Genetic Variants Linked to Myocardial Infarction in Individuals with Non-Alcoholic Fatty Liver Disease and Their Potential Interaction with Dietary Patterns
Abstract
1. Introduction
2. Materials and Methods
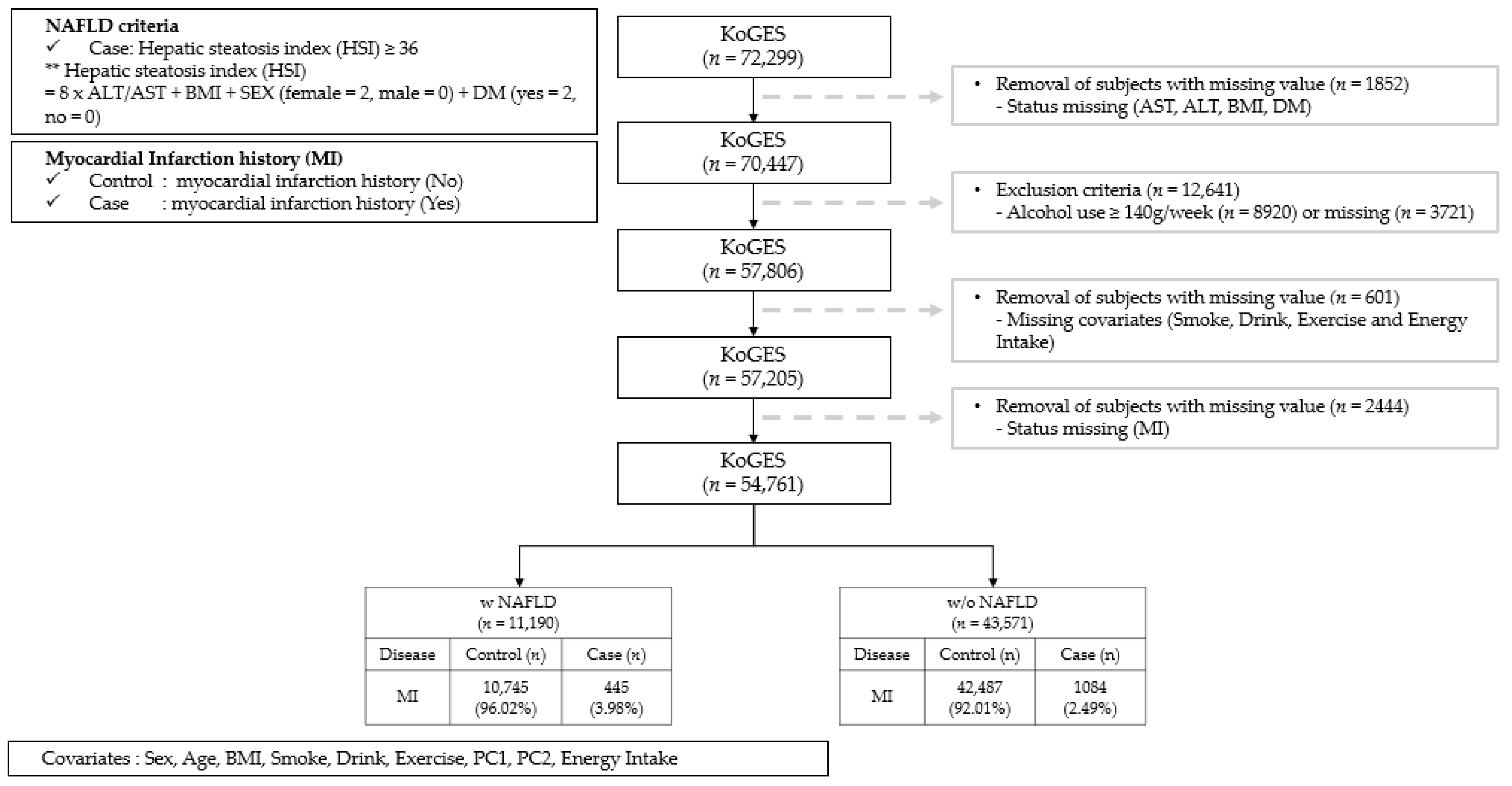
2.1. Study Design and Population
2.2. Definitions of NAFLD and MI
2.3. Definitions of Nutrition Intake
2.4. Genotyping
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Association between Genetic Polymorphisms and MI Based on NAFLD
3.3. In Silico Annotation of Linked Genes and Functional Relevance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huh, Y.; Cho, Y.J.; Nam, G.E. Recent epidemiology and risk factors of nonalcoholic fatty liver disease. J. Obes. Metab. Syndr. 2022, 31, 17. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. Ectopic fat, insulin resistance, and nonalcoholic fatty liver disease: Implications for cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1155–1161. [Google Scholar] [CrossRef]
- Jung, M.-H.; Ihm, S.H. Obesity-related hypertension and chronic kidney disease: From evaluation to management. Kidney Res. Clin. Pract. 2023, 42, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-J.; Jung, M.-H.; Jeong, S.-H.; Hong, Y.-P.; Kim, Y.I.; An, S.J. The association between nonalcoholic fatty liver disease and stroke: Results from the korean genome and epidemiology study (koges). Int. J. Environ. Res. Public Health 2020, 17, 9568. [Google Scholar] [CrossRef]
- Sinn, D.H.; Kang, D.; Chang, Y.; Ryu, S.; Cho, S.J.; Paik, S.W.; Song, Y.B.; Pastor-Barriuso, R.; Guallar, E.; Cho, J.; et al. Non-alcoholic fatty liver disease and the incidence of myocardial infarction: A cohort study. J. Gastroenterol. Hepatol. 2020, 35, 833–839. [Google Scholar] [CrossRef]
- Söderberg, C.; Stål, P.; Askling, J.; Glaumann, H.; Lindberg, G.; Marmur, J.; Hultcrantz, R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 2010, 51, 595–602. [Google Scholar] [CrossRef]
- Sung, K.-C.; Wild, S.H.; Kwag, H.J.; Byrne, C.D. Fatty liver, insulin resistance, and features of metabolic syndrome: Relationships with coronary artery calcium in 10,153 people. Diabetes Care 2012, 35, 2359–2364. [Google Scholar] [CrossRef] [PubMed]
- Abosheaishaa, H.; Hussein, M.; Ghallab, M.; Abdelhamid, M.; Balassiano, N.; Ahammed, M.R.; Baig, M.A.; Khan, J.; Elshair, M.; Soliman, M.Y.; et al. Association between non-alcoholic fatty liver disease and coronary artery disease outcomes: A systematic review and meta-analysis. Diabetes Metab. Syndr. Clin. Res. Rev. 2024, 18, 102938. [Google Scholar] [CrossRef]
- Cazac, G.-D.; Lăcătușu, C.-M.; Mihai, C.; Grigorescu, E.-D.; Onofriescu, A.; Mihai, B.-M. New insights into non-alcoholic fatty liver disease and coronary artery disease: The liver-heart axis. Life 2022, 12, 1189. [Google Scholar] [CrossRef]
- Bisaccia, G.; Ricci, F.; Khanji, M.Y.; Sorella, A.; Melchiorre, E.; Iannetti, G.; Galanti, K.; Mantini, C.; Pizzi, A.D.; Tana, C.; et al. Cardiovascular morbidity and mortality related to non-alcoholic fatty liver disease: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2023, 48, 101643. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, H.; Zhang, X.J.; Cai, J.; Li, H. NAFLD: An emerging causal factor for cardiovascular disease. Physiology 2023, 38, 255–265. [Google Scholar] [CrossRef]
- Bali, A.D.; Rosenzveig, A.; Frishman, W.H.; Aronow, W.S. Nonalcoholic fatty liver disease and cardiovascular disease: Causation or association. Cardiol. Rev. 2023, e000537. [Google Scholar] [CrossRef]
- McPherson, R.; Tybjaerg-Hansen, A. Genetics of coronary artery disease. Circ. Res. 2016, 118, 564–578. [Google Scholar] [CrossRef]
- Samani, N.J.; Erdmann, J.; Hall, A.S.; Hengstenberg, C.; Mangino, M.; Mayer, B.; Dixon, R.J.; Meitinger, T.; Braund, P.; Wichmann, H.E.; et al. Genomewide association analysis of coronary artery disease. N. Engl. J. Med. 2007, 357, 443–453. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.-G.; KoGES Group. Cohort profile: The Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- Bessembinders, K.; Wielders, J.; van de Wiel, A. Severe hypertriglyceridemia influenced by alcohol (SHIBA). Alcohol Alcohol. 2011, 46, 113–116. [Google Scholar] [CrossRef]
- Chen, J.; Mao, X.; Deng, M.; Luo, G. Validation of nonalcoholic fatty liver disease (NAFLD) related steatosis indices in metabolic associated fatty liver disease (MAFLD) and comparison of the diagnostic accuracy between NAFLD and MAFLD. Eur. J. Gastroenterol. Hepatol. 2023, 35, 394. [Google Scholar] [CrossRef]
- Kim, S.; Chung, J.; Ahn, S.; Joung, H. Development of semi-quantitative food frequency questionnaire for obese Korean adults. Proc. Nutr. Soc. 2022, 81, E214. [Google Scholar] [CrossRef]
- Oh, S.W. Current status of nutrient intake in korea: Focused on macronutrients. J. Korean Med. Assoc. 2022, 65, 801–809. [Google Scholar] [CrossRef]
- Moon, S.; Kim, Y.J.; Han, S.; Hwang, M.Y.; Shin, D.M.; Park, M.Y.; Lu, Y.; Yoon, K.; Jang, H.-M.; Kim, Y.K.; et al. The Korea biobank array: Design and identification of coding variants associated with blood biochemical traits. Sci. Rep. 2019, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Zhao, Y.; Qian, C.; Zhang, L.; Zhang, R.; Gou, J.; Liu, J.; Liu, L.; Chen, F. Statistical analysis for genome-wide association study. J. Biomed. Res. 2015, 29, 285–297. [Google Scholar] [PubMed]
- Malik, A.R.; Willnow, T.E. Vps10p domain receptors: Sorting out brain health and disease. Trends Neurosci. 2020, 43, 870–885. [Google Scholar] [CrossRef]
- Kalnytska, O.; Qvist, P.; Kunz, S.; Conrad, T.; Willnow, T.E.; Schmidt, V. Sorting receptor sorcs2 facilitates a protective stress response in pancreatic islets. iScience 2023, 27, 108725. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Shackel, N.; Gorrell, M.; McLennan, S.; Twigg, S. Diabetes and nonalcoholic fatty liver disease: A pathogenic duo. Endocr. Rev. 2013, 34, 84–129. [Google Scholar] [CrossRef]
- Møller, P.L.; Rohde, P.D.; Winther, S.; Breining, P.; Nissen, L.; Nykjaer, A.; Bøttcher, M.; Nyegaard, M.; Kjolby, M. Sortilin as a biomarker for cardiovascular disease revisited. Front. Cardiovasc. Med. 2021, 8, 652584. [Google Scholar] [CrossRef]
- Barclay, J.L.; Kerr, L.M.; Arthur, L.; Rowland, J.E.; Nelson, C.N.; Ishikawa, M.; d’Aniello, E.M.; White, M.; Noakes, P.G.; Waters, M.J. In vivo targeting of the growth hormone receptor (GHR) box1 sequence demonstrates that the GHR does not signal exclusively through JAK2. Mol. Endocrinol. 2010, 24, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Sos, B.C.; Harris, C.; Nordstrom, S.M.; Tran, J.L.; Balázs, M.; Caplazi, P.; Febbraio, M.; Applegate, M.A.; Wagner, K.-U.; Weiss, E.J. Abrogation of growth hormone secretion rescues fatty liver in mice with hepatocyte-specific deletion of JAK2. J. Clin. Investig. 2011, 121, 1412–1423. [Google Scholar] [CrossRef]
- Leopold, J.A. Inhibiting Jak2 ameliorates pulmonary hypertension: Fulfilling the promise of precision medicine. Am. J. Respir. Cell Mol. Biol. 2021, 64, 12–13. [Google Scholar] [CrossRef]
- Kirabo, A.; Kearns, P.N.; Jarajapu, Y.P.; Sasser, J.M.; Oh, S.P.; Grant, M.B.; Kasahara, H.; Cardounel, A.J.; Baylis, C.; Wagner, K.-U.; et al. Vascular smooth muscle Jak2 mediates angiotensin II-induced hypertension via increased levels of reactive oxygen species. Cardiovasc. Res. 2011, 91, 171–179. [Google Scholar] [CrossRef]
- Guilluy, C.; Brégeon, J.; Toumaniantz, G.; Rolli-Derkinderen, M.; Retailleau, K.; Loufrani, L.; Henrion, D.; Scalbert, E.; Bril, A.; Torres, R.M.; et al. The rho exchange factor arhgef1 mediates the effects of angiotensin ii on vascular tone and blood pressure. Nat. Med. 2010, 16, 183–190. [Google Scholar] [CrossRef]
- Rashid, S.; Bibi, N.; Parveen, Z.; Shafique, S. Inhibition of janus kinases by tyrosine phosphorylation inhibitor, tyrphostin ag-490. J. Biomol. Struct. Dyn. 2015, 33, 2368–2379. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.J.; Peloso, G.M.; Yu, H.; Butterworth, A.S.; Wang, X.; Mahajan, A.; Saleheen, D.; Emdin, C.; Alam, D.; Alves, A.C.; et al. Exome-wide association study of plasma lipids in >300,000 individuals. Nat. Genet. 2017, 49, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Pipal, K.V.; Mamtani, M.; Patel, A.A.; Jaiswal, S.G.; Jaisinghani, M.T.; Kulkarni, H. Susceptibility loci for type 2 diabetes in the ethnically endogamous Indian Sindhi population: A pooled blood genome-wide association study. Genes 2022, 13, 1298. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total | w NAFLD | w/o NAFLD | p |
|---|---|---|---|---|
| n | 54,761 | 11,190 | 43,571 | |
| Female (n, %) | 40,582 (74.11) | 8135 (72.7) | 32,447 (74.47) | |
| Age (mean ± sd) | 54.05 ± 8.26 | 54.54 ± 8.0 | 53.92 ± 8.32 | <0.001 |
| Alcohol Intake (g/week) | 13.48 ± 27.09 | 12.94 ± 27.0 | 13.62 ± 27.11 | 0.017 |
| Myocardial Infarction History (n, %) | 53,232 (97.21)/1529 (2.79) | 10,745 (96.02)/445 (3.98) | 42,487 (97.51)/1084 (2.49) | <0.001 |
| Anthropometric traits | ||||
| Body mass index (kg/m2) | 23.89 ± 2.92 | 27.43 ± 2.59 | 22.98 ± 2.23 | <0.001 |
| Waist circumference (cm) | 80.38 ± 8.61 | 88.52 ± 7.43 | 78.29 ± 7.59 | <0.001 |
| Systolic blood pressure (mmHg) | 121.46 ± 15.31 | 126.37 ± 15.2 | 120.19 ± 15.08 | <0.001 |
| Diastolic blood pressure (mmHg) | 75.23 ± 9.88 | 78.33 ± 9.82 | 74.44 ± 9.73 | <0.001 |
| Biochemical traits | ||||
| Fasting plasma glucose (mg/dl) | 94.21 ± 19.01 | 102.42 ± 26.46 | 92.11 ± 15.9 | <0.001 |
| HbA1c | 5.71 ± 0.73 | 6.09 ± 1.03 | 5.62 ± 0.6 | <0.001 |
| hs-CRP | 0.25 ± 1.22 | 0.35 ± 1.33 | 0.22 ± 1.19 | <0.001 |
| INSUL_FBS | 7.74 ± 4.86 | 9.44 ± 4.68 | 7.15 ± 4.78 | <0.001 |
| HOMA-IR | 1.3 ± 1.22 | 1.81 ± 1.41 | 1.12 ± 1.1 | <0.001 |
| Total cholesterol (mg/dl) | 198.06 ± 35.65 | 202.59 ± 38.24 | 196.89 ± 34.86 | <0.001 |
| HDL cholesterol (mg/dl) | 53.11 ± 13.09 | 48.5 ± 11.31 | 54.29 ± 13.25 | <0.001 |
| Triglyceride (mg/dl) | 123.25 ± 80.29 | 157.06 ± 101.67 | 114.56 ± 71.25 | <0.001 |
| r-glutamyltransferase | 25.5 ± 27.01 | 36.72 ± 35.5 | 22.63 ± 23.52 | <0.001 |
| AST | 23.52 ± 22.61 | 27.48 ± 45.23 | 22.51 ± 10.59 | <0.001 |
| ALT | 21.89 ± 22.82 | 34.75 ± 43.1 | 18.58 ± 11.15 | <0.001 |
| ALP | 163.29 ± 106.68 | 173.78 ± 106.66 | 160.73 ± 106.53 | <0.001 |
| Albumin | 4.56 ± 0.47 | 4.55 ± 0.54 | 4.56 ± 0.45 | 0.020 |
| Blood Urea Nitrogen | 14.46 ± 4.03 | 14.8 ± 4.04 | 14.37 ± 4.03 | <0.001 |
| Creatinine | 0.8 ± 0.2 | 0.81 ± 0.21 | 0.8 ± 0.2 | <0.001 |
| Uric Acid | 4.55 ± 1.2 | 4.94 ± 1.26 | 4.46 ± 1.17 | <0.001 |
| Disease History | ||||
| Hypertension (n, %) | 10,663 (19.47) | 3579 (31.98) | 7084 (16.26) | <0.001 |
| Type 2 Diabetes (n, %) | 3551 (6.48) | 1823 (16.29) | 1728 (3.97) | <0.001 |
| Dyslipidemia (n, %) | 5387 (9.84) | 1589 (14.2) | 3798 (8.72) | <0.001 |
| Nutrient | ||||
| Energy (kcal) | 1729.34 ± 557.38 | 1756.92 ± 561.31 | 1722.26 ± 556.15 | <0.001 |
| Protein (%) | 13.24 ± 2.52 | 13.25 ± 2.54 | 13.24 ± 2.52 | 0.687 |
| Fat (%) | 13.46 ± 5.35 | 13.27 ± 5.3 | 13.5 ± 5.36 | <0.001 |
| Carbohydrate (%) | 72.28 ± 6.87 | 72.41 ± 6.83 | 72.25 ± 6.88 | 0.032 |
| Calcium (mg) | 442.38 ± 256.28 | 443.49 ± 255.15 | 442.1 ± 256.57 | 0.606 |
| Phosphorus (mg) | 881.69 ± 354.26 | 895.05 ± 350.59 | 878.26 ± 355.12 | <0.001 |
| Iron (mg) | 9.83 ± 4.85 | 9.92 ± 4.83 | 9.8 ± 4.85 | 0.021 |
| Potassium (mg) | 2216.09 ± 1041.95 | 2249.75 ± 1050.08 | 2207.45 ± 1039.69 | <0.001 |
| Vitamin A (R.E) | 468.36 ± 336.24 | 476.29 ± 342.78 | 466.33 ± 334.51 | 0.006 |
| Sodium (mg) | 2443.04 ± 1382.47 | 2535.32 ± 1434.55 | 2419.34 ± 1367.78 | <0.001 |
| Vitamin B1 (mg) | 0.99 ± 0.44 | 1.0 ± 0.44 | 0.98 ± 0.44 | <0.001 |
| Vitamin B2 (mg) | 0.88 ± 0.44 | 0.89 ± 0.44 | 0.88 ± 0.44 | 0.005 |
| Niacin (mg) | 14.13 ± 6.04 | 14.35 ± 5.92 | 14.07 ± 6.07 | <0.001 |
| Vitamin C (mg) | 106.98 ± 70.01 | 108.21 ± 71.45 | 106.66 ± 69.63 | 0.041 |
| Zinc (μg) | 7.79 ± 3.57 | 7.89 ± 3.44 | 7.76 ± 3.61 | <0.001 |
| Vitamin B6 (mg) | 1.56 ± 0.68 | 1.59 ± 0.68 | 1.56 ± 0.68 | <0.001 |
| Folate (μg) | 215.48 ± 119.14 | 217.69 ± 120.41 | 214.91 ± 118.81 | 0.029 |
| Fiber (g) | 5.75 ± 2.88 | 5.84 ± 2.94 | 5.73 ± 2.86 | <0.001 |
| Vitamin E (mg) | 8.02 ± 4.47 | 8.13 ± 4.57 | 7.99 ± 4.45 | <0.001 |
| Cholesterol (mg) | 163.12 ± 123.01 | 161.78 ± 118.25 | 163.46 ± 124.2 | 0.184 |
| Lifestyle | ||||
| Drinking status: Never/Quit/Current (n, %) | 34,071 (62.22)/78 (0.14)/20,612 (37.64) | 7198 (64.33)/26 (0.23)/3966 (35.44) | 26,873 (61.68)/52 (0.12)/16,646 (38.2) | <0.001 |
| Smoking status: Never/Quit/Current (n, %) | 44,283 (80.87)/6123 (11.18)/4355 (7.95) | 8888 (79.43)/1276 (11.4)/1026 (9.17) | 35,395 (81.24)/4847 (11.12)/3329 (7.64) | <0.001 |
| Exercise status: No/Yes (n, %) | 26,984 (49.28)/27,777 (50.72) | 5974 (53.39)/5216 (46.61) | 21,010 (48.22)/22,561 (51.78) | <0.001 |
| Macronutrient Intake | ||||
| Protein (Low/High) | 45,560 (79.64)/11,645 (20.36) | 9364 (79.32)/2441 (20.68) | 36,196 (79.73)/9204 (20.27) | 0.421 |
| Carbohydrate (Low/High) | 7680 (13.43)/49,525 (86.57) | 1573 (13.32)/10,232 (86.68) | 6107 (13.45)/39,293 (86.55) | 0.650 |
| Fat (Low/High) | 50,968 (89.1)/6237 (10.9) | 10,559 (89.45)/1246 (10.55) | 40,409 (89.01)/4991 (10.99) | 0.183 |
| High-Carbohydrate–Low-Protein | 44,163 (87.55) | 9093 (87.47) | 35,070 (87.56) | 0.880 |
| Low-Carbohydrate–High-Protein | 6283 (12.45) | 1302 (12.53) | 4981 (12.44) |
| SNP | CHR | BP | Feature | Gene | ALT | REF | 1KG Phase1 ALT freq. | A1 | Myocardial Infraction History (MI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EAS | EUR | AMR | Total | w NAFLD | Control | |||||||||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |||||||||||
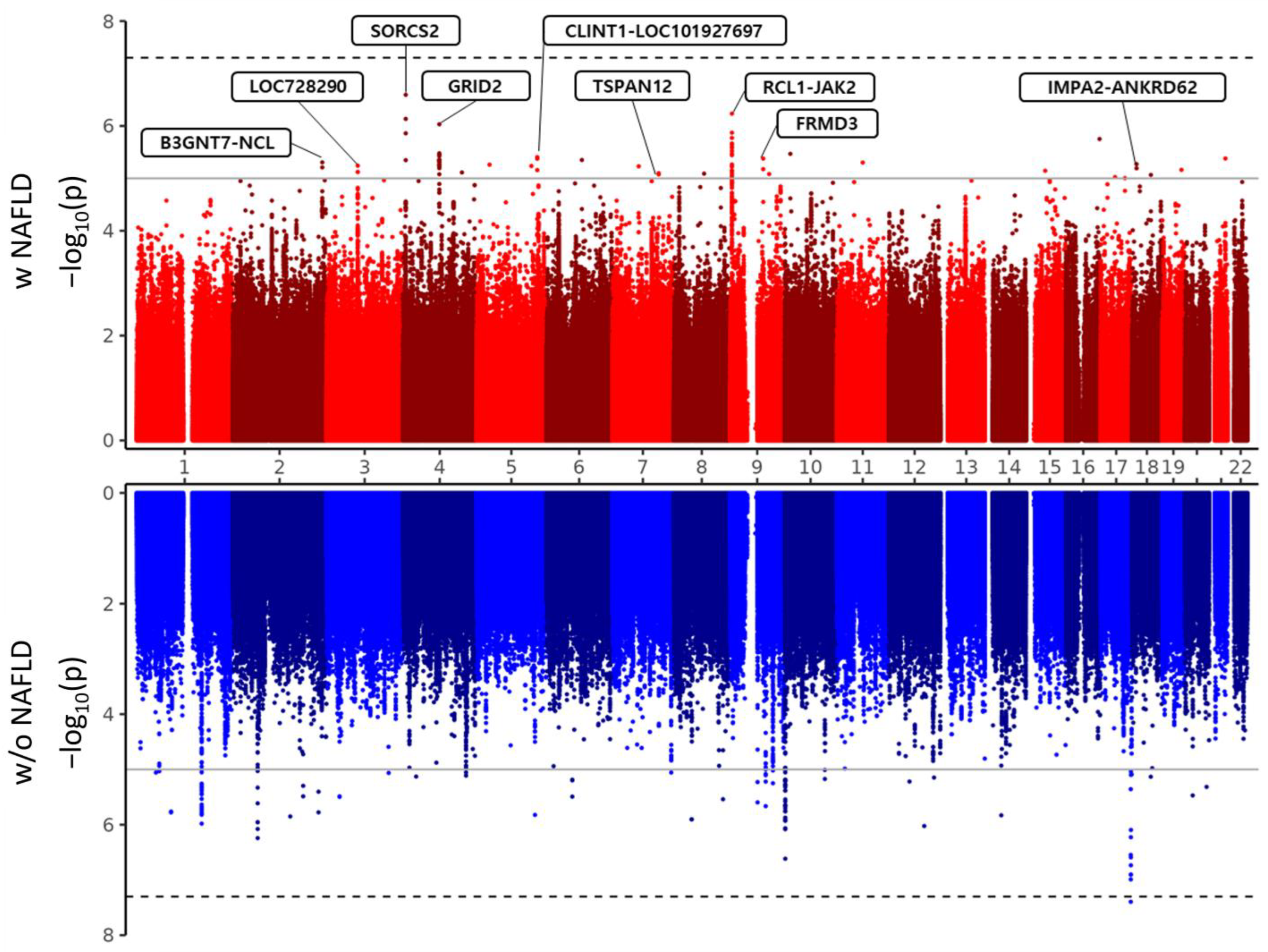
| rs11891202 | 2 | 232276827 | intergenic_region | B3GNT7-NCL | T | C | 0.197 | 0.144 | 0.114 | T | 1.15 (1.06–1.26) | 1.49 × 10−3 | 1.44 (1.23–1.68) | 4.95 × 10−6 | 1.05 (0.94–1.16) | 3.94 × 10−1 |
| rs2278549 | 3 | 81144666 | non_coding_transcript_exon_variant | LOC728290 | G | T | 0.396 | 0.100 | 0.138 | G | 0.93 (0.86–1.01) | 6.56 × 10−2 | 0.70 (0.61–0.82) | 5.74 × 10−6 | 1.04 (0.95–1.14) | 4.43 × 10−1 |
| rs13146480 | 4 | 7483798 | intron_variant | SORCS2 | C | T | 0.044 | 0.577 | 0.484 | T | 1.24 (1.05–1.47) | 1.11 × 10−2 | 1.97 (1.52–2.56) | 2.55 × 10−7 | 0.94 (0.76–1.18) | 6.01 × 10−1 |
| rs17293047 | 4 | 94588105 | intron_variant | GRID2 | A | G | 0.073 | 0.128 | 0.068 | A | 1.37 (1.11–1.68) | 2.72 × 10−3 | 2.20 (1.60–3.01) | 9.30 × 10−7 | 1.03 (0.78–1.36) | 8.24 × 10−1 |
| rs184257317 | 5 | 157464382 | intergenic_region | CLINT1-LOC101927697 | T | C | 0.051 | 0.000 | 0.027 | T | 1.31 (1.09–1.59) | 4.92 × 10−3 | 2.04 (1.51–2.77) | 3.93 × 10−6 | 1.05 (0.82–1.34) | 7.20 × 10−1 |
| rs183081683 | 7 | 120478350 | intron_variant | TSPAN12 | A | C | 0.019 | 0.000 | 0.000 | A | 1.20 (0.97–1.50) | 9.39 × 10−2 | 2.09 (1.51–2.89) | 7.98 × 10−6 | 0.86 (0.64–1.16) | 3.19 × 10−1 |
| rs1887427 | 9 | 4979730 | intergenic_region | RCL1-JAK2 | G | A | 0.162 | 0.259 | 0.193 | G | 1.13 (1.03–1.24) | 1.07 × 10−2 | 1.51 (1.28–1.77) | 5.83 × 10−7 | 0.99 (0.88–1.11) | 8.71 × 10−1 |
| rs146939423 | 9 | 86095179 | intron_variant | FRMD3 | G | A | 0.039 | 0.000 | 0.000 | G | 1.24 (1.01–1.52) | 4.19 × 10−2 | 2.09 (1.53–2.86) | 4.20 × 10−6 | 0.92 (0.70–1.21) | 5.64 × 10−1 |
| rs76662689 | 18 | 12079732 | intergenic_region | IMPA2-ANKRD62 | A | G | 0.034 | 0.000 | 0.001 | A | 1.42 (1.11–1.81) | 5.34 × 10−3 | 2.48 (1.68–3.67) | 5.35 × 10−6 | 1.08 (0.78–1.49) | 6.52 × 10−1 |
| SNP | CHR | BP | Nearby Genes | Description | Expected Function |
|---|---|---|---|---|---|
| rs11891202 | 2 | 232276827 | B3GNT7 | UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 7 | Preventing cells from migrating out of the original tissues and invading surrounding tissues. |
| NCL | nucleolin | It induces chromatin decondensation by binding to histone H1. It is thought to play a role in pre-rRNA transcription and ribosome assembly. | |||
| rs2278549 | 3 | 81144666 | LINC02027 | long intergenic non-protein coding RNA 2027 | Not known |
| rs13146480 | 4 | 7483798 | SORCS2 | sortilin-related VPS10 domain-containing receptor 2 | These genes are strongly expressed in the central nervous system |
| rs17293047 | 4 | 94588105 | GRID2 | glutamate ionotropic receptor delta type subunit 2 | Synapse organization between parallel fibers and Purkinje cells. |
| rs184257317 | 5 | 157464382 | 5q33.3 | Intergenic | |
| rs183081683 | 7 | 120478350 | TSPAN12 | tetraspanin 12 | The regulation of cell development, activation, growth, and motility. |
| rs1887427 | 9 | 4979730 | JAK2 | tyrosine-protein kinase JAK2 | Involved in a specific subset of cytokine receptor signaling pathways. |
| rs146939423 | 9 | 86095179 | FRMD3 | FERM domain containing 3 | Determine the shape of red blood cells and the function of the encoded protein. |
| rs76662689 | 18 | 12079732 | ANKRD62 | ankyrin repeat domain 62 | Not known |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-B.; Choi, J.-E.; Hong, K.-W.; Jung, D.-H. Genetic Variants Linked to Myocardial Infarction in Individuals with Non-Alcoholic Fatty Liver Disease and Their Potential Interaction with Dietary Patterns. Nutrients 2024, 16, 602. https://doi.org/10.3390/nu16050602
Lee S-B, Choi J-E, Hong K-W, Jung D-H. Genetic Variants Linked to Myocardial Infarction in Individuals with Non-Alcoholic Fatty Liver Disease and Their Potential Interaction with Dietary Patterns. Nutrients. 2024; 16(5):602. https://doi.org/10.3390/nu16050602
Chicago/Turabian StyleLee, Sung-Bum, Ja-Eun Choi, Kyung-Won Hong, and Dong-Hyuk Jung. 2024. "Genetic Variants Linked to Myocardial Infarction in Individuals with Non-Alcoholic Fatty Liver Disease and Their Potential Interaction with Dietary Patterns" Nutrients 16, no. 5: 602. https://doi.org/10.3390/nu16050602
APA StyleLee, S.-B., Choi, J.-E., Hong, K.-W., & Jung, D.-H. (2024). Genetic Variants Linked to Myocardial Infarction in Individuals with Non-Alcoholic Fatty Liver Disease and Their Potential Interaction with Dietary Patterns. Nutrients, 16(5), 602. https://doi.org/10.3390/nu16050602






