Consistency and Variability of the Human Milk Oligosaccharide Profile in Repeat Pregnancies
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Sample Collection
2.2. Quantification of HMOs
2.3. Statistical Analysis
3. Results
3.1. Participants and HMO Profiles
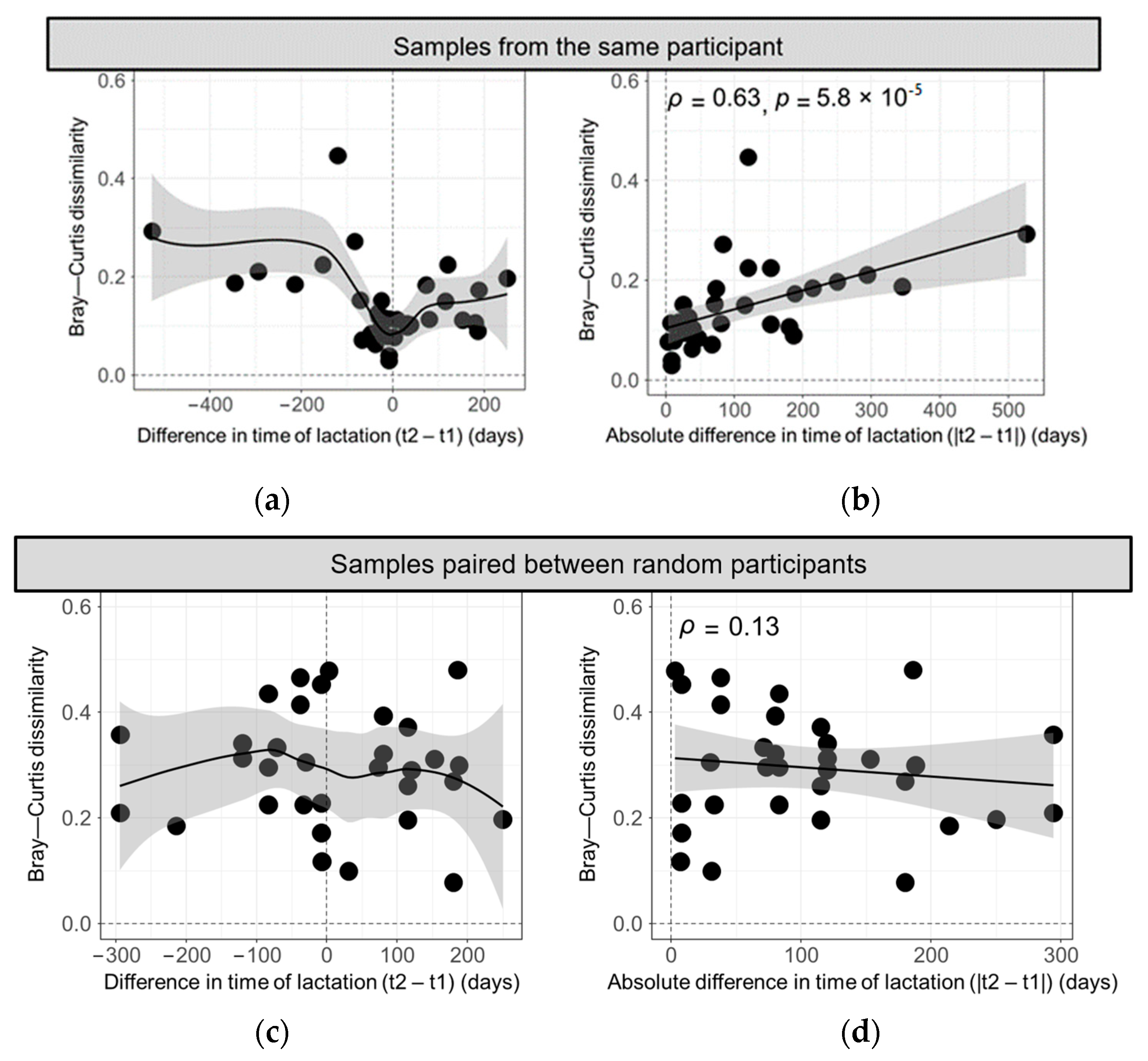
3.2. Bray–Curtis Dissimilarity in HMO Profiles across Different Deliveries
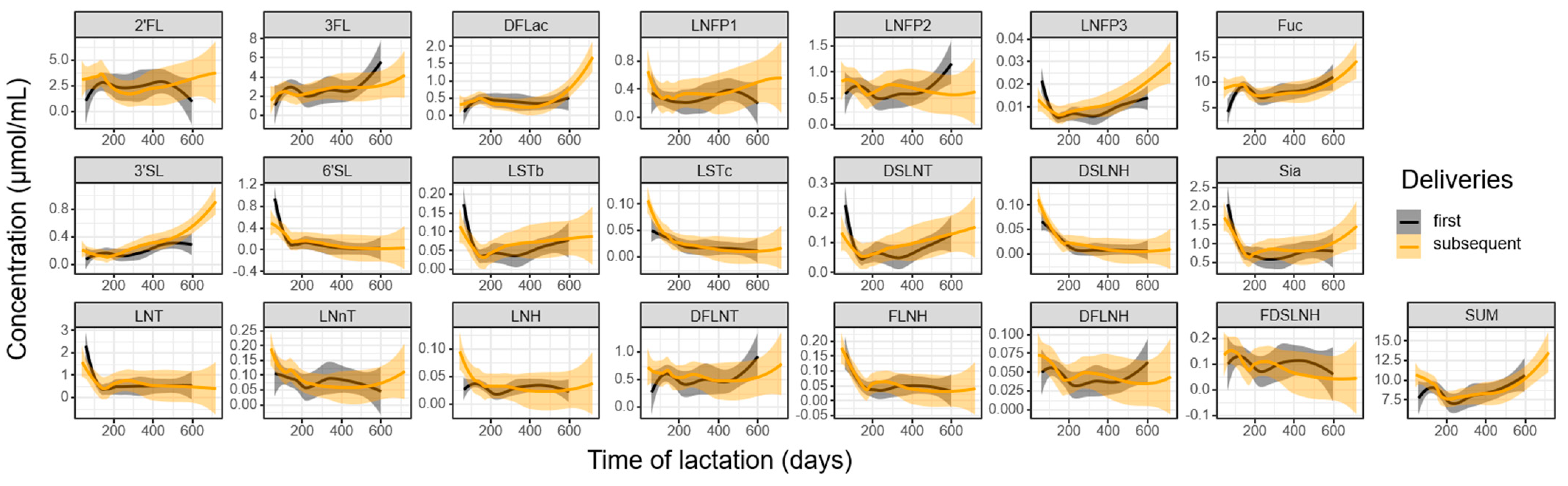
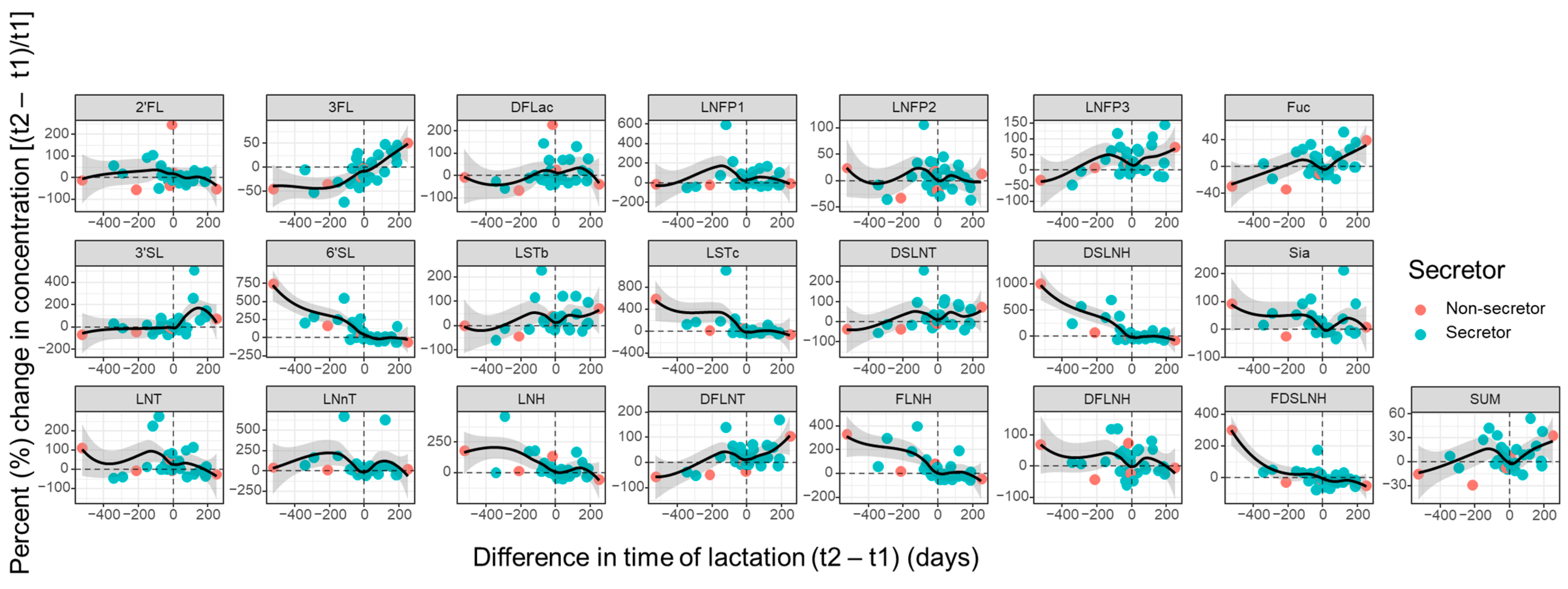
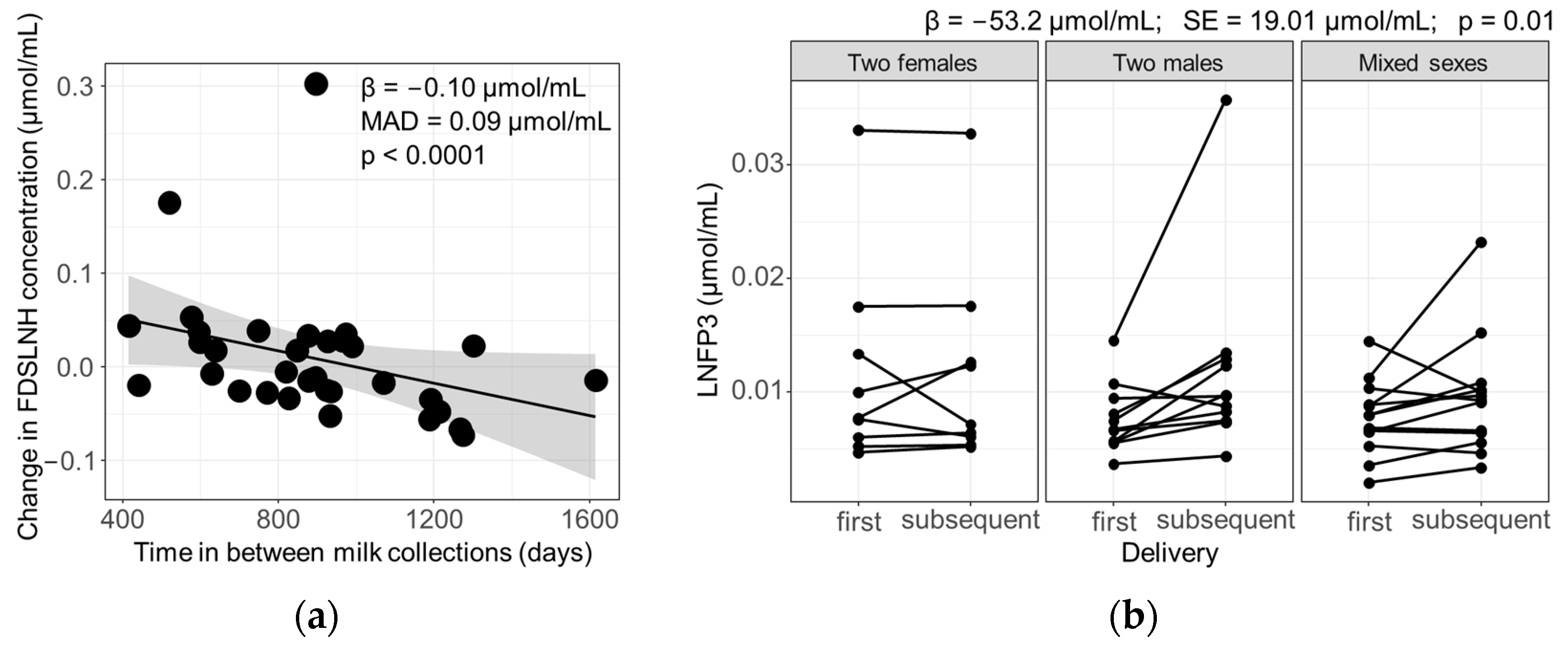
3.3. Influence of Parity and Lactation Time on Individual HMOs
3.4. Influence of Maternal and Infant Factors on HMO Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bode, L. Human Milk Oligosaccharides: Every Baby Needs a Sugar Mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of Human Milk Oligosaccharides in Relation to Milk Groups and Lactational Periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Ayechu-Muruzabal, V.; van Stigt, A.H.; Mank, M.; Willemsen, L.E.M.; Stahl, B.; Garssen, J.; van’t Land, B. Diversity of Human Milk Oligosaccharides and Effects on Early Life Immune Development. Front. Pediatr. 2018, 6, 239. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Yeung, C.Y. Recent Advance in Infant Nutrition: Human Milk Oligosaccharides. Pediatr. Neonatol. 2021, 62, 347–353. [Google Scholar] [CrossRef]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s Normal? Oligosaccharide Concentrations and Profiles in Milk Produced by Healthy Women Vary Geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martínez-Costa, C.; et al. Impact of Maternal Characteristics on Human Milk Oligosaccharide Composition over the First 4 Months of Lactation in a Cohort of Healthy European Mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, Z.; Zhao, A.; Zhang, J.; Wu, W.; Ren, Z.; Wang, P.; Zhang, Y. Neutral Human Milk Oligosaccharides Are Associated with Multiple Fixed and Modifiable Maternal and Infant Characteristics. Nutrients 2020, 12, 826. [Google Scholar] [CrossRef]
- Durham, S.D.; Robinson, R.C.; Olga, L.; Ong, K.K.; Chichlowski, M.; Dunger, D.B.; Barile, D. A One-Year Study of Human Milk Oligosaccharide Profiles in the Milk of Healthy UK Mothers and Their Relationship to Maternal FUT2 Genotype. Glycobiology 2021, 31, 1254–1267. [Google Scholar] [CrossRef]
- Eussen, S.R.B.M.; Mank, M.; Kottler, R.; Hoffmann, X.K.; Behne, A.; Rapp, E.; Stahl, B.; Mearin, M.L.; Koletzko, B. Presence and Levels of Galactosyllactoses and Other Oligosaccharides in Human Milk and Their Variation during Lactation and According to Maternal Phenotype. Nutrients 2021, 13, 2324. [Google Scholar] [CrossRef]
- Bandoli, G.; Bertrand, K.; Saoor, M.; Chambers, C.D. The Design and Mechanics of an Accessible Human Milk Research Biorepository. Breastfeed. Med. 2020, 15, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.K.; Hampson, H.E.; Schmidt, K.A.; Alderete, T.L.; Furst, A.; Yonemitsu, C.; Demerath, E.; Goran, M.I.; Fields, D.A.; Bode, L. Stability of Human-Milk Oligosaccharide Concentrations Over 1 Week of Lactation and Over 6 Hours Following a Standard Meal. J. Nutr. 2022, 152, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- James, K.; Bottacini, F.; Contreras, J.I.S.; Vigoureux, M.; Egan, M.; Motherway, M.O.; Holmes, E.; van Sinderen, D. Metabolism of the Predominant Human Milk Oligosaccharide Fucosyllactose by an Infant Gut Commensal. Sci. Rep. 2019, 9, 15427. [Google Scholar] [CrossRef] [PubMed]
- Salli, K.; Hirvonen, J.; Siitonen, J.; Ahonen, I.; Anglenius, H.; Maukonen, J. Selective Utilization of the Human Milk Oligosaccharides 2′-Fucosyllactose, 3-Fucosyllactose, and Difucosyllactose by Various Probiotic and Pathogenic Bacteria. J. Agric. Food Chem. 2021, 69, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, A.; Katoh, T.; Sakanaka, M.; Ling, Y.; Yamada, C.; Asakuma, S.; Urashima, T.; Tomabechi, Y.; Katayama-Ikegami, A.; Kurihara, S.; et al. Sharing of Human Milk Oligosaccharides Degradants within Bifidobacterial Communities in Faecal Cultures Supplemented with Bifidobacterium bifidum. Sci. Rep. 2018, 8, 13958. [Google Scholar] [CrossRef] [PubMed]
- Schroten, H.; Hanisch, F.G.; Hansman, G.S. Human Norovirus Interactions with Histo-Blood Group Antigens and Human Milk Oligosaccharides. J. Virol. 2016, 90, 5855–5859. [Google Scholar] [CrossRef] [PubMed]
- Koromyslova, A.; Tripathi, S.; Morozov, V.; Schroten, H.; Hansman, G.S. Human Norovirus Inhibition by a Human Milk Oligosaccharide. Virology 2017, 508, 81–89. [Google Scholar] [CrossRef]
- Zuurveld, M.; Van Witzenburg, N.P.; Garssen, J.; Folkerts, G.; Stahl, B.; Van’t Land, B.; Willemsen, L.E. Immunomodulation by Human Milk Oligosaccharides: The Potential Role in Prevention of Allergic Diseases. Front. Immunol. 2020, 11, 801. [Google Scholar] [CrossRef]
- Siziba, L.P.; Mank, M.; Stahl, B.; Gonsalves, J.; Blijenberg, B.; Rothenbacher, D.; Genuneit, J. Human Milk Oligosaccharide Profiles over 12 Months of Lactation: The Ulm SPATZ Health Study. Nutrients 2021, 13, 1973. [Google Scholar] [CrossRef]
- Tonon, K.M.; de Morais, M.B.; Abrão, A.C.F.V.; Miranda, A.; Morais, T.B. Maternal and Infant Factors Associated with Human Milk Oligosaccharides Concentrations According to Secretor and Lewis Phenotypes. Nutrients 2019, 11, 1358. [Google Scholar] [CrossRef] [PubMed]
- James, K.; Motherway, M.O.; Bottacini, F.; van Sinderen, D. Bifidobacterium breve UCC2003 Metabolises the Human Milk Oligosaccharides Lacto-N-Tetraose and Lacto-N-Neo-Tetraose through Overlapping, yet Distinct Pathways. Sci. Rep. 2016, 6, 38560. [Google Scholar] [CrossRef]
- Ferreira, A.L.; Alves, R.; Figueiredo, A.; Alves-Santos, N.; Freitas-Costa, N.; Batalha, M.; Yonemitsu, C.; Manivong, N.; Furst, A.; Bode, L.; et al. Human Milk Oligosaccharide Profile Variation Throughout Postpartum in Healthy Women in a Brazilian Cohort. Nutrients 2020, 12, 790. [Google Scholar] [CrossRef]
- Elwakiel, M.; Hageman, J.A.; Wang, W.; Szeto, I.M.; van Goudoever, J.B.; Hettinga, K.A.; Schols, H.A. Human Milk Oligosaccharides in Colostrum and Mature Milk of Chinese Mothers: Lewis Positive Secretor Subgroups. J. Agric. Food Chem. 2018, 66, 7036–7043. [Google Scholar] [CrossRef]
- Hahn, W.H.; Song, J.H.; Song, S.; Kang, N.M. Do Gender and Birth Height of Infant Affect Calorie of Human Milk? An Association Study between Human Milk Macronutrient and Various Birth Factors. J. Matern. Fetal Neonatal Med. 2017, 30, 1608–1612. [Google Scholar] [CrossRef]
- Hosseini, M.; Valizadeh, E.; Hosseini, N.; Khatibshahidi, S.; Raeisi, S. The Role of Infant Sex on Human Milk Composition. Breastfeed. Med. 2020, 15, 341–346. [Google Scholar] [CrossRef]
- Powe, C.E.; Knott, C.D.; Conklin-Brittain, N. Infant Sex Predicts Breast Milk Energy Content. Am. J. Hum. Biol. 2010, 22, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Fields, D.A.; George, B.; Williams, M.; Whitaker, K.; Allison, D.B.; Teague, A.; Demerath, E.W. Associations between Human Breast Milk Hormones and Adipocytokines and Infant Growth and Body Composition in the First 6 Months of Life. Pediatr. Obes. 2017, 12, 78–85. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renwick, S.; Rahimi, K.; Sejane, K.; Bertrand, K.; Chambers, C.; Bode, L. Consistency and Variability of the Human Milk Oligosaccharide Profile in Repeat Pregnancies. Nutrients 2024, 16, 643. https://doi.org/10.3390/nu16050643
Renwick S, Rahimi K, Sejane K, Bertrand K, Chambers C, Bode L. Consistency and Variability of the Human Milk Oligosaccharide Profile in Repeat Pregnancies. Nutrients. 2024; 16(5):643. https://doi.org/10.3390/nu16050643
Chicago/Turabian StyleRenwick, Simone, Kamand Rahimi, Kristija Sejane, Kerri Bertrand, Christina Chambers, and Lars Bode. 2024. "Consistency and Variability of the Human Milk Oligosaccharide Profile in Repeat Pregnancies" Nutrients 16, no. 5: 643. https://doi.org/10.3390/nu16050643
APA StyleRenwick, S., Rahimi, K., Sejane, K., Bertrand, K., Chambers, C., & Bode, L. (2024). Consistency and Variability of the Human Milk Oligosaccharide Profile in Repeat Pregnancies. Nutrients, 16(5), 643. https://doi.org/10.3390/nu16050643




