiota. The interactions between the host and microbes are mediated by microbial-derived metabolites [113,114]. Dietary-derived bacterial metabolites, particularly TMAO, short-chain fatty acids (SCFAs), tryptophan derivatives, bile acids, and branched-chain amino acids, play a significant role in maintaining metabolic homeostasis.
4.2. Gut Microbiota Dysbiosis and MetS Programming
An imbalance in the composition of gut microbiota, known as dysbiosis, contributes to the fundamental pathogenesis of MetS programming [10]. Numerous components of offspring MetS, such as hypertension [115], obesity [116], insulin resistance [117], and dyslipidemia [118], have been documented in association with dysbiosis.
A maternal high-fat diet is often employed to investigate MetS programming, as this model induces various MetS components in adult offspring [6]. The long-term consequences of a maternal high-fat diet include a reduction in α-diversity in the offspring’s microbiota [119]. Dysbiosis characterized by a loss of α-diversity is associated with several human diseases [120]. Additionally, a maternal high-fat diet induces offspring hypertension, linked to an elevated Firmicutes-to-Bacteroidetes (F/B) ratio. An increased F/B ratio, resulting from Firmicutes expansion and/or Bacteroidetes contraction, is widely considered a hallmark of cardiometabolic disease [121]. Gut dysbiosis may also stem from a depletion of beneficial microbes [109], with previous studies indicating a reduced abundance of beneficial bacterial strains like Lactobacillus and Akkermansia in adult offspring born to dams fed a high-fat diet [122,123]. It has been shown that a maternal high-fat diet induces offspring hypertension, accompanied by alterations in gut microbiota content, reduced fecal SCFAs, dysregulated SCFA receptors, and impaired TMAO metabolic pathways in adult rat offspring [122].
Gut dysbiosis increases intestinal permeability, leading to lipopolysaccharide (LPS) absorption, endotoxemia, and systemic inflammation [124]. Elevated circulating LPS, generated in the intestine, is implicated in the pathogenesis of insulin resistance and obesity [125]. Prenatal exposure to LPS has been shown to lead to offspring hypertension [83]. Feeding lactating dams with low-fiber diets results in offspring with microbiota dysbiosis, characterized by an impaired gut barrier, increased bacterial translocation, reduced taxonomic diversity, and an increased proportion of Proteobacteria species. This microbiota dysbiosis is associated with low-grade gut inflammation and increased adiposity in adult offspring [126].
Gut microbiota dysbiosis also affects bile acid metabolism. Bile acids act as signaling molecules that regulate lipid digestion, cholesterol metabolism, and other regulatory pathways. Dysregulated gut-microbiota-derived bile acids and their receptors are implicated in type 2 diabetes, obesity, dyslipidemia, and NAFLD [127]. As an example, a diet rich in fats has been shown to induce hyperlipidemia, linked to compromised bile acid metabolism [128]. In another investigation, it was observed that maternal high-fat consumption led to NAFLD in offspring, accompanied by alterations in bile acid composition [129].
Several tryptophan derivatives produced by gut microbes may contribute to MetS pathogenesis [130]. The gut microbiota controls the three major tryptophan metabolic pathways leading to kynurenine, indole, and serotonin derivatives. Increased kynurenine levels are correlated with obesity and hyperlipidemia [131], while decreased serotonin levels are negatively correlated with BMI and body fat in patients with MetS [132]. Additionally, indole catabolites of tryptophan in the microbial metabolism participate in MetS pathogenesis via activating the aryl hydrocarbon receptor (AHR) signaling pathway [130,133]. Impaired AHR signaling is associated with various MetS components, including obesity [134], insulin resistance [135], liver steatosis [136], and hypertension [137]. Moreover, gut microbiota regulation of the bile acid metabolism is crucial for maintaining a healthy gut microbiota, insulin sensitivity, and balanced carbohydrate/lipid metabolism [138]. Studies in both humans and animals have shown that MetS is associated with the dysregulation of bile acid homeostasis [139]. Conversely, microbiota dysbiosis may be modulated through the administration of dietary nutrients, prebiotics, or probiotics to help treat MetS [140].
5. Targeting Gut Microbiota to Reprogram MetS
Implementing early-life interventions to reprogram adult disease represents a promising strategy for disrupting adverse programming processes [141]. Given the significant role of gut microbiota in the developmental origins of MetS, targeted therapies focusing on the gut microbiota could serve as a reprogramming approach to mitigate the risk of MetS in adulthood.
5.1. Gut-Microbiota-Targeted Therapy
Considering the intricate relationship between gut microbiota and host health, concerted efforts have been directed towards implementing diverse therapies aimed at modulating the gut microbiota to manage or prevent various diseases, including MetS [142,143,144].
MetS is notably influenced by insufficient dietary habits, as demonstrated by the Western diet. Conversely, the preservation of gut microbiota and protection against MetS are closely associated with the maintenance of a well-balanced and healthy diet [145]. Diets rich in high-fiber foods, plant-based ingredients, and fermented foods are linked to a more diverse and advantageous gut microbiota, ultimately contributing to improved cardiometabolic health [145].
An illustrative example is the Mediterranean diet, which draws inspiration from the traditional eating habits of individuals in the Mediterranean region [146]. This dietary approach, while limiting the intake of processed foods, underscores the importance of a high consumption of vegetables, fruits, whole grains, seafood, and olive oil [147]. The consistent incorporation of these elements into the Mediterranean diet results in the accumulation of polyunsaturated fatty acids (PUFAs) and polyphenolic compounds in the human body. Notably, one captivating aspect of the Mediterranean diet is its positive influence on gut microbiota.
Moreover, the Dietary Approaches to Stop Hypertension (DASH) diet, characterized by a high intake of fiber-rich foods such as fruits, whole grains, and vegetables, can also nourish beneficial gut bacteria [148]. By fostering the growth of advantageous bacteria and promoting SCFA production, the DASH diet may enhance gut health and potentially reduce the risk of CVD [149]. Additionally, vegan diets rich in fiber have been linked to increased SCFA production, supporting overall cardiometabolic health [150].
Several human studies, such as the GLYNDIET study [151], METADIET study [152], and METDIET study [153], have been established to explore the intricate interactions between diet, gut microbiome, and MetS. However, a notable gap exists in human studies designed to investigate the role of maternal diets in shaping the gut microbiota to prevent MetS in offspring.
Probiotic therapy involves the deliberate introduction of beneficial microbes into the gut microbiota [154]. Prebiotics, on the other hand, refer to food ingredients that promote the growth or enhance the activity of beneficial microorganisms [155]. Probiotics and prebiotics are commonly discussed and implemented in clinical practice. The term “synbiotic” is used when a product contains both probiotics and prebiotics. Additionally, postbiotics and parabiotics serve as beneficial agents promoting human health [156]. Postbiotics refer to the metabolites of probiotics after processing, such as vitamins, secreted proteins, SCFAs, and secreted biosurfactants. Parabiotics involve crude cell extracts or inanimate microbial cells of probiotics. An alternative method for re-establishing gut microbiota is fecal microbiota transplantation (FMT). This procedure entails the transfer of fecal material from a healthy donor to a recipient with the aim of restoring or altering the recipient’s gut microbiota. While certain research indicates the potential effectiveness of FMT in addressing obesity and its linked metabolic disorders [157], the existing evidence from both animal studies and rigorously controlled human trials is limited in confirming the benefits of FMT for improving metabolic parameters. Bacterial metabolite modulation is also a targeted therapy aiming to alleviate illness, for instance via the modulation of TMAO by microbial choline TMA lyase inhibitors, such as 3,3-dimethyl-1-butanol (DMB) or iodomethylcholine (IMC) [158,159].
As discussed in other scholarly works [160], evidence derived from diverse animal models of MetS programming reinforces the idea that therapies directed at the gut microbiota could potentially prevent MetS traits in adult offspring. Nevertheless, numerous aspects still lack clarity, particularly regarding the intricate interplay between the gut microbiota and oxidative stress in the context of MetS programming. Table 2 provides insight into studies that showcase gut-microbiota-targeted therapies in animal models, specifically those associated with oxidative stress (as presented in Table 1).

Table 2.
Summary of animal models illustrating therapies targeting the gut microbiota for metabolic syndrome with developmental origins.
5.2. Targeting Gut Microbiota to Prevent Oxidative-Stress-Associated MetS Programming
Interventions targeting the gut microbiota have been explored in various models related to oxidative-stress-associated MetS programming. These models encompass scenarios such as protein restriction [161], maternal high-fructose diet [162,163], high-fat diet [72,117,122,164,165,166,167,168,169], maternal high-sucrose/fat diet [170], and maternal exposure to bisphenol A (BPA) [103,171,172].
These gut-microbiota-targeted interventions involve the utilization of probiotics, prebiotics, and postbiotics, and the modulation of bacterial metabolites. A visual representation of gut-microbiota-targeted therapies for oxidative-stress-associated MetS programming is presented in Figure 2.
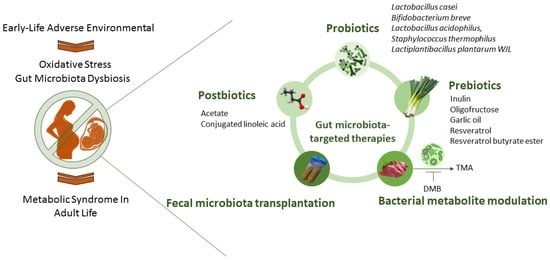
Figure 2.
An overview of potential therapies targeting the gut microbiota to prevent the developmental programming of metabolic syndrome.
Fructose-primed developmental programming is intricately associated with both oxidative stress and the gut microbiota [173]. The consumption of high levels of fructose contributes to dysbiosis in gut microbial communities. These communities play a crucial role in maintaining host–microbiota homeostasis through redox signaling, and an imbalanced redox state can trigger inflammatory responses, leading to gut microbial dysbiosis [174]. Therapies such as probiotics, prebiotics, postbiotics, and the modulation of bacterial metabolites have shown promise in preventing fructose-induced MetS programming.
Studies have indicated the beneficial effects of probiotics in MetS [175]. For instance, supplementation with Lactobacillus casei during gestation and lactation prevented hypertension in adult offspring born to dams fed a high-fructose diet [162]. Additionally, the perinatal supplementation of inulin, a well-known prebiotic, provided protection against hypertension programmed by a maternal high-fructose diet in adult progeny [162]. Inulin’s beneficial action is associated with increased plasma propionate and the restoration of reduced GPR43 expression induced by a high-fructose diet.
Postbiotics, which include various constituents such as SCFAs, have also shown promise [176]. Acetate, an abundant SCFA, can interact with its receptors to regulate BP [177]. Perinatal acetate supplementation demonstrated benefits against offspring hypertension in a rodent model of a maternal high-fructose diet [163]. Furthermore, the modulation of the microbial metabolite TMAO has been effective in protecting against fructose-induced offspring hypertension. TMAO, transformed from trimethylamine (TMA), has been linked to CVD risk [178,179]. The inhibition of microbe-dependent TMAO and TMA formation [180], achieved by using the choline analog DMB, protected adult rat offspring against maternal high-fructose diet-programmed hypertension [163].
Gut-microbiota-targeted therapies have also been investigated in the context of a high-fat diet, another commonly used model for studying MetS programming. Probiotics, such as Lactobacillus casei and Lactiplantibacillus plantarum WJL, demonstrated reprogramming effects by improving gut microbiota diversity, hypertension, lipid profile, and insulin resistance in adult offspring [117,122,164]. Prebiotics, including inulin and oligofructose, have been effective in protecting against maternal high-fat diet-primed hypertension and improving hepatic steatosis, insulin sensitivity, and glucose tolerance in adult offspring [122,170].
Certain dietary components, including garlic and resveratrol, demonstrate prebiotic properties in addition to fibers [181,182]. While numerous foods are recognized as prebiotics, Table 2 highlights that only garlic and resveratrol have been studied in the context of MetS programming. Garlic, known scientifically as Allium sativum, is abundant in polysulfides, serving as a dietary source of hydrogen sulfide (H2S) donors [183] and contributing health benefits. Maternal supplementation with garlic oil has shown positive effects against hypertension in offspring primed with a high-fat diet. This supplementation led to increased levels of acetate, butyrate, and propionate in plasma, enhanced α-diversity, and an elevated proportion of beneficial microbes such as Bifidobacterium and Lactobacillus [72].
Resveratrol, a natural polyphenol found in grapes, is widely acknowledged for its antioxidant and prebiotic properties [184]. It has been proposed as a reprogramming strategy to prevent MetS traits [185,186]. Table 2 indicates that resveratrol has beneficial effects against MetS induced by a high-fat diet, addressing issues such as obesity, hyperlipidemia, and hepatic steatosis [165,166,168,169]. In a model combining maternal high-fat diet and NO deficiency [187], resveratrol protected adult offspring from hypertension by reducing the F/B ratio, a microbial marker associated with hypertension [188]. This highlights resveratrol’s potential to reshape the gut microbiome and prevent hypertension in offspring. Another example of a postbiotic used for reprogramming in MetS programming is conjugated linoleic acid. Derived from the gut microbiota metabolism, conjugated linoleic acid is catabolized from dietary polyunsaturated fatty acids. Maternal supplementation with conjugated linoleic acid has been shown to reverse cardiometabolic dysfunction in adult offspring primed by a high-fat diet [167].
Despite the benefits, the low bioavailability of resveratrol poses a challenge in translating basic scientific findings into clinical practice [189]. To address this concern, previous efforts have involved esterifying resveratrol with butyrate to create resveratrol butyrate esters (RBEs), aiming to enhance efficacy [190]. Research has demonstrated that low-dose RBEs (30 mg/L) improved hyperlipidemia and obesity in female progeny and hepatic steatosis in male offspring, both of which were primed by maternal exposure to bisphenol A (BPA) [171,172], showing sex-specific effects. Despite the known benefits of various prebiotic foods for MetS-related disorders [140], much remains unclear regarding their impact as reprogramming strategies for preventing offspring MetS.
Beyond resveratrol, recent research has highlighted the therapeutic potential of other dietary polyphenols in addressing obesity and related diseases [191,192]. Among these commonly consumed polyphenols are epigallocatechin [193], curcumin [194], quercetin [195], and epigallocatechin gallate [196]. Given that specific polyphenols have been identified as reprogramming agents to mitigate offspring hypertension [93], further investigation is warranted to elucidate whether they possess the capacity to prevent MetS programming.
5.3. The Interplay between Oxidative Stress and Gut Microbiota
In the preceding sections, we elucidated the pivotal roles of gut-microbiota-targeted therapy in mitigating oxidative-stress-associated MetS programming. Conversely, the utilization of antioxidant therapy in early life has exhibited promise for preventing MetS [46]. Various antioxidants administered during pregnancy and lactation in diverse models of MetS programming encompass vitamins, N-acetylcysteine (NAC), amino acids, polyphenols, melatonin, and synthetic antioxidants.
In a rodent model subjected to a high-fructose diet, perinatal supplementation with resveratrol prevented offspring hypertension while concurrently reducing oxidative stress and inducing alterations in gut microbiota composition [197]. Resveratrol therapy was found to augment the expression of nuclear factor erythroid-derived 2-related factor 2 (Nrf2) and decrease the expression of 8-OHdG in the offspring’s kidneys. Furthermore, the enduring effects of resveratrol on the offspring’s gut microbiota included an increase in the proportions of Lactobacillus and Bifidobacterium [197].
Another study demonstrated that antioxidant therapy with NAC in pregnant spontaneously hypertensive rats (SHRs) successfully prevented offspring hypertension [198]. The positive impact of maternal NAC therapy on hypertension was associated with a high abundance of the phylum Actinobacteria and the genera Bifidobacterium and Allobaculum, along with a low abundance of the phylum Verrucomicrobia and the genera Akkermansia and Turicibacter.
Additionally, L-malic acid, an antioxidant component commonly found in food additives, was investigated. A maternal diet supplemented with L-malic acid augmented antioxidant capacity, subsequently enhancing insulin sensitivity and glucose metabolism by modulating the gut microbiota of piglet offspring [199]. The increased abundance of Romboutsia, Colidextribacter, and Family_XIII_AD3011_group was associated with improved antioxidant capacity and glucose metabolism. Conversely, lower levels of Blautia, Prevotellaceae_NK3B31_group, Prevotella, and Collinsella were linked to reduced insulin sensitivity. This evidence underscores the intricate interplay between oxidative stress and the gut microbiota in the context of MetS programming.
5.4. Bridging the Gap between Animal Models and Clinical Practice
Animal research provides support for the potential preventive effects of the early utilization of specific gut-microbiota-targeted therapies against MetS programming. However, the translation of this growing body of evidence into clinical practice is still pending. Presently, the application of probiotics or prebiotics during gestation remains limited in human studies [200]. The evidence is scarce regarding probiotic supplementation for pregnant women, but the existing literature suggests its generally safe use and potential benefits for conditions such as gestational diabetes [201], preeclampsia [202], spontaneous preterm delivery [203], vaginal infections [204], and obesity [205]. However, information regarding the utility of prebiotic-rich foods or prebiotic-like components, either alone or in combination, during pregnancy is largely lacking [206].
It is crucial to note that there is a dearth of information on the influence of probiotic or prebiotic supplementation during gestation on the long-term outcomes of offspring related to MetS in human studies. Despite ongoing trials involving more than 10 studies focusing on prebiotic or probiotic supplements in pregnant women [207], none of them primarily concentrate on the development of MetS in offspring.
Elaborating on safety considerations, it is essential to emphasize that parabiotics and postbiotics are generally considered safer alternatives compared to probiotics. However, a notable gap exists in the realm of clinical practice, where a universally accepted definition for both parabiotics and postbiotics is still lacking. In contrast, authoritative bodies such as the Food and Agriculture Organization of the United Nations-WHO (FAO-WHO) and the International Scientific Association for Probiotics and Prebiotics (ISAPP) have established clear definitions for probiotics and prebiotics.
Given the dynamic nature of microbial-based therapies, there is a pressing need for governmental regulatory bodies to establish precise definitions for parabiotics and postbiotics from a regulatory standpoint. Clarity in terminology is crucial not only for effective communication within scientific and medical communities, but also for ensuring that regulatory frameworks can thoroughly assess and oversee the safety and efficacy of these emerging interventions. The absence of standardized definitions may introduce ambiguity, impeding the progress of research and application in these promising fields. Addressing this need for precise terminology will contribute to a more informed and regulated integration of parabiotics and postbiotics into clinical practice, promoting both scientific advancement and public health.
6. Concluding Remarks
Existing evidence indicates that the gut microbiota and oxidative stress play crucial roles as pathogenic factors in the developmental programming of MetS. Our review underscores the potential of gut-microbiota-targeted therapy as a strategy for reprogramming, aiming to prevent MetS. Despite the apparent benefits observed in MetS programming with this therapy, its effectiveness awaits confirmation through future human investigations. We anticipate that novel reprogramming interventions, influencing the interaction between oxidative stress and gut microbiota, will emerge in the future, contributing to the prevention of MetS.
Author Contributions
Conceptualization, Y.-L.T. and C.-N.H.; writing—original draft, Y.-L.T. and C.-N.H.; data curation, Y.-L.T. and C.-N.H.; Funding acquisition, Y.-L.T. and C.-N.H.; writing—review and editing, Y.-L.T. and C.-N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan, under grants CORPG8M0381, CORPG8L0551, CMRPG8M0721, and CMRPG8M0722.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar]
- Schwarz, P.E.; Reimann, M.; Li, J.; Bergmann, A.; Licinio, J.; Wong, M.L.; Bornstein, S.R. The Metabolic Syndrome—A global challenge for prevention. Horm. Metab. Res. 2007, 39, 777–780. [Google Scholar] [CrossRef]
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef]
- De Gusmão Correia, M.L.; Volpato, A.M.; Águila, M.B.; Mandarim-de-Lacerda, C.A. Developmental origins of health and disease: Experimental and human evidence of fetal programming for metabolic syndrome. J. Hum. Hypertens. 2012, 26, 405–419. [Google Scholar] [CrossRef]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal programming of the metabolic syndrome. Taiwan J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef]
- Hanson, M. The birth and future health of DOHaD. J. Dev. Orig. Health Dis. 2015, 6, 434–437. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Cardoso, R.C.; Puttabyatappa, M. Developmental Programming, a Pathway to Disease. Endocrinology 2016, 157, 1328–1340. [Google Scholar] [CrossRef]
- Armitage, J.A.; Khan, I.Y.; Taylor, P.D.; Nathanielsz, P.W.; Poston, L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: How strong is the evidence from experimental models in mammals? J. Physiol. 2004, 561, 355–377. [Google Scholar] [CrossRef]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef]
- Velasquez, M.T.; Ramezani, A.; Manal, A.; Raj, D.S. Trimethylamine N-Oxide: The good, the bad and the unknown. Toxins 2016, 8, 326. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Hsu, W.H.; Tain, Y.L. Cardiovascular Diseases of Developmental Origins: Preventive Aspects of Gut Microbiota-Targeted Therapy. Nutrients 2021, 13, 2290. [Google Scholar] [CrossRef]
- Perler, B.K.; Friedman, E.S.; Wu, G.D. The Role of the Gut Microbiota in the Relationship between Diet and Human Health. Annu. Rev. Physiol. 2023, 85, 449–468. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Spahis, S.; Borys, J.M.; Levy, E. Metabolic Syndrome as a Multifaceted Risk Factor for Oxidative Stress. Antioxid. Redox Signal. 2017, 26, 445–461. [Google Scholar] [CrossRef]
- Stanner, S.A.; Yudkin, J.S. Fetal programming and the Leningrad Siege study. Twin Res. 2001, 4, 287–292. [Google Scholar] [CrossRef]
- Hult, M.; Tornhammar, P.; Ueda, P.; Chima, C.; Bonamy, A.K.; Ozumba, B.; Norman, M. Hypertension, diabetes and overweight: Looming legacies of the Biafran famine. PLoS ONE 2010, 5, e13582. [Google Scholar] [CrossRef]
- Schulz, L.C. The Dutch Hunger Winter and the Developmental Origins of Health and Disease. Proc. Natl. Acad. Sci. USA 2010, 107, 16757–16758. [Google Scholar] [CrossRef]
- Bo, S.; Cavallo-Perin, P.; Ciccone, G.; Scaglione, L.; Pagano, G. The metabolic syndrome in twins: A consequence of low birth weight or of being a twin? Exp. Clin. Endocrinol. Diabetes 2001, 109, 135–140. [Google Scholar] [CrossRef]
- Vaag, A.; Poulsen, P. Twins in metabolic and diabetes research: What do they tell us? Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 591–596. [Google Scholar] [CrossRef]
- Huxley, R.; Neil, A.; Collins, R. Unravelling the fetal origins hypothesis: Is there really an inverse association between birthweight and subsequent blood pressure? Lancet 2002, 360, 659–665. [Google Scholar] [CrossRef]
- Parsons, T.J.; Power, C.; Manor, O. Fetal and early life growth and body mass index from birth to early adulthood in 1958 British cohort: Longitudinal study. Bone Miner. J. 2001, 323, 1331–1335. [Google Scholar] [CrossRef]
- Phipps, K.; Barker, D.J.P.; Hales, C.N.; Fall, C.H.D.; Osmond, C.; Clark, P.M.S. Fetal growth and impaired glucose tolerance in men and women. Diabetologia 1993, 36, 225–228. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Panera, N.; Agostoni, C. Low birth weight and catch-up-growth associated with metabolic syndrome: A ten year systematic review. Pediatr. Endocrinol. Rev. 2008, 6, 241–247. [Google Scholar]
- Kelishadi, R.; Haghdoost, A.A.; Jamshidi, F.; Aliramezany, M.; Moosazadeh, M. Low birthweight or rapid catch-up growth: Which is more associated with cardiovascular disease and its risk factors in later life? A systematic review and cryptanalysis. Paediatr. Int. Child Health 2015, 35, 110–123. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Z.; Bartell, T.; Wang, X. Early Life Origins of Metabolic Syndrome: The Role of Environmental Toxicants. Curr. Environ. Health Rep. 2014, 1, 78–89. [Google Scholar] [CrossRef]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, B.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, e290–e296. [Google Scholar] [CrossRef]
- Tam, W.H.; Ma, R.C.W.; Ozaki, R.; Li, A.M.; Chan, M.H.M.; Yuen, L.Y.; Lao, T.T.H.; Yang, X.; Ho, C.S.; Tutino, G.E.; et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care 2017, 40, 679–686. [Google Scholar] [CrossRef]
- Rogers, J.M. Smoking and pregnancy: Epigenetics and developmental origins of the metabolic syndrome. Birth Defects Res. 2019, 111, 1259–1269. [Google Scholar] [CrossRef]
- Eberle, C.; Fasig, T.; Brüseke, F.; Stichling, S. Impact of maternal prenatal stress by glucocorticoids on metabolic and cardiovascular outcomes in their offspring: A systematic scoping review. PLoS ONE 2021, 16, e0245386. [Google Scholar] [CrossRef]
- Soubry, A. Epigenetics as a Driver of Developmental Origins of Health and Disease: Did We Forget the Fathers? Bioessays 2018, 40, 1700113. [Google Scholar] [CrossRef]
- Eberle, C.; Kirchner, M.F.; Herden, R.; Stichling, S. Paternal metabolic and cardiovascular programming of their offspring: A systematic scoping review. PLoS ONE 2020, 15, e0244826. [Google Scholar] [CrossRef]
- Lakshmy, R. Metabolic syndrome: Role of maternal undernutrition and fetal programming. Rev. Endocr. Metab. Disord. 2013, 14, 229–240. [Google Scholar] [CrossRef]
- Hsu, C.N.; Yang, H.W.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Melatonin Prevents Chronic Kidney Disease-Induced Hypertension in Young Rat Treated with Adenine: Implications of Gut Microbiota-Derived Metabolites. Antioxidants 2021, 10, 1211. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N.; Lee, C.T.; Lin, Y.J.; Tsai, C.C. N-Acetylcysteine Prevents Programmed Hypertension in Male Rat Offspring Born to Suramin-Treated Mothers. Biol. Reprod. 2016, 95, 8. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hung, C.H.; Hou, C.Y.; Chang, C.I.; Tain, Y.L. Perinatal Resveratrol Therapy to Dioxin-Exposed Dams Prevents the Programming of Hypertension in Adult Rat Offspring. Antioxidants 2021, 10, 1393. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Resveratrol Butyrate Ester Supplementation Blunts the Development of Offspring Hypertension in a Maternal Di-2-ethylhexyl Phthalate Exposure Rat Model. Nutrients 2023, 15, 697. [Google Scholar] [CrossRef]
- Slabiak-Blaz, N.; Adamczak, M.; Gut, N.; Grajoszek, A.; Nyengaard, J.R.; Ritz, E.; Wiecek, A. Administration of cyclosporine a in pregnant rats—The effect on blood pressure and on the glomerular number in their offspring. Kidney Blood Press. Res. 2015, 40, 413–423. [Google Scholar] [CrossRef]
- Nyirenda, M.J.; Lindsay, R.S.; Kenyon, C.J.; Burchell, A.; Seckl, J.R. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J. Clin. Investig. 1998, 101, 2174–2181. [Google Scholar] [CrossRef]
- Ito, J.; Nakagawa, K.; Kato, S.; Miyazawa, T.; Kimura, F.; Miyazawa, T. The combination of maternal and offspring high-fat diets causes marked oxidative stress and development of metabolic syndrome in mouse offspring. Life Sci. 2016, 151, 70–75. [Google Scholar] [CrossRef]
- Pankey, C.L.; Walton, M.W.; Odhiambo, J.F.; Smith, A.M.; Ghnenis, A.B.; Nathanielsz, P.W.; Ford, S.P. Intergenerational impact of maternal overnutrition and obesity throughout pregnancy in sheep on metabolic syndrome in grandsons and granddaughters. Domest. Anim. Endocrinol. 2017, 60, 67–74. [Google Scholar] [CrossRef]
- Arentson-Lantz, E.J.; Buhman, K.K.; Ajuwon, K.; Donkin, S.S. Excess pregnancy weight gain leads to early indications of metabolic syndrome in a swine model of fetal programming. Nutr. Res. 2014, 34, 241–249. [Google Scholar] [CrossRef]
- Rousseau-Ralliard, D.; Richard, C.; Hoarau, P.; Lallemand, M.S.; Morillon, L.; Aubrière, M.C.; Valentino, S.A.; Dahirel, M.; Guinot, M.; Fournier, N.; et al. Prenatal air pollution exposure to diesel exhaust induces cardiometabolic disorders in adulthood in a sex-specific manner. Environ. Res. 2021, 200, 111690. [Google Scholar] [CrossRef]
- Puppala, S.; Li, C.; Glenn, J.P.; Saxena, R.; Gawrieh, S.; Quinn, A.; Palarczyk, J.; Dick, E.J., Jr.; Nathanielsz, P.W.; Cox, L.A. Primate fetal hepatic responses to maternal obesity: Epigenetic signalling pathways and lipid accumulation. J. Physiol. 2018, 596, 5823–5837. [Google Scholar] [CrossRef]
- Thompson, L.P.; Al-Hasan, Y. Impact of oxidative stress in fetal programming. J. Pregnancy 2012, 2012, 582748. [Google Scholar] [CrossRef]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef]
- Jašarevi’c, E.; Bale, T.L. Prenatal and postnatal contributions of the maternal microbiome on offspring programming. Front. Neuroendocrinol. 2019, 55, 100797. [Google Scholar] [CrossRef]
- Jansson, T.; Powell, T.L. Role of placental nutrient sensing in developmental programming. Clin. Obstet. Gynecol. 2013, 56, 591–601. [Google Scholar] [CrossRef]
- Block, T.; El-Osta, A. Epigenetic programming, early life nutrition and the risk of metabolic disease. Atherosclerosis 2017, 266, 31–40. [Google Scholar] [CrossRef]
- Bogdarina, I.; Welham, S.; King, P.J.; Burns, S.P.; Clark, A.J. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007, 100, 520–526. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. The NOS/NO System in Renal Programming and Reprogramming. Antioxidants 2023, 12, 1629. [Google Scholar] [CrossRef]
- Cottrell, E.C.; Seckl, J.R. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 2009, 3, 19. [Google Scholar] [CrossRef]
- Dennery, P.A. Oxidative stress in development: Nature or nurture? Free Radic. Biol. Med. 2010, 49, 1147–1151. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Myatt, L. Placental adaptive responses and fetal programming. J. Physiol. 2006, 572, 25–30. [Google Scholar] [CrossRef]
- Tran, C.T.; Leiper, J.M.; Vallance, P. The DDAH/ADMA/NOS pathway. Atheroscler. Suppl. 2003, 4, 33–40. [Google Scholar] [CrossRef]
- Fickling, S.A.; Williams, D.; Vallance, P.; Nussey, S.S.; Whitley, G.S. Plasma of endogenous inhibitor of nitric oxide synthesis in normal pregnancy and pre-eclampsia. Lancet 1993, 342, 242–243. [Google Scholar] [CrossRef]
- Holden, D.P.; Fickling, S.A.; Whitley, G.S.; Nussey, S.S. Plasma concentrations of asymmetric dimethylarginine, a natural inhibitor of nitric oxide synthase, in normal pregnancy and preeclampsia. Am. J. Obstet. Gynecol. 1998, 178, 551–556. [Google Scholar] [CrossRef]
- Akturk, M.; Altinova, A.; Mert, I.; Dincel, A.; Sargin, A.; Buyukkagnici, U.; Arslan, M.; Danisman, N. Asymmetric dimethylarginine concentrations are elevated in women with gestational diabetes. Endocrine 2010, 38, 134–141. [Google Scholar] [CrossRef]
- Vida, G.; Sulyok, E.; Ertl, T.; Martens-Lobenhoffer, J.; Bode-Böger, S.M. Birth by cesarean section is associated with elevated neonatal plasma levels of dimethylarginines. Pediatr. Int. 2012, 54, 476–479. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef]
- Franco Mdo, C.; Ponzio, B.F.; Gomes, G.N.; Gil, F.Z.; Tostes, R.; Carvalho, M.H.; Fortes, Z.B. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009, 85, 327–333. [Google Scholar] [CrossRef]
- Holemans, K.; Verhaeghe, J.; Dequeker, J.; Van Assche, F.A. Insulin sensitivity in adult female rats subjected to malnutrition during the perinatal period. J. Soc. Gynecol. Investig. 1996, 3, 71–77. [Google Scholar] [CrossRef]
- Cambonie, G.; Comte, B.; Yzydorczyk, C.; Ntimbane, T.; Germain, N.; Lê, N.L.; Pladys, P.; Gauthier, C.; Lahaie, I.; Abran, D.; et al. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1236–R1245. [Google Scholar] [CrossRef]
- Ozanne, S.E.; Smith, G.D.; Tikerpae, J.; Hales, C.N. Altered regulation of hepatic glucose output in the male offspring of protein-malnourished rat dams. Am. J. Physiol. 1996, 270, E559–E564. [Google Scholar] [CrossRef]
- Tain, Y.L.; Wu, K.L.; Lee, W.C.; Leu, S.; Chan, J.Y. Maternal fructose-intake-induced renal programming in adult male offspring. J. Nutr. Biochem. 2015, 26, 642–650. [Google Scholar] [CrossRef]
- Chao, Y.M.; Tain, Y.L.; Leu, S.; Wu, K.L.; Lee, W.C.; Chan, J.Y. Developmental programming of the metabolic syndrome: Next generation sequencing analysis of transcriptome expression in a rat model of maternal high fructose intake. Sheng Li Xue Bao 2016, 68, 557–567. [Google Scholar]
- Saad, A.F.; Dickerson, J.; Kechichian, T.B.; Yin, H.; Gamble, P.; Salazar, A.; Patrikeev, I.; Motamedi, M.; Saade, G.R.; Costantine, M.M. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. Am. J. Obstet. Gynecol. 2016, 215, 378.e1–378.e6. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose-fed male adult rat offspring. J. Nutr. Biochem. 2016, 38, 86–92. [Google Scholar] [CrossRef]
- Chao, Y.M.; Wu, K.L.H.; Tsai, P.C.; Tain, Y.L.; Leu, S.; Lee, W.C.; Chan, J.Y.H. Anomalous AMPK-regulated angiotensin AT1R expression and SIRT1-mediated mitochondrial biogenesis at RVLM in hypertension programming of offspring to maternal high fructose exposure. J. Biomed. Sci. 2020, 27, 68. [Google Scholar] [CrossRef]
- Tsai, P.C.; Chao, Y.M.; Chan, J.Y.H. Sympathetic activation of splenic T-lymphocytes in hypertension of adult offspring programmed by maternal high fructose exposure. Chin. J. Physiol. 2020, 63, 263–275. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal Garlic Oil Supplementation Prevents High-Fat Diet-Induced Hypertension in Adult Rat Offspring: Implications of H2S-Generating Pathway in the Gut and Kidneys. Mol. Nutr. Food Res. 2021, 65, e2001116. [Google Scholar] [CrossRef]
- Tsai, T.A.; Tsai, C.K.; Huang, L.T.; Sheen, J.M.; Tiao, M.M.; Tain, Y.L.; Chen, C.C.; Lin, I.C.; Lai, Y.J.; Tsai, C.C.; et al. Maternal Resveratrol Treatment Re-Programs and Maternal High-Fat Diet-Induced Retroperitoneal Adiposity in Male Offspring. Int. J. Environ. Res. Public Health 2020, 17, 2780. [Google Scholar] [CrossRef] [PubMed]
- Sheen, J.M.; Yu, H.R.; Tain, Y.L.; Tsai, W.L.; Tiao, M.M.; Lin, I.C.; Tsai, C.C.; Lin, Y.J.; Huang, L.T. Combined maternal and postnatal high-fat diet leads to metabolic syndrome and is effectively reversed by resveratrol: A multiple-organ study. Sci. Rep. 2018, 8, 5607. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, J.; Xu, H.; Lyv, Y.; Feng, X.; Fang, Y.; Xu, Y. Maternal quercetin administration during gestation and lactation decrease endoplasmic reticulum stress and related inflammation in the adult offspring of obese female rats. Eur. J. Nutr. 2014, 53, 1669–1683. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.L.; Hsu, C.N.; Tain, Y.L. Whether AICAR in Pregnancy or Lactation Prevents Hypertension Programmed by High Saturated Fat Diet: A Pilot Study. Nutrients 2020, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, L.C.P.; Neto, J.P.R.C.; de Andrade Braga, V.; Lagranha, C.J.; de Brito Alves, J.L. Maternal exposure to high-fat and high-cholesterol diet induces arterial hypertension and oxidative stress along the gut-kidney axis in rat offspring. Life Sci. 2020, 261, 118367. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS ONE 2013, 8, e55420. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.C.; Andreotti, S.; Chimin, P.; Sertié, R.A.; Farias Tda, S.; Torres-Leal, F.L.; de Proença, A.R.; Campaña, A.B.; D’Avila, L.S.; Oliveira, K.A.; et al. Neonatal streptozotocin-induced diabetes in mothers promotes metabolic programming of adipose tissue in male rat offspring. Life Sci. 2015, 136, 151–156. [Google Scholar] [CrossRef]
- Thaeomor, A.; Teangphuck, P.; Chaisakul, J.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents Metabolic and Cardiovascular Effects of Maternal Diabetes in Adult Rat Offspring. Adv. Exp. Med. Biol. 2017, 975, 295–305. [Google Scholar]
- Martínez Gascón, L.E.; Ortiz, M.C.; Galindo, M.; Sanchez, J.M.; Sancho-Rodriguez, N.; Albaladejo Otón, M.D.; Rodriguez Mulero, M.D.; Rodriguez, F. Role of heme oxygenase in the regulation of the renal hemodynamics in a model of sex dependent programmed hypertension by maternal diabetes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 322, R181–R191. [Google Scholar] [CrossRef]
- Yu, C.; Chen, S.; Wang, X.; Wu, G.; Zhang, Y.; Fu, C.; Hu, C.; Liu, Z.; Luo, X.; Wang, J.; et al. Exposure to maternal diabetes induces endothelial dysfunction and hypertension in adult male rat offspring. Microvasc. Res. 2021, 133, 104076. [Google Scholar] [CrossRef]
- Wang, J.; Yin, N.; Deng, Y.; Wei, Y.; Huang, Y.; Pu, X.; Li, L.; Zheng, Y.; Guo, J.; Yu, J.; et al. Ascorbic Acid Protects against Hypertension through Downregulation of ACE1 Gene Expression Mediated by Histone Deacetylation in Prenatal Inflammation Induced Offspring. Sci. Rep. 2016, 6, 39469. [Google Scholar] [CrossRef]
- Tsosura, T.V.S.; Chiba, F.Y.; Mattera, M.S.L.C.; Pereira, R.F.; Cintra, L.T.A.; Conti, L.C.; Santos, R.M.D.; Mateus, J.H.P.; Garbin, C.A.S.; Sumida, D.H. Maternal apical periodontitis is associated with insulin resistance in adult offspring. Int. Endod. J. 2019, 52, 1040–1050. [Google Scholar] [CrossRef]
- Vieira, L.D.; Farias, J.S.; de Queiroz, D.B.; Cabral, E.V.; Lima-Filho, M.M.; Sant’Helena, B.R.M.; Aires, R.S.; Ribeiro, V.S.; SantosRocha, J.; Xavier, F.E.; et al. Oxidative stress induced by prenatal LPS leads to endothelial dysfunction and renal haemodynamic changes through angiotensin II/NADPH oxidase pathway: Prevention by early treatment with α-tocopherol. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Wlodek, M.E.; Westcott, K.; Siebel, A.L.; Owens, J.A.; Moritz, K.M. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int. 2008, 74, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Nüsken, K.D.; Dötsch, J.; Rauh, M.; Rascher, W.; Schneider, H. Uteroplacental insufficiency after bilateral uterine artery ligation in the rat: Impact on postnatal glucose and lipid metabolism and evidence for metabolic programming of the offspring by sham operation. Endocrinology 2008, 149, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, N.B.; Hennington, B.S.; Williamson, D.T.; Hill, M.L.; Betson, N.E.; Sartori-Valinotti, J.C.; Reckelhoff, J.F.; Royals, T.P.; Alexander, B.T. Oxidative stress contributes to sex differences in blood pressure in adult growth-restricted offspring. Hypertension 2012, 60, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Tiao, M.M.; Sheen, J.M.; Huang, L.T.; Tain, Y.L.; Lin, I.C.; Lin, Y.J.; Lai, Y.J.; Chen, C.C.; Chang, K.A.; et al. Obesity programmed by prenatal dexamethasone and postnatal high-fat diet leads to distinct alterations in nutrition sensory signals and circadian-clock genes in visceral adipose tissue. Lipids Health Dis. 2019, 18, 19. [Google Scholar] [CrossRef]
- Tiao, M.M.; Huang, L.T.; Chen, C.J.; Sheen, J.M.; Tain, Y.L.; Chen, C.C.; Kuo, H.C.; Huang, Y.H.; Tang, K.S.; Chu, E.W.; et al. Melatonin in the regulation of liver steatosis following prenatal glucocorticoid exposure. BioMed Res. Int. 2014, 2014, 942172. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, J.; Khurana, S.; Tharmalingam, S.; Williamson, C.; Byrne, C.J.; Lees, S.J.; Khaper, N.; Kumar, A.; Tai, T.C. Oxidative Stress Mediates the Fetal Programming of Hypertension by Glucocorticoids. Antioxidants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, Y.J.; Lu, P.C.; Tain, Y.L. Maternal resveratrol therapy protects male rat offspring against programmed hypertension induced by TCDD and dexamethasone exposures: Is it relevant to aryl hydrocarbon receptor? Int. J. Mol. Sci. 2018, 19, 2459. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lin, Y.J.; Chan, J.Y.H.; Lee, C.T.; Hsu, C.N. Maternal melatonin or agomelatine therapy prevents programmed hypertension in male offspring of mother exposed to continuous light. Biol. Reprod. 2017, 97, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.S.; Amaral, F.G.; Mesquita, C.C.; Barbosa, A.P.; Lellis-Santos, C.; Turati, A.O.; Santos, L.R.; Sollon, C.S.; Gomes, P.R.; Faria, J.A.; et al. Maternal melatonin programs the daily pattern of energy metabolism in adult offspring. PLoS ONE 2012, 7, e38795. [Google Scholar] [CrossRef] [PubMed]
- Voiculescu, S.E.; Le Duc, D.; Roșca, A.E.; Zeca, V.; Chiţimuș, D.M.; Arsene, A.L.; Drăgoi, C.M.; Nicolae, A.C.; Zăgrean, L.; Schöneberg, T.; et al. Behavioral and molecular effects of prenatal continuous light exposure in the adult rat. Brain Res. 2016, 1650, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Huang, X.; Yang, S.; Zhang, L. Antenatal nicotine induces heightened oxidative stress and vascular dysfunction in rat offspring. Br. J. Pharmacol. 2011, 164, 1400–1409. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, X.; Li, Y.; Dasgupta, C.; Wang, L.; Zhang, L. Antenatal Antioxidant Prevents Nicotine-Mediated Hypertensive Response in Rat Adult Offspring. Biol. Reprod. 2015, 93, 66. [Google Scholar] [CrossRef]
- Conceição, E.P.; Peixoto-Silva, N.; Pinheiro, C.R.; Oliveira, E.; Moura, E.G.; Lisboa, P.C. Maternal nicotine exposure leads to higher liver oxidative stress and steatosis in adult rat offspring. Food Chem. Toxicol. 2015, 78, 52–59. [Google Scholar] [CrossRef]
- Gray, S.P.; Denton, K.M.; Cullen-McEwen, L.; Bertram, J.F.; Moritz, K.M. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J. Am. Soc. Nephrol. 2010, 21, 1891–1902. [Google Scholar] [CrossRef]
- Nguyen, T.M.T.; Steane, S.E.; Moritz, K.M.; Akison, L.K. Prenatal alcohol exposure programmes offspring disease: Insulin resistance in adult males in a rat model of acute exposure. J. Physiol. 2019, 597, 5619–5637. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.L.; de la Fuente-Ortega, E.; Vargas-Roberts, S.; Muñoz, D.C.; Goic, C.A.; Haeger, P.A. NADPH Oxidase Isoform 2 (NOX2) Is Involved in Drug Addiction Vulnerability in Progeny Developmentally Exposed to Ethanol. Front. Neurosci. 2017, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Lin, Y.J.; Tain, Y.L. Maternal exposure to bisphenol A combined with high-fat diet-induced programmed hypertension in adult male rat offspring: Effects of resveratrol. Int. J. Mol. Sci. 2019, 20, 4382. [Google Scholar] [CrossRef] [PubMed]
- Galyon, K.D.; Farshidi, F.; Han, G.; Ross, M.G.; Desai, M.; Jellyman, J.K. Maternal bisphenol A exposure alters rat offspring hepatic and skeletal muscle insulin signaling protein abundance. Am. J. Obstet. Gynecol. 2017, 216, 290.e1–290.e9. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Song, L.; Wei, J.; Chen, T.; Chen, J.; Lin, Y.; Xia, W.; Xu, B.; Li, X.; Chen, X.; et al. Maternal exposure to di-(2-ethylhexyl) phthalate alters kidney development through the renin-angiotensin system in offspring. Toxicol. Lett. 2012, 212, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, G.; Bhaskaran, R.S.; Karundevi, B. Maternal di-(2-ethylhexyl) phthalate exposure alters hepatic insulin signal transduction and glucoregulatory events in rat F1 male offspring. J. Appl. Toxicol. 2019, 39, 751–763. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.P.; Chen, L.; Wang, X.J.; Jiang, Q.H.; Bei, X.Y.; Sun, W.L.; Xia, S.J.; Jiang, J.T. Maternal exposure to di-n-butyl phthalate (DBP) induces renal fibrosis in adult rat offspring. Oncotarget 2017, 8, 31101–31111. [Google Scholar] [CrossRef]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Xiao, L.; Zhao, F. Microbial transmission, colonisation and succession: From pregnancy to infancy. Gut 2023, 72, 772–786. [Google Scholar] [CrossRef]
- Matamoros, S.; Gras-Leguen, C.; Le Vacon, F.; Potel, G.; De La Cochetiere, M.-F. Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 2013, 21, 167–173. [Google Scholar] [CrossRef]
- Tamburini, S.; Shen, N.; Wu, H.C.; Clemente, J.C. The microbiome in early life: Implications for health outcomes. Nat. Med. 2016, 22, 713–722. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell 2018, 9, 416–431. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Zheng, T.; Meric, G.; Marques, F.Z. The gut microbiome and hypertension. Nat. Rev. Nephrol. 2023, 19, 153–167. [Google Scholar] [CrossRef]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Thakali, K.M.; Shankar, K. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS ONE 2017, 12, e0175675. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, K.S.L.; Braga, V.A.; Noronha, S.I.S.R.; Costa, W.K.A.D.; Makki, K.; Cruz, J.C.; Brandão, L.R.; Chianca Junior, D.A.; Meugnier, E.; Leulier, F.; et al. Lactiplantibacillus plantarum WJL administration during pregnancy and lactation improves lipid profile, insulin sensitivity and gut microbiota diversity in dyslipidemic dams and protects male offspring against cardiovascular dysfunction in later life. Food Funct. 2020, 11, 8939–8950. [Google Scholar] [CrossRef]
- De Oliveira, Y.; Cavalcante, R.G.S.; Cavalcanti Neto, M.P.; Magnani, M.; Braga, V.A.; de Souza, E.L.; de Brito Alves, J.L. Oral administration of Lactobacillus fermentum post-weaning improves the lipid profile and autonomic dysfunction in rat offspring exposed to maternal dyslipidemia. Food Funct. 2020, 11, 5581–5594. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Seki, Y.; Vuguin, P.M.; Charron, M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. Acta 2014, 1842, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Leclerc, M.; Hugot, J.P. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front. Microbiol. 2016, 7, 455. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chan, J.Y.H.; Lee, C.T.; Tain, Y.L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients 2019, 11, 2908. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Lee, C.T.; Chan, J.Y.H.; Tain, Y.L. The Interplay between Maternal and Post-Weaning High-Fat Diet and Gut Microbiota in the Developmental Programming of Hypertension. Nutrients 2019, 11, 1982. [Google Scholar] [CrossRef] [PubMed]
- Pussinen, P.J.; Havulinna, A.S.; Lehto, M.; Sundvall, J.; Salomaa, V. Endotoxemia Is Associated with an Increased Risk of Incident Diabetes. Diabetes Care 2011, 34, 392–397. [Google Scholar] [CrossRef]
- Jia, X.; Xu, W.; Zhang, L.; Li, X.; Wang, R.; Wu, S. Impact of Gut Microbiota and Microbiota-Related Metabolites on Hyperlipidemia. Front. Cell. Infect. Microbiol. 2021, 11, 634780. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Ngo, V.L.; Wang, Y.; Wang, Y.; Gewirtz, A.T. Maternal fiber deprivation alters microbiota in offspring, resulting in low-grade inflammation and predisposition to obesity. Cell Host Microbe 2023, 31, 45–57.e7. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Talavera, O.; Tailleux, A.; Lefebvre, P.; Staels, B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology 2017, 152, 1679–1694.e3. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Guan, X.; Huang, K.; Zhang, Y.; Li, S.; Xia, J.; Shen, M. Flavonoids from Whole-Grain Oat Alleviated High-Fat Diet-Induced Hyperlipidemia via Regulating Bile Acid Metabolism and Gut Microbiota in Mice. J. Agric. Food Chem. 2021, 69, 7629–7640. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Derse, A.; Ferey, J.; Reid, M.; Xie, Y.; Christ, M.; Chatterjee, D.; Nguyen, C.; Harasymowicz, N.; Guilak, F.; et al. Transgenerational impact of maternal obesogenic diet on offspring bile acid homeostasis and nonalcoholic fatty liver disease. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E674–E686. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Mallmann, N.H.; Lima, E.S.; Lalwani, P. Dysregulation of Tryptophan Catabolism in Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hodge, S.; Bunting, B.P.; Carr, E.; Strain, J.J.; Stewart-Knox, B.J. Obesity, whole blood serotonin and sex differences in healthy volunteers. Obes. Facts 2012, 5, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Natividad, J.M.; Agus, A.; Planchais, J.; Lamas, B.; Jarry, A.C.; Martin, R.; Michel, M.L.; Chong-Nguyen, C.; Roussel, R.; Straube, M.; et al. Impaired Aryl Hydrocarbon Receptor Ligand Production by the Gut Microbiota Is a Key Factor in Metabolic Syndrome. Cell Metab. 2018, 28, 737–749.e4. [Google Scholar] [CrossRef] [PubMed]
- Girer, N.G.; Tomlinson, C.R.; Elferink, C.J. The Aryl Hydrocarbon Receptor in Energy Balance: The Road from Dioxin-Induced Wasting Syndrome to Combating Obesity with Ahr Ligands. Int. J. Mol. Sci. 2020, 22, 49. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Song, J.; Gao, J.; Cheng, J.; Xie, H.; Zhang, L.; Wang, Y.H.; Gao, Z.; Wang, Y.; Wang, X.; et al. Adipocyte-derived kynurenine promotes obesity and insulin resistance by activating the AhR/STAT3/IL-6 signaling. Nat. Commun. 2022, 13, 3489. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Lee, J.H.; Febbraio, M.; Xie, W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp. Biol. Med. 2011, 236, 1116–1121. [Google Scholar] [CrossRef]
- Woon, P.Y.; Kaisaki, P.J.; Bragança, J.; Bihoreau, M.T.; Levy, J.C.; Farrall, M.; Gauguier, D. Aryl hydrocarbon receptor nuclear translocator-like (BMAL1) is associated with susceptibility to hypertension and type 2 diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 14412–14417. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- McGlone, E.R.; Bloom, S.R. Bile acids and the metabolic syndrome. Ann. Clin. Biochem. 2019, 56, 326–337. [Google Scholar] [CrossRef]
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef]
- Paauw, N.D.; van Rijn, B.B.; Lely, A.T.; Joles, J.A. Pregnancy as a critical window for blood pressure regulation in mother and child: Programming and reprogramming. Acta Physiol. 2017, 219, 241–259. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics-A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef] [PubMed]
- Barengolts, E. Gut microbiota, prebiotics, probiotics, and synbiotics in management of obesity and prediabetes: Review of randomized controlled trials. Endocr. Pract. 2016, 22, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Zółkiewicz, J.; Marzec, A.; Ruszczy’nski, M.; Feleszko, W. Postbiotics-A step beyond pre- and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Hussain, N.; Hameed, Z.; Lin, L. Elucidating the role of diet in maintaining gut health to reduce the risk of obesity, cardiovascular and other age-related inflammatory diseases: Recent challenges and future recommendations. Gut Microbes 2024, 16, 2297864. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, Á.D.; Hernández-Contreras, M.E.; Rodríguez-Gutiérrez, R. Mediterranean diet as a complementary therapy in adults with chronic liver disease: A review. Nutrients 2020, 12, 1436. [Google Scholar]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.N.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and potential health benefits of the Mediterranean diet: Views from experts around the world. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef]
- Zimmer, J.; Lange, B.; Frick, J.S.; Sauer, H.; Zimmermann, K.; Schwiertz, A.; Rusch, S.; Klosterhalfen, S.; Enck, P. A vegan or vegetarian diet substantially alters the human colonic faecal microbiota. Eur. J. Clin. Nutr. 2012, 66, 53–60. [Google Scholar] [CrossRef]
- Giardina, S.; Hernández-Alonso, P.; Díaz-López, A.; Salas-Huetos, A.; Salas-Salvadó, J.; Bulló, M. Changes in circulating miRNAs in healthy overweight and obese subjects: Effect of diet composition and weight loss. Clin. Nutr. 2019, 38, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; Papandreou, C.; Arcelin, P.; Garcia, D.; Palau-Galindo, A.; Gutiérrez-Tordera, L.; Folch, À.; Bulló, M. Examining the Interaction of the Gut Microbiome with Host Metabolism and Cardiometabolic Health in Metabolic Syndrome. Nutrients 2021, 13, 4318. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; García-Gavilán, J.; Papandreou, C.; Camacho-Barcía, L.; Arcelin, P.; Palau-Galindo, A.; Rabassa, A.; Bulló, M. Effects of Mediterranean Diet on plasma metabolites and their relationship with insulin resistance and gut microbiota composition in a crossover randomized clinical trial. Clin. Nutr. 2021, 40, 3798–3806. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). Guidelines for the Evaluation of Probiotics in Food. In Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; WHO: London, ON, Canada, 2002. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics (isapp) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Amp. Hepatol. 2017, 14, 491. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-parabiotics: The new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Marotz, C.A.; Zarrinpar, A. Treating Obesity and Metabolic Syndrome with Fecal Microbiota Transplantation. Yale J. Biol. Med. 2016, 89, 383–388. [Google Scholar] [PubMed]
- Zixin, Y.; Lulu, C.; Xiangchang, Z.; Qing, F.; Binjie, Z.; Chunyang, L.; Tai, R.; Dongsheng, O. TMAO as a potential biomarker and therapeutic target for chronic kidney disease: A review. Front. Pharmacol. 2022, 13, 929262. [Google Scholar] [CrossRef] [PubMed]
- Organ, C.L.; Li, Z.; Sharp, T.E.; Polhemus, D.J.; Guptam, N.; Goodchild, T.T.; Tang, W.H.W.; Hazen, S.L.; Lefer, D.J. Nonlethal Inhibition of Gut Microbial Trimethylamine N-oxide Production Improves Cardiac Function and Remodeling in a Murine Model of Heart Failure. J. Am. Heart Assoc. 2020, 9, e016223. [Google Scholar] [CrossRef]
- Huang, Y.H.; Tain, Y.L.; Hsu, C.N. Maternal Supplementation of Probiotics, Prebiotics or Postbiotics to Prevent Offspring Metabolic Syndrome: The Gap between Preclinical Results and Clinical Translation. Int. J. Mol. Sci. 2022, 23, 10173. [Google Scholar] [CrossRef]
- Vega, C.C.; Reyes-Castro, L.A.; Rodríguez-González, G.L.; Bautista, C.J.; Vázquez-Martínez, M.; Larrea, F.; Chamorro-Cevallos, G.A.; Nathanielsz, P.W.; Zambrano, E. Resveratrol partially prevents oxidative stress and metabolic dysfunction in pregnant rats fed a low protein diet and their offspring. J. Physiol. 2016, 594, 1483–1499. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lin, Y.J.; Hou, C.Y.; Tain, Y.L. Maternal administration of probiotic or prebiotic prevents male adult rat offspring against developmental programming of hypertension induced by high fructose consumption in pregnancy and lactation. Nutrients 2018, 10, 1229. [Google Scholar] [CrossRef]
- Hsu, C.N.; Chang-Chien, G.P.; Lin, S.; Hou, C.Y.; Tain, Y.L. Targeting on gut microbial metabolite trimethylamine-N-Oxide and short-chain fatty acid to prevent maternal high-fructose-diet-induced developmental programming of hypertension in adult male offspring. Mol. Nutr. Food Res. 2019, 63, e1900073. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Z.; Chen, L.; Tang, L.; Wen, S.; Liu, Y.; Yuan, J. Diet induced maternal obesity affects offspring gut microbiota and persists into young adulthood. Food Funct. 2018, 9, 4317–4327. [Google Scholar] [CrossRef] [PubMed]
- Ros, P.; Díaz, F.; Freire-Regatillo, A.; Argente-Arizón, P.; Barrios, V.; Argente, J.; Chowen, J.A. Resveratrol Intake during Pregnancy and Lactation Modulates the Early Metabolic Effects of Maternal Nutrition Differently in Male and Female Offspring. Endocrinology 2018, 159, 810–825. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Chen, D.; Yang, Q.; Wang, B.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J. Physiol. 2017, 595, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.; Vickers, M.H.; Segovia, S.A.; Zhang, X.D.; Reynolds, C.M. A maternal high fat diet programmes endothelial function and cardiovascular status in adult male offspring independent of body weight, which is reversed by maternal conjugated linoleic acid (CLA) supplementation. PLoS ONE 2015, 10, e0115994. [Google Scholar]
- Hsu, M.H.; Sheen, J.M.; Lin, I.C.; Yu, H.R.; Tiao, M.M.; Tain, Y.L.; Huang, L.T. Effects of Maternal Resveratrol on Maternal High-Fat Diet/Obesity with or without Postnatal High-Fat Diet. Int. J. Mol. Sci. 2020, 21, 3428. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Yu, H.R.; Tsai, C.C.; Huang, L.T.; Chen, C.C.; Sheen, J.M.; Tiao, M.M.; Tain, Y.L.; Lin, I.C.; Lai, Y.J.; et al. Resveratrol intake during pregnancy and lactation re-programs adiposity and ameliorates leptin resistance in male progeny induced by maternal high-fat/high sucrose plus postnatal high-fat/high sucrose diets via fat metabolism regulation. Lipids Health Dis. 2020, 19, 174. [Google Scholar] [CrossRef] [PubMed]
- Paul, H.A.; Collins, K.H.; Nicolucci, A.C.; Urbanski, S.J.; Hart, D.A.; Vogel, H.J.; Reimer, R.A. Maternal prebiotic supplementation reduces fatty liver development in offspring through altered microbial and metabolomic profiles in rats. FASEB J. 2019, 33, 5153–5167. [Google Scholar] [CrossRef]
- Shih, M.K.; Tain, Y.L.; Chen, Y.W.; Hsu, W.H.; Yeh, Y.T.; Chang, S.K.C.; Liao, J.X.; Hou, C.Y. Resveratrol Butyrate Esters Inhibit Obesity Caused by Perinatal Exposure to Bisphenol A in Female Offspring Rats. Molecules 2021, 26, 4010. [Google Scholar] [CrossRef]
- Liao, J.X.; Chen, Y.W.; Shih, M.K.; Tain, Y.L.; Yeh, Y.T.; Chiu, M.H.; Chang, S.K.C.; Hou, C.Y. Resveratrol Butyrate Esters Inhibit BPA-Induced Liver Damage in Male Offspring Rats by Modulating Antioxidant Capacity and Gut Microbiota. Int. J. Mol. Sci. 2021, 22, 5273. [Google Scholar] [CrossRef]
- Hsu, C.N.; Yu, H.R.; Chan, J.Y.H.; Wu, K.L.H.; Lee, W.C.; Tain, Y.L. The Impact of Gut Microbiome on Maternal Fructose Intake-Induced Developmental Programming of Adult Disease. Nutrients 2022, 14, 1031. [Google Scholar] [CrossRef]
- Campbell, E.L.; Colgan, S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 106–120. [Google Scholar] [CrossRef]
- Tenorio-Jiménez, C.; Martínez-Ramírez, M.J.; Gil, Á.; Gómez-Llorente, C. Effects of Probiotics on Metabolic Syndrome: A Systematic Review of Randomized Clinical Trials. Nutrients 2020, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L. Microbial short-chain fatty acids and blood pressure regulation. Curr. Hypertens. Rep. 2017, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Sannino, A.; Toscano, E.; Giugliano, G.; Gargiulo, G.; Franzone, A.; Trimarco, B.; Esposito, G.; Perrino, C. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and doseresponse meta-analysis. Eur. Heart J. 2017, 38, 2948–2956. [Google Scholar] [CrossRef] [PubMed]
- Guasti, L.; Galliazzo, S.; Molaro, M.; Visconti, E.; Pennella, B.; Gaudio, G.V.; Lupi, A.; Grandi, A.M.; Squizzato, A. TMAO as a biomarker of cardiovascular events: A systematic review and meta-analysis. Intern. Emerg. Med. 2021, 16, 201–207. [Google Scholar] [CrossRef]
- Wang, Z.; Roberts, A.B.; Buffa, J.A.; Levison, B.S.; Zhu, W.; Org, E.; Gu, X.; Huang, Y.; Zamanian-Daryoush, M.; Culley, M.K.; et al. Non-lethal Inhibition of Gut Microbial Trimethylamine Production for the Treatment of Atherosclerosis. Cell 2015, 163, 1585–1595. [Google Scholar] [CrossRef]
- Sunu, P.; Sunartim, D.; Mahfudz, L.D.; Yunianto, V.D. Prebiotic activity of garlic Allium sativum extract on Lactobacillus acidophilus. Vet. World 2019, 12, 2046–2051. [Google Scholar] [CrossRef]
- Garcia-Alonso, A.; Sánchez-Paniagua López, M.; Manzanares-Palenzuela, C.L.; Redondo-Cuenca, A.; López-Ruíz, B. Edible plant by-products as source of polyphenols: Prebiotic effect and analytical methods. Crit. Rev. Food Sci. Nutr. 2022, 6, 10814–10835. [Google Scholar] [CrossRef] [PubMed]
- Iciek, M.; Kwiecien, I.; Wlodek, L. Biological properties of garlic and garlic-derived Organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247. [Google Scholar] [CrossRef] [PubMed]
- Kursvietiene, L.; Staneviciene, I.; Mongirdiene, A.; Bernatoniene, J. Multiplicity of effects and health benefits of resveratrol. Medicina 2016, 52, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Carpéné, C.; Mercader, J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Karimi, R.; Momtaz, S.; Naseri, R.; Farzaei, M.H. Effect of resveratrol on metabolic syndrome components: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2019, 20, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.E.; Lin, Y.J.; Lin, I.C.; Yu, H.R.; Sheen, J.M.; Tsai, C.C.; Huang, L.T.; Tain, Y.L. Resveratrol prevents combined prenatal NG-nitro-L-arginine-methyl ester (L-NAME) treatment plus postnatal high-fat diet induced programmed hypertension in adult rat offspring: Interplay between nutrient-sensing signals, oxidative stress and gut microbiota. J. Nutr. Biochem. 2019, 70, 28–37. [Google Scholar] [CrossRef]
- Khodor, S.A.; Reichert, B.; Shatat, I.F. The Microbiome and Blood Pressure: Can Microbes Regulate Our Blood Pressure? Front. Pediatr. 2017, 5, 138. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef]
- Tain, Y.L.; Chang, S.K.C.; Liao, J.X.; Chen, Y.W.; Huang, H.T.; Li, Y.L.; Hou, C.Y. Synthesis of Short-Chain-Fatty-Acid Resveratrol Esters and Their Antioxidant Properties. Antioxidants 2021, 10, 420. [Google Scholar] [CrossRef]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Badimon, L. Effects of Polyphenol Intake on Metabolic Syndrome: Current Evidences from Human Trials. Oxid. Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef]
- Cremonini, E.; Iglesias, D.E.; Kang, J.; Lombardo, G.E.; Mostofinejad, Z.; Wang, Z.; Zhu, W.; Oteiza, P.I. (-)-Epicatechin and the comorbidities of obesity. Arch. Biochem. Biophys. 2020, 690, 108505. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.G. Curcumin and obesity. Biofactors 2013, 39, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pozo, C.; Cires, M.J.; Gotteland, M. Quercetin and Epigallocatechin Gallate in the Prevention and Treatment of Obesity: From Molecular to Clinical Studies. J. Med. Food 2019, 22, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Wu, K.L.H.; Leu, S.; Chan, J.Y.H. Resveratrol Prevents the Development of Hypertension Programmed by Maternal Plus Post-Weaning High-Fructose Consumption through Modulation of Oxidative Stress, Nutrient-Sensing Signals, and Gut Microbiota. Mol. Nutr. Food Res. 2018, 30, e1800066. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Maternal N-Acetylcysteine Therapy Prevents Hypertension in Spontaneously Hypertensive Rat Offspring: Implications of Hydrogen Sulfide-Generating Pathway and Gut Microbiota. Antioxidants 2020, 9, 856. [Google Scholar] [CrossRef]
- Zhang, P.; Jiang, G.; Wang, Y.; Yan, E.; He, L.; Guo, J.; Yin, J.; Zhang, X. Maternal consumption of l-malic acid enriched diets improves antioxidant capacity and glucose metabolism in offspring by regulating the gut microbiota. Redox Biol. 2023, 67, 102889. [Google Scholar] [CrossRef]
- Gomez Arango, L.F.; Barrett, H.L.; Callaway, L.K.; Nitert, M.D. Probiotics and pregnancy. Curr. Diabet. Rep. 2015, 15, 567. [Google Scholar] [CrossRef]
- Luoto, R.; Laitinen, K.; Nermes, M.; Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: A double-blind, placebo controlled study. Br. J. Nutr. 2010, 103, 1792–1799. [Google Scholar] [CrossRef]
- Brantsaeter, A.L.; Myhre, R.; Haugen, M.; Myking, S.; Sengpiel, V.; Magnus, P.; Jacobsson, B.; Meltzer, H.M. Intake of probiotic food and risk of preeclampsia in primiparous women: The norwegian mother and child cohort study. Am. J. Epidemiol. 2011, 174, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.; Neilson, J.P.; Alfirevic, Z. Probiotics for preventing preterm labour. Cochrane Database Syst. Rev. 2007, CD005941. [Google Scholar] [CrossRef] [PubMed]
- Vitali, B.; Cruciani, F.; Baldassarre, M.E.; Capursi, T.; Spisni, E.; Valerii, M.C.; Candela, M.; Turroni, S.; Brigidi, P. Dietary supplementation with probiotics during late pregnancy: Outcome on vaginal microbiota and cytokine secretion. BMC Microbiol. 2012, 12, 236. [Google Scholar] [CrossRef] [PubMed]
- Wiedmer, E.B.; Herter-Aeberli, I. The Potential of Prebiotic and Probiotic Supplementation During Obese Pregnancy to Improve Maternal and Offspring’s Metabolic Health and Reduce Obesity Risk-A Narrative Review. Front. Nutr. 2022, 9, 819882. [Google Scholar] [CrossRef]
- Jinno, S.; Toshimitsu, T.; Nakamura, Y.; Kubota, T.; Igoshi, Y.; Ozawa, N.; Suzuki, S.; Nakano, T.; Morita, Y.; Arima, T.; et al. Maternal prebiotic ingestion increased the number of fecal bifidobacteria in pregnant women but not in their neonates aged one month. Nutrients 2017, 9, 196. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. 2022. Available online: https://clinicaltrials.gov/ (accessed on 27 November 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).