Abstract
The food insulin index (FII) is a novel algorithm used to determine insulin responses of carbohydrates, proteins, and fats. This scoping review aimed to provide an overview of all scientifically relevant information presented on the application of the FII in the prevention and management of insulin resistance and diabetes. The Arksey and O’Malley framework and the PRISMA Extension for Scoping Reviews 22-item checklist were used to ensure that all areas were covered in the scoping review. Our search identified 394 articles, of which 25 articles were included. Three main themes emerged from the included articles: 1. the association of FII with the development of metabolic syndrome, insulin resistance, and diabetes, 2. the comparison of FII with carbohydrate counting (CC) for the prediction of postprandial insulin response, and 3. the effect of metabolic status on the FII. Studies indicated that the FII can predict postprandial insulin response more accurately than CC, and that a high DII and DIL diet is associated with the development of metabolic syndrome, insulin resistance, and diabetes. The FII could be a valuable tool to use in the prevention and management of T1DM, insulin resistance, and T2DM, but more research is needed in this field.
1. Introduction
Insulin resistance is a disease classified by the resistance of cells to the function of insulin and may lead to the development of type 2 diabetes (T2DM) and heart disease [1,2]. In T2DM, the chronic hypersecretion of insulin as brought about by cellular insulin resistance may lead to beta-cell exhaustion and impaired insulin release, causing impaired glucose tolerance [3]. In type 1 diabetes (T1DM), the beta-cells are unable to produce insulin, and exogenous insulin needs to be administered via injections or an insulin pump, where the miscalculation of dosage and amounts and types of carbohydrates consumed often leads to hyper- or hypoglycaemia [3].
Carbohydrate counting (CC) has been used since the 1920s in dietary management of diabetes to determine carbohydrate amounts prescribed in diabetic diets [4,5]. CC rests on two main principles, namely, carbohydrates are the nutrients responsible for rises in blood glucose levels, and when any carbohydrates (fruit, pasta, rice, etc.) are eaten in certain amounts, blood glucose levels will rise in similar degrees (regardless of the type of carbohydrate eaten) [6]. However, this concept has been challenged by studies, showing that the equivalent amounts of carbohydrates from different carbohydrate sources elicit glycaemic responses that vary over a 4–5-fold range [7,8,9,10,11,12,13,14,15]. The glycaemic index (GI) of carbohydrates, a concept developed in 1980 by Jenkins and colleagues, has been researched by several studies, confirming that not all carbohydrates yield similar glycaemic or insulinemic responses [9,11,12,16,17,18,19,20,21,22]. While the GI proved a valuable tool to predict the postprandial effect of a single (carbohydrate) food on blood glucose levels, it cannot predict the effect a meal has on postprandial blood glucose levels. This led to the development and use of glycaemic load (GL) that takes both the amount of carbohydrate and the GI of the carbohydrate consumed in a meal or day into account [14,22,23]. A low GL diet has proven to decrease the risk of T2DM in many studies [17,22,24], and showed that the GL of a meal or food item will directly affect the amount of postprandial insulin secreted [9,13,15,25,26].
The food insulin index (FII) was first introduced by Holt and colleagues in 1997 when the postprandial insulin responses of thirty-eight isoenergetic foods (including carbohydrate-, protein-, and fat-rich food) were compared [27]. Other than CC, GI, or GL (where only carbohydrates are considered), the FII also takes the postprandial insulinemic effect of protein and fat into account [27]. Procedures for testing the FII are similar to the international standards for GI testing [28]. An insulin score (IS) is used to determine postprandial insulin responses to isoenergetic food (relative to white bread): a higher IS indicates an increased postprandial insulin response compared to a lower IS that indicates a reduced postprandial insulin response [27,29,30]. A glucose score (GS) for each test food can be calculated similar to the GI. The GS differs from GI by using 1000 kJ food portions compared to GI, where 50 g glycaemic carbohydrate portions are tested [7,12,27]. As the FII provides numeric values of IS and GS for each tested food [19], it can be used as a valuable tool during meal planning in the dietary treatment of insulin resistance to identify foods that will produce lower rises in postprandial blood insulin levels compared to other tested food (also consumed in 1000 kJ portions) and to determine portion size suggestions [27].
The dietary management of insulin resistance or diabetes should include limiting postprandial insulin levels. Therefore, there is a vital need to predict insulin secretion (to prevent or manage insulin resistance or T2DM) or insulin demand, in the case of T1DM. The aim of this scoping review was to produce an overview of all scientifically relevant information and research presented on the application of the FII in the management of insulin resistance and diabetes.
2. Materials and Methods
This review followed the Arksey and O’Malley (2005) [31] framework for scoping reviews. The methodology manual by the Joanna Briggs Institute for scoping reviews and recommendations by Levac et al. [32] were also consulted. The PRISMA Extension for Scoping Reviews (PRISMA-SCR) [33] 22-item checklist was used to ensure that all areas were covered in the scoping review. This approach includes the formulation of a research question, the selection of studies addressing the research question, and the summarisation and reporting of the results. The research question guiding the research was as follows: what is known about the FII in the prevention and management of insulin resistance and diabetes? The Population, Concept, Context (PCC) [34] eligibility criteria were as follows:
Population: all children, adolescents, and adults (from any ethnical group), healthy or diabetic, that received FII testing or dietary intervention involving the use of the FII.
Concept: dietary management and intervention specifically involving a calculated FII value of a meal or food item that was ingested, and postprandial glucose and insulin levels were monitored.
Context: data from all countries were included.
2.1. Search Strategy
Peer-reviewed, published literature as well as grey literature was searched with the assistance of a scoping review expert librarian. A comprehensive literature search was performed using the following electronic databases: Academic Search Complete, CINAHL, Cochrane CENTRAL, PUBMED, and SCOPUS. The terms “insulin index (II)”, “food insulin index (FII)”, and “dietary insulin index (DII)” were searched. Reference lists of relevant articles and peer-reviewed literature were hand-searched to identify relevant studies that were not listed in the electronic databases.
2.2. Eligibility Criteria
Original research articles were included if they reported on how the implementation of FII was linked to the prevention and management of either insulin resistance or diabetes. Articles were excluded if research was not performed on humans (animal studies) and if articles were published before 1997. Review papers, editorials, case studies, and opinion pieces were excluded.
2.3. Selection of Sources of Evidence
Records identified by the electronic databases were exported to Endnote 20, and imported into Rayyan (available at: https://rayyan.ai/ (accessed on 14 April 2023)), which was used as an electronic screening tool. Duplicates were removed. Two reviewers (H.S. and E.D.) independently conducted level 1 screening (screening of titles and abstracts) and level 2 screening (full-text screening) on all records for inclusion in the review. Discrepancies were discussed and resolved without the need to consult a third reviewer.
2.4. Charting the Data
A data extraction form was developed to chart data of all articles included in this study. The data that were extracted from each article included: author(s) and year of publication, city, and country where the study was performed, aim, study design, sample size and population, total number of participants, and main findings.
2.5. Summarizing and Reporting the Results
Data extracted from the included articles were thematically analysed and summarised. These themes were as follows: the association of FII with the development of metabolic syndrome, insulin resistance, and diabetes; the comparison of FII with CC for the prediction of postprandial insulin response; and the effect of metabolic status on the FII.
2.6. Quality Appraisal
The mixed methods appraisal tool (MMAT) version 2018 was used to evaluate the quality of included articles [35]. Two reviewers (H.S. and J.M.) independently appraised the methodological quality of the articles according to five categories of research: qualitative research, quantitative randomised controlled trials, quantitative non-randomised studies, quantitative descriptive studies, and mixed methods studies [35]. A score of ≤50% represented low-quality evidence, 50–75% represented average-quality evidence, and 76–100% presented high-quality evidence [35].
3. Results
3.1. Selection of the Included Articles
Our search returned a total of 394 results, which consisted of 393 articles listed on electronic databases and 1 article from grey literature (Figure 1). After duplicates were removed, 205 articles remained, of which 134 articles were excluded because studies were not performed on humans (n = 101), and because FII was linked to other illnesses (not diabetes or insulin resistance) (n = 32). Full-text screening was performed on 72 articles of which 47 were excluded. Data extraction was performed on the remaining 25 articles [27,29,30,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57].
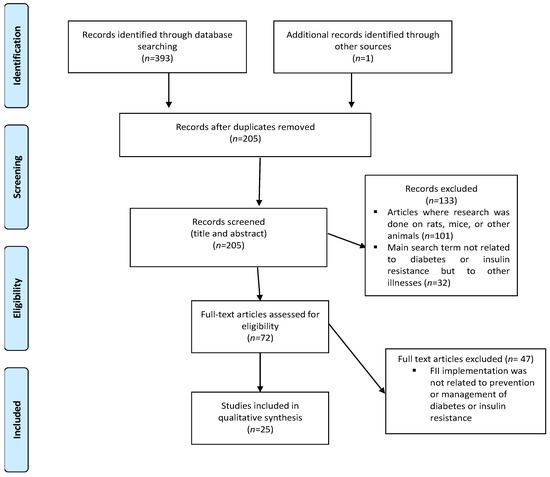
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses-Scoping Review (PRISMA-ScR) flow chart of literature search and selection of included articles.
3.2. Characteristics of the Included Articles
Of the twenty-five included articles, eleven were cross-sectional [29,36,37,38,39,48,50,51,54,55,56], six were crossover studies [30,41,43,46,47,52], four were randomised controlled trials (RCTs) [27,40,44], three were prospective studies [49,53,57], and one was a regression analysis [45]. Most of the studies were conducted in Australia (n = 11) and Iran (n = 10). Two of the articles were from Australia with co-authors from the US. The remainder of the studies were conducted in the UK (n = 2), Turkey (n = 2), Canada (n = 1), Italy (n = 1), and the USA (n = 1). Most of the articles (n = 13) were published between 2019 and 2023. The study populations of the articles included the following: four studies on type 2 diabetic individuals [36,37,38,39], seven studies on type 1 diabetic individuals [40,41,42,43,44,47,52], one study on obese adolescents with insulin resistance [46], eleven on healthy participants [27,29,45,48,49,51,53,54,55,56,57], one on both healthy and type 2 diabetic participants [30], and one study used healthy participants as well as participants with insulin resistance and T2DM [50]. Characteristics and findings of the included studies are summarised in Table 1.

Table 1.
Characteristics and findings of included studies per theme.
3.3. Quality of Evidence
All 25 included articles were appraised with Mixed Methods Appraisal Tool (MMAT) version 2018 for methodological quality [35] (Table S1). One study scored 40%, representing low-quality evidence [52]. Five studies scored 50–75%. representing average-quality evidence [48,53,54,55,57], and 19 studies scored 76–100%, representing high-quality evidence [27,29,30,36,37,38,39,40,41,42,43,44,45,46,47,49,50,51,56].
3.4. Main Findings
All the articles that were included examined the effect of incorporating the FII to predict insulin response or how it is linked to diabetes or insulin resistance development or management. The findings are reported according to the identified main themes (Table 1). Figure 2 includes a visual summary of the findings of the scoping review.
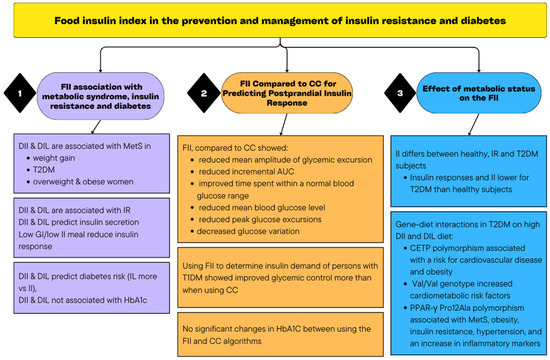
Figure 2.
Summary of the findings of the scoping review.
3.4.1. The Association of FII with the Development of Metabolic Syndrome, Insulin Resistance, and Diabetes
Ten studies examined the association of the FII with the development of insulin resistance, metabolic syndrome, and diabetes [39,46,48,49,51,53,54,55,56,57]. In most of these studies, the FII was used to determine insulin responses to meals by calculating dietary insulin index (DII) and dietary insulin load (DIL) [39,48,49,51,54,55,56].
Four studies in Iran examined whether an increased DII was associated with the development of metabolic syndrome [39,48,49,56]. One study found no significant association between DIL and DII and the risk of developing obesity and metabolic syndrome [48]. In contrast, a prospective study included a diet with a high DIL and DII, and it was significantly associated with higher risk for weight gain, and in women with weight stability, it was positively associated with the risk of developing metabolic syndrome. A higher risk of metabolic syndrome was seen with a diet high in DIL and DII in the group that gained weight and had low activity levels [49]. Sadeghi et al. studied healthy adults and found that higher DII and DIL were positively associated with the development of metabolic syndrome in women, while a moderate DIL was associated with increased risk of metabolic syndrome in men, whereas no significant association between high DII and metabolic syndrome was seen in men [56]. Anjom-Shoae et al., in a study conducted among people with T2DM, found that higher DIL and DII were positively associated with the risk of developing metabolic syndrome [39].
An association between DII and obesity was found in three studies, two in Iran [39,55] and one in Turkey [46]. Anjom-Shoae et al. [39] showed that higher DIL and DII were positively associated with abdominal obesity and that a higher DIl was associated with general obesity. Noori et al. also found a significant association between metabolically healthy overweight and obese women and DII and that inflammatory markers, e.g., IL-1β, may affect this association [55]. In addition, an association between a high II and increased appetite was reported by Caferoglu et al. [46]. In a randomised, single-blind, cross-over study, obese adolescents with insulin resistance received two test meals on different days [46]. The GI and macronutrient value of the meals were matched, but there was a two-fold difference in FII. Serum glucose, insulin, and C-peptide levels as well as appetite scores were recorded for early (0–30 min), late (45–240 min), and total (0–240 min) stages. Results showed a decrease of 25.8% and 27.5%, respectively, in the feeling of hunger in the late and total stages after the low-GI, low-II meal compared to the low-GI, high-II meal (p < 0.05). Postprandial insulin responses were lowered by 56.1% in the early stage, 34.6% in the late stage, and 35.6% in the total stage after a low-GI, low-II meal, compared to those of the low-GI, high-II meal (p < 0.05) [46].
In a 3-year prospective study in Iran on healthy adults, increased DIL was associated with an increased risk of insulin resistance, and increased DII had a borderline positive association with the development of insulin resistance [53].
Three studies examined whether DII and DIL were associated with an increased risk of developing diabetes [51,54,57]. C-peptide concentrations are seen to be a more valid indication of insulin resistance than insulin secretion [51]. Two studies in the USA examined how DII and DIL were associated with C-peptide concentrations [51,54]. Nimptsch et al. found no significant associations between DII and DIL and plasma HbA1C and C-peptide in healthy adults, indicating no significant association between DII and DIL and risk for diabetes [54]. In contrast, Lee et al. found that higher DII and DIL scores were associated with increased 24 h urinary C-peptide concentrations (and insulin secretion) in
Healthy men [51]. In a cohort study in Iran, to investigate the association of II, GI, IL, and GL per day with the risk of developing diabetes among healthy adults, it was found that although all four dietary scores were significantly associated with an increased risk of diabetes, IL and GL per day showed the strongest association (increasing the risk of diabetes by 70 and 84%, respectively). II was associated with a 33% increased risk for developing diabetes, and GI a 28% increased risk [57].
3.4.2. The FII Compared to CC for Predicting Postprandial Insulin Response
Ten articles (nine Australian, one Turkish) examined whether using CC compared to the FII produced a better predicting value for postprandial insulin response [27,29,40,41,42,43,44,45,47,52].
In the first FII testing study, Holt et al. found that GS and IS were significantly correlated for most food; however, food high in protein as well as food high in both fat and refined carbohydrates (bakery products) elicited insulin responses that were much higher than their glucose responses [27]. High-protein food items often produced insulin secretions similar to the amounts of insulin secreted by carbohydrate-rich food and food with similar nutrient values elicited different insulin secretions. For example, food with similar carbohydrate contents produced different insulin responses. Therefore, the authors hypothesised that a meal’s insulinemic effect rather than the carbohydrate content should be used to predict postprandial response [27].
In order to predict the postprandial insulin demand using the FII, some of the studies [29,43,44,52] used the food insulin demand (FID) as prescribed by Bell et al. [43] (FID = FII × kJ per serving/1000) [43]. Prandial insulin dosage was determined using individualised insulin, i.e., FID ratio, which is similar to the insulin/carbohydrate ratio [29,43,44,52].
Two studies examined whether the FII could predict postprandial insulin response better than using CC, GL, or GI [29,45]. In a study by Bao et al. [29], GL, CC, and the FII were used to predict an insulin response of 13 isoenergetic meals consumed by healthy adults. The usage of the FID showed the highest correlation with the observed postprandial insulin responses (p = 0.0016). GL per meal also strongly correlated with the observed insulin response (p = 0.01) (although lesser than FII), while CC was not a significant predictor of the observed insulin responses (p = 0.064). The study also showed that two meals with similar observed insulin responses had markedly different carbohydrate contents (37 g and 63 g, respectively) [29]. Also, a meal with 40 g carbohydrates produced double the insulin response of a meal with a similar carbohydrate content of 37 g [29]. A study where mathematical algorithms were generated to improve the prediction of postprandial insulinemia found that GL per serving, GI, and glycaemic carbohydrate content were the strongest predictors of FII, but that glycaemic carbohydrate only accounted for 47% of the variation. Moreover, the FII could not be calculated by carbohydrate content alone, but all nutrients in the meal as well as their interactions are responsible for the postprandial insulin response [45].
Six studies that used both CC and the FII to determine the insulin demand of people with T1DM all found improved glycaemic control when using the FII (compared to using CC) [40,41,42,43,44,47]. In a study at the University of Sidney, Australia, among T1DM people on insulin pump therapy, the insulin requirements of six protein-containing single foods were determined once by using CC and once by using the FII to calculate an estimated food insulin demand [43]. Mean blood glucose levels at 180 min and mean change in blood glucose levels over 3 h were significantly lower when using the FII algorithm compared to those with CC (p = 0.003 and p = 0.001, respectively). In addition, the time to reach peak blood glucose levels were almost halved, demonstrating that the application of the FII (compared to CC) to single-protein-containing foods improved hyperglycaemia. The maximum amplitude of glycaemic excursion was significantly larger (4.4 ± 0.2 vs. 3.7 ± 0.2 mmol/L, p = 0.02) using the FII, and high rates of mild hypoglycaemia occurred during both treatments [43].
In a RCT, 26 people with T1DM on insulin pump therapy were assigned to either CC or FID counting, to calculate their prandial insulin demand over a 12-week period [44]. Results showed no significant changes in glycated haemoglobin (HbA1c) from baseline to 12 weeks in either group. The mean amplitude of glycaemic excursion, as well as the 120 min incremental area under the curve (AUC) following breakfast, was significantly reduced in the FID counters. The number of hyperglycaemic episodes, as well as time spent within normal blood glucose range, was similar for both groups, but the FID counters showed a reduced risk for hypoglycaemia [44]. Bao et al. [40] showed that when people with T1DM used either CC or FID counting to determine the prandial insulin dosage of breakfast meals, compared to carbohydrate counters, FII counters significantly improved the time spent within the normal blood glucose range, produced a significantly lower incremental AUC, reduced the time to reestablish the fasting blood glucose level, and caused a smaller peak glucose excursion. [40].
In a study in Turkey among adolescents with T1DM, participants consumed two meals with similar energy, macronutrient content, and FII, but with a two-fold difference in GI [47]. Insulin dosage for each meal was calculated once by using CC and once by using FID calculation. Results showed that for the high GI meal, compared to CC, the FII algorithm significantly decreased peak glucose excursion (−57%, p = 0.02) as well as the incremental AUC (−65%, p = 0.02), and the coefficient of glucose variation was decreased by 37% (p = 0.03). No significant difference was seen between using the CC and FII algorithms for the low GI meal, and no significant difference in the occurrence of hypoglycaemia was seen between any of the insulin-dosing algorithms in low- or high-GI meals [47].
Bell et al. used CC compared to the FII algorithm to estimate insulin dosage for six single foods in adults with T1DM and found, compared with CC, the FII algorithm significantly reduced mean blood glucose levels, produced a smaller mean change in blood glucose levels, and a smaller peak change in blood glucose excursion, without causing a significant risk of hypoglycaemia [41]. The same authors compared CC to the FII algorithm to predict insulin dosages for people with T1DM on insulin pump therapy over a 12-week period [42]. They found the FII counters showed a 43% reduction in hypoglycaemia at 12 weeks, while carbohydrate counters showed no change in hypoglycaemia. Both groups showed similar changes in HbA1c and postprandial glucose levels [42].
The only study that showed no significant difference in glucose excursions between CC and FII was a cross-over trial of children and adolescents with T1DM who consumed meals with similar carbohydrate content but with a high-protein or high-fat content using either CC or the FII algorithm to determine prandial insulin dose [52]. However, this study was the only one with a low-quality rating.
3.4.3. The Effect of Metabolic Status on the FII
Two studies investigated whether II can differ among individuals based on their metabolic status [30,50]. In an Australian study, Bell et al. provided two diets matched for macronutrient and fibre content as well as GI, but with a two-fold difference in FII, to healthy and T2DM.adults. Postprandial plasma insulinemia and glycaemia was measured over 8 h. Results showed no difference in postprandial glycemia in either group or between the diets. However, the mean postprandial insulin response (over eight hours) was 53% lower in the healthy subjects on the low-FII diet compared to the high-FII diet, and 41% lower in the T2DM group on the low-FII diet compared to the high-FII diet [30]. In a cross-sectional study in Canada, GI and II were compared in healthy, hyperinsulinemic and T2DM subjects to investigate whether GI and II were dependent of the subjects’ metabolic status. The study found that GI values did not differ significantly between subject groups but that II values were higher in the T2DM group than in the healthy- or hyperinsulinemic groups and that II was inversely associated with insulin sensitivity [50].
Apart from differences in II due to individuals’ metabolic status, gene–diet interactions can further affect the metabolic status of individuals with T2DM, as seen in three Iranian studies [36,37,38]. One study found a significant interaction between cholesteryl ester transfer protein (CETP) polymorphism and DIL and DII and their effect on obesity indices (waist circumference and BMI), lipid profiles (triglycerides, high-density lipoproteins (HDL) and low-density lipoproteins (LDL) to high density lipoprotein ratio, inflammatory markers (interleukin-18, C-reactive protein, and prostaglandin F2-α), and antioxidant markers (total antioxidant capacity and superoxide dis-mutases)) in patients with T2DM. The study suggested that CETP polymorphism may be associated with a risk for cardiovascular disease in patients following an increased DII and DIL diet [36]. In another study, it was found that individuals with T2DM and the Val/Val genotype of the BDNF (brain-derived neurotropic factor) gene were more likely to be at risk of cardiovascular disease, compared to subjects carrying Met-alleles. Individuals with the Met/Val or Met/Met genotypes had lower BMI, serum leptin, and total cholesterol levels, even when they consumed diets higher in the DIL index compared to individuals with the Val/Val genotype. The highest quartile of DIL, showed an increase in waist circumference and LDL/HDL for the Val/Val homozygotes compared with Met-allele carriers. The study recommended that T2DM people with the Val/Val genotype should especially follow a low GI, daily low GL, low DII and DIL diet for protection from reduced insulin sensitivity and cardiometabolic risk factors [37]. A cross-sectional study in Iran among patients with T2DM showed that PPAR-γ Pro12Ala polymorphism was able to increase the effect of DII and DIL and that it is associated with metabolic syndrome, obesity, insulin resistance, hypertension, and an increase in inflammatory markers [38].
4. Discussion
This scoping review was conducted to summarise the evidence available on how the FII can be implemented in the dietary prevention and management of diabetes and insulin resistance. We identified 25 studies published between 1997 and 2023 addressing this topic with three main research themes.
The World Health Organization (WHO) has reported a continued rise in numbers, with a reported 1.5 million deaths caused directly by diabetes in 2019. These alarming figures emphasise the importance of evidence-based intervention in not only the management but also the prevention of insulin resistance and T2DM. As insulin resistance is caused by chronic high concentrations of insulin, intervention should focus on limiting postprandial insulin secretion. Our findings indicate that the majority of the included studies showed an association between an increased DII and DIL and the development of metabolic syndrome (including insulin resistance) as well as weight gain [39,49,53,56], and the development of T2DM [51,57]. Therefore, the FII could potentially be used as a guide to choose food with a lower DII and DIL to lower the risk for the development of insulin resistance and T2DM. More research and guidelines are needed on how the FII can be practically implemented to achieve this.
When the ability of FII to predict postprandial insulin response was compared to CC, FII showed a significant correlation to observed insulin responses while CC was found not to be a significant predictor of observed insulin responses [29]. In addition, studies showed that the FII, compared to CC, reduced the mean amplitude of glycaemic excursion [41,43,44], reduced the incremental AUC [40,44,47], improved time spent within a normal blood glucose range [40], reduced mean blood glucose levels [41], reduced peak glucose excursions [40,41,47], and decreased glucose variation, These findings indicate that, if high GI carbohydrates are consumed as part of a mixed meal, FII should preferably be used instead of CC for better control of blood glucose levels. However, for a low GI meal, studies showed no significant differences between using CC and the FII algorithms, indicating that if the carbohydrate content of a mixed meal has a low GI value, CC could be used and should be effective in predicting the insulin demand of the meal [47]. Although no significant changes were seen in HbA1C levels between using the FII and CC algorithms [42,44], a reduced risk of hypoglycaemia was observed [42,44]. Contrary to that, one study found that the peak glucose excursion was significantly larger for the FII group compared to that of the CC group, and high incidences of mild hypoglycaemia were found in both the FII and CC groups [44]. In contrast, another study found no difference in glucose excursion between FII and CC groups [49], even though the majority of the included studies found the FII to be more beneficial in predicting postprandial insulin levels and/or improving glycemia and insulinemia. However, CC is still considered the gold standard in the calculation of insulin dosages and is compulsory to initiate insulin pump therapy [43,44].
Results from FII testing showed that foods containing similar carbohydrate amounts produced very different insulin scores [27,43]. Also, foods with a high-carbohydrate content can elicit low levels of insulin secretion (this could be due to the difference in their GI values and GL values per serving), and that ingestion of high-protein and high-fat food items can induce insulin responses similar to those of a high-carbohydrate meal [27,29,43]. These results challenge the validity of CC, as CC treats all carbohydrates consumed in similar amounts to elicit equal insulin responses and disregards their GI values and GL values per serving/meal, as well as the effects that protein and fat may have on postprandial insulin responses. As studies have shown that in order to rank food according to their insulin demand, the knowledge of glycaemic response together with nutrient composition should be considered [27,45], and using the FID to calculate insulin demand proved to be a more reliable option [29,43,44], since it takes into account the GI values of the carbohydrate foods, the mean GI value and the GL value of the carbohydrate component of the meal, and the protein and fat content of the meal. However, the FII needs to be expanded to include more foods. Our study highlights the need for studies focusing on evidence using the FII as a tool to calculate insulin demand (and determine exogenous insulin dosage) of mixed meals, especially for individuals with T1DM. This should assist greatly in the prevention of hypoglycaemia and hyperglycaemia at different times, the reduction and stabilisation of the insulin dosage taken, and the stabilisation of blood glucose levels.
This scoping review identified two other factors that need consideration when implementing the II, namely, metabolic status and gene interactions. Firstly, results showed that the postprandial effect of the II could differ depending on metabolic status. Subjects with T2DM showed lower insulin responses and lower subsequent II values than healthy subjects or subjects with insulin resistance [30,50]. This contradicts studies on GI, which do not differ according to metabolic status [30,50], suggesting a need to adjust II tables to be specific for metabolic status such as healthy and T1DM or T2DM. Secondly, the presence of certain genes was associated with an increased risk for heart disease and metabolic syndrome in patients following an increased DII and DIL [36,37,38]. This indicates that gene testing and a patient’s gene profile could be considered together with their diet, but more research is needed before recommendations can be made for practise.
Strengths and Limitations
The notable strength of this scoping review is the robust methodology used whose implementation was ensured in each step using standards and guidelines from several recognised organisations. The inclusion of grey literature as part of the search strategy also reduced publication bias, by increasing the comprehensiveness of the available evidence reviewed for inclusion in this scoping review.
Included studies were evaluated for their quality of evidence based on their methodological quality using the MMAT, with majority (76%) of included studies representing high-quality evidence. In addition, this scoping review provided a summary of the evidence supporting the role of FII in the prevention and management of insulin resistance and diabetes, thereby identifying gaps and future research needs of selected themes to add to the existing body of knowledge, with the aim of formulating practise guidelines for the use of the FII in diabetes management.
A limitation of this review is that because most articles included in this study are limited to two countries (Australia or Iran), it may limit the representation of results to a wider population. However, this highlights a gap in research that could urge researchers from other countries to contribute research in this field as well.
5. Conclusions
This scoping review indicates that the FII can be used to predict postprandial insulin response and determine insulin dosage for individuals with T1DM more accurately than CC. Increased DII and DIL are linked to the development of insulin resistance and T2DM, and more research is needed to determine how the FII can be implemented practically. Factors such as metabolic status and the presence of specific genes should also be considered in the treatment of metabolic syndrome and diabetes. The FII could ultimately be a valuable tool for use in the dietary prevention and management of T1DM, insulin resistance, and T2DM, but more research is needed in this field.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16050584/s1, Table S1. Quality appraisal of included studies.
Author Contributions
The authors’ responsibilities were as follows. H.S., Z.W. and J.M.: study design and conceptualization; H.S.: first reviewer; E.D.: second reviewer; H.S.: developed the search strategy and conducted the systematic search; H.S.: drafted the original manuscript in close communication with the team; H.S., Z.W., J.M. and E.D.: edited and reviewed original draft; H.S. and J.M.: appraisal of included studies; H.S. and Z.W.: had primary responsibility for final content. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Acknowledgments
We acknowledge librarian Glenda Makate for her contribution to the search strategy.
Conflicts of Interest
Elizabeth Delport is a co-founder of the GI foundation of South Africa. The authors declare no other conflicts of interest.
References
- Jeffery, A. Insulin resistance. Nurs. Stand. R. Coll. Nurs. Great Br. 1987 2003, 17, 47–53. [Google Scholar]
- Krentz, A. Insulin Resistance: A Clinical Handbook; John Wiley & Sons: Chichester, UK, 2002. [Google Scholar]
- LeRoith, D.; Taylor, S.I.; Olefsky, J.M. Diabetes Mellitus: A Fundamental and Clinical Text, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004. [Google Scholar]
- Meade, L.T.; Rushton, W.E. Accuracy of Carbohydrate Counting in Adults. Clin. Diabetes A Publ. Am. Diabetes Assoc. 2016, 34, 142–147. [Google Scholar] [CrossRef]
- Gillespie, S.J.; Kulkarni, K.D.; Daly, A.E. Using carbohydrate counting in diabetes clinical practice. J. Am. Diet. Assoc. 1998, 98, 897–905. [Google Scholar] [CrossRef]
- Warshaw, H.S.; Bolderman, K.M. American Diabetes A. In Practical Carbohydrate Counting: A How-to-Teach Guide for Health Professionals, 2nd ed.; American Diabetes Association: Alexandria, VA, USA, 2008. Available online: http://catdir.loc.gov/catdir/toc/ecip0818/2008020554.html (accessed on 20 September 2023).
- Wolever, T.M.; Jenkins, D.J.; Jenkins, A.L.; Josse, R.G. The glycemic index: Methodology and clinical implications. Am. J. Clin. Nutr. 1991, 54, 846–854. [Google Scholar] [CrossRef]
- Villegas, R.; Liu, S.; Gao, Y.; Yang, G.; Li, H.; Zheng, W.; Shu, X.O. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch. Intern. Med. 2007, 167, 2310–2316. [Google Scholar] [CrossRef]
- Slabber, M.; Barnard, H.C. Effects of a low-insulin-response, energy-restricted diet on weight loss and plasma insulin. Am. J. Clin. Nutr. 1994, 60, 48–53. [Google Scholar] [CrossRef]
- Rizkalla, S.W.; Taghrid, L.; Laromiguiere, M.; Huet, D.; Boillot, J.; Rigoir, A.; Elgrably, F.; Slama, G. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: A randomized controlled trial. Diabetes Care 2004, 27, 1866–1872. [Google Scholar] [CrossRef]
- Liese, A.D.; Schulz, M.; Fang, F.; Wolever, T.M.; D’Agostino, R.B., Jr.; Sparks, K.C.; Mayer-Davis, E.J. Dietary glycemic index and glycemic load, carbohydrate and fiber intake, and measures of insulin sensitivity, secretion, and adiposity in the Insulin Resistance Atherosclerosis Study. Diabetes Care 2005, 28, 2832–2838. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Taylor, R.H.; Barker, H.; Fielden, H.; Baldwin, J.M.; Bowling, A.C.; Newman, H.C.; Jenkins, A.L.; Goff, D.V. Glycemic index of foods: A physiological basis for carbohydrate exchange. Am. J. Clin. Nutr. 1981, 34, 362–366. [Google Scholar] [CrossRef]
- Bao, J.; Atkinson, F.; Petocz, P.; Willett, W.C.; Brand-Miller, J.C. Prediction of postprandial glycemia and insulinemia in lean, young, healthy adults: Glycemic load compared with carbohydrate content alone. Am. J. Clin. Nutr. 2011, 93, 984–996. [Google Scholar] [CrossRef]
- Brand-Miller, J.C.; Thomas, M.; Swan, V.; Ahmad, Z.I.; Petocz, P.; Colagiuri, S. Physiological validation of the concept of glycemic load in lean young adults. J. Nutr. 2003, 133, 2728–2732. [Google Scholar] [CrossRef]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. Dis. 2015, 25, 795–815. [Google Scholar] [CrossRef]
- Steenkamp, G.; Delport, L. The Smart Carb Guide. In The South African GI and GL Guide; The Glycemic Index foundation of SA: Nelspruit, South Africa, 2016. [Google Scholar]
- Salmerón, J.; Ascherio, A.; Rimm, E.B.; Colditz, G.A.; Spiegelman, D.; Jenkins, D.J.; Stampfer, M.J.; Wing, A.L.; Willett, W.C. Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 1997, 20, 545–550. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Thorne, M.J.; Jenkins, A.L.; Wong, G.S.; Josse, R.G.; Csima, A. The relationship between glycemic response, digestibility, and factors influencing the dietary habits of diabetics. Am. J. Clin. Nutr. 1984, 40, 1175–1191. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Jenkins, A.L. Starchy foods and glycemic index. Diabetes Care 1988, 11, 149–159. [Google Scholar] [CrossRef]
- Crapo, P.A.; Kolterman, O.G.; Waldeck, N.; Reaven, G.M.; Olefsky, J.M. Postprandial hormonal responses to different types of complex carbohydrate in individuals with impaired glucose tolerance. Am. J. Clin. Nutr. 1980, 33, 1723–1728. [Google Scholar] [CrossRef]
- Crapo, P.A.; Insel, J.; Sperling, M.; Kolterman, O.G. Comparison of serum glucose, insulin, and glucagon responses to different types of complex carbohydrate in noninsulin-dependent diabetic patients. Am. J. Clin. Nutr. 1981, 34, 184–190. [Google Scholar] [CrossRef]
- Salmeron, J. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA J. Am. Med. Assoc. 1997, 277, 472–477. [Google Scholar] [CrossRef]
- Solomon, T.P.; Haus, J.M.; Kelly, K.R.; Rocco, M.; Kashyap, S.R.; Kirwan, J.P. Improved pancreatic beta-cell function in type 2 diabetic patients after lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care 2010, 33, 1561–1566. [Google Scholar] [CrossRef]
- Barclay, A.; Petocz, P.; McMillan-Price, J.; Flood, V.; Prvan, T.; Mitchell, P.; Brand-Miller, J.; Wellington New, Z. CD1-1 Glycemic index, glycemic load and diabetes risk: A meta-analysis. Diabetes Res. Clin. Pract. 2008, 79, S30–S31. [Google Scholar] [CrossRef]
- Livesey, G.; Taylor, R.; Livesey, H.; Liu, S. Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2013, 97, 584–596. [Google Scholar] [CrossRef]
- Brownley, K.A.; Heymen, S.; Hinderliter, A.L.; Galanko, J.; Macintosh, B. Low-glycemic load decreases postprandial insulin and glucose and increases postprandial ghrelin in white but not black women. J. Nutr. 2012, 142, 1240–1245. [Google Scholar] [CrossRef]
- Holt, S.H.; Miller, J.C.; Petocz, P. An insulin index of foods: The insulin demand generated by 1000-kJ portions of common foods. Am. J. Clin. Nutr. 1997, 66, 1264–1276. [Google Scholar] [CrossRef]
- ISO. Determination of the Glycaemic Index (GI) and Recommendation for Food Classification. Available online: https://www.iso.org/standard/43633.html (accessed on 6 November 2023).
- Bao, J.; de Jong, V.; Atkinson, F.; Petocz, P.; Brand-Miller, J.C. Food insulin index: Physiologic basis for predicting insulin demand evoked by composite meals. Am. J. Clin. Nutr. 2009, 90, 986–992. [Google Scholar] [CrossRef]
- Bell, K.J.; Bao, J.; Petocz, P.; Colagiuri, S.; Brand-Miller, J.C. Validation of the food insulin index in lean, young, healthy individuals, and type 2 diabetes in the context of mixed meals: An acute randomized crossover trial. Am. J. Clin. Nutr. 2015, 102, 801–806. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Danielle, L.; Heather, C.; Kelly, K.O.B. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Peters, M.; Godfrey, C.; McInerney, P.; Soares, C.; Khalil, H.; Parker, D. Methodology for JBI Scoping Reviews. In The Joanna Briggs Institute Reviewers’ Manual 2015; The Joanna Briggs Institute: Adelaide, South Australia, 2015; pp. 1–24. [Google Scholar]
- Hong, Q.N.; Fàbregues, S.; Bartlett, G.; Boardman, F.; Cargo, M.; Dagenais, P.; Gagnon, M.-P.; Griffiths, F.; Nicolau, B.; O’Cathain, A.; et al. The Mixed Methods Appraisal Tool (MMAT) Version 2018 for Information Professionals and Researchers. Educ. Inf. 2018, 34, 285–291. [Google Scholar] [CrossRef]
- Abaj, F.; Rafiee, M.; Koohdani, F. Interaction between CETP polymorphism and dietary insulin index and load in relation to cardiovascular risk factors in diabetic adults. Sci. Rep. 2021, 11, 15906. [Google Scholar] [CrossRef]
- Abaj, F.; Rafiee, M.; Koohdani, F. Interactions of dietary insulin index and dietary insulin load with brain-derived neurotrophic factor (BDNF) Val66Met polymorphism in relation to cardiometabolic markers in Iranian diabetic patients: A cross-sectional study. Br. J. Nutr. 2022, 128, 785–792. [Google Scholar] [CrossRef]
- Abaj, F.; Rafiee, M.; Koohdani, F. A personalised diet approach study: Interaction between PPAR-γ Pro12Ala and dietary insulin indices on metabolic markers in diabetic patients. J. Hum. Nutr. Diet. 2022, 35, 663–674. [Google Scholar] [CrossRef]
- Anjom-Shoae, J.; Namazi, N.; Ayati, M.H.; Darbandi, M.; Najafi, F.; Pasdar, Y. Dietary insulin index and load in relation to cardiometabolic risk factors in patients with type 2 diabetes mellitus: A cross-sectional study on the RaNCD cohort study. Nutrition 2023, 105, 111830. [Google Scholar] [CrossRef]
- Bao, J.; Gilbertson, H.R.; Gray, R.; Munns, D.; Howard, G.; Petocz, P.; Colagiuri, S.; Brand-Miller, J.C. Improving the estimation of mealtime insulin dose in adults with type 1 diabetes: The Normal Insulin Demand for Dose Adjustment (NIDDA) study. Diabetes Care 2011, 34, 2146–2151. [Google Scholar] [CrossRef]
- Bell, K.; Gray, R.; Munns, D.; Howard, G.; Petocz, P.; Brand-Miller, J.C.; Colagiuri, S. Estimating prandial insulin dose for single foods using the food insulin index improves post-prandial glycemic control in type 1 diabetes: A randomized controlled trial. Diabetes 2013, 62, A42. [Google Scholar] [CrossRef][Green Version]
- Bell, K.J.; Gray, R.; Munns, D.; Howard, G.; Colagiuri, S.; Brand-Miller, J.C. Food Insulin Index (FII) vs. traditional carbohydrate counting for glycemic control in adults with type 1 diabetes: A 3-month pilot study. Diabetes 2014, 63, A189. [Google Scholar] [CrossRef][Green Version]
- Bell, K.J.; Gray, R.; Munns, D.; Petocz, P.; Howard, G.; Colagiuri, S.; Brand-Miller, J.C. Estimating insulin demand for protein-containing foods using the food insulin index. Eur. J. Clin. Nutr. 2014, 68, 1055–1059. [Google Scholar] [CrossRef]
- Bell, K.J.; Gray, R.; Munns, D.; Petocz, P.; Steil, G.; Howard, G.; Colagiuri, S.; Brand-Miller, J.C. Clinical Application of the Food Insulin Index for Mealtime Insulin Dosing in Adults with Type 1 Diabetes: A Randomized Controlled Trial. Diabetes Technol. Ther. 2016, 18, 218–225. [Google Scholar] [CrossRef]
- Bell, K.J.; Petocz, P.; Colagiuri, S.; Brand-Miller, J.C. Algorithms to Improve the Prediction of Postprandial Insulinaemia in Response to Common Foods. Nutrients 2016, 8, 210. [Google Scholar] [CrossRef]
- Caferoglu, Z.; Hatipoglu, N.; Gokmen Ozel, H. Does food insulin index in the context of mixed meals affect postprandial metabolic responses and appetite in obese adolescents with insulin resistance? A randomised cross-over trial. Br. J. Nutr. 2019, 122, 942–950. [Google Scholar] [CrossRef]
- Erdal, B.; Caferoglu, Z.; Hatipoglu, N. The comparison of two mealtime insulin dosing algorithms for high and low glycaemic index meals in adolescents with type 1 diabetes. Diabet. Med. 2021, 38, e14444. [Google Scholar] [CrossRef]
- Ghorbaninejad, P.; Imani, H.; Sheikhhossein, F.; Tijani Jibril, A.; Mohammadpour, S.; Shab-Bidar, S. Higher dietary insulin load and index are not associated with the risk of metabolic syndrome and obesity in Iranian adults. Int. J. Clin. Pract. 2021, 75, e14229. [Google Scholar] [CrossRef]
- Khoshnoudi-Rad, B.; Hosseinpour-Niazi, S.; Javadi, M.; Mirmiran, P.; Azizi, F. Relation of dietary insulin index and dietary insulin load to metabolic syndrome depending on the lifestyle factors: Tehran lipid and glucose study. Diabetol. Metab. Syndr. 2022, 14, 198. [Google Scholar] [CrossRef]
- Lan-Pidhainy, X.; Wolever, T.M.S. Are the glycemic and insulinemic index values of carbohydrate foods similar in healthy control, hyperinsulinemic and type 2 diabetic patients. Eur. J. Clin. Nutr. 2011, 65, 727–734. [Google Scholar] [CrossRef][Green Version]
- Lee, D.H.; Giovannucci, E.L.; Tabung, F.K. Insulin-related dietary indices predict 24-h urinary C-peptide in adult men. Br. J. Nutr. 2020, 1–8. [Google Scholar] [CrossRef]
- Lopez, P.E.; Evans, M.; King, B.R.; Jones, T.W.; Bell, K.; McElduff, P.; Davis, E.A.; Smart, C.E. A randomized comparison of three prandial insulin dosing algorithms for children and adolescents with Type 1 diabetes. Diabet. Med. 2018, 35, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Esfandiari, S.; Bahadoran, Z.; Tohidi, M.; Azizi, F. Dietary insulin load and insulin index are associated with the risk of insulin resistance: A prospective approach in tehran lipid and glucose study. J. Diabetes Metab. Disord. 2015, 15, 23. [Google Scholar] [CrossRef]
- Nimptsch, K.; Brand-Miller, J.C.; Franz, M.; Sampson, L.; Willett, W.C.; Giovannucci, E. Dietary insulin index and insulin load in relation to biomarkers of glycemic control, plasma lipids, and inflammation markers. Am. J. Clin. Nutr. 2011, 94, 182–190. [Google Scholar] [CrossRef]
- Noori, S.; Mirzababaei, A.; Shiraseb, F.; Bagheri, R.; Clark, C.C.T.; Wong, A.; Suzuki, K.; Mirzaei, K. The Association of Inflammatory Markers, IL-1 α and TGF-β, with Dietary Insulin Load and Dietary Insulin Index in Overweight and Obese Women with Healthy and Unhealthy Metabolic Phenotypes: A Cross-Sectional Study. Int. J. Clin. Pract. 2022, 2022, 3407320. [Google Scholar] [CrossRef]
- Sadeghi, O.; Hasani, H.; Mozaffari-Khosravi, H.; Maleki, V.; Lotfi, M.H.; Mirzaei, M. Dietary Insulin Index and Dietary Insulin Load in Relation to Metabolic Syndrome: The Shahedieh Cohort Study. J. Acad. Nutr. Diet. 2020, 120, 1672–1686.e1674. [Google Scholar] [CrossRef]
- Teymoori, F.; Farhadnejad, H.; Moslehi, N.; Mirmiran, P.; Mokhtari, E.; Azizi, F. The association of dietary insulin and glycemic indices with the risk of type 2 diabetes. Clin. Nutr. 2021, 40, 2138–2144. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).