Associations of Triglycerides and Atherogenic Index of Plasma with Brain Structure in the Middle-Aged and Elderly Adults
Abstract
1. Introduction
2. Materials and Methods
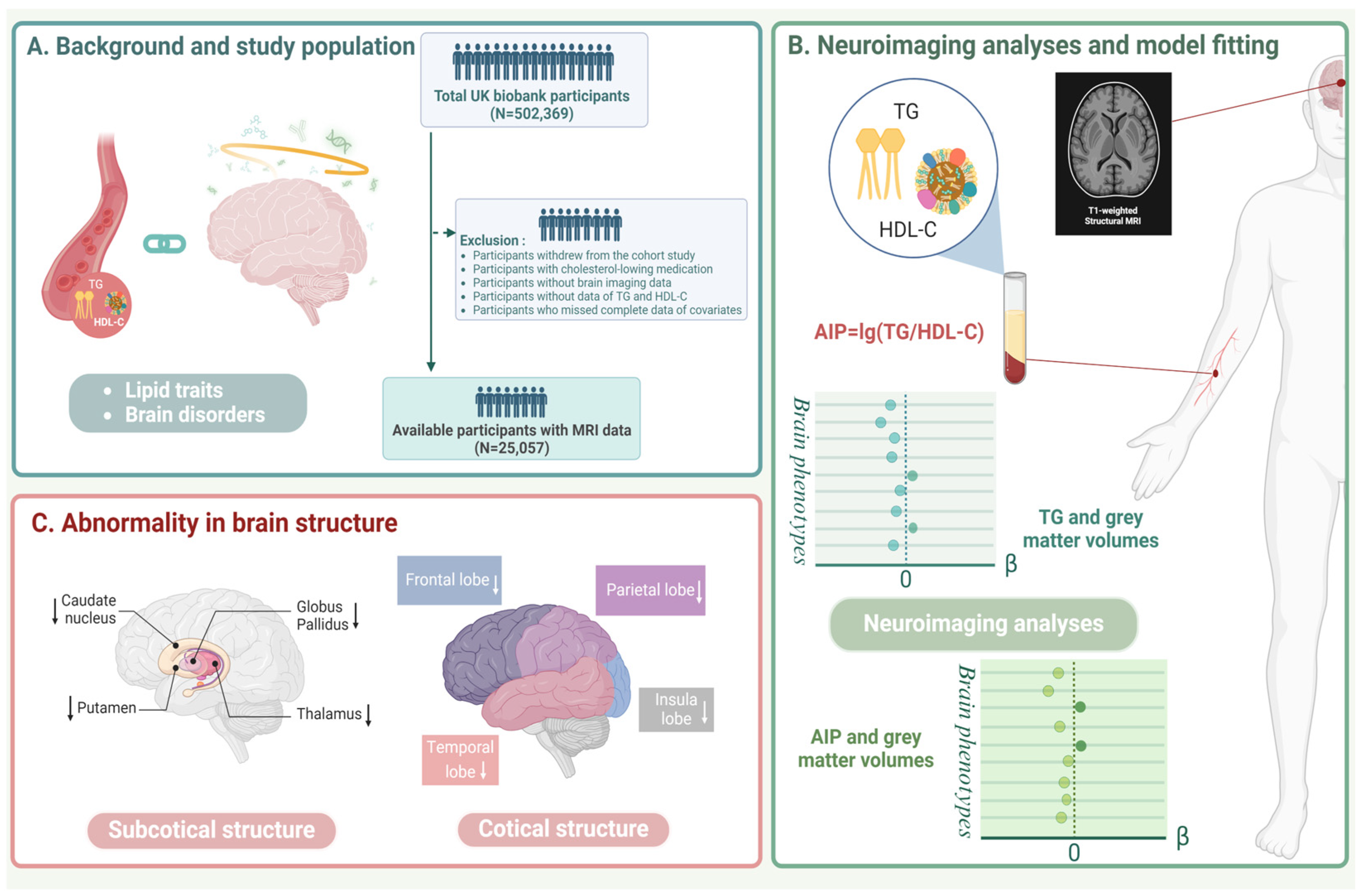
2.1. Study Population
2.2. Lipid-Related Exposures
2.3. Outcomes
2.4. Covariates
2.5. Statistical Analysis
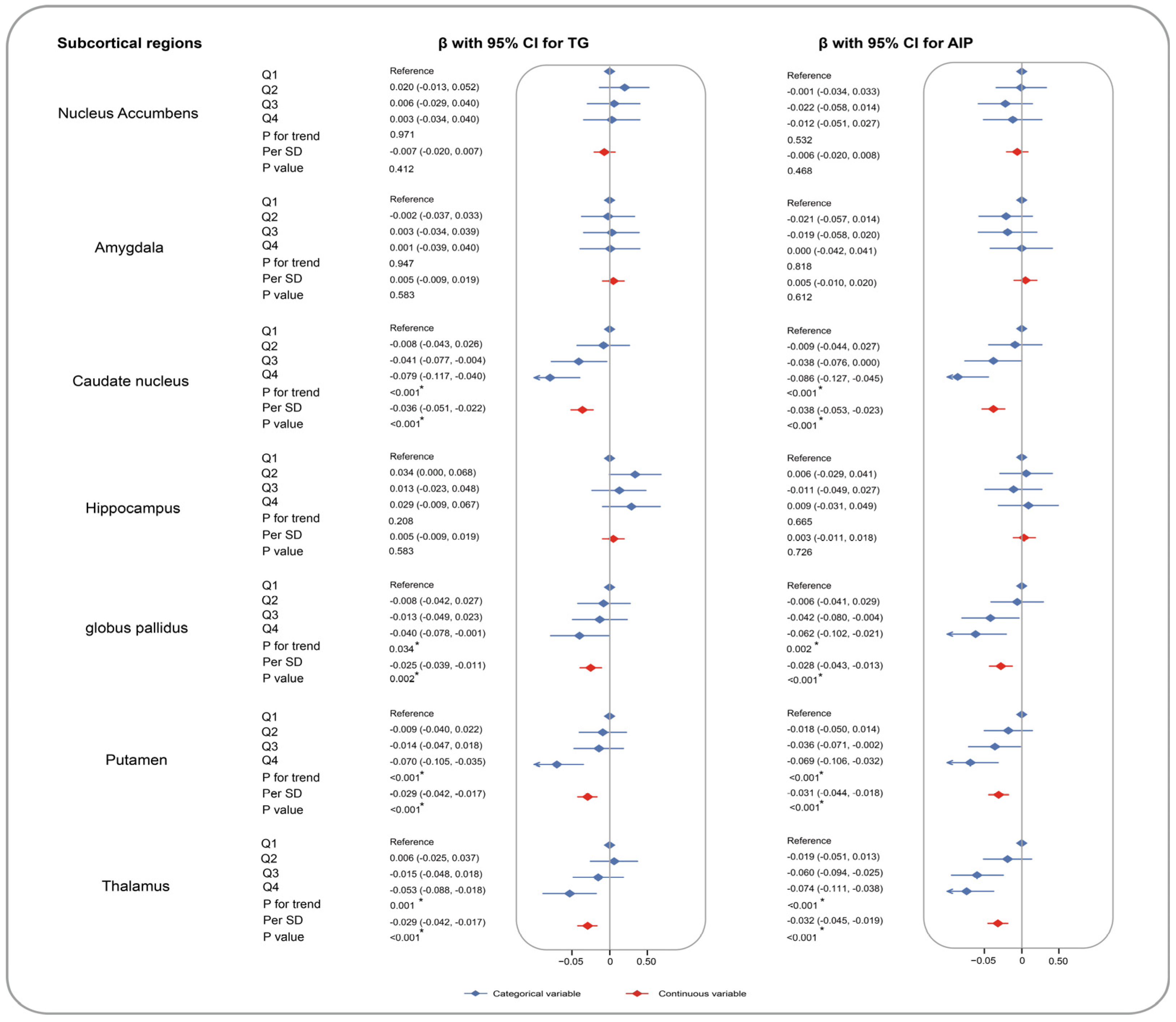
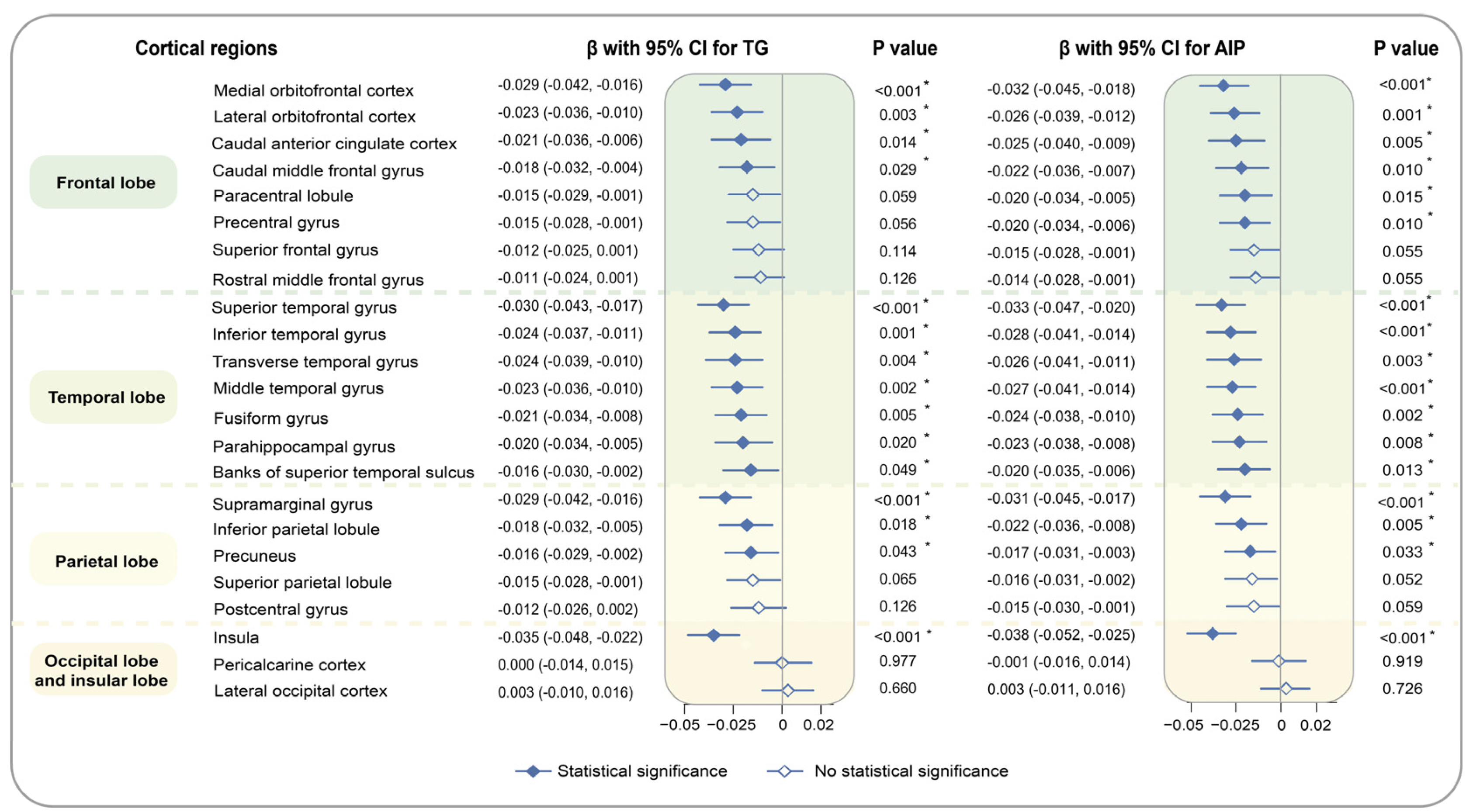
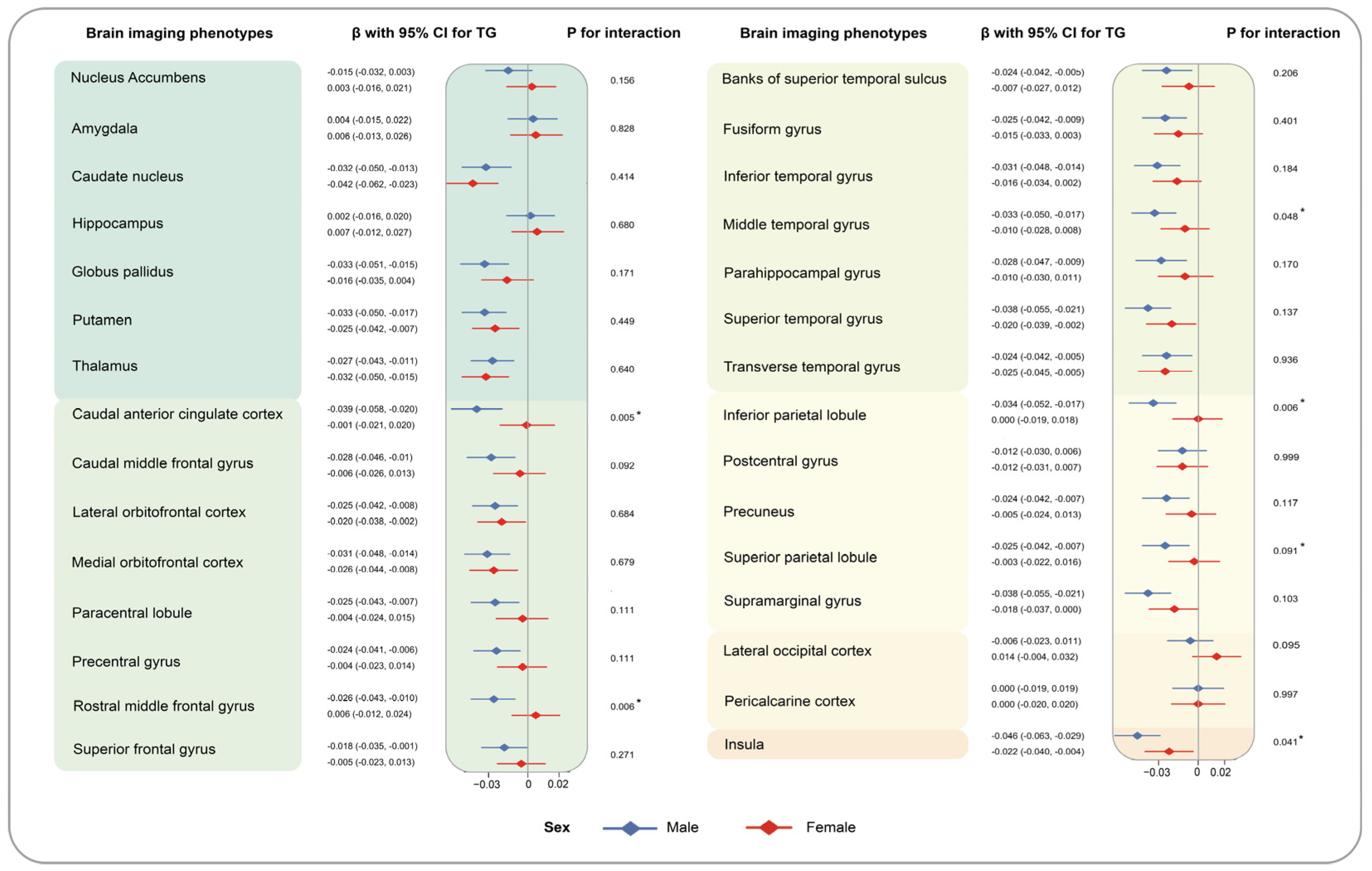
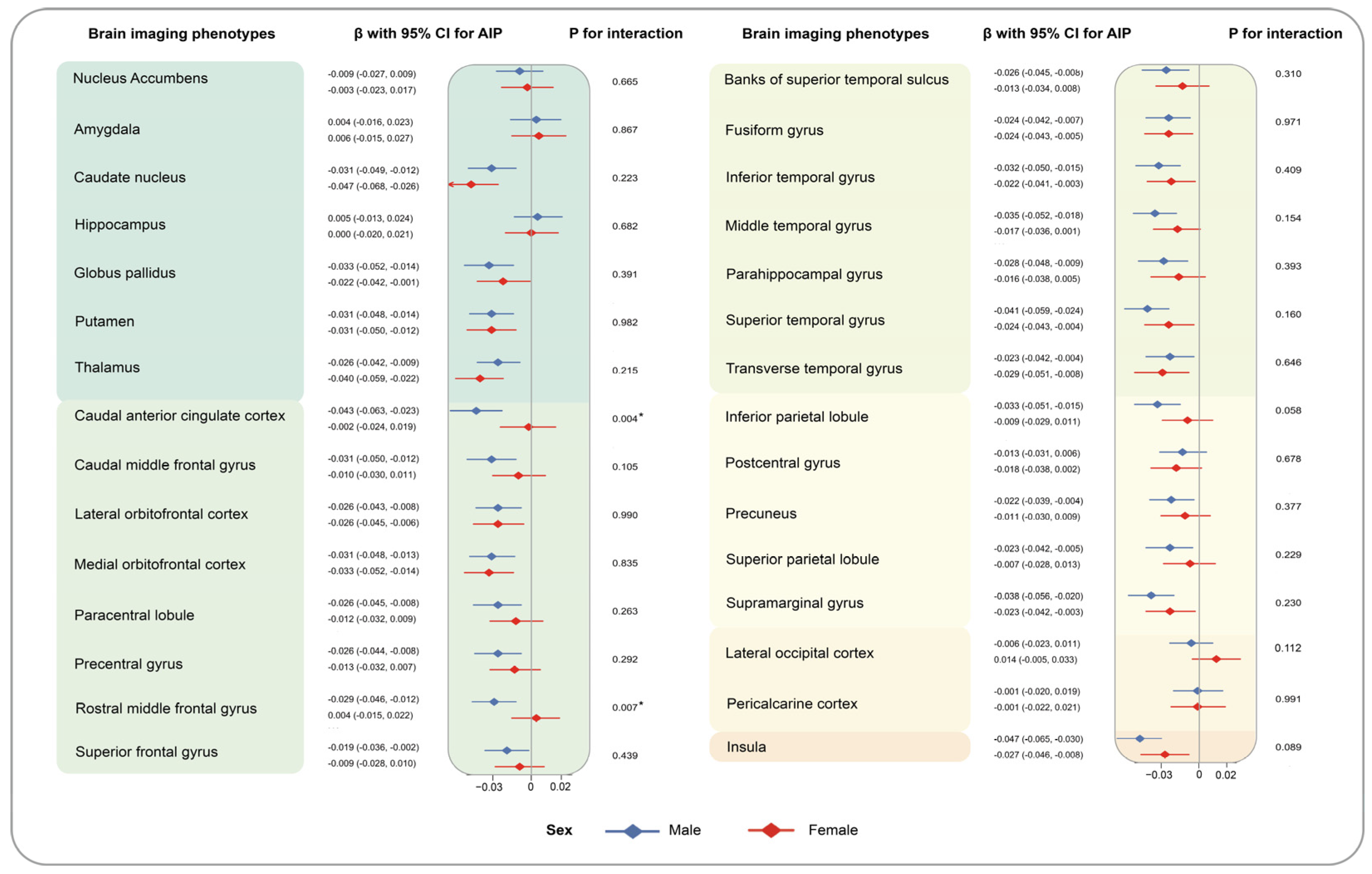
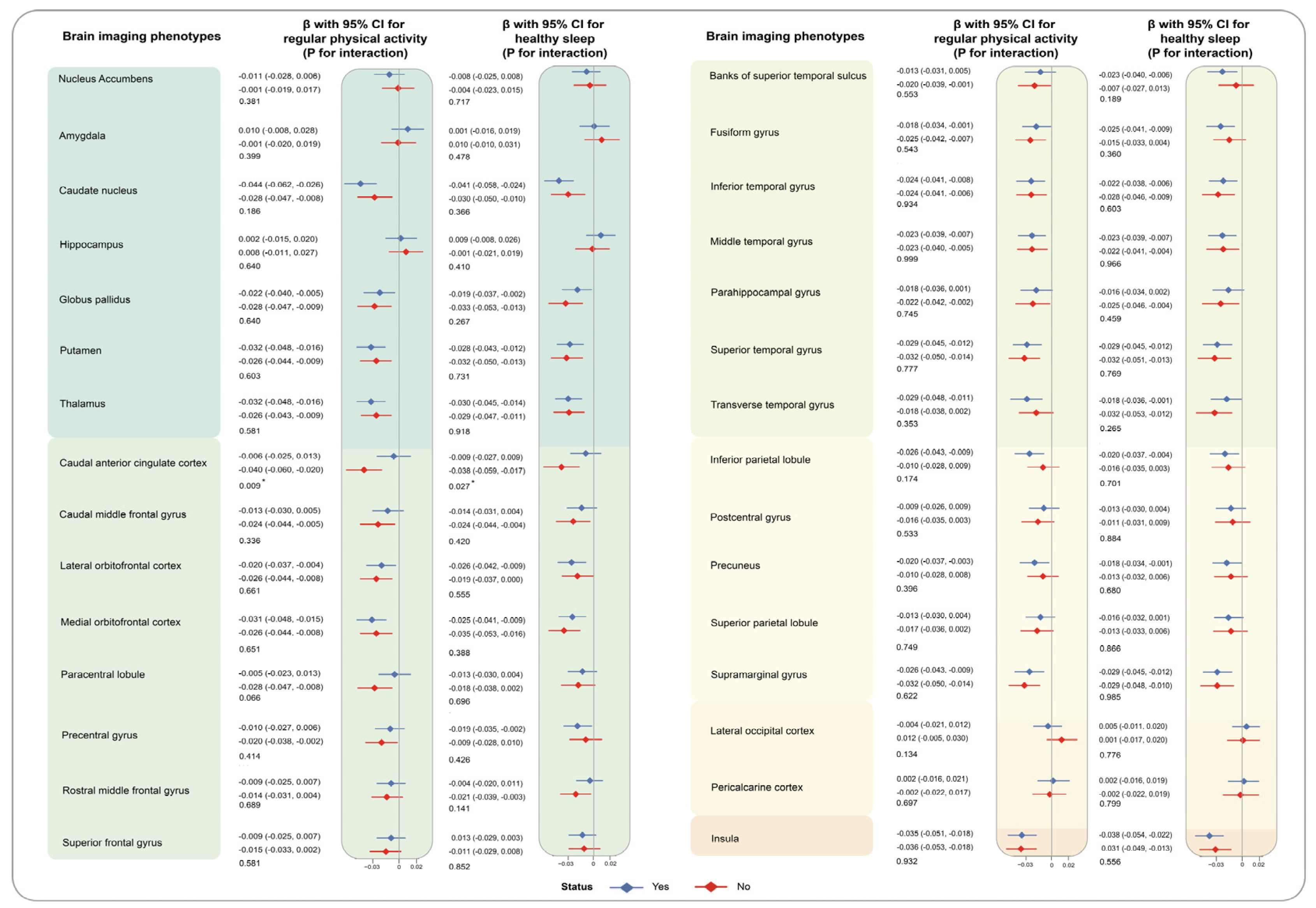
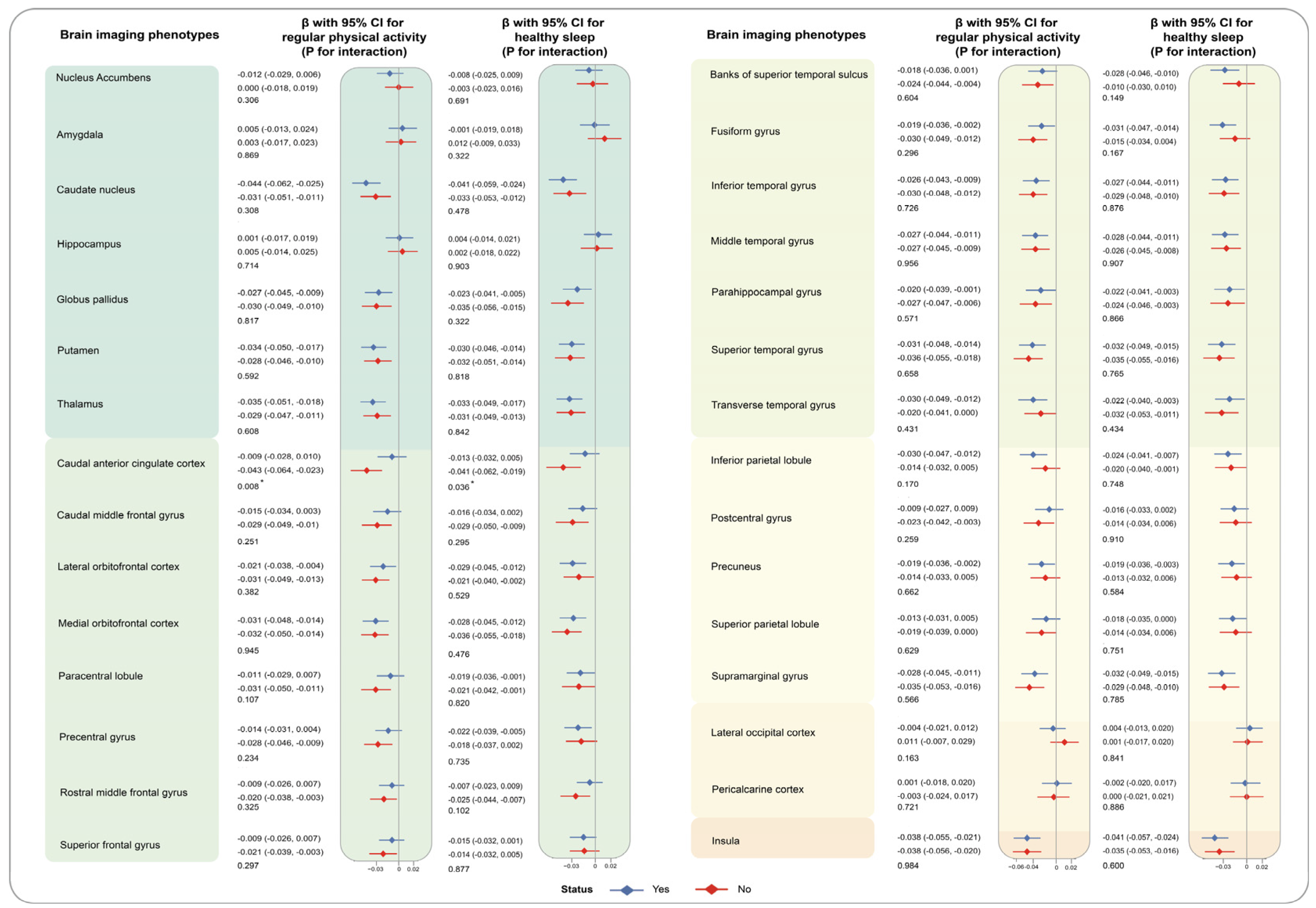
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137–150. [Google Scholar] [CrossRef]
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D.; et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American heart association/American stroke association. Stroke 2011, 42, 2672–2713. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Kennedy, K.G.; Islam, A.H.; Grigorian, A.; Fiksenbaum, L.; Mitchell, R.H.; McCrindle, B.W.; MacIntosh, B.J.; Goldstein, B.I. Elevated lipids are associated with reduced regional brain structure in youth with bipolar disorder. Acta Psychiatr. Scand. 2021, 143, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Chou, K.H.; Peng, L.N.; Liu, L.K.; Lee, W.J.; Chen, L.K.; Lin, C.P.; Wang, P.N. Associations between low circulatory low-density lipoprotein cholesterol level and brain health in non-stroke non-demented subjects. Neuroimage 2018, 181, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, N.F.; Nordstrom, L.K.; Pagen, L.H.; Palombo, D.J.; Salat, D.H.; Milberg, W.P.; McGlinchey, R.E.; Leritz, E.C. Differential associations of metabolic risk factors on cortical thickness in metabolic syndrome. Neuroimage Clin. 2018, 17, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, R.N.; Rossetti, H.C.; Gupta, M.K.; Rosenberg, R.N.; Weiner, M.F.; Peshock, R.M.; McColl, R.W.; Hynan, L.S.; Lucarelli, R.T.; King, K.S. Cardiovascular Risk Factors Associated with Smaller Brain Volumes in Regions Identified as Early Predictors of Cognitive Decline. Radiology 2016, 278, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, A.M.; Coutu, J.P.; Lindemer, E.R.; Rosas, H.D.; Rosen, B.R.; Salat, D.H. Differential associations between systemic markers of disease and cortical thickness in healthy middle-aged and older adults. Neuroimage 2017, 146, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, M.M.; Ajilore, O.; Charlton, R.C.; Cohen, J.; Yang, S.; Sieg, E.; Bhaumik, D.K.; Kumar, A.; Lamar, M. Divergent Influences of Cardiovascular Disease Risk Factor Domains on Cognition and Gray and White Matter Morphology. Psychosom. Med. 2017, 79, 541–548. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Z.; Wei, M.; Huang, Q.; Feng, J.; Liu, Z.; Xia, J. The atherogenic index of plasma and carotid atherosclerosis in a community population: A population-based cohort study in China. Cardiovasc. Diabetol. 2023, 22, 125. [Google Scholar] [CrossRef]
- Fernández-Macías, J.C.; Ochoa-Martínez, A.C.; Varela-Silva, J.A.; Pérez-Maldonado, I.N. Atherogenic Index of Plasma: Novel Predictive Biomarker for Cardiovascular Illnesses. Arch. Med. Res. 2019, 50, 285–294. [Google Scholar] [CrossRef]
- Qi, X.; Jia, Y.; Pan, C.; Li, C.; Wen, Y.; Hao, J.; Liu, L.; Cheng, B.; Cheng, S.; Yao, Y.; et al. Index of multiple deprivation contributed to common psychiatric disorders: A systematic review and comprehensive analysis. Neurosci. Biobehav. Rev. 2022, 140, 104806. [Google Scholar] [CrossRef]
- Tyrrell, J.; Jones, S.E.; Beaumont, R.; Astley, C.M.; Lovell, R.; Yaghootkar, H.; Tuke, M.; Ruth, K.S.; Freathy, R.M.; Hirschhorn, J.N.; et al. Height, body mass index, and socioeconomic status: Mendelian randomisation study in UK Biobank. BMJ 2016, 352, i582. [Google Scholar] [CrossRef]
- Lourida, I.; Hannon, E.; Littlejohns, T.J.; Langa, K.M.; Hypponen, E.; Kuzma, E.; Llewellyn, D.J. Association of Lifestyle and Genetic Risk with Incidence of Dementia. JAMA 2019, 322, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, T.; Ma, H.; Huang, T.; Gao, X.; Manson, J.E.; Qi, L. Healthy Sleep Patterns and Risk of Incident Arrhythmias. J. Am. Coll. Cardiol. 2021, 78, 1197–1207. [Google Scholar] [CrossRef]
- Han, H.; Cao, Y.; Feng, C.; Zheng, Y.; Dhana, K.; Zhu, S.; Shang, C.; Yuan, C.; Zong, G. Association of a Healthy Lifestyle with All-Cause and Cause-Specific Mortality Among Individuals with Type 2 Diabetes: A Prospective Study in UK Biobank. Diabetes Care 2022, 45, 319–329. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [PubMed]
- Zhai, S.; Zhang, H.; Mehrotra, D.V.; Shen, J. Pharmacogenomics polygenic risk score for drug response prediction using PRS-PGx methods. Nat. Commun. 2022, 13, 5278. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A.; Wierzbicki, A.S. Investigating mixed hyperlipidaemia. BMJ 2011, 343, d5146. [Google Scholar] [CrossRef]
- Oh, R.C.; Trivette, E.T.; Westerfield, K.L. Management of Hypertriglyceridemia: Common Questions and Answers. Am. Fam. Physician 2020, 102, 347–354. [Google Scholar]
- Gong, J.; Harris, K.; Peters, S.A. Serum lipid traits and the risk of dementia: A cohort study of 254,575 women and 214,891 men in the UK Biobank. EClinicalMedicine 2022, 54, 101695. [Google Scholar] [CrossRef] [PubMed]
- Wysokiński, A.; Strzelecki, D.; Kłoszewska, I. Levels of triglycerides, cholesterol, LDL, HDL and glucose in patients with schizophrenia, unipolar depression and bipolar disorder. Diabetes Metab. Syndr. Clin. Res. Rev. 2015, 9, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Geng, T.; Li, N.; Wu, L.; Wang, Y.; Zheng, D.; Guo, B.; Wang, B. Associations of Lipids and Lipid-Lowering Drugs with Risk of Vascular Dementia: A Mendelian Randomization Study. Nutrients 2022, 15, 69. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.C.; Ho, P.C.; Tu, Y.K.; Jou, I.M.; Tsai, K.J. Lipids and Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 1505. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.; Villeneuve, S.; Mack, W.; DeCarli, C.; Chui, H.C.; Jagust, W. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol. 2014, 71, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Leritz, E.C.; Salat, D.H.; Williams, V.J.; Schnyer, D.M.; Rudolph, J.L.; Lipsitz, L.; Fischl, B.; McGlinchey, R.E.; Milberg, W.P. Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage 2011, 54, 2659–2671. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.N.; Stanford, M.; Jiang, X. Low Cholesterol Level Linked to Reduced Semantic Fluency Performance and Reduced Gray Matter Volume in the Medial Temporal Lobe. Front. Aging Neurosci. 2020, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Tohid, H.; Faizan, M.; Faizan, U. Alterations of the occipital lobe in schizophrenia. Neurosciences 2015, 20, 213–224. [Google Scholar] [CrossRef]
- Moazzami, K.; Power, M.C.; Gottesman, R.; Mosley, T.; Lutsey, P.L.; Jack, C.R., Jr.; Hoogeveen, R.C.; West, N.; Knopman, D.S.; Alonso, A. Association of mid-life serum lipid levels with late-life brain volumes: The atherosclerosis risk in communities neurocognitive study (ARICNCS). Neuroimage 2020, 223, 117324. [Google Scholar] [CrossRef]
- Liu, C.; Dhindsa, D.; Almuwaqqat, Z.; Ko, Y.-A.; Mehta, A.; Alkhoder, A.A.; Alras, Z.; Desai, S.R.; Patel, K.J.; Hooda, A.; et al. Association between High-Density Lipoprotein Cholesterol Levels and Adverse Cardiovascular Outcomes in High-risk Populations. JAMA Cardiol. 2022, 7, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Rudge, J.D. The Lipid Invasion Model: Growing Evidence for This New Explanation of Alzheimer’s Disease. J. Alzheimers Dis. 2023, 94, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Kaddoumi, A.; Denney, T.S., Jr.; Deshpande, G.; Robinson, J.L.; Beyers, R.J.; Redden, D.T.; Praticò, D.; Kyriakides, T.C.; Lu, B.; Kirby, A.N.; et al. Extra-Virgin Olive Oil Enhances the Blood-Brain Barrier Function in Mild Cognitive Impairment: A Randomized Controlled Trial. Nutrients 2022, 14, 5102. [Google Scholar] [CrossRef]
- Pflanz, C.P.; Tozer, D.J.; Harshfield, E.L.; Tay, J.; Farooqi, S.; Markus, H.S. Central Obesity is Selectively Associated with Cerebral Gray Matter Atrophy in 15,634 Subjects in the UK Biobank. Int. J. Obes. 2022, 46, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, A.; Rebernick, R.J.; Kuppa, A.; Pant, A.; Chen, Y.; Du, X.; Cushing, K.C.; Bell, H.N.; Raut, C.; Prabhu, P.; et al. Comprehensive genetic study of the insulin resistance marker TG:HDL-C in the UK Biobank. Nat. Genet. 2024, 56, 212–221. [Google Scholar] [CrossRef]
- Woo, M.H.; Lee, K.O.; Chung, D.; Choi, J.-W.; Kim, S.-H.; Oh, S.-H. Triglyceride/HDL-Cholesterol Ratio as an Index of Intracranial Atherosclerosis in Nonstroke Individuals. Front. Neurol. 2020, 11, 504219. [Google Scholar] [CrossRef]
- Wang, Q.; Zang, F.; He, C.; Zhang, Z.; Xie, C.; Alzheimer’s Disease Neuroimaging Initiative. Dyslipidemia induced large-scale network connectivity abnormality facilitates cognitive decline in the Alzheimer’s disease. J. Transl. Med. 2022, 20, 567. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S. Clinical Implications for Dopaminergic and Functional Neuroimage Research in Cognitive Symptoms of Parkinson’s Disease. Mol. Med. 2021, 27, 40. [Google Scholar] [CrossRef]
- Castro, D.C.; Berridge, K.C. Advances in the Neurobiological Bases for Food ‘Liking’ versus ‘Wanting’. Physiol. Behav. 2014, 136, 22–30. [Google Scholar] [CrossRef]
- Pang, K.; Liu, C.; Tong, J.; Ouyang, W.; Hu, S.; Tang, Y. Higher Total Cholesterol Concentration May Be Associated with Better Cognitive Performance among Elderly Females. Nutrients 2022, 14, 4198. [Google Scholar] [CrossRef]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 4198. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Beedie, C.; Jimenez, A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med. 2014, 44, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Fry, A.; Littlejohns, T.J.; Sudlow, C.; Doherty, N.; Adamska, L.; Sprosen, T.; Collins, R.; Allen, N.E. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants with Those of the General Population. Am. J. Epidemiol. 2017, 186, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | All (N = 25,057) | Q1 a (N = 6264) | Q2 (N = 6264) | Q3 (N = 6264) | Q4 (N = 6265) |
|---|---|---|---|---|---|
| Age, year | 54.3 (7.5) | 53.3 (7.4) | 54.3 (7.5) | 54.9 (7.5) | 54.6 (7.4) |
| Female population | 13,416 (53.5%) | 4754 (75.9%) | 3901 (62.3%) | 3012 (48.1%) | 1749 (27.9%) |
| IMD b | 14.7 (12.0) | 14.0 (11.5) | 14.4 (11.8) | 14.8 (12.2) | 15.4 (12.6) |
| WHR c | |||||
| Ideal | 15,140 (60.4%) | 5334 (85.2%) | 4436 (70.8%) | 3357 (53.6%) | 2013 (32.1%) |
| Poor | 9917 (39.6%) | 930 (14.8%) | 1828 (29.2%) | 2907 (46.4%) | 4252 (67.9%) |
| Lifestyle | |||||
| Never smoking | 23,499 (93.8%) | 5971 (95.3%) | 5936 (94.8%) | 5848 (93.4%) | 5744 (91.7%) |
| Never drinking | 1109 (4.4%) | 214 (3.4%) | 254 (4.1%) | 283 (4.5%) | 358 (5.7%) |
| Regular physical activity d | 13,745 (54.9%) | 3823 (61.0%) | 3483 (55.6%) | 3331 (53.2%) | 3108 (49.6%) |
| Healthy sleep pattern e | 14,822 (59.2%) | 4112 (65.6%) | 3859 (61.6%) | 3514 (56.1%) | 3337 (53.3%) |
| Healthy diet f | 10,058 (40.1%) | 2884 (46.0%) | 2703 (43.2%) | 2359 (37.7%) | 2112 (33.7%) |
| Cardiometabolic factors | |||||
| SBP (mmHg) | 134.0 (17.6) | 130.0 (17.6) | 132.0 (17.7) | 136.0 (17.5) | 138.0 (16.5) |
| DBP (mmHg) | 81.2 (10.0) | 78.3 (9.6) | 80.1 (9.7) | 82.2 (9.8) | 84.2 (9.6) |
| HbA1c (mmol/mol) | 34.5 (4.3) | 33.7 (3.6) | 34.2 (3.7) | 34.7 (4.3) | 35.4 (5.3) |
| TC (mmol/L) | 5.8 (1.0) | 5.7 (1,0) | 5.8 (1.0) | 5.9 (1.0) | 6.1 (1.0) |
| LDL-C (mmol/L) | 3.7 (0.8) | 3.3 (0.7) | 3.6 (0.8) | 3.8 (0.8) | 3.9 (0.8) |
| Prevalence of comorbidities | |||||
| Hypertension | 3834 (15.3%) | 601 (9.6%) | 835 (13.3%) | 1070 (17.1%) | 1328 (21.2%) |
| Diabetes | 224 (0.9%) | 31 (0.5%) | 34 (0.5%) | 63 (1.0%) | 96 (1.5%) |
| Atrial fibrillation | 112 (0.5%) | 18 (0.3%) | 31 (0.5%) | 33 (0.5%) | 30 (0.5%) |
| Stroke | 76 (0.3%) | 12 (0.2%) | 21 (0.3%) | 17 (0.3%) | 26 (0.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Bao, Y.; Zhao, J.; Wang, Z.; Gao, Q.; Ma, M.; Xie, Z.; He, M.; Deng, X.; Ran, J. Associations of Triglycerides and Atherogenic Index of Plasma with Brain Structure in the Middle-Aged and Elderly Adults. Nutrients 2024, 16, 672. https://doi.org/10.3390/nu16050672
Chen X, Bao Y, Zhao J, Wang Z, Gao Q, Ma M, Xie Z, He M, Deng X, Ran J. Associations of Triglycerides and Atherogenic Index of Plasma with Brain Structure in the Middle-Aged and Elderly Adults. Nutrients. 2024; 16(5):672. https://doi.org/10.3390/nu16050672
Chicago/Turabian StyleChen, Xixi, Yujia Bao, Jiahao Zhao, Ziyue Wang, Qijing Gao, Mingyang Ma, Ziwen Xie, Mu He, Xiaobei Deng, and Jinjun Ran. 2024. "Associations of Triglycerides and Atherogenic Index of Plasma with Brain Structure in the Middle-Aged and Elderly Adults" Nutrients 16, no. 5: 672. https://doi.org/10.3390/nu16050672
APA StyleChen, X., Bao, Y., Zhao, J., Wang, Z., Gao, Q., Ma, M., Xie, Z., He, M., Deng, X., & Ran, J. (2024). Associations of Triglycerides and Atherogenic Index of Plasma with Brain Structure in the Middle-Aged and Elderly Adults. Nutrients, 16(5), 672. https://doi.org/10.3390/nu16050672






