Glycemic Changes Related to Arsenic Exposure: An Overview of Animal and Human Studies
Abstract
1. Introduction
2. Materials and Methods
3. Results
| Author/Year | Region | Study Design | Sample Size | Age (Years) | Main Results |
|---|---|---|---|---|---|
| Coronado-González et al., 2007 [48] | Mexico | Case-control | Men and women (n = 400) | ≥30 | Dose-response relationship between As concentrations in urine and T2DM. |
| Navas-Acien et al., 2008 [35] | USA | Cross-sectional | Men and women (n = 788) | ≥20 | Association between exposure to As and the prevalence of T2DM. |
| Kim and Lee 2011 [46] | Korea | Cohort | Men and women (n = 1677) | ≥20 | Urinary associations increased the risk of DM, mainly in females. |
| Gribble et al., 2012 [3] | USA | Cohort | Men and women (n = 3925) | 45–74 | As was positively associated with hemoglobin A1c concentrations in participants with DM. |
| Rhee et al., 2013 [10] | Korea | Cross-sectional | Men and women (n = 3602) | ≥20 | Significantly higher total urinary As concentration in females, the elderly, and residents of urban areas. |
| Drobná, Del Razo, and García–Vargas 2013 [33] | Mexico | Cross-sectional | Men and women (n = 255) | ≥5 | Individuals with the AS3MT/M287T and G4965C variants had higher concentrations of DMAIII. |
| Kim, Mason, and Nelson 2013 [38] | USA | Cross-sectional | Men and women (n = 300) | ≥25 | Fasting plasma glucose was negatively correlated with % MMA and positively correlated with total As. |
| Pan et al., 2013 [52] | Bangladesh | Case-control | Men and women (n = 919) | DM: 40.0 (14.0) C: 33.0 (18.0) | Genetic susceptibility to T2DM likely induced by As. |
| Pan et al., 2013 [53] | Bangladesh | Case-control | Men and women (n = 933) | DM: 33.0 (18.0) C: 40.0 (13.5) | Synergistic effect between As exposure, smoking, and BMI resulted in the highest risk of T2DM. |
| Bailey et al., 2013 [32] | Mexico | Cross-sectional | Women (n = 16) | * | Methylation patterns of DM-related genes were associated with urinary concentrations of iAs metabolites. |
| Jovanovic et al., 2013 [37] | Serbia | Cross-sectional | Population of Middle Banat region, Serbia (*) | Men: Exposed: 60.1 (10.9) Not exposed: 60.8 (11.2) Women: Exposed: 61.7 (9.8) Not exposed: 63.5 (10.7) | Higher incidence rates of T2DM in the population exposed to As. |
| Díaz-Villaseñor et al., 2013 [50] | Mexico | Case-control | Men and women (n = 72) | 35–65 | Chronic exposure to iAs reduced β cell function. |
| Huang et al., 2014 [36] | Cambodia | Cross-sectional | Men and women (n = 142) | 40.4 | Water intake with As concentrations above the median (907.25 μg/L) was associated with an increased risk of DM. |
| Peng, Harlow, and Park 2015 [43] | USA | Cross-sectional | Men and women (n = 835) | 12–19 | No associations between HOMA-IR and As, iAs, or DMA. |
| Martin, González-Horta, and Rager 2015 [5] | Mexico | Cohort | Men and women (n = 1165) | ≥18 | Difference in the metabolites found in the urine of individuals with or without DM. |
| Feseke et al., 2015 [16] | Canada | Cross-sectional | Men and women (n = 3151) | 20–79 | Urinary As concentration was positively associated with the prevalence of T2DM and prediabetes. |
| Park et al., 2016 [41] | USA | Cross-sectional | Men and women (n = 221) | 52.5 | Total urine was associated with high concentrations of fasting blood glucose. |
| Grau-Perez et al., 2017 [58] | USA | Cohort | Men and women (n = 1838) | 24–47 | Interaction of one-carbon metabolism nutrients and % MMA with an AS3MT genetic variant. |
| Grau-Perez, Navas-Acien, and Galan-Chilet 2018 [6] | Spain | Cross-sectional | Men and women (n = 1451) | ≥20 | Positive association between total As in urine and the prevalence of DM. |
| Spratlen et al., 2018 [47] | USA | Cohort | Men and women (1458) | >14 | Participants who developed DM were older, had higher % DMA, BMI, HOMA-IR, and waist circumference and lower % MMA. |
| Yang et al., 2019 [15] | USA | Cohort | Men and women n = (4102) | 20–32 | Low to moderate concentrations of As in the nails were not associated with the risk of developing DM. |
| Spratlen et al., 2019 [44] | USA | Cross-sectional | Men and women (n = 935) | 14–23 | Association of lower % MMA and higher % DMA with DM-related outcomes may be influenced by carbon metabolism status. |
| Paul et al., 2019 [42] | Bangladesh | Cross-sectional | Men and women (n = 641) | 18–60 | Dose-dependent association between As exposure and hyperglycemia, especially in females. |
| Rehman, Fatima, and Akash 2019 [55] | Pakistan | Case-control | Men and women (n = 150) | ≥18 | As was positively associated with increased risk of DM when adjusted for sex, age ≥ 60 years, education, and smoking. |
| Zhang et al., 2020 [57] | China | Case-control | Men and women (n = 1248) | ≥18 | Patients with higher urinary % As were more likely to have DM. |
| Lucio, Barbir, and Vučić Lovrenčić 2020 [4] | Croatia | Case-control | Men and women (n = 201) | East—C: 49 (14); PD: 64 (7); DM: 64 (10) West—C: 45 (11); PD: 57 (6); DM: 57 (7) | Total As metabolites in urine were positively correlated with hemoglobin A1c. |
| Idrees and Batool 2020 [51] | Pakistan | Case-control | Men and women (n = 200) | 26–80 | Association between As exposure and T2DM development. |
| Wu et al., 2021 [56] | USA | Case-control | Men and women (n = 190) | 56 (51–64) | Increase in % MMA was positively associated with prediabetes and DM. |
| Arab, Arbabi, and Ziarati 2021 [48] | Iran | Case-control | Men and women (n = 200) | >40 | Urinary As concentration was four times higher in patients with T2DM. |
| Li, Wang, and Park 2021 [39] | USA | Cross-sectional | Men and women (n = 5469) | ≥20 | Rice consumption was positively associated with higher urinary DMA concentration but inversely associated with MMA. |
| Liu et al., 2022 [40] | China | Cross-sectional | Men and women (n = 436) | >18 | As exposure had a disruptive effect on glucose homeostasis and resulted in an elevated inflammatory response. |
| Rangel-Moreno et al., 2022 [54] | Mexico | Case-control | Women (n = 681) | 36–88 | T2DM prevalence was associated with iAs metabolism but not with urinary As concentration. |
| Fan et al., 2022 [34] | China | Cross-sectional | Men and women (n = 938) | >20 | Age ≥ 60 years, the female gender, and high level of urinary iAs were correlated with a risk of T2DM, whereas the A allele and AA genotype of the KEAP1 SNP rs11545829 may be a protective factor. |
| Zhou, Zhao, and Huang 2022 [45] | USA | Cross-sectional | Men and women (n = 815) | 20–79 | Total As exposure was positively correlated with insulin resistance. |
4. Discussion
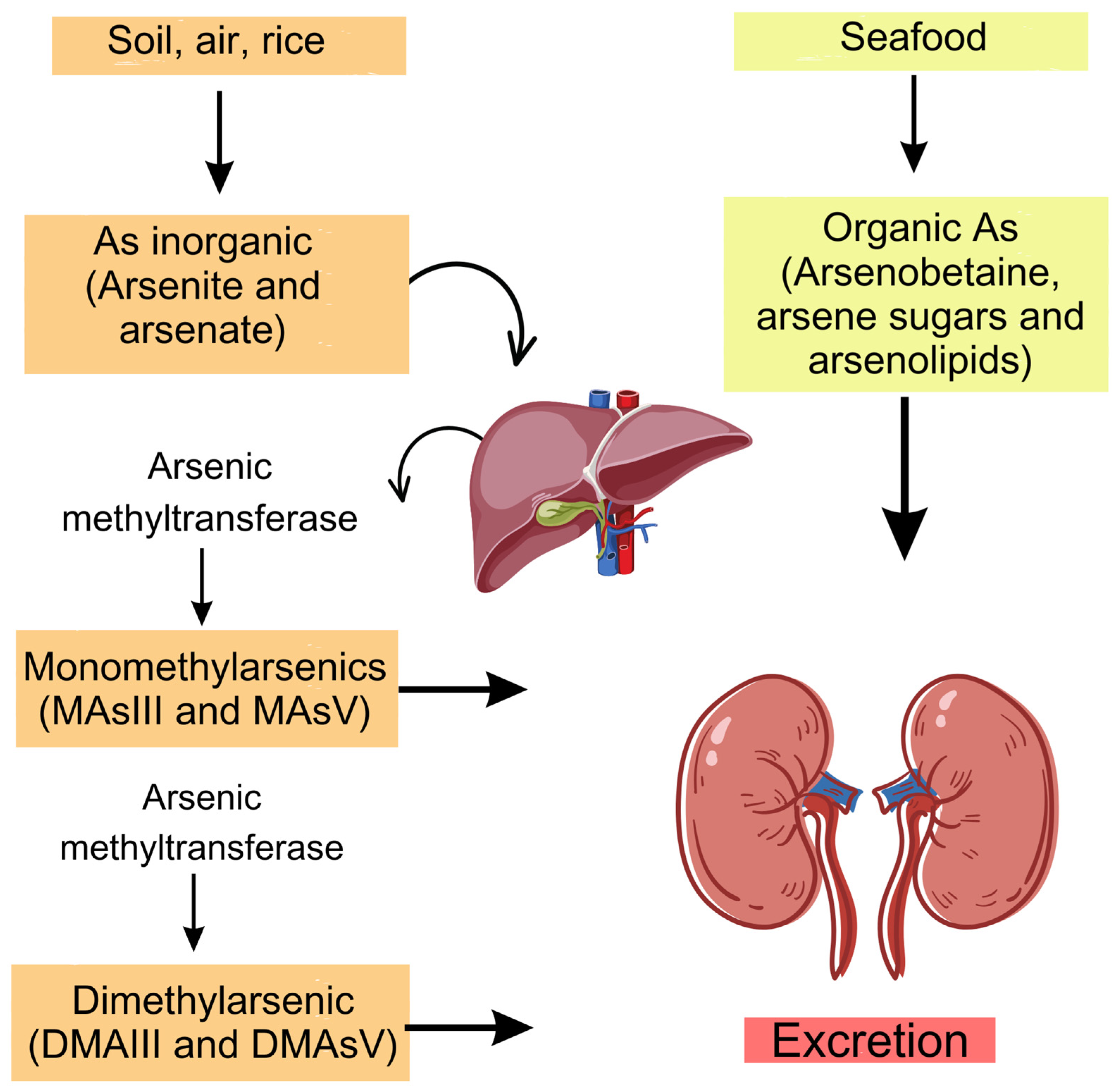
4.1. Forms and Sources of As
4.2. As Absorption, Metabolism, and Excretion
4.3. Exposure to As and Glycemic Alterations
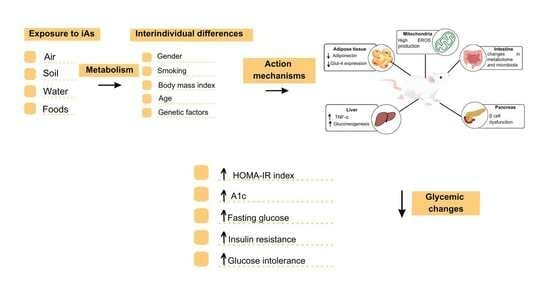
4.4. Mechanism of Action for As-Induced Glycemic Changes
4.4.1. Mitochondrial Dysfunction and Expression of Pro-Inflammatory Factors
4.4.2. Damage Caused to DNA
4.4.3. Reduced GLUT4 Expression and Reduced PPARγ Expression
4.4.4. Increased Gluconeogenesis and Pancreatic β-Cell Dysfunction
4.4.5. Changes in the Metabolome and Intestinal Microbiome
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rana, S.V.S. Perspectives in endocrine toxicity of heavy metals—A Review. Biol. Trace Element Res. 2014, 160, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Akash, M.S.H.; Fiayyaz, F.; Saleem, U.; Mehmood, M.H.; Rehman, K. Role of cadmium and arsenic as endocrine disruptors in the metabolism of carbohydrates: Inserting the association into perspectives. Biomed. Pharmacother. 2019, 114, 108802. [Google Scholar] [CrossRef] [PubMed]
- Gribble, M.O.; Howard, B.V.; Umans, J.G.; Shara, N.M.; Francesconi, K.A.; Goessler, W.; Crainiceanu, C.M.; Silbergeld, E.K.; Guallar, E.; Navas-Acien, A. Arsenic exposure, diabetes prevalence, and diabetes control in the strong heart study. Am. J. Epidemiol. 2012, 176, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Lucio, M.; Barbir, R.; Lovrenčić, M.V.; Varžić, S.C.; Ljubić, S.; Duvnjak, L.S.; Šerić, V.; Milić, M.; Lovaković, B.T.; Krivohlavek, A.; et al. Association between arsenic exposure and biomarkers of type 2 diabetes mellitus in a Croatian population: A comparative observational pilot study. Sci. Total Environ. 2020, 720, 137575. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; González-Horta, C.; Rager, J.; Bailey, K.A.; Sánchez-Ramírez, B.; Ballinas-Casarrubias, L.; Ishida, M.C.; Gutiérrez-Torres, D.S.; Cerón, R.H.; Morales, D.V.; et al. Metabolomic characteristics of arsenic-associated diabetes in a prospective cohort in Chihuahua, Mexico. Toxicol. Sci. 2015, 144, 338–346. [Google Scholar] [CrossRef]
- Grau-Perez, M.; Navas-Acien, A.; Galan-Chilet, I.; Briongos-Figuero, L.S.; Morchon-Simon, D.; Bermudez, J.D.; Crainiceanu, C.M.; de Marco, G.; Rentero-Garrido, P.; Garcia-Barrera, T.; et al. Arsenic exposure, diabetes-related genes and diabetes prevalence in a general population from Spain. Environ. Pollut. 2018, 235, 948–955. [Google Scholar] [CrossRef]
- Teixeira, M.C.; Santos, A.C.; Fernandes, C.S.; Ng, J.C. Arsenic contamination assessment in Brazil–Past, present and future concerns: A historical and critical review. Sci. Total Environ. 2020, 730, 138217. [Google Scholar] [CrossRef]
- Madhyastha, H.; Madhyastha, R.; Nakajima, Y.; Maruyama, M. Deciphering the molecular events during arsenic induced transcription signal cascade activation in cellular milieu. BioMetals 2018, 31, 7–15. [Google Scholar] [CrossRef]
- Kuo, C.C.; Howard, B.V.; Umans, J.G.; Gribble, M.O.; Best, L.G.; Francesconi, K.A.; Goessler, W.; Lee, E.; Guallar, E.; Navas-Acien, A. Arsenic Exposure, Arsenic Metabolism, and Incident Diabetes in the Strong Heart Study. Diabetes Care 2015, 38, 620–627. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Hwang, Y.-C.; Woo, J.T.; Chin, S.O.; Chon, S.; Kim, Y.S. Arsenic Exposure and Prevalence of Diabetes Mellitus in Korean Adults. J. Korean Med. Sci. 2013, 28, 861–868. [Google Scholar] [CrossRef]
- Martin, E.M.; Stýblo, M.; Fry, R.C. Genetic and epigenetic mechanisms underlying arsenic-associated diabetes mellitus: A perspective of the current evidence. Epigenomics 2017, 9, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Keshtzar, E.; Khodayar, M.J.; Javadipour, M. SirT3 regulates diabetogenic effects caused by arsenic: An implication for mitochondrial complex II modification. Toxicol. Lett. 2019, 301, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Kirkley, A.G.; Carmean, C.M.; Ruiz, D.; Ye, H.; Regnier, S.M.; Poudel, A.; Hara, M.; Kamau, W.; Johnson, D.N.; Roberts, A.A.; et al. Arsenic exposure induces glucose intolerance and alters global energy metabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R294–R303. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, X.; Wu, B.; Yu, H.; Zhang, X.; Li, M. Arsenic induces diabetic effects through beta-cell dysfunction and increased gluconeogenesis in mice. Sci. Rep. 2014, 4, 6894. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xun, P.; Carnethon, M.; Carson, A.P.; Lu, L.; Zhu, J.; He, K. Low to moderate toenail arsenic levels in young adulthood and incidence of diabetes later in life: Findings from the CARDIA Trace Element study. Environ. Res. 2019, 171, 321–327. [Google Scholar] [CrossRef]
- Feseke, S.K.; St-Laurent, J.; Anassour-Sidi, E.; Ayotte, P.; Bouchard, M.; Levallois, P. Arsenic exposure and type 2 diabetes: Results from the 2007–2009 Canadian Health Measures Survey. Health Promot. Chronic Dis. Prev. Can. 2015, 35, 63–72. [Google Scholar] [CrossRef]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal database combinations for literature searches in systematic reviews: A prospective exploratory study. Syst. Rev. 2017, 6, 245. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Yin, J.; Liu, S.; Yu, J.; Wu, B. Differential toxicity of arsenic on renal oxidative damage and urinary metabolic profiles in normal and diabetic mice. Environ. Sci. Pollut. Res. 2017, 24, 17485–17492. [Google Scholar] [CrossRef]
- Song, X.; Li, Y.; Liu, J.; Ji, X.; Zhao, L.; Wei, Y. Changes in Serum Adiponectin in Mice Chronically Exposed to Inorganic Arsenic in Drinking Water. Biol. Trace Element Res. 2017, 179, 140–147. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, Z.; Gao, T.; Yin, Y.; Wang, Z.; Hou, Y.; Fu, J.; Liu, S.; Wang, H.; Xu, Y.; et al. Prolonged inorganic arsenic exposure via drinking water impairs brown adipose tissue function in mice. Sci. Total Environ. 2019, 668, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Brejnrod, A.D.; Ernst, M.; Rykær, M.; Herschend, J.; Olsen, N.M.C.; Dorrestein, P.C.; Rensing, C.; Sørensen, S.J. Heavy metal exposure causes changes in the metabolic health-associated gut microbiome and metabolites. Environ. Int. 2019, 126, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Liu, J.; Xue, Y.; Zhuang, Z.; Qian, S.; Zhou, W.; Li, X.; Qian, J.; Ding, G.; Sun, Z. Non-monotonic dose-response effects of arsenic on glucose metabolism. Toxicol. Appl. Pharmacol. 2019, 377, 114605. [Google Scholar] [CrossRef] [PubMed]
- Castriota, F.; Zushin, P.-J.H.; Sanchez, S.S.; Phillips, R.V.; Hubbard, A.; Stahl, A.; Smith, M.T.; Wang, J.-C.; La Merrill, M.A. Chronic arsenic exposure impairs adaptive thermogenesis in male C57BL/6J mice. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E667–E677. [Google Scholar] [CrossRef]
- Li, W.; Wu, L.; Sun, Q.; Yang, Q.; Xue, J.; Shi, M.; Tang, H.; Zhang, J.; Liu, Q. MicroRNA-191 blocking the translocation of GLUT4 is involved in arsenite-induced hepatic insulin resistance through inhibiting the IRS1/AKT pathway. Ecotoxicol. Environ. Saf. 2021, 215, 112130. [Google Scholar] [CrossRef]
- Liu, P.; Dodson, M.; Li, H.; Schmidlin, C.J.; Shakya, A.; Wei, Y.; Garcia, J.G.; Chapman, E.; Kiela, P.R.; Zhang, Q.Y.; et al. Non-canonical NRF2 activation promotes a pro-diabetic shift in hepatic glucose metabolism. Mol. Metab. 2021, 51, 101243. [Google Scholar] [CrossRef]
- Patel, H.V.; Kalia, K. Role of hepatic and pancreatic oxidative stress in arsenic induced diabetic condition in Wistar rats. J. Environ. Biol. 2013, 34, 231–236. [Google Scholar]
- Souza, A.C.F.; Bastos, D.S.S.; Santos, F.C.; Sertorio, M.N.; Ervilha, L.O.G.; Gonçalves, R.V.; de Oliveira, L.L.; Machado-Neves, M. Arsenic aggravates oxidative stress causing hepatic alterations and inflammation in diabetic rats. Life Sci. 2018, 209, 472–480. [Google Scholar] [CrossRef]
- Izquierdo-Vega, J.A.; Soto, C.A.; Sanchez-Peña, L.C.; De Vizcaya-Ruiz, A.; Del Razo, L.M. Diabetogenic effects and pancreatic oxidative damage in rats subchronically exposed to arsenite. Toxicol. Lett. 2006, 160, 135–142. [Google Scholar] [CrossRef]
- Rezaei, M.; Khodayar, M.J.; Seydi, E.; Soheila, A.; Parsi, I.K. Acute, but not Chronic, Exposure to Arsenic Provokes Glucose Intolerance in Rats: Possible Roles for Oxidative Stress and the Adrenergic Pathway. Can. J. Diabetes 2017, 41, 273–280. [Google Scholar] [CrossRef]
- Xenakis, J.G.; Douillet, C.; Bell, T.A.; Hock, P.; Farrington, J.; Liu, T.; Murphy, C.E.Y.; Saraswatula, A.; Shaw, G.D.; Nativio, G.; et al. An interaction of inorganic arsenic exposure with body weight and composition on type 2 diabetes indicators in Diversity Outbred mice. Mamm. Genome 2022, 33, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.A.; Wu, M.C.; Ward, W.O.; Smeester, L.; Rager, J.E.; García-Vargas, G.; Del Razo, L.-M.; Drobná, Z.; Stýblo, M.; Fry, R.C. Arsenic and the epigenome: Interindividual differences in arsenic metabolism related to distinct patterns of DNA methylation. J. Biochem. Mol. Toxicol. 2013, 27, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Drobná, Z.; Del Razo, L.M.; García-Vargas, G.G.; Sánchez-Peña, L.C.; Barrera-Hernández, A.; Stýblo, M.; Loomis, D. Environmental exposure to arsenic, AS3MT polymorphism and prevalence of diabetes in Mexico. J. Expo. Sci. Environ. Epidemiol. 2012, 23, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zhan, Z.; Zhang, X.; Lou, Q.; Guo, N.; Su, M.; Gao, Y.; Qin, M.; Wu, L.; Huang, W.; et al. Research for type 2 diabetes mellitus in endemic arsenism areas in central China: Role of low level of arsenic exposure and KEAP1 rs11545829 polymorphism. Arch. Toxicol. 2022, 96, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Silbergeld, E.K.; Pastor-Barriuso, R.; Guallar, E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 2008, 300, 814–822. [Google Scholar] [CrossRef]
- Huang, J.-W.; Cheng, Y.-Y.; Sung, T.-C.; Guo, H.-R.; Sthiannopkao, S. Association between arsenic exposure and diabetes mellitus in Cambodia. BioMed Res. Int. 2014, 2014, 683124. [Google Scholar] [CrossRef]
- Jovanovic, D.; Rasic-Milutinovic, Z.; Paunovic, K.; Jakovljevic, B.; Plavsic, S.; Milosevic, J. Low levels of arsenic in drinking water and type 2 diabetes in Middle Banat region, Serbia. Int. J. Hyg. Environ. Health 2013, 216, 50–55. [Google Scholar] [CrossRef]
- Kim, N.H.; Mason, C.C.; Nelson, R.G.; Afton, S.E.; Essader, A.S.; Medlin, J.E.; Levine, K.E.; Hoppin, J.A.; Lin, C.; Knowler, W.C.; et al. Arsenic exposure and incidence of type 2 diabetes in southwestern American Indians. Am. J. Epidemiol. 2013, 177, 962–969. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Park, S.K. Associations between rice consumption, arsenic metabolism, and insulin resistance in adults without diabetes. Int. J. Hyg. Environ. Health 2021, 237, 113834. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Zou, Z.; Sun, B.; Liang, B.; Zhang, A. Association and risk of circulating inflammatory markers with hyperglycemia in coal-burning arsenicosis. Ecotoxicol. Environ. Saf. 2022, 247, 114208. [Google Scholar] [CrossRef]
- Park, S.K.; Peng, Q.; Bielak, L.F.; Silver, K.D.; Peyser, P.A.; Mitchell, B.D. Arsenic exposure is associated with diminished insulin sensitivity in non-diabetic Amish adults. Diabetes Metab. Res. Rev. 2016, 32, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.K.; Islam, M.S.; Hasibuzzaman, M.M.; Hossain, F.; Anjum, A.; Saud, Z.A.; Haque, M.M.; Sultana, P.; Haque, A.; Andric, K.B.; et al. Higher risk of hyperglycemia with greater susceptibility in females in chronic arsenic-exposed individuals in Bangladesh. Sci. Total. Environ. 2019, 668, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Harlow, S.D.; Park, S.K. Urinary arsenic and insulin resistance in US adolescents. Int. J. Hyg. Environ. Health 2015, 218, 407–413. [Google Scholar] [CrossRef]
- Spratlen, M.J.; Grau-Perez, M.; Umans, J.G.; Yracheta, J.; Best, L.G.; Francesconi, K.; Goessler, W.; Bottiglieri, T.; Gamble, M.V.; Cole, S.A.; et al. A Targeted metabolomics to understand the association between arsenic metabolism and diabetes-related outcomes: Preliminary evidence from the Strong Heart Family Study. Environ. Res. 2019, 168, 146–157. [Google Scholar] [CrossRef]
- Zhou, M.; Zhao, E.; Huang, R. Association of urinary arsenic with insulin resistance: Cross-sectional analysis of the National Health and Nutrition Examination Survey, 2015–2016. Ecotoxicol. Environ. Saf. 2022, 231, 113218. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, B.K. Association between urinary arsenic and diabetes mellitus in the Korean general population according to KNHANES 2008. Sci. Total Environ. 2011, 409, 4054–4062. [Google Scholar] [CrossRef]
- Spratlen, M.J.; Grau-Perez, M.; Umans, J.G.; Yracheta, J.; Best, L.G.; Francesconi, K.; Goessler, W.; Balakrishnan, P.; Cole, S.A.; Gamble, M.V.; et al. Arsenic, one carbon metabolism and diabetes-related outcomes in the Strong Heart Family Study. Environ. Int. 2018, 121 Pt 1, 728–740. [Google Scholar] [CrossRef]
- YarMohammadi, A.A.; Bidgoli, S.A.; Ziarati, P. Increased urinary arsenic concentration in newly diagnosed type 2 diabetes mellitus: A gender-independent, smoking-dependent exposure biomarker in older adults in Tehran. Environ. Sci. Pollut. Res. 2021, 28, 27769–27777. [Google Scholar] [CrossRef]
- Coronado-González, J.A.; Del Razo, L.M.; García-Vargas, G.; Sanmiguel-Salazar, F.E.-D.; la Peña, J. Inorganic arsenic exposure and type 2 diabetes mellitus in Mexico. Environ. Res. 2007, 104, 383–389. [Google Scholar] [CrossRef]
- Díaz-Villaseñor, A.; Cruz, L.; Cebrián, A.; Hernández-Ramírez, R.U.; Hiriart, M.; García-Vargas, G.; Bassol, S.; Sordo, M.; Gandolfi, A.J.; Klimecki, W.T.; et al. Arsenic exposure and calpain-10 polymorphisms impair the function of pancreatic beta-cells in humans: A pilot study of risk factors for T2DM. PLoS ONE 2013, 8, e51642. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S. Environmental risk assessment of chronic arsenic in drinking water and prevalence of type-2 diabetes mellitus in Pakistan. Environ. Technol. 2020, 41, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.-C.; Kile, M.L.; Seow, W.J.; Lin, X.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Mostofa, G.; Lu, Q.; Christiani, D.C. Genetic susceptible locus in NOTCH2 interacts with arsenic in drinking water on risk of type 2 diabetes. PLoS ONE 2013, 8, e70792. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.C.; Seow, W.J.; Kile, M.L.; Hoffman, E.B.; Quamruzzaman, Q.; Rahman, M.; Mahiuddin, G.; Mostofa, G.; Lu, Q.; Christiani, D.C. Association of low to moderate levels of arsenic exposure with risk of type 2 diabetes in Bangladesh. Am. J. Epidemiol. 2013, 178, 1563–1570. [Google Scholar] [CrossRef]
- Rangel-Moreno, K.; Gamboa-Loira, B.; López-Carrillo, L.; Cebrián, M.E. Prevalence of type 2 diabetes mellitus in relation to arsenic exposure and metabolism in Mexican women. Environ. Res. 2022, 210, 112948. [Google Scholar] [CrossRef] [PubMed]
- Rehman, K.; Fatima, F.; Akash, M. Biochemical investigation of association of arsenic exposure with risk factors of diabetes mellitus in Pakistani population and its validation in animal model. Environ. Monit. Assess. 2019, 191, 511. [Google Scholar] [CrossRef]
- Wu, F.; Chen, Y.; Navas-Acien, A.; Garabedian, M.L.; Coates, J.; Newman, J.D. Arsenic Exposure, Arsenic Metabolism, and Glycemia: Results from a Clinical Population in New York City. Int. J. Environ. Res. Public Health 2021, 18, 3749. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, Y.; Wang, D.; Xu, Y.; Wang, H.; Liu, J.; Xia, L.; Li, Y.; Tang, N.; Zheng, Q.; et al. Interactions of arsenic metabolism with arsenic exposure and individual factors on diabetes occurrence: Baseline findings from Arsenic and Non-Communicable disease cohort (AsNCD) in China. Environ. Pollut. 2020, 265 Pt A, 114968. [Google Scholar] [CrossRef]
- Grau-Perez, M.; Kuo, C.-C.; Gribble, M.O.; Balakrishnan, P.; Spratlen, M.J.; Vaidya, D.; Francesconi, K.A.; Goessler, W.; Guallar, E.; Silbergeld, E.K.; et al. Association of Low-Moderate Arsenic Exposure and Arsenic Metabolism with Incident Diabetes and Insulin Resistance in the Strong Heart Family Study. Environ. Health Perspect. 2017, 125, 127004. [Google Scholar] [CrossRef]
- Walton, F.S.; Harmon, A.W.; Paul, D.S.; Drobná, Z.; Patel, Y.M.; Styblo, M. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: Possible mechanism of arsenic-induced diabetes. Toxicol. Appl. Pharmacol. 2004, 198, 424–433. [Google Scholar] [CrossRef]
- Velmurugan, G.; Swaminathan, K.; Veerasekar, G.; Purnell, J.Q.; Mohanraj, S.; Dhivakar, M.; Avula, A.K.; Cherian, M.; Palaniswami, N.G.; Alexander, T.; et al. Metals in urine in relation to the prevalence of pre-diabetes, diabetes and atherosclerosis in rural India. Occup. Environ. Med. 2018, 75, 661–667. [Google Scholar] [CrossRef]
- Food and Agriculture Organization on the United Nations. In Proceedings of the Codex Alimentarius Commission. JOINT FAO/WHO Food Standards Programme CODEX Committeee on Contaminants in Foods, 12th Session, Utrecht, The Netherlands, 12–16 March 2018.
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Madhyastha, H.; Madhyastha, R.; Maruyama, M.; Arunachlam, S.; Abilash, V.G. Role of arsenic exposure in adipose tissue dysfunction and its possible implication in diabetes pathophysiology. Toxicol. Lett. 2018, 284, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. A review on environmental factors regulating arsenic methylation in humans. Toxicol. Appl. Pharmacol. 2009, 235, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Vahter, M.; Marafante, E. Effects of low dietary intake of methionine, choline or proteins on the biotransformation of arsenite in the rabbit. Toxicol. Lett. 1987, 37, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Grashow, R.; Zhang, J.; Fang, S.C.; Weisskopf, M.G.; Christiani, D.C.; Kile, M.L.; Cavallari, J.M. Inverse association between toenail arsenic and body mass index in a population of welders. Environ. Res. 2014, 131, 131–133. [Google Scholar] [CrossRef]
- Hughes, M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002, 133, 1–16. [Google Scholar] [CrossRef]
- Jomova, K.; Jenisova, Z.; Feszterova, M.; Baros, S.; Liska, J.; Hudecova, D.; Rhodes, C.J.; Valko, M. Arsenic: Toxicity, oxidative stress and human disease. J. Appl. Toxicol. 2011, 31, 95–107. [Google Scholar] [CrossRef]
- Darenskaya, M.A.; Kolesnikova, L.I.; Kolesnikov, S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021, 171, 179–189. [Google Scholar] [CrossRef]
- Prakash, C.; Chhikara, S.; Kumar, V. Mitochondrial Dysfunction in Arsenic-Induced Hepatotoxicity: Pathogenic and Therapeutic Implications. Biol. Trace Element Res. 2022, 200, 261–270. [Google Scholar] [CrossRef]
- Wei, X.; Che, W.; Wang, Q.; Yu, L. Evaluation of adiponectin and TNF-α expression in diabetic patients and its relationship with cardiovascular diseases. Cell. Mol. Biol. 2023, 69, 75–79. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Wang, H.; Xue, P.; Li, X.; Li, B.; Zheng, Q.; Sun, G. Arsenic induces mitochondria-dependent apoptosis by reactive oxygen species generation rather than glutathione depletion in Chang human hepatocytes. Arch. Toxicol. 2009, 83, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Akram, Z.; Mahjabeen, I.; Umair, M.; Fahim, M.; Kayani, M.A.; Fatima, L.; Ahmad, M.W.; Jahan, S.; Afsar, T.; Almajwal, A.; et al. Expression variation of OGG1 and HPRT gene and DNA damage in arsenic exposed industrial workers. PLoS ONE 2022, 17, e0273211. [Google Scholar] [CrossRef]
- Bozack, A.K.; Domingo-Relloso, A.; Haack, K.; Gamble, M.V.; Tellez-Plaza, M.; Umans, J.G.; Best, L.G.; Yracheta, J.; Gribble, M.O.; Cardenas, A.; et al. Locus-Specific Differential DNA Methylation and Urinary Arsenic: An Epigenome-Wide Association Study in Blood among Adults with Low-to-Moderate Arsenic Exposure. Environ. Health Perspect. 2020, 128, 67015. [Google Scholar] [CrossRef] [PubMed]
- Javaid, A.; Akbar, I.; Javed, H.; Khan, U.; Iftikhar, H.; Zahra, D.; Rashid, F.; Ashfaq, U.A. Role of Heavy Metals in Diabetes: Mechanisms and Treatment Strategies. Crit. Rev. Eukaryot. Gene Expr. 2021, 31, 65–80. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Arun, K.B.; Madhavan, A.; Sindhu, R.; Emmanual, S.; Binod, P.; Pugazhendhi, A.; Sirohi, R.; Reshmy, R.; Awasthi, M.K.; Gnansounou, E.; et al. Probiotics and gut microbiome—Prospects and challenges in remediating heavy metal toxicity. J. Hazard Mater. 2021, 420, 126676. [Google Scholar] [CrossRef] [PubMed]
- Moffa, S.; Mezza, T.; Cefalo, C.M.A.; Cinti, F.; Impronta, F.; Sorice, G.P.; Santoro, A.; Di Giuseppe, G.; Pontecorvi, A.; Giaccari, A. The Interplay between Immune System and Microbiota in Diabetes. Mediat. Inflamm. 2019, 2019, 9367404. [Google Scholar] [CrossRef]
| Author/Year | Experimental Model | Treatment/Duration | Main Results |
|---|---|---|---|
| Izquierdo-Vega et al., 2006 [29] | Male Wistar rats | Water or sodium arsenite; 1.7 mg/kg. 12 h. 90 days | Hyperglycemia, hyperinsulinemia, and low insulin sensitivity. Increased total glutathione and lipoperoxidation in the pancreas of the group exposed to iAs |
| Patel and Kalia 2013 [27] | Albino Wistar Rats | Distilled water, 1.5 mg/kg−1 b. wt or 5.0 mg/kg−1 b. wt of sodium arsenite. 4 weeks | Increased superoxide dismutase (SOD), catalase, and glutathione-S-transferase activity |
| Liu et al., 2014 [14] | Healthy (C57BLKS/J) and diabetic (C57BKS/Leprdb) mice | Deionized water or 3 mg/L iAs. 16 weeks | iAs increased oxidative stress and inflammation in liver and pancreas of healthy mice. It also increased gluconeogenesis and reduced gene expression of GLUT4. |
| Rezaei et al., 2017 [30] | Male Sprague-Dawley rats | Control, As, As + N-acetylcysteine, carvedilol, carvedilol + As, propranolol, or propranolol + As. Acute exposure: 2, 4, or 8 mg/L of As for 15 to 120 min. Chronic exposure: 0.20, 40, or 60 ppm for 8 weeks (0.20 mg/L, 40 mg/L, 60 mg/L) or 200, 400, or 800 ppm for 20 weeks (200 mg/L, 400 mg/L, 800 mg/L) | Acute exposure to As-induced glucose intolerance. Preventive role of N-acetylcysteine against glycemic changes caused by As. |
| Yin et al., 2017 [19] | Diabetic and healthy mice (C57BLKS/J), age—7 weeks | Deionized water or sodium arsenite 3 mg/L. 16 weeks | Increased glutathione peroxidase concentration in diabetic mice exposed to iAs. |
| Song et al., 2017 [20] | Healthy mice (C57BL/6), age—4 weeks | Water, water + 5 ppm (5 mg/L) of iAs, or water + 50 ppm (50 mg/L). 18 weeks | No changes in serum insulin and glucose concentrations. Adiponectin reduction. |
| Souza et al., 2018 [28] | Healthy and diabetic male Wistar rats, age—70 days | Diabetes was induced using streptozotocin. Exposed to saline solution (0.9% NaCl) or 10 mg/L of sodium arsenate. 40 days | iAs exposure increased SOD and glutathione s-transferase activity in healthy and diabetic rats. iAs caused a hepatic inflammatory reaction with increased TNF-α. |
| Kirkley et al., 2018 [13] | Male mice (C57BL/6J), age—7 to 8 weeks | Water or water + 50 mg L of sodium arsenite. 8 weeks | Mice exposed to As exhibited glucose intolerance without altering overall insulin sensitivity. 28% reduction in HOMA-IR. |
| Zuo et al., 2019 [21] | Female mice (C57BL/6), 10 weeks | Mice exposed to 0, 5, or 20 ppm (5 or 20 mg/L) iAs in drinking water. 17 weeks | Prolonged exposure to iAs caused glucose intolerance, insulin resistance, and lower PPARγ. |
| Li et al., 2019 [22] | Mice (C57BL/6), 5 weeks | Water, water + 50 ppm cadmium chloride, or water + 50 ppm (50 mg/L) sodium arsenite. 2 weeks | Exposure to iAs caused overall changes in the intestinal metabolome and microbiome. |
| Gong et al., 2019 [23] | Mice (C57BL/6), 8 weeks | Deionized water + 0.25 ppm (0.25 mg/L) sodium arsenite or deionized water + 2.5 ppm (2.5 mg/L) sodium arsenite. 15 weeks | Exposure to 0.25 ppm iAs caused glucose intolerance. Exposure to 2.5 ppm iAs not significant for glucose tolerance. |
| Rezaei et al., 2019 [12] | Male Wistar rats, 10 weeks | Normal diet, diet + As trioxide (7 mg/kg), varying with or without the presence of metformin or berberine. Every 2 days for 8 days | iAs increased fasting glucose and insulin compared to the control group. Increased SIRT3 concentration and mitochondrial dysfunction due to exposure to iAs. |
| Castriota et al., 2020 [24] | Mice (C57BL/6J), 5 weeks | Drinking water + 300 μg/L (0.3 mg/L) of sodium metaarsenite. 9 weeks | Exposure to iAs caused the dysregulation of mitochondrial processes. |
| Li et al., 2021 [25] | Male mice (C57BL/6J), age—7 to 8 weeks | 0 or 20 mg/L (0 or 20 ppm) sodium arsenite. 12 months | iAs exposure induced systemic and hepatic insulin resistance and decreased liver GLUT4 concentrations. |
| Liu et al., 2021 [26] | Mice (C57BL/6J), age—8 to 10 weeks | Drinking water or drinking water + 25 ppm sodium arsenite. 20 weeks | NRF2 and p62 are associated with iAs-mediated insulin resistance |
| Xenakis et al., 2022 [31] | Diversity Outbred male mice (J:DO JAX stock number 009376) generation 35, age—26 to 32 days | 100 ppb iAs in drinking water for 26 weeks | Associations between iAs consumption and fasting blood glucose, plasma insulin, β-cell function, and insulin resistance manifested as significant interactions between iAs and body weight/composition. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosendo, G.B.O.; Ferreira, R.L.U.; Aquino, S.L.S.; Barbosa, F.; Pedrosa, L.F.C. Glycemic Changes Related to Arsenic Exposure: An Overview of Animal and Human Studies. Nutrients 2024, 16, 665. https://doi.org/10.3390/nu16050665
Rosendo GBO, Ferreira RLU, Aquino SLS, Barbosa F, Pedrosa LFC. Glycemic Changes Related to Arsenic Exposure: An Overview of Animal and Human Studies. Nutrients. 2024; 16(5):665. https://doi.org/10.3390/nu16050665
Chicago/Turabian StyleRosendo, Geovanna Beatriz Oliveira, Rannapaula Lawrynhuk Urbano Ferreira, Séphora Louyse Silva Aquino, Fernando Barbosa, and Lucia Fatima Campos Pedrosa. 2024. "Glycemic Changes Related to Arsenic Exposure: An Overview of Animal and Human Studies" Nutrients 16, no. 5: 665. https://doi.org/10.3390/nu16050665
APA StyleRosendo, G. B. O., Ferreira, R. L. U., Aquino, S. L. S., Barbosa, F., & Pedrosa, L. F. C. (2024). Glycemic Changes Related to Arsenic Exposure: An Overview of Animal and Human Studies. Nutrients, 16(5), 665. https://doi.org/10.3390/nu16050665