Dietary Isoeugenol Supplementation Attenuates Chronic UVB-Induced Skin Photoaging and Modulates Gut Microbiota in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and UVB Radiation
2.3. Cell Viability Assay
2.4. Assessment of the HA Levels, PIP, and MMP-1 in Cells
2.5. Animal Experiments
2.6. Evaluation of Wrinkle Formation
2.7. Evaluation of the Skin Hydration
2.8. Determination of the HA Content in Skin
2.9. Masson and Hematoxylin and Eosin (HE) Staining
2.10. Fecal DNA Extraction and 16S rRNA Gene Sequencing
2.11. Microbiota Data Analysis
2.12. Statistical Assessment
3. Results
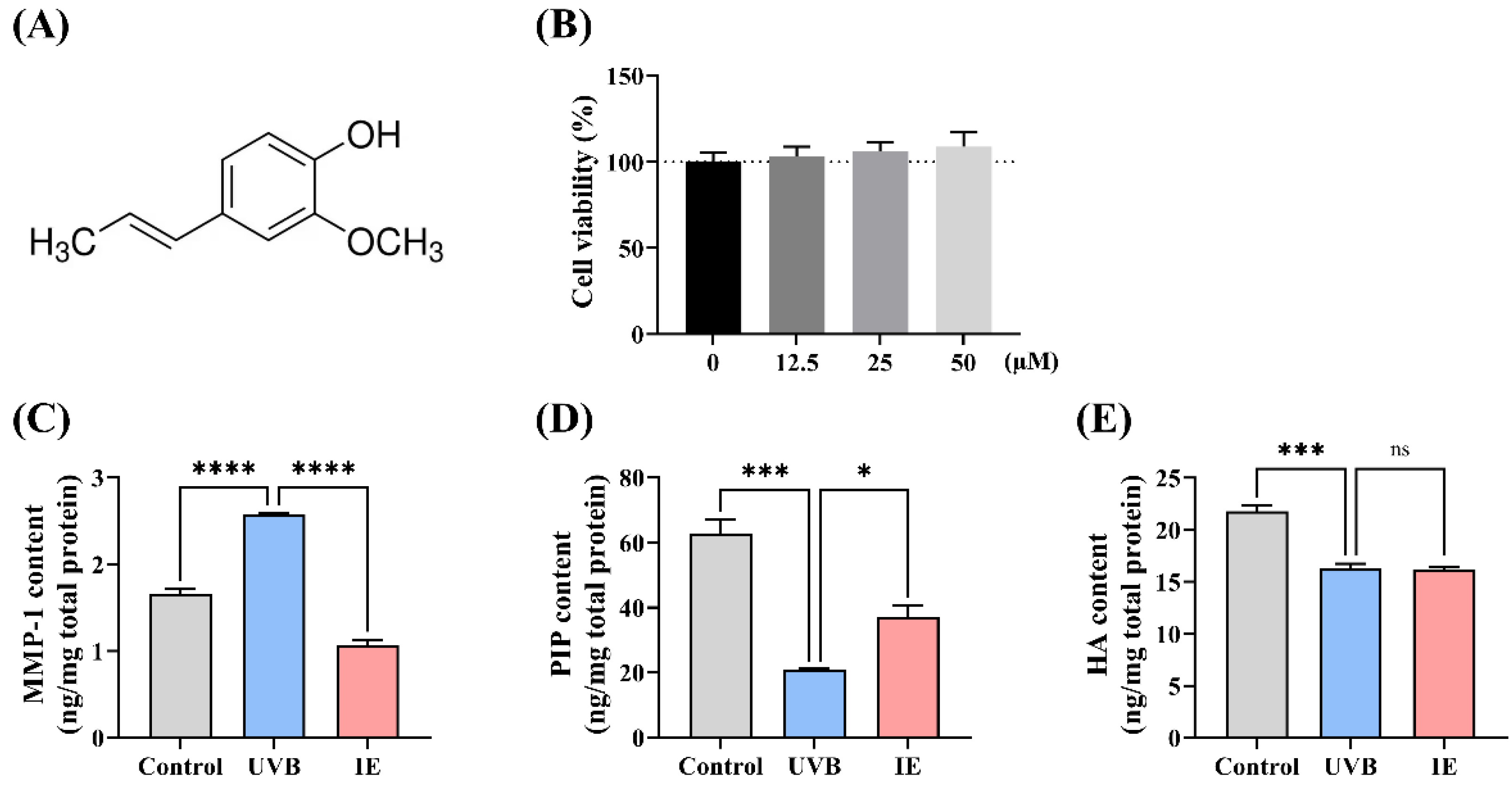
3.1. IE Mitigates UVB Irradiation-Induced Photoaging of Hs68 Cells
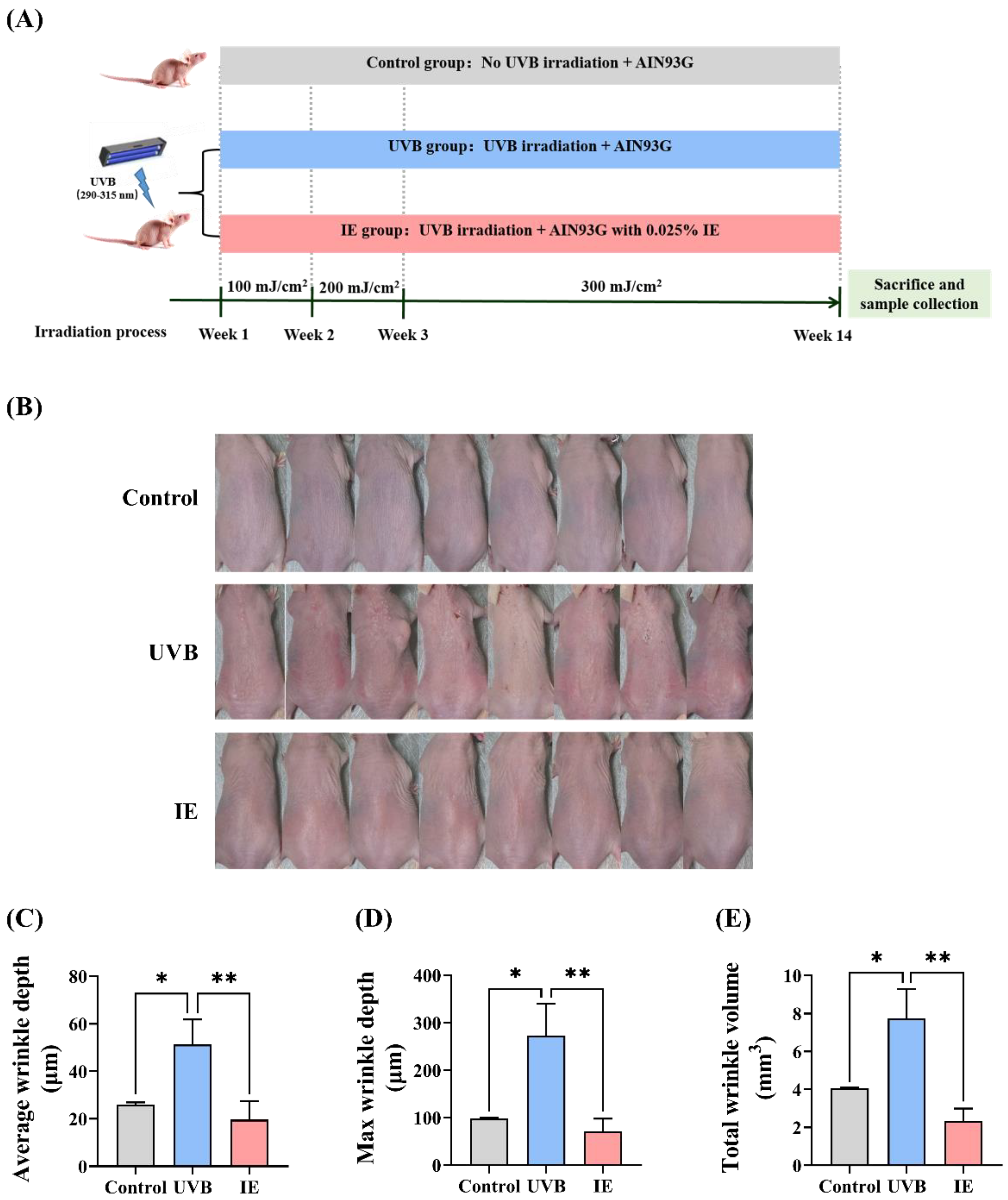
3.2. Dietary IE Alleviates Chronic UVB-Triggered Wrinkle Formation in Mice
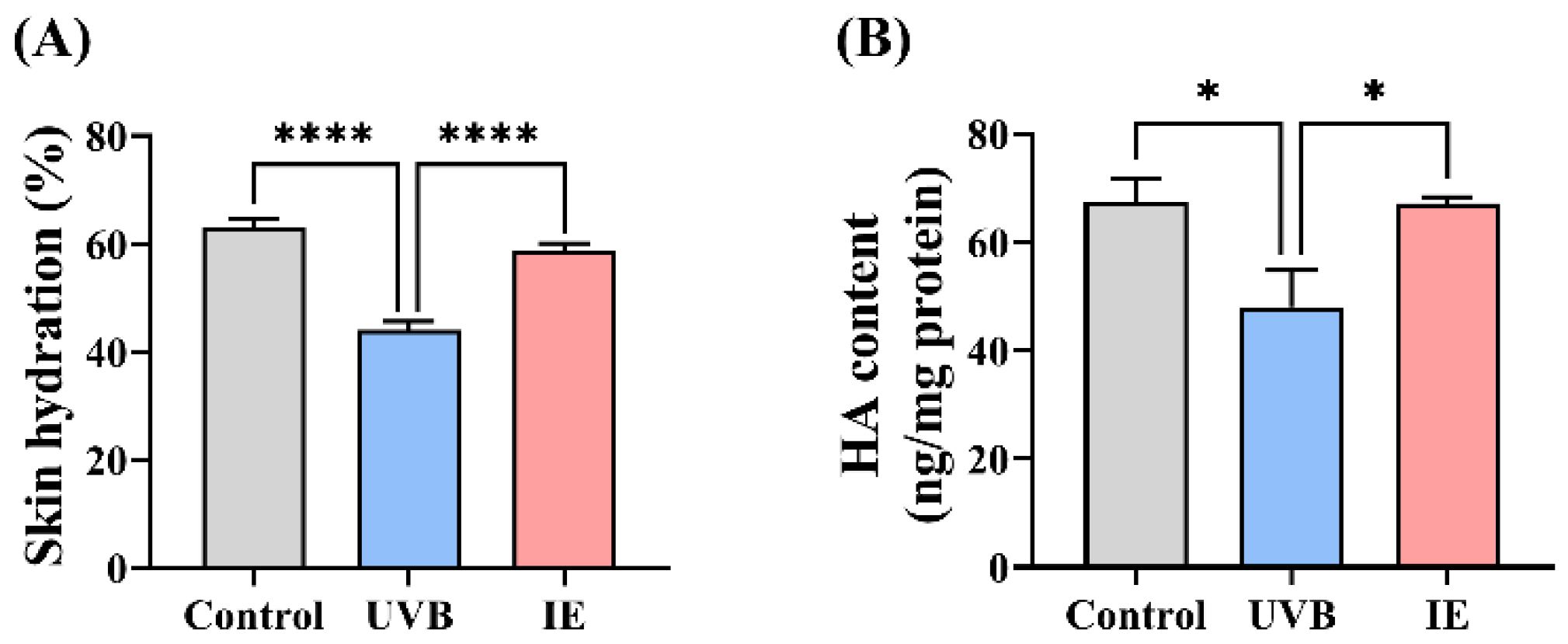
3.3. IE Relieves Skin Dryness in Photoaged Mice
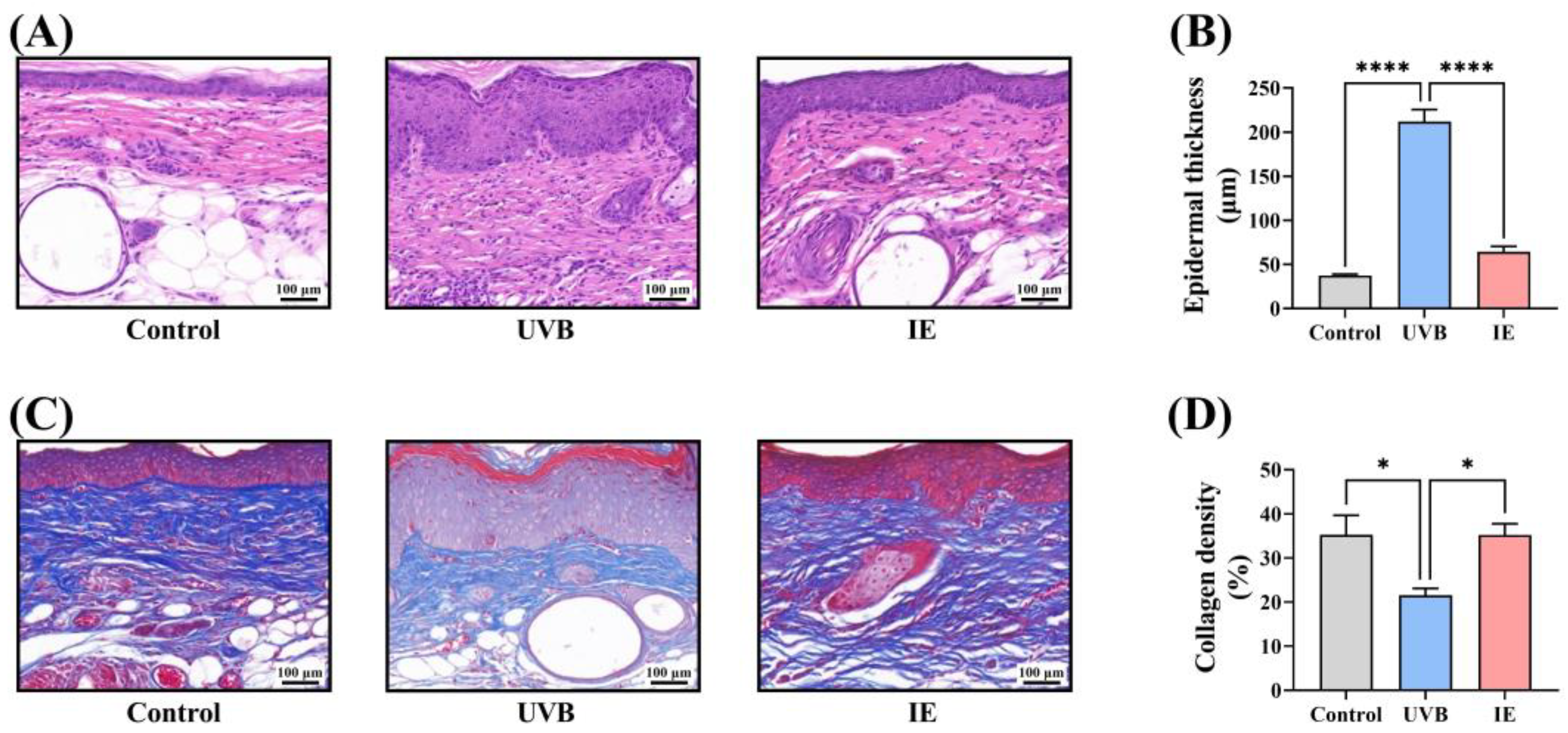
3.4. IE Inhibits Chronic UVB-Induced Epidermal Thickening in Mice
3.5. IE Prevents Chronic UVB-Induced Collagen Loss in Mouse Skin
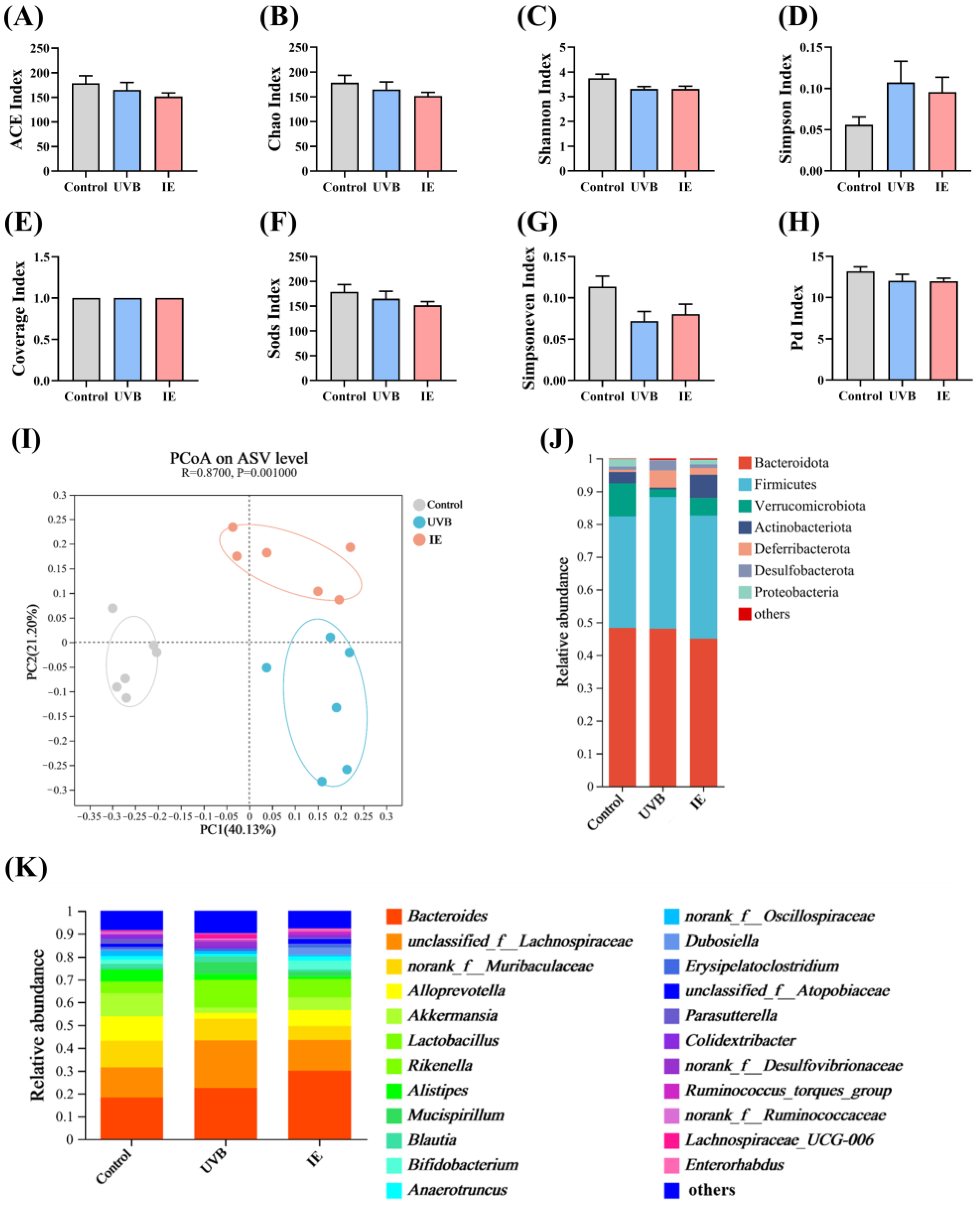
3.6. IE Induces Alterations in Both the Diversity and Composition of the Intestinal Microbiota in Mice Chronically Exposed to UVB
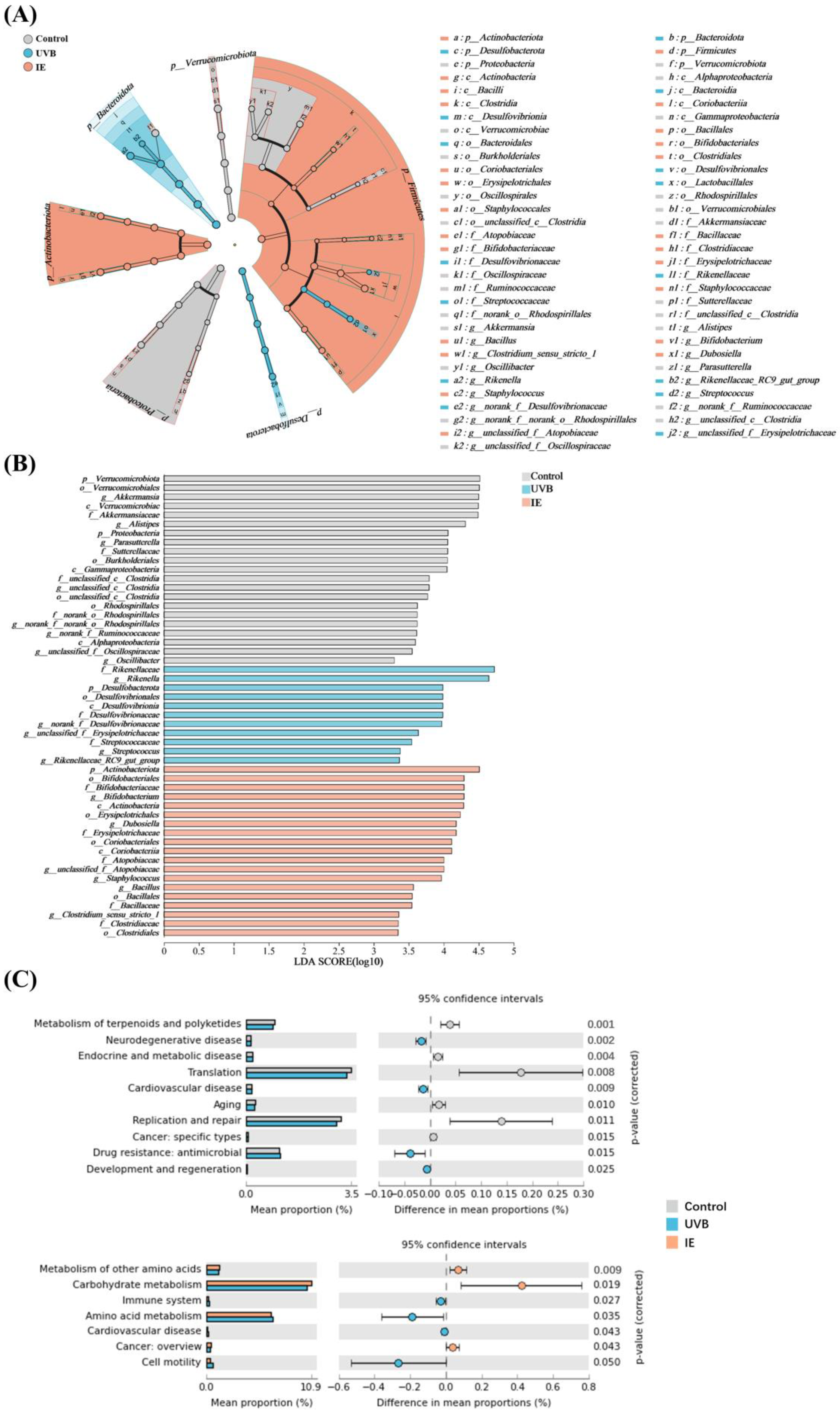
3.7. The Species Difference Analysis and Functional Prediction Analysis after IE Supplementation
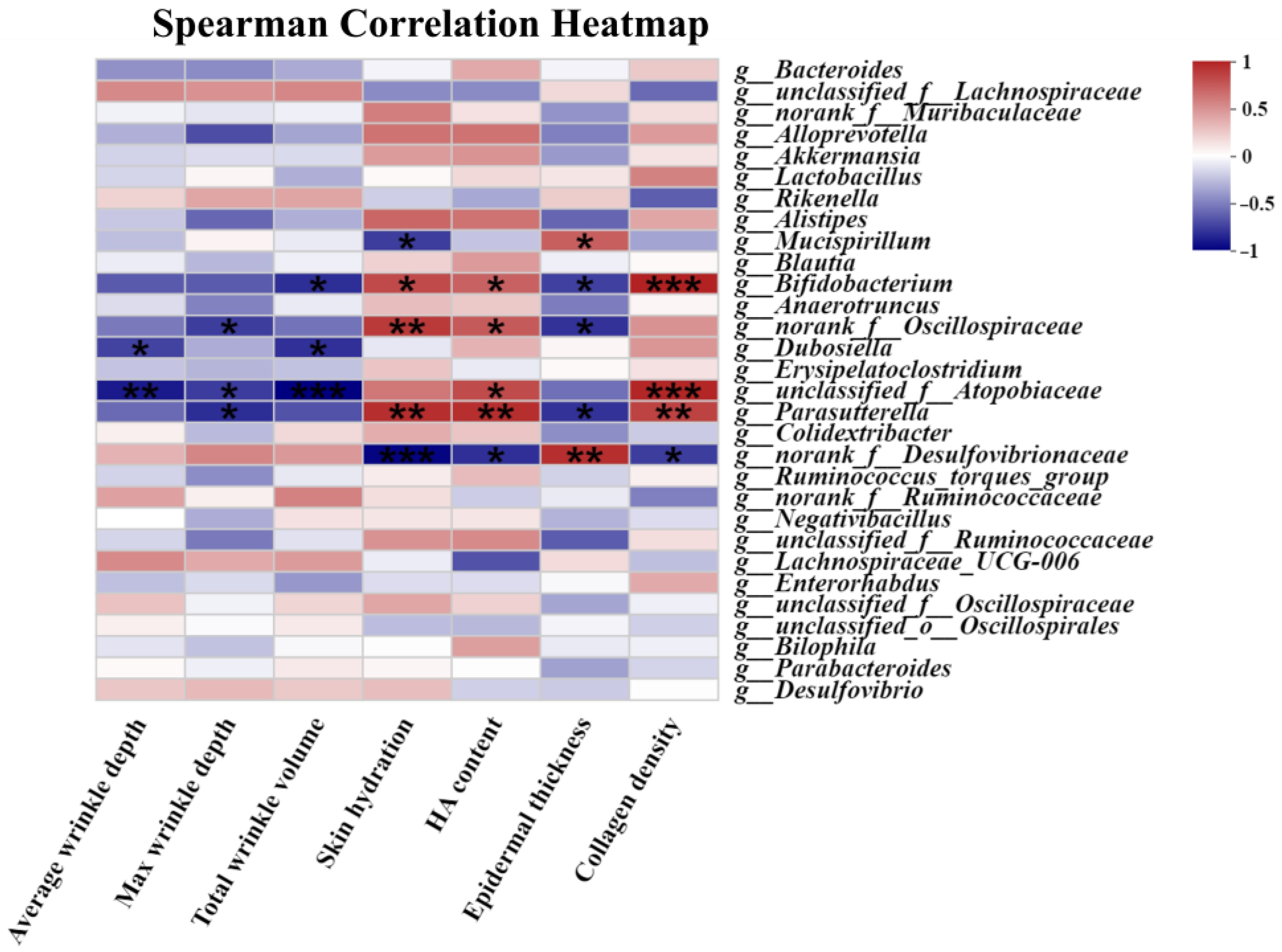
3.8. The Correlation Assessment between the Relative Abundance of Gut Microbiota and Skin Photoaging Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geng, R.; Wang, Y.; Fang, J.; Zhao, Y.; Li, M.; Kang, S.G.; Huang, K.; Tong, T. Ectopic odorant receptors responding to flavor compounds in skin health and disease: Current insights and future perspectives. Crit. Rev. Food Sci. 2023, 63, 9392–9408. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Schikowski, T.; Morita, A.; Berneburg, M. Environmentally-induced (extrinsic) skin aging: Exposomal factors and underlying mechanisms. J. Investig. Dermatol. 2021, 141, 1096–1103. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Kang, S.; Huang, K.; Tong, T. Boosting the photoaged skin: The potential role of dietary components. Nutrients 2021, 13, 1691. [Google Scholar] [CrossRef]
- Huang, A.H.; Chien, A.L. Photoaging: A review of current literature. Curr. Dermatol. Rep. 2020, 9, 22–29. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Leduc, C.; Verbeke, A.; Toussaint, O. UV, stress and aging. Derm. Endocrinol. 2012, 4, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Choi, D.; Park, T. Dietary suberic acid protects against UVB-induced skin photoaging in hairless mice. Nutrients 2019, 11, 2948. [Google Scholar] [CrossRef]
- Renz, H.; Brandtzaeg, P.; Hornef, M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat. Rev. Immunol. 2011, 12, 9–23. [Google Scholar] [CrossRef]
- Pascal, M.; Perez-Gordo, M.; Caballero, T.; Escribese, M.M.; Lopez, L.M.; Luengo, O.; Manso, L.; Matheu, V.; Seoane, E.; Zamorano, M.; et al. Microbiome and allergic diseases. Front. Immunol. 2018, 9, 1584. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, E.; Park, Y.M.; Hong, S.J. Microbiome in the gut-skin axis in atopic dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.; Ramser, A.; Isham, N.; Ghannoum, M.A. The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 2018, 9, 1459. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.A.; Monteleone, G.; McLaughlin, J.T.; Paus, R. The gut-skin axis in health and disease: A paradigm with therapeutic implications. Bioessays 2016, 38, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.A.; Rashid, H.; Tasduq, S.A. Molecular basis of skin photoaging and therapeutic interventions by plant-derived natural product ingredients: A comprehensive review. Heliyon 2023, 9, e13580. [Google Scholar] [CrossRef] [PubMed]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Xue, Q.; Xiang, Z.; Wang, S.; Cong, Z.; Gao, P.; Liu, X. Recent advances in nutritional composition, phytochemistry, bioactive, and potential applications of Syzygium aromaticum L. (Myrtaceae). Front. Nutr. 2022, 9, 1002147. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Xu, J.; Hu, Q. Comparison study on antioxidant, DNA damage protective and antibacterial activities of eugenol and isoeugenol against several foodborne pathogens. Food Nutr. Res. 2017, 61, 1353356. [Google Scholar] [CrossRef]
- GB2760-2014; National Food Safety Standard Food Additive Usage Standard. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2014.
- Hennen, J.; Kalmes, M.; Blömeke, B. Eugenol and isoeugenol as antiproliferative agents in skin cancer cells. Cancer Res. 2009, 69, 3321. [Google Scholar]
- Alharthy, K.M.; Balaha, M.F.; Devi, S.; Altharawi, A.; Yusufoglu, H.S.; Aldossari, R.M.; Alam, A.; Giacomo, V.D. Ameliorative effects of isoeugenol and eugenol against impaired nerve function and inflammatory and oxidative mediators in diabetic neuropathic rats. Biomedicines 2023, 11, 1203. [Google Scholar] [CrossRef]
- Yarosh, D.B.; Yee, V. SKH-1 hairless mice repair UV-induced pyrimidine dimers in epidermal DNA. J. Photochem. Photobiol. B Biol. 1990, 7, 173–179. [Google Scholar] [CrossRef]
- Gao, T.; Li, Y.; Wang, X.; Ren, F. The melatonin-mitochondrial axis: Engaging the repercussions of ultraviolet radiation photoaging on the skin’s circadian rhythm. Antioxidants 2023, 12, 1000. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Kang, S.; Huang, K.; Tong, T. A-ionone protects against UVB-induced photoaging in epidermal keratinocytes. Chin. Herb. Med. 2022, 15, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Geng, R.; Fang, J.; Kang, S.G.; Huang, K.; Tong, T. Chronic exposure to UVB induces nephritis and gut microbiota dysbiosis in mice based on the integration of renal transcriptome profiles and 16S rRNA sequencing data. Environ. Pollut. 2023, 333, 122035. [Google Scholar] [CrossRef] [PubMed]
- Fitsiou, E.; Pulido, T.; Campisi, J.; Alimirah, F.; Demaria, M. Cellular senescence and the senescence-associated secretory phenotype as drivers of skin photoaging. J. Investig. Dermatol. 2021, 141, 1119–1126. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Bhatia, S.; Bruze, M.; Calow, P.; Dagli, M.L.; Dekant, W.; Fryer, A.D.; Kromidas, L.; La Cava, S.; et al. RIFM fragrance ingredient safety assessment, isoeugenol, CAS Registry Number 97-54-1. Food Chem. Toxicol. 2016, 97S, S49–S56. [Google Scholar] [CrossRef]
- Quentin, T.; Franke, H.; Lachenmeier, D.W. Risk assessment of isoeugenol in food based on benchmark dose—Response modeling. Toxics 2023, 11, 991. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.P.; Fuciarelli, A.F.; Johnson, J.D.; Graves, S.W.; Bates, D.J.; Smith, C.S.; Waidyanatha, S. Toxicokinetics of isoeugenol in F344 rats and B6C3F1 mice. Xenobiotica 2013, 43, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, S.; Kaakoush, N.O.; Lloyd, F.; Gordon, L.; Forest, C.; Lawrance, I.C.; Hart, P.H. Ultraviolet irradiation of skin alters the faecal microbiome independently of vitamin d in mice. Nutrients 2018, 10, 1069. [Google Scholar] [CrossRef]
- Boyajian, J.L.; Ghebretatios, M.; Schaly, S.; Islam, P.; Prakash, S. Microbiome and human aging: Probiotic and prebiotic potentials in longevity, skin health and cellular senescence. Nutrients 2021, 13, 4550. [Google Scholar] [CrossRef]
- Vaiserman, A.; Romanenko, M.; Piven, L.; Moseiko, V.; Lushchak, O.; Kryzhanovska, N.; Guryanov, V.; Koliada, A. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population. BMC Microbiol. 2020, 20, 221. [Google Scholar] [CrossRef]
- Thevaranjan, N.; Puchta, A.; Schulz, C.; Naidoo, A.; Szamosi, J.C.; Verschoor, C.P.; Loukov, D.; Schenck, L.P.; Jury, J.; Foley, K.P.; et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 2018, 23, 570. [Google Scholar] [CrossRef]
- Patel, B.K.; Patel, K.H.; Huang, R.Y.; Lee, C.N.; Moochhala, S.M. The gut-skin microbiota axis and its role in diabetic wound healing-A review based on current literature. Int. J. Mol. Sci. 2022, 23, 2375. [Google Scholar] [CrossRef]
- Visser, M.; Kell, D.B.; Pretorius, E. Bacterial dysbiosis and translocation in psoriasis vulgaris. Front. Cell. Infect. Microbiol. 2019, 9, 7. [Google Scholar] [CrossRef]
- Okada, K.; Matsushima, Y.; Mizutani, K.; Yamanaka, K. The role of gut microbiome in psoriasis: Oral administration of staphylococcus aureus and streptococcus danieliae exacerbates skin inflammation of imiquimod-induced psoriasis-like dermatitis. Int. J. Mol. Sci. 2020, 21, 3303. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, W.; Zhang, Y.; Zeng, Y. Curcumin alleviates imiquimod-induced psoriasis-like inflammation and regulates gut microbiota of mice. Immun. Inflamm. Dis. 2023, 11, e967. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Ma, W.; Pan, Z.; Mao, B.; Tang, X.; Zhang, Q.; Zhao, J.; Chen, W.; Cui, S. Ameliorative effect of Lactobacillus plantarum CCFM8661 on oleic acid-induced acne: Integrated gut microbiota link to acne pathogenesis. J. Sci. Food Agric. 2023, 8, 13. [Google Scholar]
- Fang, Z.; Pan, T.; Li, L.; Wang, H.; Zhu, J.; Zhang, H.; Zhao, J.; Chen, W.; Lu, W. Bifidobacterium longum mediated tryptophan metabolism to improve atopic dermatitis via the gut-skin axis. Gut Microbes 2022, 14, 2044723. [Google Scholar] [CrossRef]
- Gao, T.; Li, Y.; Wang, X.; Tao, R.; Ren, F. Bifidobacterium longum 68S mediated gut-skin axis homeostasis improved skin barrier damage in aging mice. Phytomedicine 2023, 120, 155051. [Google Scholar] [CrossRef]
- Fang, Z.; Pan, T.; Wang, H.; Zhu, J.; Zhang, H.; Zhao, J.; Chen, W.; Lu, W. Limosilactobacillus reuteri attenuates atopic dermatitis via changes in gut bacteria and indole derivatives from tryptophan metabolism. Int. J. Mol. Sci. 2022, 23, 7735. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, J.; Tsui, J.C.; Wang, L.; Zhou, J.; Chan, U.K.; Lo, C.; Siu, P.; Loo, S.; Tsui, S. Unique gut microbiome signatures among adult patients with moderate to severe atopic dermatitis in southern chinese. Int. J. Mol. Sci. 2023, 24, 12856. [Google Scholar] [CrossRef] [PubMed]
- Skopelja-Gardner, S.; Tai, J.; Sun, X.; Tanaka, L.; Kuchenbecker, J.A.; Snyder, J.M.; Kubes, P.; Mustelin, T.; Elkon, K.B. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2019097118. [Google Scholar] [CrossRef] [PubMed]
- Reeve, V.E.; Allanson, M.; Domanski, D.; Painter, N. Gender differences in UV-induced inflammation and immunosuppression in mice reveal male unresponsiveness to UVA radiation. Photochem. Photobiol. Sci. 2012, 11, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.Y.; Lin, B.; Chen, Y.T.; Huang, Y.P.; Feng, W.P.; Wu, Y.; Long, G.H.; Zou, Y.N.; Liu, Y.; Lin, B.Q.; et al. Gender differences in UV-induced skin inflammation, skin carcinogenesis and systemic damage. Environ. Toxicol. Pharmacol. 2021, 81, 103512. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Control and UVB Groups (AIN93G Diet, g/kg) | IE Group (AIN93G Diet Supplemented with 0.025% IE, g/kg) |
|---|---|---|
| Corn starch | 397.486 | 397.236 |
| Casein | 200 | 200 |
| Maltodextrin | 132 | 132 |
| Sucrose | 100 | 100 |
| Soybean oil | 70 | 70 |
| Cellulose | 50 | 50 |
| Mineral mix 1 | 35 | 35 |
| Vitamin mix 2 | 10 | 10 |
| L-Cystine | 3 | 3 |
| Choline bitartrate | 2.5 | 2.5 |
| Tert-Butylhydroquinone | 0.014 | 0.014 |
| IE | - | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, R.; Kang, S.-G.; Huang, K.; Tong, T. Dietary Isoeugenol Supplementation Attenuates Chronic UVB-Induced Skin Photoaging and Modulates Gut Microbiota in Mice. Nutrients 2024, 16, 481. https://doi.org/10.3390/nu16040481
Geng R, Kang S-G, Huang K, Tong T. Dietary Isoeugenol Supplementation Attenuates Chronic UVB-Induced Skin Photoaging and Modulates Gut Microbiota in Mice. Nutrients. 2024; 16(4):481. https://doi.org/10.3390/nu16040481
Chicago/Turabian StyleGeng, Ruixuan, Seong-Gook Kang, Kunlun Huang, and Tao Tong. 2024. "Dietary Isoeugenol Supplementation Attenuates Chronic UVB-Induced Skin Photoaging and Modulates Gut Microbiota in Mice" Nutrients 16, no. 4: 481. https://doi.org/10.3390/nu16040481
APA StyleGeng, R., Kang, S.-G., Huang, K., & Tong, T. (2024). Dietary Isoeugenol Supplementation Attenuates Chronic UVB-Induced Skin Photoaging and Modulates Gut Microbiota in Mice. Nutrients, 16(4), 481. https://doi.org/10.3390/nu16040481







