Correlation between Selenium and Zinc Levels and Survival among Prostate Cancer Patients
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Cohort
2.2. Methodology for Measurements
2.2.1. Sample Storage and Collection
2.2.2. Measurement Methodology
2.2.3. Quality Control
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Trama, A.; Foschi, R.; Larrañaga, N.; Sant, M.; Fuentes-Raspall, R.; Serraino, D.; Tavilla, A.; Van Eycken, L.; Nicolai, N.; Hackl, M.; et al. Survival of Male Genital Cancers (Prostate, Testis and Penis) in Europe 1999–2007: Results from the EUROCARE-5 Study. Eur. J. Cancer 2015, 51, 2206–2216. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sears, S.P.; Carr, G.; Bime, C. Acute and Chronic Respiratory Failure in Cancer Patients. In Oncologic Critical Care; Springer International Publishing: Cham, Switzerland, 2020; pp. 445–475. [Google Scholar] [CrossRef]
- Sukiennicki, G.M.; Marciniak, W.; Muszyńska, M.; Baszuk, P.; Gupta, S.; Białkowska, K.; Jaworska-Bieniek, K.; Durda, K.; Lener, M.; Pietrzak, S.; et al. Iron Levels, Genes Involved in Iron Metabolism and Antioxidative Processes and Lung Cancer Incidence. PLoS ONE 2019, 14, e0208610. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Bao, X.; Li, J.; Pan, C.; Qian, J.; Lin, L.; Qiu, Y.; Shi, B.; Liu, F.; Chen, F.; et al. Correlation between Serum Iron Level and Overall Survival of Oral Cancer. Wei Sheng Yan Jiu 2021, 50, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Rowińska, K.; Baszuk, P.; Rogoża-Janiszewska, E.; Deptuła, J.; Marciniak, W.; Derkacz, R.; Lener, M.; Cybulski, C.; Kiedrowicz, M.; Boer, M.; et al. Serum Iron Level and 10-Year Survival after Melanoma. Biomedicines 2022, 10, 3018. [Google Scholar] [CrossRef] [PubMed]
- Pietrzak, S.; Wójcik, J.; Baszuk, P.; Marciniak, W.; Wojtyś, M.; Dębniak, T.; Cybulski, C.; Gronwald, J.; Alchimowicz, J.; Masojć, B.; et al. Influence of the Levels of Arsenic, Cadmium, Mercury and Lead on Overall Survival in Lung Cancer. Biomolecules 2021, 11, 1160. [Google Scholar] [CrossRef] [PubMed]
- Lubiński, J.; Marciniak, W.; Muszynska, M.; Jaworowska, E.; Sulikowski, M.; Jakubowska, A.; Kaczmarek, K.; Sukiennicki, G.; Falco, M.; Baszuk, P.; et al. Serum Selenium Levels and the Risk of Progression of Laryngeal Cancer. PLoS ONE 2018, 1, e0184873. [Google Scholar] [CrossRef] [PubMed]
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Debniak, T.; Falco, M.; Kladny, J.; et al. Serum Selenium Levels Predict Survival after Breast Cancer. Breast Cancer Res. Treat. 2018, 16, 591–598. [Google Scholar] [CrossRef]
- Pietrzak, S.; Wójcik, J.; Scott, R.J.; Kashyap, A.; Grodzki, T.; Baszuk, P.; Bielewicz, M.; Marciniak, W.; Wójcik, N.; Dębniak, T.; et al. Influence of the Selenium Level on Overall Survival in Lung Cancer. J. Trace Elem. Med. Biol. 2019, 56, 46–51. [Google Scholar] [CrossRef]
- Rogoża-Janiszewska, E.; Malińska, K.; Baszuk, P.; Marciniak, W.; Derkacz, R.; Lener, M.; Jakubowska, A.; Cybulski, C.; Huzarski, T.; Masojć, B.; et al. Serum Selenium Level and 10-Year Survival after Melanoma. Biomedicines 2021, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Szwiec, M.; Marciniak, W.; Derkacz, R.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Dębniak, T.; Jakubowska, A.; Lener, M.; Falco, M.; et al. Serum Selenium Level Predicts 10-Year Survival after Breast Cancer. Nutrients 2021, 13, 953. [Google Scholar] [CrossRef] [PubMed]
- Kornitzer, M.; Valente, F.; De Bacquer, D.; Neve, J.; De Backer, G. Serum Selenium and Cancer Mortality: A Nested Case–Control Study within an Age- and Sex-Stratified Sample of the Belgian Adult Population. Eur. J. Clin. Nutr. 2004, 58, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Sandsveden, M.; Nilsson, E.; Borgquist, S.; Rosendahl, A.H.; Manjer, J. Prediagnostic Serum Selenium Levels in Relation to Breast Cancer Survival and Tumor Characteristics. Int. J. Cancer 2020, 147, 2424–2436. [Google Scholar] [CrossRef]
- Baker, J.R.; Umesh, S.; Jenab, M.; Schomburg, L.; Tjønneland, A.; Olsen, A.; Boutron-Ruault, M.C.; Rothwell, J.A.; Severi, G.; Katzke, V.; et al. Prediagnostic Blood Selenium Status and Mortality among Patients with Colorectal Cancer in Western European Populations. Biomedicines 2021, 9, 1521. [Google Scholar] [CrossRef]
- Lubiński, J.; Jaworowska, E.; Derkacz, R.; Marciniak, W.; Białkowska, K.; Baszuk, P.; Scott, R.J.; Lubiński, J.A. Survival of Laryngeal Cancer Patients Depending on Zinc Serum Level and Oxidative Stress Genotypes. Biomolecules 2021, 11, 865. [Google Scholar] [CrossRef]
- Psathakis, D.; Wedemeyer, N.; Oevermann, E.; Krug, F.; Siegers, C.P.; Bruch, H.P. Blood Selenium and Glutathione Peroxidase Status in Patients with Colorectal Cancer. Dis. Colon Rectum 1998, 41, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.A.; Endermann, T.; Stephan, C.; Stoedter, M.; Behrends, T.; Wolff, I.; Jung, K.; Schomburg, L. Selenoprotein P Status Correlates to Cancer-Specific Mortality in Renal Cancer Patients. PLoS ONE 2012, 7, e46644. [Google Scholar] [CrossRef][Green Version]
- Lubiński, J.; Lener, M.R.; Marciniak, W.; Pietrzak, S.; Derkacz, R.; Cybulski, C.; Gronwald, J.; Dębniak, T.; Jakubowska, A.; Huzarski, T.; et al. Serum Essential Elements and Survival after Cancer Diagnosis. Nutrients 2023, 15, 2611. [Google Scholar] [CrossRef]
- Epstein, M.M.; Kasperzyk, J.L.; Andrén, O.; Giovannucci, E.L.; Wolk, A.; Håkansson, N.; Andersson, S.O.; Johansson, J.E.; Fall, K.; Mucci, L.A. Dietary Zinc and Prostate Cancer Survival in a Swedish Cohort. Am. J. Clin. Nutr. 2011, 93, 586–593. [Google Scholar] [CrossRef]
- Maret, W. Metallothionein Redox Biology in the Cytoprotective and Cytotoxic Functions of Zinc. Exp. Gerontol. 2008, 43, 363–369. [Google Scholar] [CrossRef]
- Zhang, Y.; Stopsack, K.H.; Wu, K.; Song, M.; Mucci, L.A.; Giovannucci, E. Post-Diagnostic Zinc Supplement Use and Prostate Cancer Survival Among Men With Nonmetastatic Prostate Cancer. J. Urol. 2023, 209, 549–556. [Google Scholar] [CrossRef]
- Sayehmiri, K.; Azami, M.; Mohammadi, Y.; Soleymani, A.; Tardeh, Z. The Association between Selenium and Prostate Cancer: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2018, 19, 1431–1437. [Google Scholar] [CrossRef]
- Meyer, F.; Galan, P.; Douville, P.; Bairati, I.; Kegle, P.; Bertrais, S.; Estaquio, C.; Hercberg, S. Antioxidant Vitamin and Mineral Supplementation and Prostate Cancer Prevention in the SU.VI.MAX Trial. Int. J. Cancer 2005, 116, 182–186. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Stampfer, M.J.; Wu, K.; Colditz, G.A.; Willett, W.C.; Giovannucci, E.L. Zinc Supplement Use and Risk of Prostate Cancer. JNCI J. Natl. Cancer Inst. 2003, 95, 1004–1007. [Google Scholar] [CrossRef]
- Kenfield, S.A.; Van Blarigan, E.L.; DuPre, N.; Stampfer, M.J.; Giovannucci, E.L.; Chan, J.M. Selenium Supplementation and Prostate Cancer Mortality. JNCI J. Natl. Cancer Inst. 2014, 107, dju360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, M.; Mucci, L.A.; Giovannucci, E.L. Zinc Supplement Use and Risk of Aggressive Prostate Cancer: A 30-Year Follow-up Study. Eur. J. Epidemiol. 2022, 37, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Rashtchizadeh, N.; Karimi, P.; Dehgan, P.; Salimi Movahed, M. Effects of Selenium in the MAPK Signaling Cascade. J. Cardiovasc. Thorac. Res. 2015, 7, 107–112. [Google Scholar] [CrossRef][Green Version]
- Marranci, A.; Prantera, A.; Masotti, S.; De Paolo, R.; Baldanzi, C.; Podda, M.S.; Mero, S.; Vitiello, M.; Franchin, C.; Laezza, M.; et al. PARP1 Negatively Regulates MAPK Signaling by Impairing BRAF-X1 Translation. J. Hematol. Oncol. 2023, 16, 33. [Google Scholar] [CrossRef]
- Spick, M.; Muazzam, A.; Pandha, H.; Michael, A.; Gethings, L.A.; Hughes, C.J.; Munjoma, N.; Plumb, R.S.; Wilson, I.D.; Whetton, A.D.; et al. Multi-Omic Diagnostics of Prostate Cancer in the Presence of Benign Prostatic Hyperplasia. Heliyon 2023, 9, e22604. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium in Cancer Prevention: A Review of the Evidence and Mechanism of Action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Jang, S.Y.; Choi, D.H.; Jung, C.H.; Mok, J.O.; Kim, C.H. Anti-Inflammatory and Antioxidant Effects of Selenium on Orbital Fibroblasts of Patients with Graves Ophthalmopathy. Ophthalmic Plast. Reconstr. Surg. 2021, 37, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Kelleher, S.L. Cellular Mechanisms of Zinc Dysregulation: A Perspective on Zinc Homeostasis as an Etiological Factor in the Development and Progression of Breast Cancer. Nutrients 2012, 4, 875–903. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [PubMed]
- Leone, N.; Courbon, D.; Ducimetiere, P.; Zureik, M. Zinc, Copper, and Magnesium and Risks for All-Cause, Cancer, and Cardiovascular Mortality. Epidemiology 2006, 17, 308–314. [Google Scholar] [CrossRef]
- Demircan, K.; Bengtsson, Y.; Sun, Q.; Brange, A.; Vallon-Christersson, J.; Rijntjes, E.; Malmberg, M.; Saal, L.H.; Rydén, L.; Borg, Å.; et al. Serum Selenium, Selenoprotein P and Glutathione Peroxidase 3 as Predictors of Mortality and Recurrence Following Breast Cancer Diagnosis: A Multicentre Cohort Study. Redox Biol. 2021, 47, 102145. [Google Scholar] [CrossRef]
- Chang, C.; Worley, B.L.; Phaëton, R.; Hempel, N. Extracellular Glutathione Peroxidase GPx3 and Its Role in Cancer. Cancers 2020, 12, 2197. [Google Scholar] [CrossRef]
- Selenius, M.; Rundlöf, A.K.; Olm, E.; Fernandes, A.P.; Björnstedt, M. Selenium and the Selenoprotein Thioredoxin Reductase in the Prevention, Treatment and Diagnostics of Cancer. Antioxid. Redox Signal. 2010, 12, 867–880. [Google Scholar] [CrossRef]
- Gao, K.; Zhang, Y.; Niu, J.; Nie, Z.; Liu, Q.; Lv, C. Zinc Promotes Cell Apoptosis via Activating the Wnt-3a/β-Catenin Signaling Pathway in Osteosarcoma. J. Orthop. Surg. Res. 2020, 15, 57. [Google Scholar] [CrossRef]
- To, P.K.; Do, M.H.; Cho, J.H.; Jung, C. Growth Modulatory Role of Zinc in Prostate Cancer and Application to Cancer Therapeutics. Int. J. Mol. Sci. 2020, 21, 2991. [Google Scholar] [CrossRef]
- Fernandes, A.P.; Gandin, V. Selenium Compounds as Therapeutic Agents in Cancer. Biochim. Biophys. Acta BBA—Gen. Subj. 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Bera, S.; De Rosa, V.; Rachidi, W.; Diamond, A.M. Does a Role for Selenium in DNA Damage Repair Explain Apparent Controversies in Its Use in Chemoprevention? Mutagenesis 2013, 28, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S.; Beck, F.W.J.; Snell, D.C.; Kucuk, O. Zinc in Cancer Prevention. Nutr. Cancer 2009, 61, 879–887. [Google Scholar] [CrossRef]
- Falchuk, K.H. The Molecular Basis for the Role of Zinc in Developmental Biology. Mol. Cell. Biochem. 1998, 188, 41–48. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc Deficiency in Humans: A Neglected Problem. J. Am. Coll. Nutr. 1998, 17, 542–543. [Google Scholar] [CrossRef] [PubMed]
- Ho, E. Zinc Deficiency, DNA Damage and Cancer Risk. J. Nutr. Biochem. 2004, 15, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Song, Y.; Wong, C.P.; Hardin, K.; Ho, E. Zinc Deficiency Alters DNA Damage Response Genes in Normal Human Prostate Epithelial Cells3. J. Nutr. 2008, 138, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Lancia, J.K.; Mercer, T.I.; Ip, C. Selenium Compounds Regulate P53 by Common and Distinctive Mechanisms. Anticancer Res. 2004, 24, 1401–1408. [Google Scholar] [PubMed]
- Qi, L.; Wang, Y.; Su, S.; Wang, M.; Jablonska, E.; Jia, Y.; Wang, R.; Hao, S.; Feng, C.; Li, G.; et al. Sodium Selenite Inhibits Cervical Cancer Growth via ROS Mediated AMPK/FOXO3a /GADD45a Axis. Chem. Biol. Interact. 2022, 367, 110171. [Google Scholar] [CrossRef]
- Zhu, Y.; Pu, Q.; Zhang, Q.; Liu, Y.; Ma, Y.; Yuan, Y.; Liu, L.; Zhu, W. Selenium-binding Protein 1 Inhibits Malignant Progression and Induces Apoptosis via Distinct Mechanisms in Non-small Cell Lung Cancer. Cancer Med. 2023, 12, 17149–17170. [Google Scholar] [CrossRef]
- Guo, C.H.; Wang, S.Y.; Chung, C.H.; Shih, M.Y.; Li, W.C.; Chen, P.C.; Lee, S.Y.; Hsia, S. Selenium Modulates AR/IGF-1R/EGFR and TROP2 Signaling Pathways and Improves Anticancer Efficacy in Murine Mammary Carcinoma 4T1. J. Nutr. Biochem. 2023, 120, 109417. [Google Scholar] [CrossRef]
- Cheng, Z.; Yu, S.; He, W.; Li, J.; Xu, T.; Xue, J.; Shi, P.; Chen, S.; Li, Y.; Hong, S.; et al. Selenite Induces Cell Cycle Arrest and Apoptosis via Reactive Oxygen Species-Dependent Inhibition of the AKT/MTOR Pathway in Thyroid Cancer. Front. Oncol. 2021, 11, 668424. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, G.; Zhu, X.; Jiang, K.; Wu, H.; Deng, G.; Qiu, C. Sodium Selenite Induces Apoptosis via ROS-mediated NF-κB Signaling and Activation of the Bax–Caspase-9–Caspase-3 Axis in 4T1 Cells. J. Cell. Physiol. 2019, 234, 2511–2522. [Google Scholar] [CrossRef]
- Ye, Q.; Liu, J.; Xie, K. Zinc Finger Proteins and Regulation of the Hallmarks of Cancer. Histol. Histopathol. 2019, 34, 1097–1109. [Google Scholar] [CrossRef]
- Takatani-Nakase, T. Zinc Transporters and the Progression of Breast Cancers. Biol. Pharm. Bull. 2018, 41, 1517–1522. [Google Scholar] [CrossRef]
- Franklin, R.B.; Costello, L.C. Zinc as an Anti-Tumor Agent in Prostate Cancer and in Other Cancers. Arch. Biochem. Biophys. 2007, 463, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chung, C.S.; Bruno, R.S.; Traber, M.G.; Brown, K.H.; King, J.C.; Ho, E. Dietary Zinc Restriction and Repletion Affects DNA Integrity in Healthy Men. Am. J. Clin. Nutr. 2009, 90, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Joray, M.L.; Yu, T.W.; Ho, E.; Clarke, S.L.; Stanga, Z.; Gebreegziabher, T.; Hambidge, K.M.; Stoecker, B.J. Zinc Supplementation Reduced DNA Breaks in Ethiopian Women. Nutr. Res. 2015, 35, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Bevinakoppamath, S.; Saleh Ahmed, A.M.; Ramachandra, S.C.; Vishwanath, P.; Prashant, A. Chemopreventive and Anticancer Property of Selenoproteins in Obese Breast Cancer. Front. Pharmacol. 2021, 12, 618172. [Google Scholar] [CrossRef]
- Mou, D.; Ding, D.; Yang, M.; Jiang, X.; Zhao, L.; Che, L.; Fang, Z.; Xu, S.; Lin, Y.; Zhuo, Y.; et al. Maternal Organic Selenium Supplementation during Gestation Improves the Antioxidant Capacity and Reduces the Inflammation Level in the Intestine of Offspring through the NF-ΚB and ERK/Beclin-1 Pathways. Food Funct. 2021, 12, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, Q.; Yan, L.; Ren, Y.; Fan, J.; Zhang, X.; Zhu, S. Phytosomal Tripterine with Selenium Modification Attenuates the Cytotoxicity and Restrains the Inflammatory Evolution via Inhibiting NLRP3 Inflammasome Activation and Pyroptosis. Int. Immunopharmacol. 2022, 108, 108871. [Google Scholar] [CrossRef] [PubMed]
- Makita, S.; Takatori, H.; Nakajima, H. Post-Transcriptional Regulation of Immune Responses and Inflammatory Diseases by RNA-Binding ZFP36 Family Proteins. Front. Immunol. 2021, 12, 711633. [Google Scholar] [CrossRef] [PubMed]
- Kiremidjian-Schumacher, L.; Roy, M.; Glickman, R.; Schneider, K.; Rothstein, S.; Cooper, J.; Hochster, H.; Kim, M.; Newman, R. Selenium and Immunocompetence in Patients with Head and Neck Cancer. Biol. Trace Elem. Res. 2000, 73, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, K.; Birdi, A.; Tomo, S.; Sreenivasulu, K.; Charan, J.; Yadav, D.; Purohit, P.; Sharma, P. Trace Elements as Immunoregulators in SARS-CoV-2 and Other Viral Infections. Indian J. Clin. Biochem. 2021, 36, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishna, R.; Gundimeda, U. Antioxidant Regulation of Protein Kinase C in Cancer Prevention. J. Nutr. 2002, 132, 3819S–3823S. [Google Scholar] [CrossRef]
- Nasir, A.; Bullo, M.M.H.; Ahmed, Z.; Imtiaz, A.; Yaqoob, E.; Safdar, M.; Ahmed, H.; Afreen, A.; Yaqoob, S. Nutrigenomics: Epigenetics and Cancer Prevention: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1375–1387. [Google Scholar] [CrossRef]
- Davis, C.D.; Uthus, E.O. Dietary Folate and Selenium Affect Dimethylhydrazine-Induced Aberrant Crypt Formation, Global DNA Methylation and One-Carbon Metabolism in Rats. J. Nutr. 2003, 133, 2907–2914. [Google Scholar] [CrossRef]
- Azimi, Z.; Isa, M.R.; Khan, J.; Wang, S.M.; Ismail, Z. Association of Zinc Level with DNA Methylation and Its Consequences: A Systematic Review. Heliyon 2022, 8, e10815. [Google Scholar] [CrossRef]
- Mandaviya, P.R.; Joehanes, R.; Brody, J.; Castillo-Fernandez, J.E.; Dekkers, K.F.; Do, A.N.; Graff, M.; Hänninen, I.K.; Tanaka, T.; de Jonge, E.A.L.; et al. Association of Dietary Folate and Vitamin B-12 Intake with Genome-Wide DNA Methylation in Blood: A Large-Scale Epigenome-Wide Association Analysis in 5841 Individuals. Am. J. Clin. Nutr. 2019, 110, 437–450. [Google Scholar] [CrossRef]
- McGee, M.; Bainbridge, S.; Fontaine-Bisson, B. A Crucial Role for Maternal Dietary Methyl Donor Intake in Epigenetic Programming and Fetal Growth Outcomes. Nutr. Rev. 2018, 76, 469–478. [Google Scholar] [CrossRef]
- Łoboś, P.; Regulska-Ilow, B. Link between Methyl Nutrients and the DNA Methylation Process in the Course of Selected Diseases in Adults. Rocz. Państwowego Zakładu Hig. 2021, 72, 123–136. [Google Scholar] [CrossRef]
- Jiang, C.; Kim, K.H.; Wang, Z.; Lu, J. Methyl Selenium-Induced Vascular Endothelial Apoptosis Is Executed by Caspases and Principally Mediated by P38 MAPK Pathway. Nutr. Cancer 2004, 49, 174–183. [Google Scholar] [CrossRef]
- Davis, C.D.; Finley, J.W. Chemical Versus Food Forms of Selenium in Cancer Prevention. In Functional Foods & Nutraceuticals in Cancer Prevention; Iowa State Press: Ames, IA, USA, 2003; pp. 55–86. [Google Scholar] [CrossRef]
- Rello-Varona, S. Mitotic Catastrophe Induced in HeLa Cells by Photodynamic Treatment with Zn(II)-Phthalocyanine. Int. J. Oncol. 1992, 32, 1189–1196. [Google Scholar] [CrossRef]
- Shu, Y.; Wu, M.; Yang, S.; Wang, Y.; Li, H. Association of Dietary Selenium Intake with Telomere Length in Middle-Aged and Older Adults. Clin. Nutr. 2020, 39, 3086–3091. [Google Scholar] [CrossRef]
- Shi, H.; Li, X.; Yu, H.; Shi, W.; Lin, Y.; Zhou, Y. Potential Effect of Dietary Zinc Intake on Telomere Length: A Cross-Sectional Study of US Adults. Front. Nutr. 2022, 9, 993425. [Google Scholar] [CrossRef]
- Thvilum, M.; Brandt, F.; Almind, D.; Christensen, K.; Hegedüs, L.; Brix, T.H. Excess Mortality in Patients Diagnosed With Hypothyroidism: A Nationwide Cohort Study of Singletons and Twins. J. Clin. Endocrinol. Metab. 2013, 98, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Lauria, G.; Catalano, A.; Sinicropi, M.S.; Carocci, A. Biological Activity of Selenium and Its Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 2633. [Google Scholar] [CrossRef] [PubMed]
- Binitha, M.; Sarita, S.; Betsy, A. Zinc Deficiency Associated with Hypothyroidism: An Overlooked Cause of Severe Alopecia. Int. J. Trichol. 2013, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Blanco Mejia, S.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.; et al. Selenium, Antioxidants, Cardiovascular Disease, and All-Cause Mortality: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2020, 112, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chang, C.; Zhang, Y.; Chai, Z.; Li, J.; Qiu, C. The Association between Serum Selenium Concentration and Prognosis in Patients with Heart Failure in a Chinese Population. Sci. Rep. 2021, 11, 14533. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, S.; Mori, K.; Shoji, T.; Emoto, M. Association of Zinc Deficiency with Development of CVD Events in Patients with CKD. Nutrients 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Akbari, G. Role of Zinc Supplementation on Ischemia/Reperfusion Injury in Various Organs. Biol. Trace Elem. Res. 2020, 196, 1–9. [Google Scholar] [CrossRef] [PubMed]
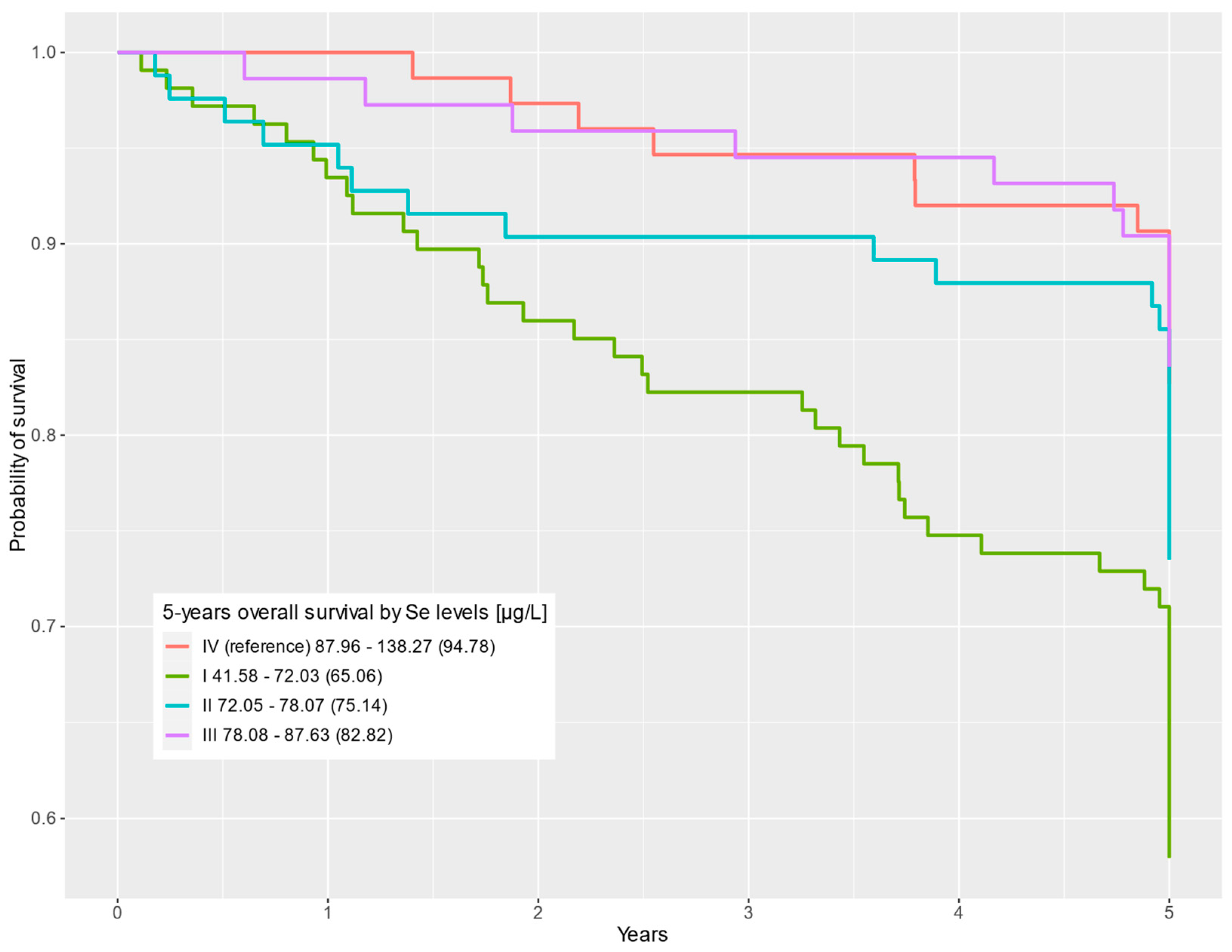
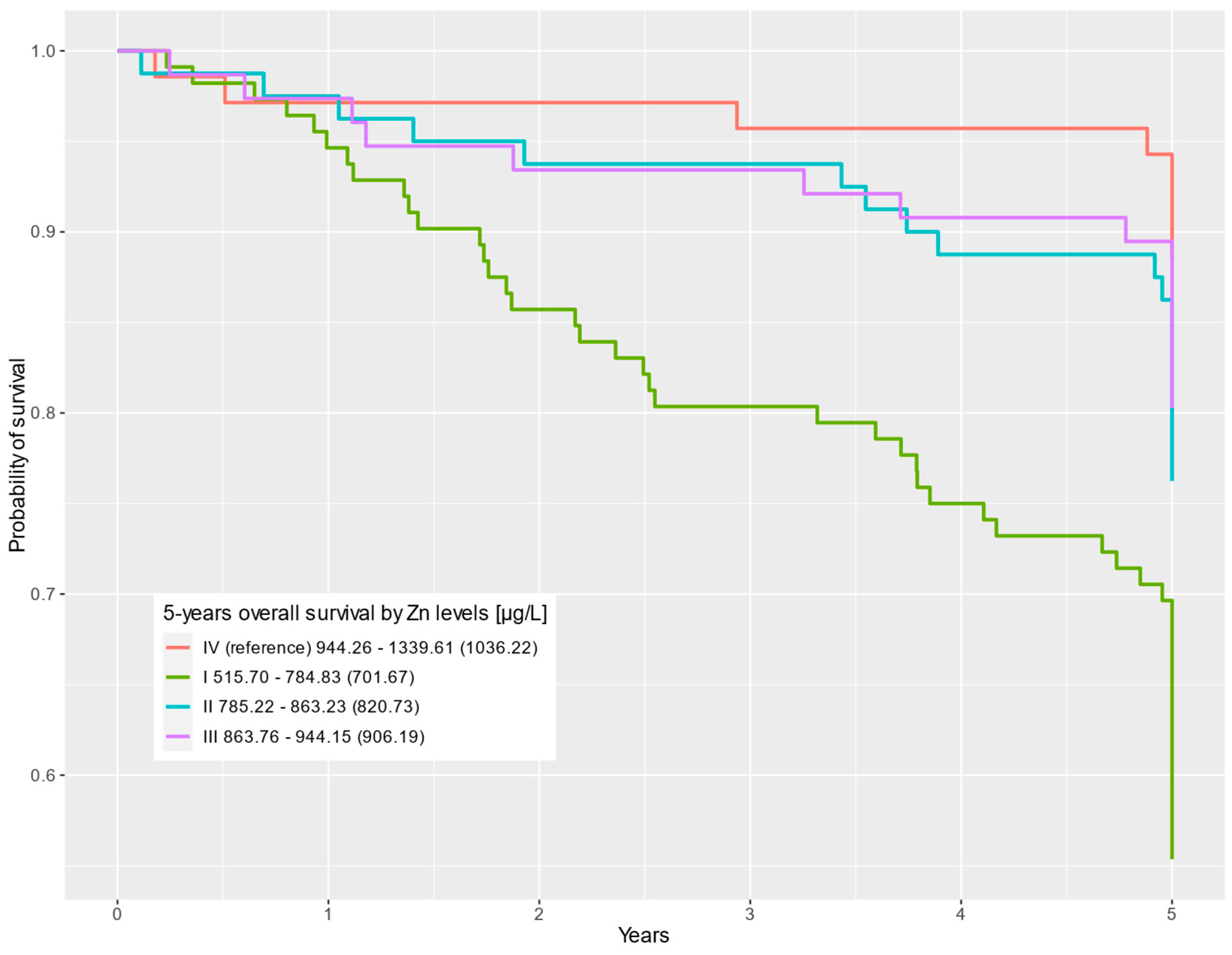
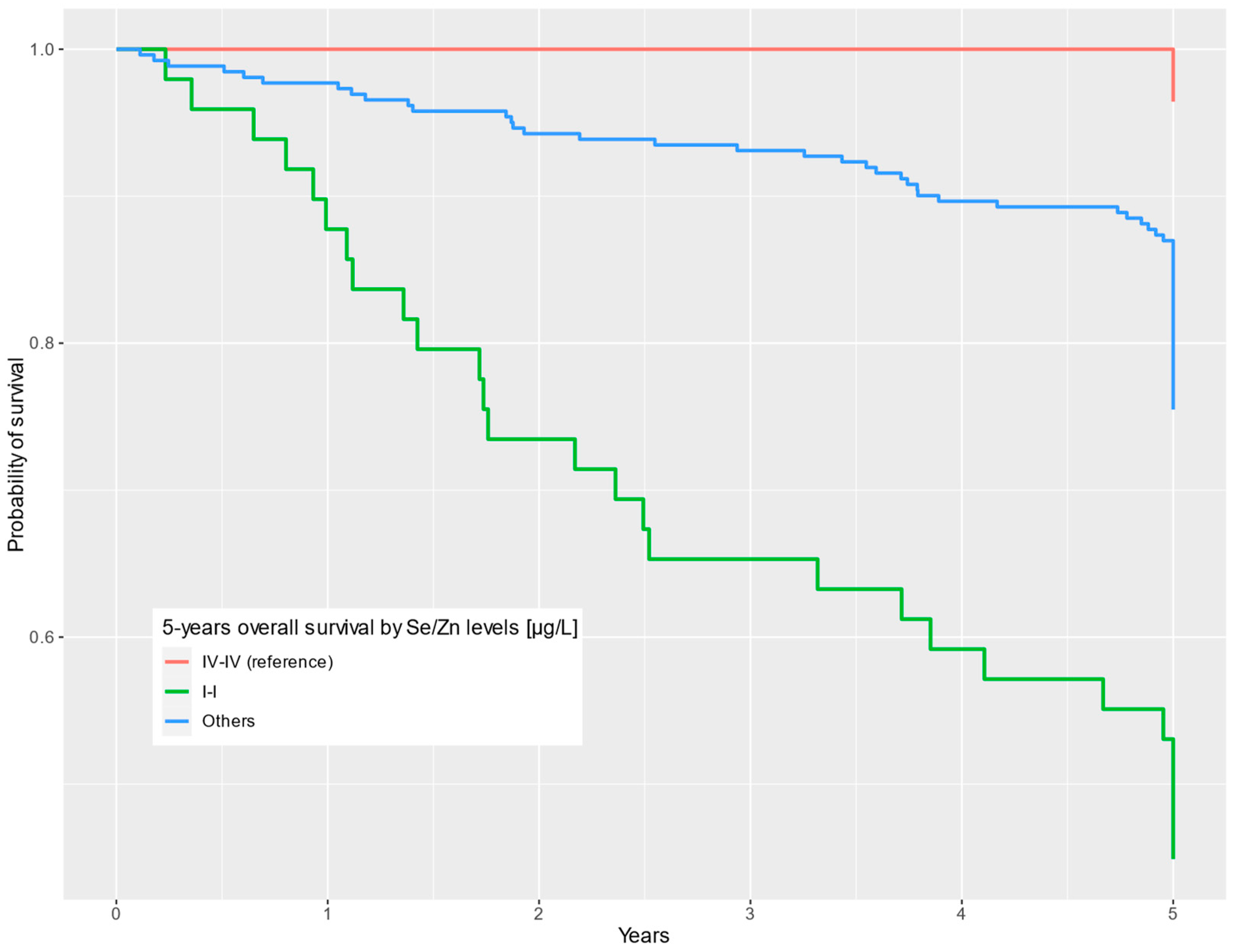
| Se | Zn | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Overall, n = 338 | QI 41.58–72.03 (65.06), n = 107 | QII 72.05–78.07 (75.14), n = 83 | QIII 78.08–87.63 (82.82), n = 73 | QIV (Reference) 87.96–138.27 (94.78), n = 75 | p | QI 515.70–784.83 (701.67), n = 112 | QII 785.22–863.23 (820.73), n = 80 | QIII 863.76–944.15 (906.19), n = 76 | QIV (Reference) 944.26–1339.61 (1036.22), n = 70 | p |
| Status | <0.001 | <0.001 | |||||||||
| Alive | 246 (73%) | 62 (58%) | 61 (73%) | 61 (84%) | 62 (83%) | 62 (55%) | 61 (76%) | 61 (80%) | 62 (89%) | ||
| Dead | 92 (27%) | 45 (42%) | 22 (27%) | 12 (16%) | 13 (17%) | 50 (45%) | 19 (24%) | 15 (20%) | 8 (11%) | ||
| Age | 0.025 | 0.059 | |||||||||
| ≤60 (reference) 41.00–60.00 (56.57) | 77 (23%) | 17 (16%) | 22 (27%) | 13 (18%) | 25 (33%) | 16 (14%) | 20 (25%) | 20 (26%) | 21 (30%) | ||
| >60 61.00–86.00 (68.45) | 261 (77%) | 90 (84%) | 61 (73%) | 60 (82%) | 50 (67%) | 96 (86%) | 60 (75%) | 56 (74%) | 49 (70%) | ||
| Gleason | 0.3 | 0.5 | |||||||||
| <7 | 114 (34%) | 43 (40%) | 22 (27%) | 28 (38%) | 21 (28%) | 43 (38%) | 25 (31%) | 27 (36%) | 19 (27%) | ||
| 7 | 166 (49%) | 46 (43%) | 49 (59%) | 33 (45%) | 38 (51%) | 47 (42%) | 41 (51%) | 37 (49%) | 41 (59%) | ||
| >7 | 58 (17%) | 18 (17%) | 12 (14%) | 12 (16%) | 16 (21%) | 22 (20%) | 14 (18%) | 12 (16%) | 10 (14%) | ||
| PSA | 0.2 | 0.2 | |||||||||
| <4 | 19 (5.6%) | 5 (4.7%) | 5 (6.0%) | 5 (6.8%) | 4 (5.3%) | 6 (5.4%) | 3 (3.8%) | 7 (9.2%) | 3 (4.3%) | ||
| 4–10 | 185 (55%) | 48 (45%) | 47 (57%) | 47 (64%) | 43 (57%) | 53 (47%) | 44 (55%) | 47 (62%) | 41 (59%) | ||
| >10 | 134 (40%) | 54 (50%) | 31 (37%) | 21 (29%) | 28 (37%) | 53 (47%) | 33 (41%) | 22 (29%) | 26 (37%) | ||
| Prostatectomy | 0.001 | <0.001 | |||||||||
| No | 68 (20%) | 31 (29%) | 16 (19%) | 11 (15%) | 10 (13%) | 34 (30%) | 17 (21%) | 12 (16%) | 5 (7.1%) | ||
| Yes | 250 (74%) | 63 (59%) | 64 (77%) | 60 (82%) | 63 (84%) | 65 (58%) | 60 (75%) | 62 (82%) | 63 (90%) | ||
| Missing | 20 (5.9%) | 13 (12%) | 3 (3.6%) | 2 (2.7%) | 2 (2.7%) | 13 (12%) | 3 (3.8%) | 2 (2.6%) | 2 (2.9%) | ||
| Radiotherapy | 0.092 | 0.023 | |||||||||
| No | 149 (44%) | 39 (36%) | 40 (48%) | 32 (44%) | 38 (51%) | 45 (40%) | 31 (39%) | 40 (53%) | 33 (47%) | ||
| Yes | 146 (43%) | 46 (43%) | 34 (41%) | 35 (48%) | 31 (41%) | 43 (38%) | 41 (51%) | 30 (39%) | 32 (46%) | ||
| Missing | 43 (13%) | 22 (21%) | 9 (11%) | 6 (8.2%) | 6 (8.0%) | 24 (21%) | 8 (10%) | 6 (7.9%) | 5 (7.1%) | ||
| Chemotherapy | 0.011 | 0.007 | |||||||||
| No | 257 (76%) | 68 (64%) | 66 (80%) | 57 (78%) | 66 (88%) | 75 (67%) | 61 (76%) | 62 (82%) | 59 (84%) | ||
| Yes | 20 (5.9%) | 11 (10%) | 4 (4.8%) | 4 (5.5%) | 1 (1.3%) | 4 (3.6%) | 7 (8.8%) | 6 (7.9%) | 3 (4.3%) | ||
| Missing | 61 (18%) | 28 (26%) | 13 (16%) | 12 (16%) | 8 (11%) | 33 (29%) | 12 (15%) | 8 (11%) | 8 (11%) | ||
| Hormonotherapy | 0.035 | 0.10 | |||||||||
| No | 197 (58%) | 49 (46%) | 50 (60%) | 48 (66%) | 50 (67%) | 55 (49%) | 46 (58%) | 51 (67%) | 45 (64%) | ||
| Yes | 107 (32%) | 41 (38%) | 24 (29%) | 21 (29%) | 21 (28%) | 39 (35%) | 27 (34%) | 20 (26%) | 21 (30%) | ||
| Missing | 34 (10%) | 17 (16%) | 9 (11%) | 4 (5.5%) | 4 (5.3%) | 18 (16%) | 7 (8.8%) | 5 (6.6%) | 4 (5.7%) | ||
| Frequency | Univariable Cox Regression | Multivariable Cox Regression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Overall, n = 338 | Alive, n = 246 | Dead, n = 92 | HR | 95% CI | p | HR | 95% CI | p |
| Se | |||||||||
| Se level | |||||||||
| QI 41.58–72.03 (65.06) | 107 (32%) | 62 (25%) | 45 (49%) | 2.94 | 1.59, 5.45 | <0.001 | 2.43 | 1.29, 4.57 | 0.006 |
| QII 72.05–78.07 (75.14) | 83 (25%) | 61 (25%) | 22 (24%) | 1.63 | 0.82–3.24 | 0.2 | 1.84 | 0.92–3.67 | 0.085 |
| QIII 78.08–87.63 (82.82) | 73 (22%) | 61 (25%) | 12 (13%) | 0.95 | 0.43–2.08 | 0.9 | 1.01 | 0.45–2.23 | >0.9 |
| QIV (reference) 87.96–138.27 (94.78) | 75 (22%) | 62 (25%) | 13 (14%) | — | — | — | — | ||
| Age | |||||||||
| ≤60 (reference) 41.00–60.00 (56.57) | 77 (23%) | 66 (27%) | 11 (12%) | — | — | — | — | ||
| >60 61.00–86.00 (68.45) | 261 (77%) | 180 (73%) | 81 (88%) | 2.37 | 1.26–4.45 | 0.007 | 2.04 | 1.07–3.87 | 0.030 |
| Gleason | |||||||||
| <7 | 114 (34%) | 80 (33%) | 34 (37%) | — | — | — | — | ||
| 7 | 166 (49%) | 135 (55%) | 31 (34%) | 0.60 | 0.37–0.98 | 0.040 | 0.62 | 0.38–1.01 | 0.056 |
| >7 | 58 (17%) | 31 (13%) | 27 (29%) | 1.84 | 1.11–3.04 | 0.018 | 1.54 | 0.92–2.59 | 0.10 |
| PSA | |||||||||
| <4 | 19 (5.6%) | 13 (5.3%) | 6 (6.5%) | — | — | — | — | ||
| 4–10 | 185 (55%) | 156 (63%) | 29 (32%) | 0.45 | 0.19–1.09 | 0.076 | 0.50 | 0.21–1.21 | 0.12 |
| >10 | 134 (40%) | 77 (31%) | 57 (62%) | 1.46 | 0.63–3.38 | 0.4 | 1.32 | 0.56–3.08 | 0.5 |
| Zn | |||||||||
| Zn level | |||||||||
| QI 515.70–784.83 (701.67) | 112 (33%) | 62 (25%) | 50 (54%) | 4.91 | 2.33–10.4 | <0.001 | 4.11 | 1.93–8.74 | <0.001 |
| QII 785.22–863.23 (820.73) | 80 (24%) | 61 (25%) | 19 (21%) | 2.22 | 0.97–5.08 | 0.058 | 2.08 | 0.91–4.75 | 0.084 |
| QIII 863.76–944.15 (906.19) | 76 (22%) | 61 (25%) | 15 (16%) | 1.81 | 0.77–4.27 | 0.2 | 1.87 | 0.79–4.42 | 0.2 |
| QIV (reference) 944.26–1339.61 (1036.22) | 70 (21%) | 62 (25%) | 8 (8.7%) | — | — | — | — | ||
| Age | |||||||||
| ≤60 (reference) 41.00–60.00 (56.57) | 77 (23%) | 66 (27%) | 11 (12%) | — | — | — | — | ||
| >60 61.00–86.00 (68.45) | 261 (77%) | 180 (73%) | 81 (88%) | 2.37 | 1.26–4.45 | 0.007 | 1.87 | 0.99–3.54 | 0.053 |
| Gleason | |||||||||
| <7 | 114 (34%) | 80 (33%) | 34 (37%) | — | — | — | — | ||
| 7 | 166 (49%) | 135 (55%) | 31 (34%) | 0.60 | 0.37–0.98 | 0.040 | 0.66 | 0.41–1.08 | 0.10 |
| >7 | 58 (17%) | 31 (13%) | 27 (29%) | 1.84 | 1.11–3.04 | 0.018 | 1.52 | 0.91–2.53 | 0.11 |
| PSA | |||||||||
| <4 | 19 (5.6%) | 13 (5.3%) | 6 (6.5%) | — | — | — | — | ||
| 4–10 | 185 (55%) | 156 (63%) | 29 (32%) | 0.45 | 0.19–1.09 | 0.076 | 0.53 | 0.22–1.30 | 0.2 |
| >10 | 134 (40%) | 77 (31%) | 57 (62%) | 1.46 | 0.63–3.38 | 0.4 | 1.54 | 0.66–3.58 | 0.3 |
| Univariable Cox Regression Models | Multivariable Cox Regression Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quartile No. | Se Level (µg/L) | Zn Level (µg/L) | Alive | Dead | HR | 95% CI | p | HR | 95% CI | p |
| SeQI-ZnQI vs. SeQIV-ZnQIV | ||||||||||
| SeQI-ZnQI | 41.58–71.79 | 515.70–784.83 | 22 | 27 | 24.5 | 3.32–180 | 0.002 | 20.9 | 2.80–156 | 0.003 |
| Others | 52.77–138.27 | 541.75–1339.61 | 197 | 64 | 7.71 | 1.07–55.6 | 0.043 | 6.52 | 0.90–47.2 | 0.063 |
| SeQIV-ZnQIV | 88.78–104.22 | 946.99–1225.28 | 27 | 1 | — | — | — | — | ||
| Study | Group (n) | Element | Survival | Cancer | Sample |
|---|---|---|---|---|---|
| Kornitzer et al. 2003 [14] | 139 | Se (≤72 vs. ≥85 μg/L *) | HR = 2.2; 95% CI = 1.3–3.7; p = 0.011 | All | Blood serum |
| Lubiński et al. 2018 [9] | 296 | Se (<50 vs. >66.8 μg/L *) | HR = 3.07; 95% CI = 1.59–5.94; p = 0.0009 | Laryngeal | Blood serum |
| Lubiński et al. 2018 [10] | 546 | Se (<81.0 vs. >81.0 μg/L *) | HR = 2.49; 95% CI = 1.53–4.04; p = 0.0002 | Breast | Blood serum |
| Pietrzak et al. 2019 [11] | 302 | Se (<57.91 vs. >69 μg/L *) | HR = 2.73; 95% CI = 1.21–6.11; p = 0.01) | Lung | Blood serum |
| Sandsveden et al. 2020 [15] | 1066 | Se (≤81 vs. ≥100.01 μg/L *) | HR = 1.67; 95% CI = 0.37–0.98 | Breast | Blood serum |
| Baker et al. 2021 [16] | 995 | Se (≤67.5 vs. ≥100 μg/L *) | HR = 1.37; 95% CI = 0.98–1.92; p = 0.06 | Colorectal | Blood serum |
| Rogoża-Janiszewska et al. 2021 [12] | 375 | Se (<76.23 vs. >96.15 μg/L *) | HR = 5.83; 95% CI = 1.32–25.8; p = 0.02 | Melanoma | Blood serum |
| Szwiec et al. 2021 [13] | 538 | Se (52.1–76.7 vs. 94.7–171.5 μg/L *) | HR = 2.31; 95% CI = 1.24–4.31; p = 0.008 | Breast | Blood serum |
| Lubiński et al. 2021 [17] | 300 | Zn (<579 vs. >688 μg/L *) | HR = 2.32;95% CI = 1.47–3.69; p < 0.01 | Laryngeal | Blood serum |
| Sukiennicki et al. 2021 [5] | 200 | Fe (<959.92 vs. >1628.62μg/L *) | HR = 1.67; 95% CI = 0.96–2.86; p = 0.07 | Lung | Blood serum |
| Lin et al. 2021 [6] | 747 | Fe (≤15.3 vs. >15.3 μmol/L *) | HR = 1.39; 95% CI = 1.11–1.92 | Oral | Blood serum |
| Rowińska et al. 2022 [7] | 375 | Fe (<893.05 vs. ≥1348.63 μg/L *) | HR = 4.66; 95% CI = 1.28–16.9; p = 0.019 | Melanoma | Blood serum |
| Pietrzak et al. 2021 [8] | 336 | Cd (<0.57 * vs. >1.97 μg/L) | HR = 7.36; 95% CI: 2.14–25.25; p < 0.01 | Lung | Blood |
| Hg (<0.44 vs. >1.30 μg/L *) | HR = 1.55; 95% CI = 1.03–2.34; p = 0.04 |
| Mechanism of Action | Se | Zn |
|---|---|---|
| Generating oxygen free radicals/involved in oxidative stress/antioxidant |
|
|
| Neoplastic growth |
| |
| DNA repair |
|
|
| Apoptosis and cell signaling |
|
|
| Maintaining DNA integrity in humans |
|
|
| Inflammation suppression |
| |
| Immune response enhancement |
| |
| Protein kinase C inactivation |
| |
| DNA methylation alteration |
|
|
| Angiogenesis inhibition |
| |
| Cell cycle blockage |
| |
| Telomere length—preserving telomere length leads to a decrease in the occurrence of age-related chronic diseases and cancers |
|
|
| Regulation of thyroid function |
| |
| Cardiovascular disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrzak, S.; Marciniak, W.; Derkacz, R.; Matuszczak, M.; Kiljańczyk, A.; Baszuk, P.; Bryśkiewicz, M.; Sikorski, A.; Gronwald, J.; Słojewski, M.; et al. Correlation between Selenium and Zinc Levels and Survival among Prostate Cancer Patients. Nutrients 2024, 16, 527. https://doi.org/10.3390/nu16040527
Pietrzak S, Marciniak W, Derkacz R, Matuszczak M, Kiljańczyk A, Baszuk P, Bryśkiewicz M, Sikorski A, Gronwald J, Słojewski M, et al. Correlation between Selenium and Zinc Levels and Survival among Prostate Cancer Patients. Nutrients. 2024; 16(4):527. https://doi.org/10.3390/nu16040527
Chicago/Turabian StylePietrzak, Sandra, Wojciech Marciniak, Róża Derkacz, Milena Matuszczak, Adam Kiljańczyk, Piotr Baszuk, Marta Bryśkiewicz, Andrzej Sikorski, Jacek Gronwald, Marcin Słojewski, and et al. 2024. "Correlation between Selenium and Zinc Levels and Survival among Prostate Cancer Patients" Nutrients 16, no. 4: 527. https://doi.org/10.3390/nu16040527
APA StylePietrzak, S., Marciniak, W., Derkacz, R., Matuszczak, M., Kiljańczyk, A., Baszuk, P., Bryśkiewicz, M., Sikorski, A., Gronwald, J., Słojewski, M., Cybulski, C., Gołąb, A., Huzarski, T., Dębniak, T., Lener, M. R., Jakubowska, A., Kluz, T., Scott, R. J., & Lubiński, J. (2024). Correlation between Selenium and Zinc Levels and Survival among Prostate Cancer Patients. Nutrients, 16(4), 527. https://doi.org/10.3390/nu16040527







