Not Only Metabolic Complications of Childhood Obesity
Abstract
1. Introduction
2. Metabolic Complications of Obesity in Children
2.1. Insulin Resistance
2.2. Diabetes Mellitus
2.3. Dyslipidemia
2.4. Hypertension
2.5. Metabolic Syndrome
2.6. NAFLD/MAFLD
3. Non-Metabolic Complications of Obesity in Children
3.1. Cardiovascular Consequences
3.2. Cardiomyopathy
3.3. Endocrine and Gynecological Consequences
3.3.1. Precocious Puberty
3.3.2. Polycystic Ovary Syndrome
3.3.3. Thyroid Function in Childhood Obesity
3.3.4. Obesity-Induced Hypogonadism
3.4. Other Non-Metabolic Consequences
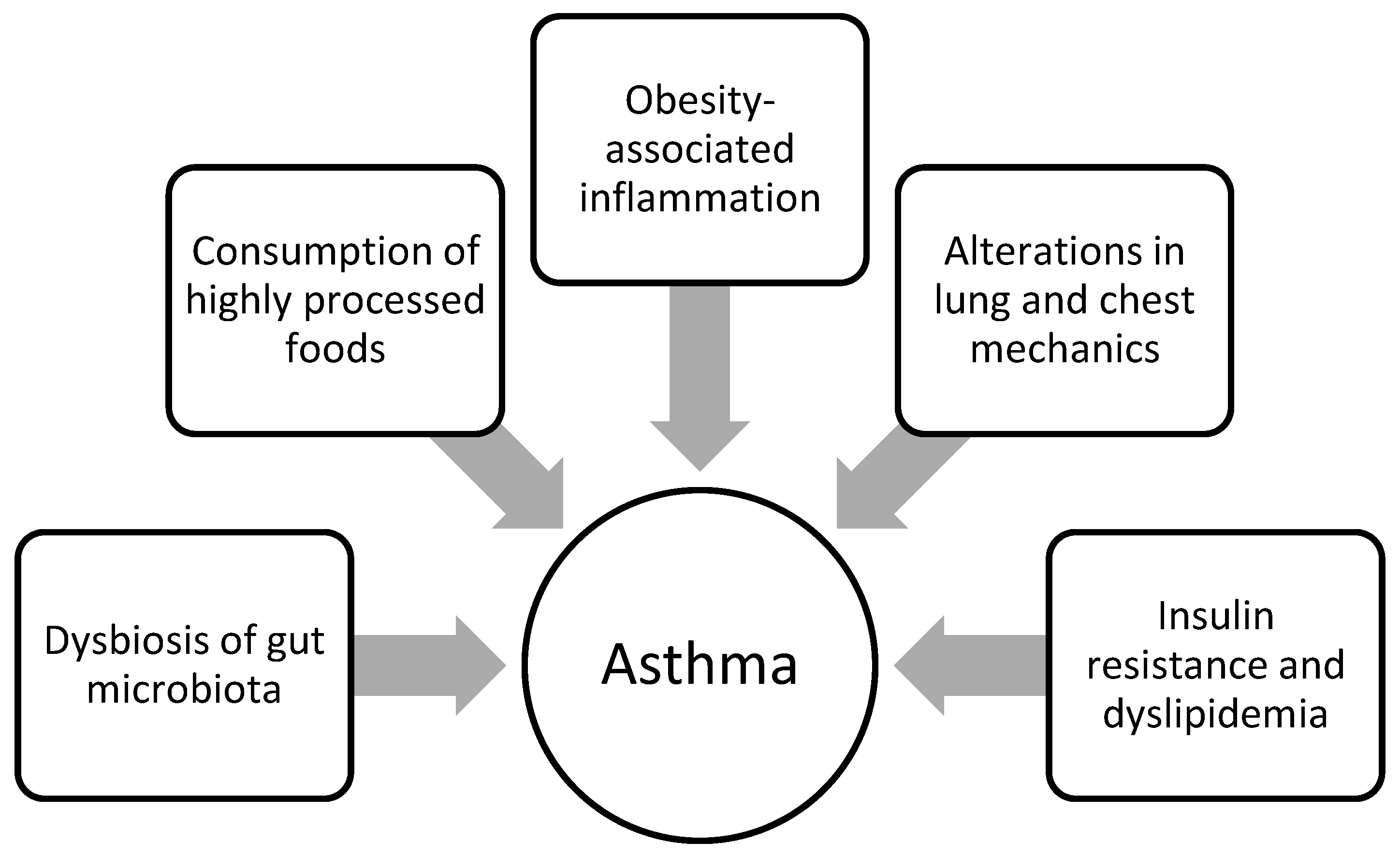
3.4.1. Respiratory Complications
Asthma
Obstructive Sleep Apnea
3.4.2. Other Allergic Diseases
3.4.3. Neurological Complications
Pseudotumor Cerebri
Migraine
3.4.4. Oral Health Disorders
3.4.5. Immunologic Diseases
3.4.6. Renal Consequences
3.4.7. Gastrointestinal Disturbances
Biliary Duct Diseases
Gastroesophageal Reflux
3.4.8. Musculoskeletal Disorders
3.4.9. Dermatologic Disturbances
4. Mental Health Problems in Children with Obesity
4.1. Psychosocial Aspects
4.2. Depression
4.3. Eating Disorders
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Fabin-Czepiel, K.; Pieczyńska-Chapuła, K.; Deja, G. “The obesity pandemic” in the COVID-19 pandemic—New treatment for an old problem. Pediatr. Endocrinol. Diabetes Metab. 2023, 29, 104–111. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report on the Fifth Round of Data Collection, 2018–2020; WHO Regional Office for Europe: Copenhagen, Denmark, 2022. [Google Scholar]
- Davies, S.C.; Department of Health and Social Care, Great Britain. Time to Solve Childhood Obesity: An Independent Report by the Chief Medical Officer, 2019; Department of Health and Social Care, APS Group: Cheshire, UK, 2019. [Google Scholar]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Tagi, V.M.; Giannini, C.; Chiarelli, F. Insulin Resistance in Children. Front. Endocrinol. 2019, 10, 342. [Google Scholar] [CrossRef]
- Redondo, M.J.; Foster, N.C.; Libman, I.M.; Mehta, S.N.; Hathway, J.M.; Bethin, K.E.; Nathan, B.M.; Ecker, M.A.; Shah, A.C.; DuBose, S.N.; et al. Prevalence of cardiovascular risk factors in youth with type 1 diabetes and elevated body mass index. Acta Diabetol. 2016, 53, 271–277. [Google Scholar] [CrossRef]
- Blüher, M. Metabolically Healthy Obesity. Endocr. Rev. 2020, 41, bnaa004. [Google Scholar] [CrossRef]
- Al-Beltagi, M.; Bediwy, A.S.; Saeed, N.K. Insulin-resistance in paediatric age: Its magnitude and implications. World J. Diabetes 2022, 13, 282–307. [Google Scholar] [CrossRef]
- Tagi, V.M.; Samvelyan, S.; Chiarelli, F. An update of the consensus statement on insulin resistance in children 2010. Front. Endocrinol. 2022, 13, 1061524. [Google Scholar] [CrossRef]
- Marcus, C.; Danielsson, P.; Hagman, E. Pediatric obesity-Long-term consequences and effect of weight loss. J. Intern. Med. 2022, 292, 870–891. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zheng, X.; Wen, X.; Zhong, J.; Zhou, Y.; Xu, L. Visceral fat correlates with insulin secretion and sensitivity independent of BMI and subcutaneous fat in Chinese with type 2 diabetes. Front. Endocrinol. 2023, 14, 1144834. [Google Scholar] [CrossRef]
- Xu, H.; Verre, M.C. Type 2 Diabetes Mellitus in Children. Am. Fam. Physician 2018, 98, 590–594. [Google Scholar]
- Chylińska-Frątczak, A.; Michalak, A.; Baranowska-Jaźwiecka, A.; Mianowska, B.; Szadkowska, A. Incidence of hyperglycaemic disorders in children and adolescents with obesity. Pediatr. Endocrinol. Diabetes Metab. 2022, 28, 274–280. [Google Scholar] [CrossRef]
- Sinha, R.; Fisch, G.; Teague, B.; Tamborlane, W.V.; Banyas, B.; Allen, K.; Savoye, M.; Rieger, V.; Taksali, S.; Barbetta, G.; et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N. Engl. J. Med. 2002, 346, 802–810. [Google Scholar] [CrossRef]
- Abbasi, A.; Juszczyk, D.; van Jaarsveld, C.H.M.; Gulliford, M.C. Body Mass Index and Incident Type 1 and Type 2 Diabetes in Children and Young Adults: A Retrospective Cohort Study. J. Endocr. Soc. 2017, 1, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Pinhas-Hamiel, O.; Zeitler, P. Acute and chronic complications of type 2 diabetes mellitus in children and adolescents. Lancet 2007, 369, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Maffeis, C.; Birkebaek, N.H.; Konstantinova, M.; Schwandt, A.; Vazeou, A.; Casteels, K.; Jali, S.; Limbert, C.; Pundziute-Lycka, A.; Toth-Heyn, P.; et al. Prevalence of underweight, overweight, and obesity in children and adolescents with type 1 diabetes: Data from the international SWEET registry. Pediatr. Diabetes 2018, 19, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Abela, A.G.; Fava, S. Why is the Incidence of Type 1 Diabetes Increasing? Curr. Diabetes Rev. 2021, 17, e030521193110. [Google Scholar] [CrossRef] [PubMed]
- Chylińska-Frątczak, A.; Pietrzak, I.; Michalak, A.; Wyka, K.; Szadkowska, A. Autoimmune reaction against pancreatic beta cells in children and adolescents with simple obesity. Front. Endocrinol. 2022, 13, 1061671. [Google Scholar] [CrossRef] [PubMed]
- Ferrara-Cook, C.; Geyer, S.M.; Evans-Molina, C.; Libman, I.M.; Becker, D.J.; Gitelman, S.E.; Redondo, M.J.; Group, T.D.T.S. Excess BMI Accelerates Islet Autoimmunity in Older Children and Adolescents. Diabetes Care 2020, 43, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Kurpiewska, E.; Ciężki, S.; Jamiołkowska-Sztabkowska, M.; Polkowska, A.; Starosz, A.; Grubczak, K.; Moniuszko, M.; Bossowski, A.; Głowińska-Olszewska, B. Excessive BMI is associated with higher C-peptide level at recognition but also with its greater loss in two years clinical observation in children with new onset type 1 diabetes. Front. Immunol. 2023, 14, 1176403. [Google Scholar] [CrossRef]
- Dathan-Stumpf, A.; Vogel, M.; Hiemisch, A.; Thiery, J.; Burkhardt, R.; Kratzsch, J.; Kiess, W. Pediatric reference data of serum lipids and prevalence of dyslipidemia: Results from a population-based cohort in Germany. Clin. Biochem. 2016, 49, 740–749. [Google Scholar] [CrossRef]
- Brzeziński, M.; Metelska, P.; Myśliwiec, M.; Szlagatys-Sidorkiewicz, A. Lipid disorders in children living with overweight and obesity- large cohort study from Poland. Lipids Health Dis. 2020, 19, 47. [Google Scholar] [CrossRef]
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P.; Innes, K.E.; Lilly, C.L. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 683. [Google Scholar] [CrossRef]
- Nielsen, T.R.H.; Lausten-Thomsen, U.; Fonvig, C.E.; Bøjsøe, C.; Pedersen, L.; Bratholm, P.S.; Hansen, T.; Pedersen, O.; Holm, J.C. Dyslipidemia and reference values for fasting plasma lipid concentrations in Danish/North-European White children and adolescents. BMC Pediatr. 2017, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.J.; Choi, M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Zhao, X.; An, X.; Yang, C.; Sun, W.; Ji, H.; Lian, F. The crucial role and mechanism of insulin resistance in metabolic disease. Front. Endocrinol. 2023, 14, 1149239. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Soliman, A.T.; Yasin, M.; Kassem, A. Leptin in pediatrics: A hormone from adipocyte that wheels several functions in children. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. 3), S577–S587. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.; Kułaga, Z. Obesity, metabolic syndrome, and primary hypertension. Pediatr. Nephrol. 2021, 36, 825–837. [Google Scholar] [CrossRef]
- Maximova, K.; O’Loughlin, J.; Paradis, G.; Hanley, J.A.; Lynch, J. Changes in anthropometric characteristics and blood pressure during adolescence. Epidemiology 2010, 21, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, A.R.; Faria, A.P.; Barbaro, N.R.; Gordo, W.M.; Modolo, R.G.; Pinho, C.; Fontana, V.; Moreno, H. Deregulation of adipokines related to target organ damage on resistant hypertension. J. Hum. Hypertens 2014, 28, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Christian Flemming, G.M.; Bussler, S.; Körner, A.; Kiess, W. Definition and early diagnosis of metabolic syndrome in children. J. Pediatr. Endocrinol. Metab. 2020, 33, 821–833. [Google Scholar] [CrossRef]
- Reinehr, T.; de Sousa, G.; Toschke, A.M.; Andler, W. Comparison of metabolic syndrome prevalence using eight different definitions: A critical approach. Arch. Dis. Child. 2007, 92, 1067–1072. [Google Scholar] [CrossRef]
- Pinhas-Hamiel, O.; Levek-Motola, N.; Kaidar, K.; Boyko, V.; Tisch, E.; Mazor-Aronovitch, K.; Graf-Barel, C.; Landau, Z.; Lerner-Geva, L.; Frumkin Ben-David, R. Prevalence of overweight, obesity and metabolic syndrome components in children, adolescents and young adults with type 1 diabetes mellitus. Diabetes Metab. Res. Rev. 2015, 31, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Tropeano, A.; Corica, D.; Li Pomi, A.; Pepe, G.; Morabito, L.A.; Curatola, S.L.; Casto, C.; Aversa, T.; Wasniewska, M. The metabolic syndrome in pediatrics: Do we have a reliable definition? A systematic review. Eur. J. Endocrinol. 2021, 185, 265–278. [Google Scholar] [CrossRef]
- Yki-Järvinen, H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014, 2, 901–910. [Google Scholar] [CrossRef]
- Nehus, E.; Mitsnefes, M. Childhood Obesity and the Metabolic Syndrome. Pediatr. Clin. N. Am. 2019, 66, 31–43. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Valenti, L.; Miele, L.; Feldstein, A.E.; Alkhouri, N. NAFLD in children: New genes, new diagnostic modalities and new drugs. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 517–530. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- Fan, R.; Wang, J.; Du, J. Association between body mass index and fatty liver risk: A dose-response analysis. Sci. Rep. 2018, 8, 15273. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chang, P.F.; Liu, K.; Chang, M.H.; Ni, Y.H. Predictors for incidence and remission of nonalcoholic fatty liver disease in obese children and adolescents. J. Formos. Med. Assoc. 2022, 121 Pt 1, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Loomis, A.K.; Kabadi, S.; Preiss, D.; Hyde, C.; Bonato, V.; St Louis, M.; Desai, J.; Gill, J.M.; Welsh, P.; Waterworth, D.; et al. Body Mass Index and Risk of Nonalcoholic Fatty Liver Disease: Two Electronic Health Record Prospective Studies. J. Clin. Endocrinol. Metab. 2016, 101, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Haczeyni, F.; Bell-Anderson, K.S.; Farrell, G.C. Causes and mechanisms of adipocyte enlargement and adipose expansion. Obes. Rev. 2018, 19, 406–420. [Google Scholar] [CrossRef]
- Grønbæk, H.; Lange, A.; Birkebæk, N.H.; Holland-Fischer, P.; Solvig, J.; Hørlyck, A.; Kristensen, K.; Rittig, S.; Vilstrup, H. Effect of a 10-week weight loss camp on fatty liver disease and insulin sensitivity in obese Danish children. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 223–228. [Google Scholar] [CrossRef]
- Campos, R.M.; de Piano, A.; da Silva, P.L.; Carnier, J.; Sanches, P.L.; Corgosinho, F.C.; Masquio, D.C.; Lazaretti-Castro, M.; Oyama, L.M.; Nascimento, C.M.; et al. The role of pro/anti-inflammatory adipokines on bone metabolism in NAFLD obese adolescents: Effects of long-term interdisciplinary therapy. Endocrine 2012, 42, 146–156. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef]
- Hagström, H.; Stål, P.; Hultcrantz, R.; Hemmingsson, T.; Andreasson, A. Overweight in late adolescence predicts development of severe liver disease later in life: A 39 years follow-up study. J. Hepatol. 2016, 65, 363–368. [Google Scholar] [CrossRef][Green Version]
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A.; Pecorelli, A.; Marinelli, S.; Tiribelli, C.; Bellentani, S.; Group, H.-N.I.S. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016, 63, 827–838. [Google Scholar] [CrossRef]
- Moran-Lev, H.; Cohen, S.; Webb, M.; Yerushalmy-Feler, A.; Amir, A.; Gal, D.L.; Lubetzky, R. Higher BMI predicts liver fibrosis among obese children and adolescents with NAFLD—An interventional pilot study. BMC Pediatr. 2021, 21, 385. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Alkhouri, N.; Vajro, P.; Baumann, U.; Weiss, R.; Socha, P.; Marcus, C.; Lee, W.S.; Kelly, D.; Porta, G.; et al. Defining paediatric metabolic (dysfunction)-associated fatty liver disease: An international expert consensus statement. Lancet Gastroenterol. Hepatol. 2021, 6, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mu, C.; Li, K.; Luo, H.; Liu, Y.; Li, Z. Estimating Global Prevalence of Metabolic Dysfunction-Associated Fatty Liver Disease in Overweight or Obese Children and Adolescents: Systematic Review and Meta-Analysis. Int. J. Public Health 2021, 66, 1604371. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Baik, S.K.; Kim, M.Y. Non-alcoholic fatty liver disease: Definition and subtypes. Clin. Mol. Hepatol. 2023, 29 (Suppl. 1), S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. Am. J. Med. 2007, 120 (Suppl. 1), S12–S18. [Google Scholar] [CrossRef] [PubMed]
- Bennett, N.R.; Ferguson, T.S.; Bennett, F.I.; Tulloch-Reid, M.K.; Younger-Coleman, N.O.; Jackson, M.D.; Samms-Vaughan, M.E.; Wilks, R.J. High-Sensitivity C-Reactive Protein is Related to Central Obesity and the Number of Metabolic Syndrome Components in Jamaican Young Adults. Front. Cardiovasc. Med. 2014, 1, 12. [Google Scholar] [CrossRef][Green Version]
- Cercato, C.; Fonseca, F.A. Cardiovascular risk and obesity. Diabetol. Metab. Syndr. 2019, 11, 74. [Google Scholar] [CrossRef]
- Hedvall Kallerman, P.; Hagman, E.; Edstedt Bonamy, A.K.; Zemack, H.; Marcus, C.; Norman, M.; Westerståhl, M. Obese children without comorbidities have impaired microvascular endothelial function. Acta Paediatr. 2014, 103, 411–417. [Google Scholar] [CrossRef]
- Garibay-Nieto, N.; Hernández-Morán, B.A.; Villanueva-Ortega, E.; Garcés-Hernández, M.J.; Pedraza-Escudero, K.; Arroyo-Valerio, A.; Pedraza-Helvert, C.; Herrera-Rosas, A.; Laresgoiti-Servitje, E.; León-Hernández, M.; et al. Comparison of Carotid Intima-Media Thickness in Children and Adults With and Without Obesity: A Hysteresis Model. Endocr. Pract. 2022, 28, 315–320. [Google Scholar] [CrossRef]
- Robertson, J.; Schaufelberger, M.; Lindgren, M.; Adiels, M.; Schiöler, L.; Torén, K.; McMurray, J.; Sattar, N.; Åberg, M.; Rosengren, A. Higher Body Mass Index in Adolescence Predicts Cardiomyopathy Risk in Midlife. Circulation 2019, 140, 117–125. [Google Scholar] [CrossRef]
- Alpert, M.A.; Omran, J.; Mehra, A.; Ardhanari, S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog. Cardiovasc. Dis. 2014, 56, 391–400. [Google Scholar] [CrossRef]
- Jobira, B.; Frank, D.N.; Pyle, L.; Silveira, L.J.; Kelsey, M.M.; Garcia-Reyes, Y.; Robertson, C.E.; Ir, D.; Nadeau, K.J.; Cree-Green, M. Obese Adolescents With PCOS Have Altered Biodiversity and Relative Abundance in Gastrointestinal Microbiota. J. Clin. Endocrinol. Metab. 2020, 105, e2134–e2144. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.R.; Han, M.M.; Yang, Y.Z.; Wang, X.; Hou, D.Y.; Meng, X.C.; Wang, H.; Zhao, W.S.; Zhang, L.; Xu, L. Fifteen-year mortality and prognostic factors in patients with dilated cardiomyopathy: Persistent standardized application of drug therapy and strengthened management may bring about encouraging change in an aging society. J. Geriatr. Cardiol. 2022, 19, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.H. Endocrine comorbidities of pediatric obesity. Clin. Exp. Pediatr. 2021, 64, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Brix, N.; Ernst, A.; Lauridsen, L.L.B.; Parner, E.T.; Arah, O.A.; Olsen, J.; Henriksen, T.B.; Ramlau-Hansena, C.H. Childhood overweight and obesity and timing of puberty in boys and girls: Cohort and sibling-matched analyses. Int. J. Epidemiol. 2020, 49, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A.; Zachurzok, A.; Baran, J.; Dereń, K.; Łuszczki, E.; Weres, A.; Wyszyńska, J.; Dylczyk, J.; Szczudlik, E.; Drożdż, D.; et al. Childhood Obesity: Position Statement of Polish Society of Pediatrics, Polish Society for Pediatric Obesity, Polish Society of Pediatric Endocrinology and Diabetes, the College of Family Physicians in Poland and Polish Association for Study on Obesity. Nutrients 2022, 14, 3806. [Google Scholar] [CrossRef] [PubMed]
- Ghergherehchi, R.; Hazhir, N. Thyroid hormonal status among children with obesity. Ther. Adv. Endocrinol. Metab. 2015, 6, 51–55. [Google Scholar] [CrossRef]
- Calcaterra, V.; Magenes, V.C.; Hruby, C.; Siccardo, F.; Mari, A.; Cordaro, E.; Fabiano, V.; Zuccotti, G. Links between Childhood Obesity, High-Fat Diet, and Central Precocious Puberty. Children 2023, 10, 241. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, T.; Li, X.; Pan, D.; Lai, X.; Chen, Y.; Wang, X.; Yu, X.; Fu, S.; Huang, S.; et al. Prevalence of precocious puberty among Chinese children: A school population-based study. Endocrine 2021, 72, 573–581. [Google Scholar] [CrossRef]
- Liu, G.; Guo, J.; Zhang, X.; Lu, Y.; Miao, J.; Xue, H. Obesity is a risk factor for central precocious puberty: A case-control study. BMC Pediatr. 2021, 21, 509. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Y.; Sun, W.; Chen, Y.; Jiang, Y.; Song, Y.; Lin, Q.; Zhu, L.; Zhu, Q.; Wang, X.; et al. Investigating the relationship between precocious puberty and obesity: A cross-sectional study in Shanghai, China. BMJ Open 2017, 7, e014004. [Google Scholar] [CrossRef]
- Reinehr, T.; Bosse, C.; Lass, N.; Rothermel, J.; Knop, C.; Roth, C.L. Effect of Weight Loss on Puberty Onset in Overweight Children. J. Pediatr. 2017, 184, 143–150.e141. [Google Scholar] [CrossRef] [PubMed]
- Farello, G.; Altieri, C.; Cutini, M.; Pozzobon, G.; Verrotti, A. Review of the Literature on Current Changes in the Timing of Pubertal Development and the Incomplete Forms of Early Puberty. Front. Pediatr. 2019, 7, 147. [Google Scholar] [CrossRef] [PubMed]
- Sultan, C.; Gaspari, L.; Maimoun, L.; Kalfa, N.; Paris, F. Disorders of puberty. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 48, 62–89. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Su, Z.; Pan, L.; Wang, L.; Xu, Z.; Peng, G.; Li, X. Factors affecting bone maturation in Chinese girls aged 4-8 years with isolated premature thelarche. BMC Pediatr. 2020, 20, 356. [Google Scholar] [CrossRef] [PubMed]
- Kaplowitz, P.B. For Premature Thelarche and Premature Adrenarche, the Case for Waiting before Testing. Horm. Res. Paediatr. 2020, 93, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bajpai, A. Precocious Puberty. Indian J. Pediatr. 2023, 90, 582–589. [Google Scholar] [CrossRef]
- Itriyeva, K. The effects of obesity on the menstrual cycle. Curr. Probl. Pediatr. Adolesc. Health Care 2022, 52, 101241. [Google Scholar] [CrossRef]
- Elizondo-Montemayor, L.; Hernández-Escobar, C.; Lara-Torre, E.; Nieblas, B.; Gómez-Carmona, M. Gynecologic and Obstetric Consequences of Obesity in Adolescent Girls. J. Pediatr. Adolesc. Gynecol. 2017, 30, 156–168. [Google Scholar] [CrossRef]
- Durá-Travé, T.; Gallinas-Victoriano, F. Hyper-androgenemia and obesity in early-pubertal girls. J. Endocrinol. Investig. 2022, 45, 1577–1585. [Google Scholar] [CrossRef]
- Wood, P.L.; Bauman, D. Gynaecological issues affecting the obese adolescent. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 453–465. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, J.S. Early menarche and its consequence in Korean female: Reducing fructose intake could be one solution. Clin. Exp. Pediatr. 2021, 64, 12–20. [Google Scholar] [CrossRef]
- Kang, M.J. The adiposity rebound in the 21st century children: Meaning for what? Korean J. Pediatr. 2018, 61, 375–380. [Google Scholar] [CrossRef]
- German, A.; Shmoish, M.; Hochberg, Z. Predicting pubertal development by infantile and childhood height, BMI, and adiposity rebound. Pediatr. Res. 2015, 78, 445–450. [Google Scholar] [CrossRef]
- Koivuaho, E.; Laru, J.; Ojaniemi, M.; Puukka, K.; Kettunen, J.; Tapanainen, J.S.; Franks, S.; Järvelin, M.R.; Morin-Papunen, L.; Sebert, S.; et al. Age at adiposity rebound in childhood is associated with PCOS diagnosis and obesity in adulthood-longitudinal analysis of BMI data from birth to age 46 in cases of PCOS. Int. J. Obes. 2019, 43, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.J.; Moreira, C.; Santos, A.C. Adiposity rebound and cardiometabolic health in childhood: Results from the Generation XXI birth cohort. Int. J. Epidemiol. 2021, 50, 1260–1271. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Deng, X.; Chen, Y.; Yang, B.; Huang, X.; Østbye, T. Association of prepubertal obesity with pubertal development in Chinese girls and boys: A longitudinal study. Am. J. Hum. Biol. 2018, 30, e23195. [Google Scholar] [CrossRef]
- Lee, J.M.; Wasserman, R.; Kaciroti, N.; Gebremariam, A.; Steffes, J.; Dowshen, S.; Harris, D.; Serwint, J.; Abney, D.; Smitherman, L.; et al. Timing of Puberty in Overweight Versus Obese Boys. Pediatrics 2016, 137, e20150164. [Google Scholar] [CrossRef]
- Papadimitriou, A.; Kanakis, G.; Douros, K.; Papadimitriou, D.T.; Boutsiadis, A.H.; Nicolaidou, P.; Fretzayas, A. Constitutional advancement of growth is associated with early puberty in girls. Horm. Res. Paediatr. 2011, 76, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, A.; Nicolaidou, P.; Fretzayas, A.; Chrousos, G.P. Clinical review: Constitutional advancement of growth, a.k.a. early growth acceleration, predicts early puberty and childhood obesity. J. Clin. Endocrinol. Metab. 2010, 95, 4535–4541. [Google Scholar] [CrossRef] [PubMed]
- Aris, I.M.; Perng, W.; Dabelea, D.; Ganiban, J.M.; Liu, C.; Marceau, K.; Robertson, O.C.; Hockett, C.W.; Mihalopoulos, N.L.; Kong, X.; et al. Analysis of Early-Life Growth and Age at Pubertal Onset in US Children. JAMA Netw. Open 2022, 5, e2146873. [Google Scholar] [CrossRef]
- Papadimitriou, A.; Marakaki, C.; Papadimitriou, D.T. Growth variations with opposite clinical outcomes and the emerging role of IGF-1. Trends Endocrinol. Metab. 2022, 33, 359–370. [Google Scholar] [CrossRef]
- AsghariHanjani, N.; Vafa, M. The role of IGF-1 in obesity, cardiovascular disease, and cancer. Med. J. Islam Repub. Iran 2019, 33, 56. [Google Scholar] [CrossRef]
- Baier, I.; Pereira, A.; Ferrer, P.; Iñiguez, G.; Mericq, V. Higher Prepubertal IGF-1 Concentrations Associate to Earlier Pubertal Tempo in Both Sexes. Horm. Res. Paediatr. 2023, 96, 404–411. [Google Scholar] [CrossRef]
- Kempf, E.; Vogel, M.; Vogel, T.; Kratzsch, J.; Landgraf, K.; Kühnapfel, A.; Gausche, R.; Gräfe, D.; Sergeyev, E.; Pfäffle, R.; et al. Dynamic alterations in linear growth and endocrine parameters in children with obesity and height reference values. EClinicalMedicine 2021, 37, 100977. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tian, J.; Blizzard, L.; Oddy, W.H.; Dwyer, T.; Bazzano, L.A.; Hickey, M.; Harville, E.W.; Venn, A.J. Associations of childhood adiposity with menstrual irregularity and polycystic ovary syndrome in adulthood: The Childhood Determinants of Adult Health Study and the Bogalusa Heart Study. Hum. Reprod. 2020, 35, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Ambia, A.M.; Pruszynski, J.E.; Fairchild, E.; McIntire, D.D.; Nelson, D.B. Perinatal outcomes of young adolescent pregnancies in an urban inner city. Am. J. Obstet. Gynecol. MFM 2023, 5, 100843. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.A.; Peña, A.S. Quality of life in adolescent girls with polycystic ovary syndrome. J. Paediatr. Child Health 2020, 56, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, M.K.; Bonny, A.E. Polycystic ovary syndrome in adolescence: Diagnostic and therapeutic strategies. Transl. Pediatr. 2017, 6, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, Q.; Ye, M.; He, Y.; Yao, A.; Shi, K. Metabolic effect of obesity on polycystic ovary syndrome in adolescents: A meta-analysis. J. Obstet. Gynaecol. 2017, 37, 1036–1047. [Google Scholar] [CrossRef] [PubMed]
- Licenziati, M.R.; Valerio, G.; Vetrani, I.; De Maria, G.; Liotta, F.; Radetti, G. Altered Thyroid Function and Structure in Children and Adolescents Who Are Overweight and Obese: Reversal After Weight Loss. J. Clin. Endocrinol. Metab. 2019, 104, 2757–2765. [Google Scholar] [CrossRef]
- Rumińska, M.; Witkowska-Sędek, E.; Majcher, A.; Pyrżak, B. Thyroid Function in Obese Children and Adolescents and Its Association with Anthropometric and Metabolic Parameters. Adv. Exp. Med. Biol. 2016, 912, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.J.; Cines, B.; Pogrebniak, E.; Sherafat-Kazemzadeh, R.; Demidowich, A.P.; Galescu, O.A.; Brady, S.M.; Reynolds, J.C.; Hubbard, V.S.; Yanovski, J.A. Associations between adiposity and indicators of thyroid status in children and adolescents. Pediatr. Obes. 2016, 11, 551–558. [Google Scholar] [CrossRef]
- Witkowska-Sędek, E.; Kucharska, A.; Rumińska, M.; Pyrżak, B. Thyroid dysfunction in obese and overweight children. Endokrynol. Pol. 2017, 68, 54–60. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaltenbach, T.E.; Graeter, T.; Oeztuerk, S.; Holzner, D.; Kratzer, W.; Wabitsch, M.; Denzer, C. Thyroid dysfunction and hepatic steatosis in overweight children and adolescents. Pediatr. Obes. 2017, 12, 67–74. [Google Scholar] [CrossRef]
- Metwalley, K.A.; Farghaly, H.S. Subclinical hypothyroidism in children: Updates for pediatricians. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dayal, D.; Attri, S.; Gupta, A.; Bhalla, A. Levothyroxine supplementation for obesity-associated thyroid dysfunction in children: A prospective, randomized, case control study. Pediatr. Endocrinol. Diabetes Metab. 2019, 25, 107–113. [Google Scholar] [CrossRef]
- Szeliga, K.; Antosz, A.; Skrzynska, K.; Kalina-Faska, B.; Gawlik, A. Subclinical hypothyroidism in children and adolescents as mild dysfunction of the thyroid gland: A single-center study. Pediatr. Endocrinol. Diabetes Metab. 2023, 29, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Salerno, M.; Capalbo, D.; Cerbone, M.; De Luca, F. Subclinical hypothyroidism in childhood—Current knowledge and open issues. Nat. Rev. Endocrinol. 2016, 12, 734–746. [Google Scholar] [CrossRef]
- Jin, H.Y. Prevalence of subclinical hypothyroidism in obese children or adolescents and association between thyroid hormone and the components of metabolic syndrome. J. Paediatr. Child Health 2018, 54, 975–980. [Google Scholar] [CrossRef]
- Mushannen, T.; Cortez, P.; Stanford, F.C.; Singhal, V. Obesity and Hypogonadism-A Narrative Review Highlighting the Need for High-Quality Data in Adolescents. Children 2019, 6, 63. [Google Scholar] [CrossRef]
- Rey, R.A. Biomarkers of male hypogonadism in childhood and adolescence. Adv. Lab. Med. 2020, 1, 20200024. [Google Scholar] [CrossRef]
- Hassan, M.M.; Sarry Eldin, A.M.; Musa, N.; El-Wakil, K.H.; Ali, M.; Ahmed, H.H. Insights into the implication of obesity in hypogonadism among adolescent boys. J. Pediatr. Endocrinol. Metab. 2022, 35, 1497–1504. [Google Scholar] [CrossRef]
- Reyes-Angel, J.; Kaviany, P.; Rastogi, D.; Forno, E. Obesity-related asthma in children and adolescents. Lancet Child Adolesc. Health 2022, 6, 713–724. [Google Scholar] [CrossRef]
- Deng, X.; Ma, J.; Yuan, Y.; Zhang, Z.; Niu, W. Association between overweight or obesity and the risk for childhood asthma and wheeze: An updated meta-analysis on 18 articles and 73252 children. Pediatr. Obes. 2019, 14, e12532. [Google Scholar] [CrossRef] [PubMed]
- Azizpour, Y.; Delpisheh, A.; Montazeri, Z.; Sayehmiri, K.; Darabi, B. Effect of childhood BMI on asthma: A systematic review and meta-analysis of case-control studies. BMC Pediatr. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Brüske, I.; Flexeder, C.; Heinrich, J. Body mass index and the incidence of asthma in children. Curr. Opin. Allergy Clin. Immunol. 2014, 14, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, E.; Mantzorou, M.; Tolia, M.; Antasouras, G.; Poutsidi, A.; Psara, E.; Poulios, E.; Fasoulas, A.; Vasios, G.K.; Giaginis, C. Childhood overweight and obesity and abnormal birth anthropometric measures are associated with a higher prevalence of childhood asthma in preschool age. J. Asthma. 2023, 60, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Di Genova, L.; Penta, L.; Biscarini, A.; Di Cara, G.; Esposito, S. Children with Obesity and Asthma: Which Are the Best Options for Their Management? Nutrients 2018, 10, 1634. [Google Scholar] [CrossRef] [PubMed]
- Fainardi, V.; Passadore, L.; Labate, M.; Pisi, G.; Esposito, S. An Overview of the Obese-Asthma Phenotype in Children. Int. J. Environ. Res. Public Health 2022, 19, 636. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; Farzan, S. Clinical implications of the obese-asthma phenotypes. Immunol. Allergy Clin. North Am. 2014, 34, 739–751. [Google Scholar] [CrossRef]
- Sikorska-Szaflik, H.; Połomska, J.; Sozańska, B. The Impact of Dietary Intervention in Obese Children on Asthma Prevention and Control. Nutrients 2022, 14, 4322. [Google Scholar] [CrossRef] [PubMed]
- Mangova, M.; Lipek, T.; Vom Hove, M.; Körner, A.; Kiess, W.; Treudler, R.; Prenzel, F. Obesity-associated asthma in childhood. Allergol. Select. 2020, 4, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Dooley, A.A.; Pillai, D.K. Paediatric obesity-related asthma: Disease burden and effects on pulmonary physiology. Paediatr. Respir. Rev. 2021, 37, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Han, Y.Y.; Mullen, J.; Celedón, J.C. Overweight, Obesity, and Lung Function in Children and Adults-A Meta-analysis. J. Allergy Clin. Immunol. Pract. 2018, 6, 570–581.e510. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Weiner, D.J.; Mullen, J.; Sawicki, G.; Kurland, G.; Han, Y.Y.; Cloutier, M.M.; Canino, G.; Weiss, S.T.; Litonjua, A.A.; et al. Obesity and Airway Dysanapsis in Children with and without Asthma. Am. J. Respir. Crit. Care Med. 2017, 195, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Carr, T.F.; Granell, R.; Stern, D.A.; Guerra, S.; Wright, A.; Halonen, M.; Henderson, J.; Martinez, F.D. High Insulin in Early Childhood Is Associated with Subsequent Asthma Risk Independent of Body Mass Index. J Allergy Clin. Immunol. Pract. 2022, 10, 785–792.e785. [Google Scholar] [CrossRef] [PubMed]
- Forno, E.; Han, Y.Y.; Muzumdar, R.H.; Celedón, J.C. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J. Allergy Clin. Immunol. 2015, 136, 304–311.e308. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Jeong, J.; Baeg, M.K.; Han, K.D.; Kim, H.S.; Yoon, J.S.; Kim, H.H.; Kim, J.T.; Chun, Y.H. Lipid profiles in adolescents with and without asthma: Korea National Health and nutrition examination survey data. Lipids Health Dis. 2018, 17, 158. [Google Scholar] [CrossRef]
- Lim, J.E.; Kim, H.M.; Kim, J.H.; Baek, H.S.; Han, M.Y. Association between dyslipidemia and asthma in children: A systematic review and multicenter cohort study using a common data model. Clin. Exp. Pediatr. 2023, 66, 357–365. [Google Scholar] [CrossRef]
- Brustad, N.; Bønnelykke, K.; Chawes, B. Dietary prevention strategies for childhood asthma. Pediatr. Allergy Immunol. 2023, 34, e13984. [Google Scholar] [CrossRef]
- Tobias, T.A.M.; Wood, L.G.; Rastogi, D. Carotenoids, fatty acids and disease burden in obese minority adolescents with asthma. Clin. Exp. Allergy 2019, 49, 838–846. [Google Scholar] [CrossRef]
- Lautenbacher, L.A.; Jariwala, S.P.; Markowitz, M.E.; Rastogi, D. Vitamin D and pulmonary function in obese asthmatic children. Pediatr. Pulmonol. 2016, 51, 1276–1283. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, X.; Liu, Y.; Zhou, H.; You, Y.; Xue, Z. Complex interplay of gut microbiota between obesity and asthma in children. Front. Microbiol. 2023, 14, 1264356. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Sotelo, E.; Hinojosa-Amaya, L.; Garza-Davila, C.; Botello-Hernández, E.; Garcia-Espinosa, P. Predictors of sleep pattern disturbances. Results from a third-level university hospital. Pediatr. Endocrinol. Diabetes Metab. 2022, 28, 257–262. [Google Scholar] [CrossRef]
- Savini, S.; Ciorba, A.; Bianchini, C.; Stomeo, F.; Corazzi, V.; Vicini, C.; Pelucchi, S. Assessment of obstructive sleep apnoea (OSA) in children: An update. Acta Otorhinolaryngol. Ital. 2019, 39, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Alsubie, H.S.; BaHammam, A.S. Obstructive Sleep Apnoea: Children are not little Adults. Paediatr. Respir. Rev. 2017, 21, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, E.B.; Sun, X.; Malhotra, A.; Tantisira, K.G.; Landeo-Gutierrez, J.S.; Jain, S.; Bhattacharjee, R. Association of body anthropometry and obstructive sleep apnea in children: Variations observed in Hispanic children. Obes. Sci. Pract. 2023, 9, 210–217. [Google Scholar] [CrossRef]
- Saint-Fleur, A.L.; Christophides, A.; Gummalla, P.; Kier, C. Much Ado about Sleep: Current Concepts on Mechanisms and Predisposition to Pediatric Obstructive Sleep Apnea. Children 2021, 8, 1032. [Google Scholar] [CrossRef]
- Xiao, L.; Su, S.; Liang, J.; Jiang, Y.; Shu, Y.; Ding, L. Analysis of the Risk Factors Associated With Obstructive Sleep Apnea Syndrome in Chinese Children. Front. Pediatr. 2022, 10, 900216. [Google Scholar] [CrossRef]
- Andersen, I.G.; Holm, J.C.; Homøe, P. Impact of weight-loss management on children and adolescents with obesity and obstructive sleep apnea. Int. J. Pediatr. Otorhinolaryngol. 2019, 123, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Morąg, B.; Kozubek, P.; Gomułka, K. Obesity and Selected Allergic and Immunological Diseases-Etiopathogenesis, Course and Management. Nutrients 2023, 15, 3813. [Google Scholar] [CrossRef]
- Agón-Banzo, P.J.; Sanmartin, R.; García-Malinis, A.J.; Hernández-Martín, Á.; Puzo, J.; Doste, D.; Pardos, C.; Gilaberte, Y. Body mass index and serum lipid profile: Association with atopic dermatitis in a paediatric population. Australas. J. Dermatol. 2020, 61, e60–e64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Luo, F.; Han, Y.; Lou, H.; Tang, X.; Zhang, L. Obesity/overweight and risk of allergic rhinitis: A meta-analysis of observational studies. Allergy 2020, 75, 1272–1275. [Google Scholar] [CrossRef]
- Hayashi, K.; Tsujiguchi, H.; Hori, D.; Yamada, Y.; Shimizu, Y.; Nguyen, T.T.T.; Hibino, Y.; Kambayashi, Y.; Hara, A.; Nakamura, H. The association between overweight and prevalence of food allergy in Japanese children: A cross-sectional study. Environ. Health Prev. Med. 2021, 26, 44. [Google Scholar] [CrossRef]
- Koenigsberg, R.; Gupta, S.; Slaven, J.E.; Sarin, T.; Vitalpur, G. Body mass index in relation to symptom presentation on diagnosis of eosinophilic esophagitis in children. Ann. Allergy Asthma. Immunol. 2023, 131, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Gaier, E.D.; Heidary, G. Pediatric Idiopathic Intracranial Hypertension. Semin. Neurol. 2019, 39, 704–710. [Google Scholar] [CrossRef]
- Barmherzig, R.; Szperka, C.L. Pseudotumor Cerebri Syndrome in Children. Curr. Pain Headache Rep. 2019, 23, 58. [Google Scholar] [CrossRef]
- Paley, G.L.; Sheldon, C.A.; Burrows, E.K.; Chilutti, M.R.; Liu, G.T.; McCormack, S.E. Overweight and obesity in pediatric secondary pseudotumor cerebri syndrome. Am. J. Ophthalmol. 2015, 159, 344–352.e341. [Google Scholar] [CrossRef]
- Matthews, Y.Y.; Dean, F.; Lim, M.J.; Mclachlan, K.; Rigby, A.S.; Solanki, G.A.; White, C.P.; Whitehouse, W.P.; Kennedy, C.R. Pseudotumor cerebri syndrome in childhood: Incidence, clinical profile and risk factors in a national prospective population-based cohort study. Arch. Dis. Child. 2017, 102, 715–721. [Google Scholar] [CrossRef]
- Mahajnah, M.; Genizi, J.; Zahalka, H.; Andreus, R.; Zelnik, N. Pseudotumor Cerebri Syndrome: From Childhood to Adulthood Risk Factors and Clinical Presentation. J. Child. Neurol. 2020, 35, 311–316. [Google Scholar] [CrossRef]
- Farello, G.; Ferrara, P.; Antenucci, A.; Basti, C.; Verrotti, A. The link between obesity and migraine in childhood: A systematic review. Ital. J. Pediatr. 2017, 43, 27. [Google Scholar] [CrossRef]
- Tarantino, S.; Papetti, L.; Di Stefano, A.; Messina, V.; Ursitti, F.; Ferilli, M.A.N.; Sforza, G.; Moavero, R.; Vigevano, F.; Gentile, S.; et al. Anxiety, Depression, and Body Weight in Children and Adolescents With Migraine. Front. Psychol. 2020, 11, 530911. [Google Scholar] [CrossRef]
- Walter, S.M.; Dai, Z.; Wang, K. Obesity, Migraine, and Overlapping Comorbidities in a Rural Pediatric Population. J. Neurosci. Rural. Pract. 2021, 12, 524–529. [Google Scholar] [CrossRef]
- Manohar, N.; Hayen, A.; Fahey, P.; Arora, A. Obesity and dental caries in early childhood: A systematic review and meta-analyses. Obes. Rev. 2020, 21, e12960. [Google Scholar] [CrossRef]
- Lock, N.C.; Susin, C.; Damé-Teixeira, N.; Maltz, M.; Alves, L.S. Sex differences in the association between obesity and gingivitis among 12-year-old South Brazilian schoolchildren. J. Periodontal. Res. 2020, 55, 559–566. [Google Scholar] [CrossRef]
- Goodson, J.M. Disease reciprocity between gingivitis and obesity. J. Periodontol. 2020, 91 (Suppl. 1), S26–S34. [Google Scholar] [CrossRef]
- Panagiotou, E.; Agouropoulos, A.; Vadiakas, G.; Pervanidou, P.; Chouliaras, G.; Kanaka-Gantenbein, C. Oral health of overweight and obese children and adolescents: A comparative study with a multivariate analysis of risk indicators. Eur. Arch. Paediatr. Dent. 2021, 22, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Alshihri, A.A.; Rogers, H.J.; Alqahtani, M.A.; Aldossary, M.S. Association between Dental Caries and Obesity in Children and Young People: A Narrative Review. Int. J. Dent. 2019, 2019, 9105759. [Google Scholar] [CrossRef] [PubMed]
- Guaré, R.O.; Perez, M.M.; Novaes, T.F.; Ciamponi, A.L.; Gorjão, R.; Diniz, M.B. Overweight/obese children are associated with lower caries experience than normal-weight children/adolescents. Int. J. Paediatr. Dent. 2019, 29, 756–764. [Google Scholar] [CrossRef] [PubMed]
- García Pérez, A.; Barrera Ortega, C.C.; González-Aragón Pineda, Á.; Villanueva Gutiérrez, T.; Pérez Pérez, N.G.; Calderon Uriostegui, D. An inverse relationship between obesity and dental caries in Mexican schoolchildren: A cross-sectional study. Public Health 2020, 180, 163–167. [Google Scholar] [CrossRef]
- Sapunarova, P.T.; Nihtyanova, T.I.; Petrova, S.G.; Kukleva, M.P. Oral Hygiene Status and Gingivitis in Overweight and Obese Children. Folia. Med. 2019, 61, 594–599. [Google Scholar] [CrossRef]
- Umano, G.R.; Pistone, C.; Tondina, E.; Moiraghi, A.; Lauretta, D.; Miraglia Del Giudice, E.; Brambilla, I. Pediatric Obesity and the Immune System. Front. Pediatr. 2019, 7, 487. [Google Scholar] [CrossRef]
- Räisänen, L.; Lommi, S.; Engberg, E.; Kolho, K.L.; Viljakainen, H. Central obesity in school-aged children increases the likelihood of developing paediatric autoimmune diseases. Pediatr. Obes. 2022, 17, e12857. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Genes, T.M. Obesity and Multiple Sclerosis-A Multifaceted Association. J. Clin. Med. 2021, 10, 2689. [Google Scholar] [CrossRef]
- Huppke, B.; Ellenberger, D.; Hummel, H.; Stark, W.; Röbl, M.; Gärtner, J.; Huppke, P. Association of Obesity With Multiple Sclerosis Risk and Response to First-line Disease Modifying Drugs in Children. JAMA Neurol. 2019, 76, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Harroud, A.; Mitchell, R.E.; Richardson, T.G.; Morris, J.A.; Forgetta, V.; Davey Smith, G.; Baranzini, S.E.; Richards, J.B. Childhood obesity and multiple sclerosis: A Mendelian randomization study. Mult. Scler. 2021, 27, 2150–2158. [Google Scholar] [CrossRef]
- Pakpoor, J.; Schmierer, K.; Cuzick, J.; Giovannoni, G.; Dobson, R. Estimated and projected burden of multiple sclerosis attributable to smoking and childhood and adolescent high body-mass index: A comparative risk assessment. Int. J. Epidemiol. 2021, 49, 2051–2057. [Google Scholar] [CrossRef]
- Ciężki, S.; Kurpiewska, E.; Bossowski, A.; Głowińska-Olszewska, B. Multi-Faceted Influence of Obesity on Type 1 Diabetes in Children—From Disease Pathogenesis to Complications. Front. Endocrinol. 2022, 13, 890833. [Google Scholar] [CrossRef] [PubMed]
- Zucker, I.; Zloof, Y.; Bardugo, A.; Tsur, A.M.; Lutski, M.; Cohen, Y.; Cukierman-Yaffe, T.; Minsky, N.; Derazne, E.; Tzur, D.; et al. Obesity in late adolescence and incident type 1 diabetes in young adulthood. Diabetologia 2022, 65, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Zakharova, I.; Klimov, L.; Kuryaninova, V.; Nikitina, I.; Malyavskaya, S.; Dolbnya, S.; Kasyanova, A.; Atanesyan, R.; Stoyan, M.; Todieva, A.; et al. Vitamin D Insufficiency in Overweight and Obese Children and Adolescents. Front. Endocrinol. 2019, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Corsello, A.; Macchi, M.; D’Oria, V.; Pigazzi, C.; Alberti, I.; Treglia, G.; De Cosmi, V.; Mazzocchi, A.; Agostoni, C.; Milani, G.P. Effects of vitamin D supplementation in obese and overweight children and adolescents: A systematic review and meta-analysis. Pharmacol. Res. 2023, 192, 106793. [Google Scholar] [CrossRef] [PubMed]
- Tayde, A.; Mittal, M.; Khadgawat, R.; Sharma, S.; Sreenivas, V.; Rai, A. Response to single oral dose vitamin D in obese vs non-obese vitamin D-deficient children. Eur. J. Pediatr. 2021, 180, 1043–1050. [Google Scholar] [CrossRef]
- Murdaca, G.; Tonacci, A.; Negrini, S.; Greco, M.; Borro, M.; Puppo, F.; Gangemi, S. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun. Rev. 2019, 18, 102350. [Google Scholar] [CrossRef]
- Mailhot, G.; White, J.H. Vitamin D and Immunity in Infants and Children. Nutrients 2020, 12, 1233. [Google Scholar] [CrossRef]
- Mohan, M.; Cherian, J.J.; Sharma, A. Exploring links between vitamin D deficiency and COVID-19. PLoS Pathog. 2020, 16, e1008874. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.E.; Niinistö, S.; Erlund, I.; Cuthbertson, D.; Nucci, A.M.; Honkanen, J.; Vaarala, O.; Hyöty, H.; Krischer, J.P.; Knip, M.; et al. Serum 25-hydroxyvitamin D concentration in childhood and risk of islet autoimmunity and type 1 diabetes: The TRIGR nested case-control ancillary study. Diabetologia 2020, 63, 780–787. [Google Scholar] [CrossRef]
- Mäkinen, M.; Simell, V.; Mykkänen, J.; Ilonen, J.; Veijola, R.; Hyöty, H.; Knip, M.; Simell, O.; Toppari, J.; Hermann, R. An increase in serum 25-hydroxyvitamin D concentrations preceded a plateau in type 1 diabetes incidence in Finnish children. J. Clin. Endocrinol. Metab. 2014, 99, E2353–E2356. [Google Scholar] [CrossRef]
- Mangat, G.; Nair, N.; Barat, O.; Abboud, B.; Pais, P.; Bagga, S.; Raina, R. Obesity-related glomerulopathy in children: Connecting pathophysiology to clinical care. Clin. Kidney J. 2023, 16, 611–618. [Google Scholar] [CrossRef]
- Correia-Costa, L.; Azevedo, A.; Caldas Afonso, A. Childhood Obesity and Impact on the Kidney. Nephron 2019, 143, 8–11. [Google Scholar] [CrossRef]
- Marzuillo, P.; Grandone, A.; Di Sessa, A.; Guarino, S.; Diplomatico, M.; Umano, G.R.; Polito, C.; La Manna, A.; Perrone, L.; Miraglia Del Giudice, E. Anthropometric and Biochemical Determinants of Estimated Glomerular Filtration Rate in a Large Cohort of Obese Children. J. Ren. Nutr. 2018, 28, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Vivante, A.; Golan, E.; Tzur, D.; Leiba, A.; Tirosh, A.; Skorecki, K.; Calderon-Margalit, R. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Arch. Intern. Med. 2012, 172, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.E.; Yoo, K.H. Obesity and chronic kidney disease: Prevalence, mechanism, and management. Clin. Exp. Pediatr. 2021, 64, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Radosz, A.; Obuchowicz, A. Lipid metabolism and renal function markers in obese adolescents. Pediatr. Endocrinol. Diabetes Metab. 2023, 29, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Di Sessa, A.; Guarino, S.; Umano, G.R.; Arenella, M.; Alfiero, S.; Quaranta, G.; Miraglia Del Giudice, E.; Marzuillo, P. MAFLD in Obese Children: A Challenging Definition. Children 2021, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Zdanowicz, K.; Daniluk, J.; Lebensztejn, D.M.; Daniluk, U. The Etiology of Cholelithiasis in Children and Adolescents—A Literature Review. Int. J. Mol. Sci. 2022, 23, 13376. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.B.; Freedman, N.D.; Gamborg, M.; Sørensen, T.I.; Baker, J.L. Childhood body mass index in relation to future risk of oesophageal adenocarcinoma. Br. J. Cancer 2015, 112, 601–607. [Google Scholar] [CrossRef][Green Version]
- Tambucci, R.; Quitadamo, P.; Ambrosi, M.; De Angelis, P.; Angelino, G.; Stagi, S.; Verrotti, A.; Staiano, A.; Farello, G. Association Between Obesity/Overweight and Functional Gastrointestinal Disorders in Children. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 517–520. [Google Scholar] [CrossRef]
- Krawczyk, M.; Kułaga, Z.; Niewiadomska, O.; Jankowska, I.; Lebensztejn, D.; Więcek, S.; Socha, P. Are children with gallstone disease more overweight? Results of a matched case-control analysis. Clin. Res. Hepatol. Gastroenterol. 2023, 47, 102204. [Google Scholar] [CrossRef]
- Cabrera Chamorro, C.C.; Pabón Arteaga, J.S.; Caicedo Paredes, C.A.; Cabrera Bravo, N.; Villamil Giraldo, C.E.; Chávez Betancourt, G.; Zarama Márquez, R.A.; Rincón Torres, C.A. Cholelithiasis and associated complications in pediatric patients. Cir. Pediatr. 2020, 33, 172–176. [Google Scholar]
- Zdanowicz, K.; Ryzko, J.; Bobrus-Chociej, A.; Wojtkowska, M.; Lebensztejn, D.M. The role of chemerin in the pathogenesis of cholelithiasis in children and adolescents. J. Paediatr. Child Health 2021, 57, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Ba, H.J.; Xu, L.L.; Qin, Y.Z.; Chen, H.S. Serum Chemerin Levels Correlate With Determinants of Metabolic Syndrome in Obese Children and Adolescents. Clin. Med. Insights Pediatr. 2019, 13, 1179556519853780. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, S. Gallstone Disease and Cholecystectomy in Children and Adolescents With Obesity. J. Pediatr. Surg. Nurs. 2019, 8, 55–57. [Google Scholar] [CrossRef]
- Frybova, B.; Drabek, J.; Lochmannova, J.; Douda, L.; Hlava, S.; Zemkova, D.; Mixa, V.; Kyncl, M.; Zeman, L.; Rygl, M.; et al. Cholelithiasis and choledocholithiasis in children; risk factors for development. PLoS ONE 2018, 13, e0196475. [Google Scholar] [CrossRef] [PubMed]
- Parra-Landazury, N.M.; Cordova-Gallardo, J.; Méndez-Sánchez, N. Obesity and Gallstones. Visc. Med. 2021, 37, 394–402. [Google Scholar] [CrossRef]
- Heida, A.; Koot, B.G.; vd Baan-Slootweg, O.H.; Pels Rijcken, T.H.; Seidell, J.C.; Makkes, S.; Jansen, P.L.; Benninga, M.A. Gallstone disease in severely obese children participating in a lifestyle intervention program: Incidence and risk factors. Int. J. Obes. 2014, 38, 950–953. [Google Scholar] [CrossRef]
- Wang, J.; Xu, C.; Cheng, Q.; Zhao, J.; Wu, S.; Li, W.; Ma, W.; Liu, C.; Jiang, X. RNA Sequencing Revealed Signals of Evolution From Gallbladder Stone to Gallbladder Carcinoma. Front. Oncol. 2020, 10, 823. [Google Scholar] [CrossRef]
- Hatia, R.I.; Eluri, M.; Hawk, E.T.; Shalaby, A.; Karatas, E.; Abdelhakeem, A.; Abdel-Wahab, R.; Chang, P.; Rashid, A.; Jalal, P.K.; et al. Independent of Primary Sclerosing Cholangitis and Cirrhosis, Early Adulthood Obesity Is Associated with Cholangiocarcinoma. Cancer Epidemiol. Biomarkers Prev. 2023, 32, 1338–1347. [Google Scholar] [CrossRef]
- Oze, I.; Ito, H.; Koyanagi, Y.N.; Abe, S.K.; Rahman, M.S.; Islam, M.R.; Saito, E.; Gupta, P.C.; Sawada, N.; Tamakoshi, A.; et al. Obesity is associated with biliary tract cancer mortality and incidence: A pooled analysis of 21 cohort studies in the Asia Cohort Consortium. Int. J. Cancer. 2024, 154, 1174–1190. [Google Scholar] [CrossRef]
- Morales Camacho, W.J.; Molina Díaz, J.M.; Plata Ortiz, S.; Plata Ortiz, J.E.; Morales Camacho, M.A.; Calderón, B.P. Childhood obesity: Aetiology, comorbidities, and treatment. Diabetes Metab. Res. Rev. 2019, 35, e3203. [Google Scholar] [CrossRef]
- Lang, J.E.; Hossain, J.; Holbrook, J.T.; Teague, W.G.; Gold, B.D.; Wise, R.A.; Lima, J.J. Gastro-oesophageal reflux and worse asthma control in obese children: A case of symptom misattribution? Thorax 2016, 71, 238–246. [Google Scholar] [CrossRef]
- Pogodina, A.; Romanitsa, A.; Rychkova, L. Functional Bowel Disorders and Obesity in Children: State of the Problem. Int. J. Biomed. 2020, 10, 316–323. [Google Scholar] [CrossRef]
- Molina-Garcia, P.; Migueles, J.H.; Cadenas-Sanchez, C.; Esteban-Cornejo, I.; Mora-Gonzalez, J.; Rodriguez-Ayllon, M.; Plaza-Florido, A.; Vanrenterghem, J.; Ortega, F.B. A systematic review on biomechanical characteristics of walking in children and adolescents with overweight/obesity: Possible implications for the development of musculoskeletal disorders. Obes. Rev. 2019, 20, 1033–1044. [Google Scholar] [CrossRef]
- Steinberg, N.; Nemet, D.; Pantanowitz, M.; Eliakim, A. Gait Pattern, Impact to the Skeleton and Postural Balance in Overweight and Obese Children: A Review. Sports 2018, 6, 75. [Google Scholar] [CrossRef]
- Merder-Coşkun, D.; Uzuner, A.; Keniş-Coşkun, Ö.; Çelenlioğlu, A.E.; Akman, M.; Karadağ-Saygı, E. Relationship between obesity and musculoskeletal system findings among children and adolescents. Turk. J. Phys. Med. Rehabil. 2017, 63, 207–214. [Google Scholar] [CrossRef]
- Maciałczyk-Paprocka, K.; Stawińska-Witoszyńska, B.; Kotwicki, T.; Sowińska, A.; Krzyżaniak, A.; Walkowiak, J.; Krzywińska-Wiewiorowska, M. Prevalence of incorrect body posture in children and adolescents with overweight and obesity. Eur. J. Pediatr. 2017, 176, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, F.S.; Castell, E.C.; Marco, F.C.; Ruiz, M.J.; Rico, J.A.Q.; Roca, A.P.N. Influence of weight status on bone mineral content measured by DXA in children. BMC Pediatr. 2021, 21, 185. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Ahn, J.; Kim, H.K.; Kim, J.H. Obese children experience more extremity fractures than nonobese children and are significantly more likely to die from traumatic injuries. Acta Paediatr. 2016, 105, 1152–1157. [Google Scholar] [CrossRef]
- Franceschi, R.; Radetti, G.; Soffiati, M.; Maines, E. Forearm Fractures in Overweight-Obese Children and Adolescents: A Matter of Bone Density, Bone Geometry or Body Composition? Calcif. Tissue Int. 2022, 111, 107–115. [Google Scholar] [CrossRef]
- Hirt, P.A.; Castillo, D.E.; Yosipovitch, G.; Keri, J.E. Skin changes in the obese patient. J. Am. Acad. Dermatol. 2019, 81, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M. Cutaneous manifestations of obesity in children: A prospective study. Indian J. Paediatr. Dermatol. 2017, 18, 28–30. [Google Scholar] [CrossRef]
- Hasse, L.; Jamiolkowski, D.; Reschke, F.; Kapitzke, K.; Weiskorn, J.; Kordonouri, O.; Biester, T.; Ott, H. Pediatric obesity and skin disease: Cutaneous findings and associated quality-of-life impairments in 103 children and adolescents with obesity. Endocr. Connect. 2023, 12, e230235. [Google Scholar] [CrossRef]
- Güven, M.; Anık, A.; Ünüvar, T.; İlgün Gürel, D.; Şendur, N. Cutaneous manifestations of obesity in Turkish children: A comparative study. Pediatr. Dermatol. 2022, 39, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Balgobind, A.; Finelt, N.; Strunk, A.; Garg, A. Association between obesity and hidradenitis suppurativa among children and adolescents: A population-based analysis in the United States. J. Am. Acad. Dermatol. 2020, 82, 502–504. [Google Scholar] [CrossRef]
- Jørgensen, A.R.; Aarestrup, J.; Baker, J.L.; Thomsen, S.F. Association of Birth Weight, Childhood Body Mass Index, and Height With Risk of Hidradenitis Suppurativa. JAMA Dermatol. 2020, 156, 746–753. [Google Scholar] [CrossRef]
- Phan, K.; Lee, G.; Fischer, G. Pediatric psoriasis and association with cardiovascular and metabolic comorbidities: Systematic review and meta-analysis. Pediatr. Dermatol. 2020, 37, 661–669. [Google Scholar] [CrossRef]
- Shreberk-Hassidim, R.; Galili, E.; Hassidim, A.; Ramot, Y.; Merdler, I.; Baum, S.; Zlotogorski, A.; Barzilai, A.; Astman, N. Epidemiology and Comorbidities of Psoriasis among Israeli Adolescents: A Large Cross-Sectional Study. Dermatology 2019, 235, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Darlenski, R.; Mihaylova, V.; Handjieva-Darlenska, T. The Link Between Obesity and the Skin. Front. Nutr. 2022, 9, 855573. [Google Scholar] [CrossRef]
- Sadowska-Przytocka, A.; Gruszczyńska, M.; Ostałowska, A.; Antosik, P.; Czarnecka-Operacz, M.; Adamski, Z.; Łącka, K. Insulin resistance in the course of acne—Literature review. Postepy Dermatol. Alergol. 2022, 39, 231–238. [Google Scholar] [CrossRef]
- Svoboda, S.A.; Shields, B.E. Cutaneous Manifestations of Nutritional Excess: Pathophysiologic Effects of Hyperglycemia and Hyperinsulinemia on the Skin. Cutis 2021, 107, 74–78. [Google Scholar] [CrossRef]
- Sagar, R.; Gupta, T. Psychological Aspects of Obesity in Children and Adolescents. Indian J. Pediatr. 2018, 85, 554–559. [Google Scholar] [CrossRef]
- Smith, J.D.; Fu, E.; Kobayashi, M.A. Prevention and Management of Childhood Obesity and Its Psychological and Health Comorbidities. Annu. Rev. Clin. Psychol. 2020, 16, 351–378. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.; Sutin, A.; Daly, M. Perceived weight discrimination mediates the prospective relation between obesity and depressive symptoms in U.S. and U.K. adults. Health Psychol. 2017, 36, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Pont, S.J.; Puhl, R.; Cook, S.R.; Slusser, W.; Section on Obesity; Obesity Society. Stigma Experienced by Children and Adolescents With Obesity. Pediatrics 2017, 140, e20173034. [Google Scholar] [CrossRef]
- Sheinbein, D.H.; Stein, R.I.; Hayes, J.F.; Brown, M.L.; Balantekin, K.N.; Conlon, R.P.K.; Saelens, B.E.; Perri, M.G.; Welch, R.R.; Schechtman, K.B.; et al. Factors associated with depression and anxiety symptoms among children seeking treatment for obesity: A social-ecological approach. Pediatr. Obes. 2019, 14, e12518. [Google Scholar] [CrossRef]
- Taylor, J.H.; Xu, Y.; Li, F.; Shaw, M.; Dziura, J.; Caprio, S.; Tamborlane, W.V.; Nowicka, P.; Savoye, M. Psychosocial predictors and moderators of weight management programme outcomes in ethnically diverse obese youth. Pediatr. Obes. 2017, 12, 453–461. [Google Scholar] [CrossRef]
- Hoare, E.; Skouteris, H.; Fuller-Tyszkiewicz, M.; Millar, L.; Allender, S. Associations between obesogenic risk factors and depression among adolescents: A systematic review. Obes. Rev. 2014, 15, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Rao, W.W.; Zong, Q.Q.; Zhang, J.W.; An, F.R.; Jackson, T.; Ungvari, G.S.; Xiang, Y.; Su, Y.Y.; D’Arcy, C.; Xiang, Y.T. Obesity increases the risk of depression in children and adolescents: Results from a systematic review and meta-analysis. J. Affect. Disord. 2020, 267, 78–85. [Google Scholar] [CrossRef]
- Agustí, A.; García-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Romaní-Pérez, M.; Sanz, Y. Interplay between the Gut-Brain Axis, Obesity and Cognitive Function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef]
- Gibson-Smith, D.; Bot, M.; Snijder, M.; Nicolaou, M.; Derks, E.M.; Stronks, K.; Brouwer, I.A.; Visser, M.; Penninx, B.W.J.H. The relation between obesity and depressed mood in a multi-ethnic population. The HELIUS study. Soc. Psychiatry Psychiatr. Epidemiol. 2018, 53, 629–638. [Google Scholar] [CrossRef]
- Incollingo Rodriguez, A.C.; Epel, E.S.; White, M.L.; Standen, E.C.; Seckl, J.R.; Tomiyama, A.J. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology 2015, 62, 301–318. [Google Scholar] [CrossRef]
- Mansur, R.B.; Brietzke, E.; McIntyre, R.S. Is there a “metabolic-mood syndrome”? A review of the relationship between obesity and mood disorders. Neurosci. Biobehav. Rev. 2015, 52, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Schachter, J.; Martel, J.; Lin, C.S.; Chang, C.J.; Wu, T.R.; Lu, C.C.; Ko, Y.F.; Lai, H.C.; Ojcius, D.M.; Young, J.D. Effects of obesity on depression: A role for inflammation and the gut microbiota. Brain Behav. Immun. 2018, 69, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Plant, D.T.; Pariante, C.M.; Sharp, D.; Pawlby, S. Maternal depression during pregnancy and offspring depression in adulthood: Role of child maltreatment. Br. J. Psychiatry 2015, 207, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, A.; Centorrino, F.; Jackson, J.W.; Fitzmaurice, G.; Valeri, L. The role of weight gain in explaining the effects of antipsychotic drugs on positive and negative symptoms: An analysis of the CATIE schizophrenia trial. Schizophr. Res. 2019, 206, 96–102. [Google Scholar] [CrossRef]
- Colasanto, M.; Madigan, S.; Korczak, D.J. Depression and inflammation among children and adolescents: A meta-analysis. J. Affect. Disord. 2020, 277, 940–948. [Google Scholar] [CrossRef]
- Jebeile, H.; Gow, M.L.; Baur, L.A.; Garnett, S.P.; Paxton, S.J.; Lister, N.B. Association of Pediatric Obesity Treatment, Including a Dietary Component, With Change in Depression and Anxiety: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, e192841. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.A.; Ditch, S.; Hansen, S. Identifying and Preventing Eating Disorders in Adolescent Patients with Obesity. Pediatr. Ann. 2018, 47, e232–e237. [Google Scholar] [CrossRef]
- Stabouli, S.; Erdine, S.; Suurorg, L.; Jankauskienė, A.; Lurbe, E. Obesity and Eating Disorders in Children and Adolescents: The Bidirectional Link. Nutrients 2021, 13, 4321. [Google Scholar] [CrossRef]
- Hornberger, L.L.; Lane, M.A.; Adolescence, C.O. Identification and Management of Eating Disorders in Children and Adolescents. Pediatrics 2021, 147, 1240–1253. [Google Scholar] [CrossRef] [PubMed]
- Micali, N.; Solmi, F.; Horton, N.J.; Crosby, R.D.; Eddy, K.T.; Calzo, J.P.; Sonneville, K.R.; Swanson, S.A.; Field, A.E. Adolescent Eating Disorders Predict Psychiatric, High-Risk Behaviors and Weight Outcomes in Young Adulthood. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 652–659.e651. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Simmons, W.K.; van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Tsiros, M.D.; Tian, E.J.; Shultz, S.P.; Olds, T.; Hills, A.P.; Duff, J.; Kumar, S. Obesity, the new childhood disability? An umbrella review on the association between adiposity and physical function. Obes. Rev. 2020, 21, e13121. [Google Scholar] [CrossRef]
- Lindberg, L.; Danielsson, P.; Persson, M.; Marcus, C.; Hagman, E. Association of childhood obesity with risk of early all-cause and cause-specific mortality: A Swedish prospective cohort study. PLoS Med. 2020, 17, e1003078. [Google Scholar] [CrossRef]
| Somatic Health Problems | Mental Health Problems | ||
|---|---|---|---|
| Metabolic | Non-Metabolic | Emotional Disorders | |
| Insulin resistance | Endocrine | Other | Eating disorders |
| Diabetes mellitus | Disturbed sexual maturation | CVD | Depression |
| Impaired glucose metabolism | Hyperandrogenism | Cardiomiopathy | Decreased quality of life |
| Dyslipidemia | Hirsutism | Asthma | Poor self-esteem |
| Hypertension | PCOS | Obstructive sleep apnoea | |
| Metabolic syndrome | Thyroid dysfunction | Allergic diseases | |
| NAFLD/MAFLD | Pseudotumor cerebri | ||
| Migraine | |||
| Oral health disorders | |||
| Immunologic diseases | |||
| Chronic kidney disease | |||
| Functional gastrointestinal disorders | |||
| Biliary duct diseases | |||
| GERD | |||
| Musculoskeletal problems | |||
| Dermatologic conditions | |||
| EDs Behaviors | Medical Complications |
|---|---|
| Bulimia nervosa | |
| Fluid and electrolytes | dehydration, hypokalemia, hypochloremia, metabolic alkalosis (vomiting); dehydration, hyperchloremic metabolic acidosis, hypocalcemia (laxative use) |
| Psychiatric | depressed mood or mood dysregulation; obsessive-compulsive symptoms; anxiety; laxative dependance; suicide |
| Gastrointestinal | gastroesophageal reflux, esophagitis; Mallory–Weiss tears; esophageal or gastric rupture |
| Dental | dental erosions |
| Binge eating | obesity with accompanying complications |
| Anorexia nervosa | |
| Psychiatric | depressed mood or mood dysregulation; obsessive-compulsive symptoms; anxiety; laxative dependance; suicide |
| Neurologic | cerebral cortical atrophy; cognitive deficits; seizures |
| Cardiac | decreased cardiac muscle mass, right axis deviation, low cardiac voltage; cardiac dysrhythmias, cardiac conduction delays; mitral valve prolapse; pericardial effusion; congestive heart failure; edema |
| Gastrointestinal | delayed gastric emptying, slowed gastrointestinal motility, constipation; superior mesenteric artery syndrome; pancreatitis; elevated transaminases; hypercholesterolemia |
| Endocrinologic | growth retardation; hypogonadotropic hypogonadism: amenorrhea, testicular atrophy, decreased libido; sick euthyroid syndrome; hypoglycemia/hyperglycemia, impaired glucose tolerance; hypercholesterolemia; decreased BMD |
| Hematologic | leukopenia, anemia, thrombocytopenia, elevated ferritin; depressed erythrocyte sedimentation rate |
| Fluid and electrolytes | dehydration; hypokalemia, hyponatremia |
| Refeeding | night sweats; polyuria, nocturia; refeeding syndrome: electrolyte abnormalities, edema, seizures, congestive heart failure (rare) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciężki, S.; Odyjewska, E.; Bossowski, A.; Głowińska-Olszewska, B. Not Only Metabolic Complications of Childhood Obesity. Nutrients 2024, 16, 539. https://doi.org/10.3390/nu16040539
Ciężki S, Odyjewska E, Bossowski A, Głowińska-Olszewska B. Not Only Metabolic Complications of Childhood Obesity. Nutrients. 2024; 16(4):539. https://doi.org/10.3390/nu16040539
Chicago/Turabian StyleCiężki, Sebastian, Emilia Odyjewska, Artur Bossowski, and Barbara Głowińska-Olszewska. 2024. "Not Only Metabolic Complications of Childhood Obesity" Nutrients 16, no. 4: 539. https://doi.org/10.3390/nu16040539
APA StyleCiężki, S., Odyjewska, E., Bossowski, A., & Głowińska-Olszewska, B. (2024). Not Only Metabolic Complications of Childhood Obesity. Nutrients, 16(4), 539. https://doi.org/10.3390/nu16040539






