Assessment of Individual and Mixed Effects of Six Minerals on Thyroid Hormones in Chinese Pregnant Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurement of Serum Element Concentrations
2.3. Maternal Thyroid Hormone Measurement
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Nutrient Concentrations during the First Trimester of Pregnancy
3.3. Linear Regression Analyses
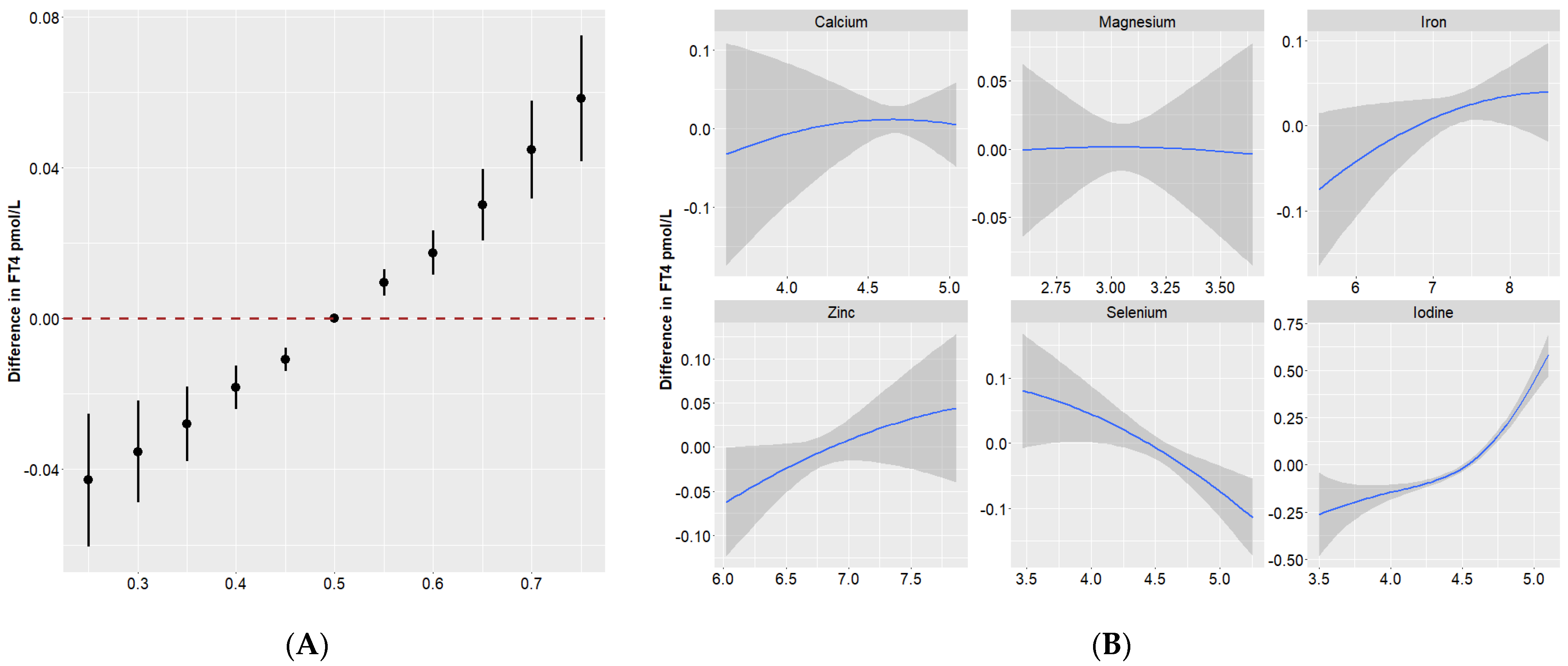
3.4. Quantile g-Computation
3.5. BKMR
4. Discussion
4.1. Effects of Iodine on Thyroid Function
4.2. Effects of Se on Thyroid Function
4.3. Effects of Zn on Thyroid Function
4.4. Effects of Ca and Mg on Thyroid Function
4.5. Effects of Fe on Thyroid Function
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senese, R.; Cioffi, F.; de Lange, P.; Goglia, F.; Lanni, A. Thyroid: Biological actions of ‘nonclassical’ thyroid hormones. J. Endocrinol. 2014, 221, R1–R12. [Google Scholar] [CrossRef]
- Zimmermann, M.B. The Importance of Adequate Iodine during Pregnancy and Infancy. World Rev. Nutr. Diet. 2016, 115, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Springer, D.; Jiskra, J.; Limanova, Z.; Zima, T.; Potlukova, E. Thyroid in pregnancy: From physiology to screening. Crit. Rev. Clin. Lab. Sci. 2017, 54, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Megier, C.; Dumery, G.; Luton, D. Iodine and Thyroid Maternal and Fetal Metabolism during Pregnancy. Metabolites 2023, 13, 633. [Google Scholar] [CrossRef] [PubMed]
- Babic, L.M.; Gunjaca, I.; Pleic, N.; Zemunik, T. Environmental Factors Affecting Thyroid-Stimulating Hormone and Thyroid Hormone Levels. Int. J. Mol. Sci. 2021, 22, 6521. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, A.; Hollenberg, A.N. New insights into thyroid hormone action. Pharmacol. Ther. 2017, 173, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, S.; Baldini, E.; Pironi, D.; Lauro, A.; D’Orazi, V.; Tartaglia, F.; Tripodi, D.; Lori, E.; Gagliardi, F.; Pratico, M.; et al. Iodine: Its Role in Thyroid Hormone Biosynthesis and Beyond. Nutrients 2021, 13, 4469. [Google Scholar] [CrossRef]
- Abel, M.H.; Korevaar, T.; Erlund, I.; Villanger, G.D.; Caspersen, I.H.; Arohonka, P.; Alexander, J.; Meltzer, H.M.; Brantsaeter, A.L. Iodine Intake is Associated with Thyroid Function in Mild to Moderately Iodine Deficient Pregnant Women. Thyroid 2018, 28, 1359–1371. [Google Scholar] [CrossRef]
- Grossklaus, R.; Liesenkotter, K.P.; Doubek, K.; Volzke, H.; Gaertner, R. Iodine Deficiency, Maternal Hypothyroxinemia and Endocrine Disrupters Affecting Fetal Brain Development: A Scoping Review. Nutrients 2023, 15, 2249. [Google Scholar] [CrossRef]
- Khanam, S. Impact of zinc on thyroid metabolism. J. Diabetes Metab. Disord. Control 2018, 5, 27–28. [Google Scholar] [CrossRef]
- Ventura, M.; Melo, M.; Carrilho, F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int. J. Endocrinol. 2017, 2017, 1297658. [Google Scholar] [CrossRef]
- Abbas, W.; Elmugabil, A.; Hamdan, H.Z.; Rayis, D.A.; Adam, I. Iron deficiency and thyroid dysfunction among sudanese women in first trimester of pregnancy: A cross-sectional study. BMC Endocr. Disord. 2023, 23, 223. [Google Scholar] [CrossRef]
- Chandra, A.K.; Goswami, H.; Sengupta, P. Dietary calcium induced cytological and biochemical changes in thyroid. Environ. Toxicol. Pharmacol. 2012, 34, 454–465. [Google Scholar] [CrossRef]
- Kolanu, B.R.; Vadakedath, S.; Boddula, V.; Kandi, V. Activities of Serum Magnesium and Thyroid Hormones in Pre-, Peri-, and Post-menopausal Women. Cureus J. Med. Sci. 2020, 12, e6554. [Google Scholar] [CrossRef]
- Darnton-Hill, I.; Mkparu, U.C. Micronutrients in pregnancy in low- and middle-income countries. Nutrients 2015, 7, 1744–1768. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets--iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Combet, E.; Gray, S.R. Nutrient-nutrient interactions: Competition, bioavailability, mechanism and function in health and diseases. Proc. Nutr. Soc. 2019, 78, 1–3. [Google Scholar] [CrossRef]
- Mayunga, K.C.; Lim-A-Po, M.; Lubberts, J.; Stoutjesdijk, E.; Touw, D.J.; Muskiet, F.; Dijck-Brouwer, D. Pregnant Dutch Women Have Inadequate Iodine Status and Selenium Intake. Nutrients 2022, 14, 3936. [Google Scholar] [CrossRef]
- Arthur, J.R.; Beckett, G.J.; Mitchell, J.H. The interactions between selenium and iodine deficiencies in man and animals. Nutr. Res. Rev. 1999, 12, 55–73. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pramesh, C.S.; Aggarwal, R. Common pitfalls in statistical analysis: Logistic regression. Perspect. Clin. Res. 2017, 8, 148–151. [Google Scholar] [CrossRef]
- Vanderpas, J.B.; Contempre, B.; Duale, N.L.; Goossens, W.; Bebe, N.; Thorpe, R.; Ntambue, K.; Dumont, J.; Thilly, C.H.; Diplock, A.T. Iodine and selenium deficiency associated with cretinism in northern Zaire. Am. J. Clin. Nutr. 1990, 52, 1087–1093. [Google Scholar] [CrossRef]
- Keil, A.P.; Buckley, J.P.; O’Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020, 128, 47004. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Valeri, L.; Claus, H.B.; Christiani, D.C.; Wright, R.O.; Mazumdar, M.; Godleski, J.J.; Coull, B.A. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015, 16, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Etemadi, A.; Amouzegar, A.; Mehran, L.; Tohidi, M.; Azizi, F.; Moradi, K.; Delshad, H. Isolated Hypothyroxinemia in Iranian Pregnant Women, the Role of Iodine Deficiency: A Population-Based Cross-Sectional Study. Thyroid 2020, 30, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Cui, T.; Chen, W.; Gao, S.; Pearce, E.N.; Wang, W.; Chen, Y.; Guo, W.; Tan, L.; Shen, J.; et al. Serum iodine concentration in pregnant women and its association with urinary iodine concentration and thyroid function. Clin. Endocrinol. 2019, 90, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yin, Y.; Cheng, Q.; Han, J.; Cheng, X.; Guo, Y.; Sun, D.; Xie, S.; Qiu, L. Validation of a simple inductively coupled plasma mass spectrometry method for detecting urine and serum iodine and evaluation of iodine status of pregnant women in Beijing. Scand. J. Clin. Lab. Investig. 2018, 78, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, S.C.; Chung, S.; Kim, S.; Yoon, J.W.; Park, Y.J. Exploring the role of copper and selenium in the maintenance of normal thyroid function among healthy Koreans. J. Trace Elem. Med. Biol. 2020, 61, 126558. [Google Scholar] [CrossRef] [PubMed]
- Kohrle, J. Selenium and the control of thyroid hormone metabolism. Thyroid 2005, 15, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Bratter, P.; Negretti, D.B.V. Influence of high dietary selenium intake on the thyroid hormone level in human serum. J. Trace Elem. Med. Biol. 1996, 10, 163–166. [Google Scholar] [CrossRef]
- Pop, V.; Krabbe, J.; Maret, W.; Rayman, M. Plasma mineral (selenium, zinc or copper) concentrations in the general pregnant population, adjusted for supplement intake, in relation to thyroid function. Br. J. Nutr. 2021, 125, 71–78. [Google Scholar] [CrossRef]
- Severo, J.S.; Morais, J.; de Freitas, T.; Andrade, A.; Feitosa, M.M.; Fontenelle, L.C.; de Oliveira, A.; Cruz, K.; Do, N.M.D. The Role of Zinc in Thyroid Hormones Metabolism. Int. J. Vitam. Nutr. Res. 2019, 89, 80–88. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, S.M.; Mulhern, M.S.; Pourshahidi, L.K.; Strain, J.J.; Yeates, A.J. Micronutrients, iodine status and concentrations of thyroid hormones: A systematic review. Nutr. Rev. 2018, 76, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Beserra, J.B.; Morais, J.; Severo, J.S.; Cruz, K.; de Oliveira, A.; Henriques, G.S.; Do, N.M.D. Relation Between Zinc and Thyroid Hormones in Humans: A Systematic Review. Biol. Trace Elem. Res. 2021, 199, 4092–4100. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Natarajan, M.; Mohanakrishnan, V. Comparison of Total and Ionic Calcium in Hypothyroidism. J. Clin. Diagn. Res. 2019, 13, BC10–BC12. [Google Scholar] [CrossRef]
- Athokpham, D.; Mohanty, S.; Pinnelli, V. Status of Serum Calcium, Phosphorus, Magnesium and Copper in Hypothyroid Patients- A Case Control Study. J. Clin. Diagn. Res. 2020, 14, BC05–BC08. [Google Scholar] [CrossRef]
- Heaton, F.W.; Humphray, H.P. Effect of magnesium status on thyroid activity and iodide metabolism. J. Endocrinol. 1974, 61, 53–61. [Google Scholar] [CrossRef]
- Ye, Y.; Li, Y.; Ma, Q.; Li, Y.; Zeng, H.; Luo, Y.; Liang, Y.; Liu, L.; Liu, L.; Lin, X.; et al. Association of multiple blood metals with thyroid function in general adults: A cross-sectional study. Front. Endocrinol. 2023, 14, 1134208. [Google Scholar] [CrossRef]
- Wang, K.; Wei, H.; Zhang, W.; Li, Z.; Ding, L.; Yu, T.; Tan, L.; Liu, Y.; Liu, T.; Wang, H.; et al. Severely low serum magnesium is associated with increased risks of positive anti-thyroglobulin antibody and hypothyroidism: A cross-sectional study. Sci. Rep. 2018, 8, 9904. [Google Scholar] [CrossRef]
- Kravchenko, V.I.; Andrusyshyna, I.M.; Luzanchuk, I.A.; Polumbryk, M.O.; Tarashchenko, Y.M. Association Between Thyroid Hormone Status and Trace Elements in Serum of Patients with Nodular Goiter. Biol. Trace Elem. Res. 2020, 196, 393–399. [Google Scholar] [CrossRef]
- Beard, J.L.; Brigham, D.E.; Kelley, S.K.; Green, M.H. Plasma thyroid hormone kinetics are altered in iron-deficient rats. J. Nutr. 1998, 128, 1401–1408. [Google Scholar] [CrossRef]
- Brigham, D.E.; Beard, J.L. Effect of thyroid hormone replacement in iron-deficient rats. Am. J. Physiol. 1995, 269, R1140–R1147. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Yuan, Z.; Li, Y.; Liu, S.; Zeng, X.; Qiu, X.; Ye, L.; Huang, D. The association between iron status and thyroid hormone levels during pregnancy. J. Trace Elem. Med. Biol. 2022, 74, 127047. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Reyes, R.; Corvilain, B.; Daelemans, C.; Wolff, F.; Fuentes, P.C.; Vandevijvere, S. Iron Deficiency Is a Risk Factor for Thyroid Dysfunction During Pregnancy: A Population-Based Study in Belgium. Thyroid 2021, 31, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Xue, S.; Zhang, L.; Chen, G. Trace elements and the thyroid. Front. Endocrinol. 2022, 13, 904889. [Google Scholar] [CrossRef] [PubMed]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Belviranli, M. Serum levels of calcium, selenium, magnesium, phosphorus, chromium, copper and iron--their relation to zinc in rats with induced hypothyroidism. Acta Clin. Croat. 2013, 52, 151–156. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Belviranli, M. L-thyroxine-induced hyperthyroidism affects elements and zinc in rats. Bratisl. Med. J. 2013, 114, 125–128. [Google Scholar] [CrossRef]
- Jain, R.B. Thyroid function and serum copper, selenium, and zinc in general U.S. population. Biol. Trace Elem. Res. 2014, 159, 87–98. [Google Scholar] [CrossRef]


| Characteristics | N = 489 |
|---|---|
| Maternal age (year, mean ± SD) | 28.90 ± 4.83 |
| Gestational week at sample collection (weeks, mean ± SD) | 9.47 ± 1.34 |
| Ethnicity [n (%)] | |
| Han | 477 (97.55) |
| Others | 12 (2.45) |
| Pre-pregnancy BMI, [n (%)] | |
| <18.5 | 79 (16.16) |
| 18.5–23.9 | 324 (66.26) |
| ≥24.0 | 86 (17.59) |
| Education, [n (%)] | |
| Middle school or lower | 116 (23.72) |
| High school | 137 (28.02) |
| Bachelor degree or higher | 236 (48.26) |
| Occupation, [n (%)] | |
| Office workers | 258 (52.76) |
| Domestic workers | 115 (23.52) |
| Others | 116 (23.72) |
| Passive smoking exposure, [n (%)] | |
| No | 397 (81.19) |
| Yes | 92 (18.81) |
| Pre-pregnancy alcohol intake, [n (%)] | |
| No | 462 (94.48) |
| Yes | 27 (5.52) |
| Thyroid function biomarkers | |
| TSH (mIU/L) | 1.11 (0.52, 1.78) |
| FT3 (pmol/L) | 4.69 (4.33, 5.15) |
| FT4 (pmol/L) | 16.37 (14.82, 17.90) |
| Element | Mean ± SD | P2.5 | P25 | P50 | P75 | P97.5 | Range |
|---|---|---|---|---|---|---|---|
| Calcium (mg/L) | 107.37 ± 13.15 | 78.58 | 101.90 | 107.90 | 114.80 | 131.28 | 37.70–154.90 |
| Magnesium (mg/L) | 21.08 ± 2.64 | 16.42 | 19.50 | 20.80 | 22.50 | 26.28 | 13.40–38.40 |
| Iron (μg/L) | 1571.1 ± 534.49 | 738.40 | 1264.00 | 1535.00 | 1807.00 | 2628.00 | 249.00–4941.00 |
| Zinc (μg/L) | 907.38 ± 240.48 | 553.80 | 777.00 | 888.00 | 989.00 | 1388.40 | 413.00–2599.00 |
| Selenium (μg/L) | 94.81 ± 21.58 | 50.88 | 81.10 | 94.20 | 106.40 | 139.92 | 32.10–191.40 |
| Iodine (μg/L) | 85.77 ± 20.29 | 54.00 | 71.50 | 84.20 | 95.80 | 132.80 | 33.10–165.20 |
| Thyroid Function Parameters | β (95%CI) | p |
|---|---|---|
| TSH | −0.43 (−0.60, −0.25) | <0.001 |
| FT3 | 0.06 (0.04, 0.08) | <0.001 |
| FT4 | 0.08 (0.06, 0.10) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; Mo, Z.; Chen, Z.; Li, X.; Jiang, Y.; Liu, C.; Guo, F.; Li, Y.; Mao, G.; Huang, X.; et al. Assessment of Individual and Mixed Effects of Six Minerals on Thyroid Hormones in Chinese Pregnant Women. Nutrients 2024, 16, 450. https://doi.org/10.3390/nu16030450
Gu S, Mo Z, Chen Z, Li X, Jiang Y, Liu C, Guo F, Li Y, Mao G, Huang X, et al. Assessment of Individual and Mixed Effects of Six Minerals on Thyroid Hormones in Chinese Pregnant Women. Nutrients. 2024; 16(3):450. https://doi.org/10.3390/nu16030450
Chicago/Turabian StyleGu, Simeng, Zhe Mo, Zhijian Chen, Xueqing Li, Yujie Jiang, Chenyang Liu, Fanjia Guo, Yahui Li, Guangming Mao, Xuemin Huang, and et al. 2024. "Assessment of Individual and Mixed Effects of Six Minerals on Thyroid Hormones in Chinese Pregnant Women" Nutrients 16, no. 3: 450. https://doi.org/10.3390/nu16030450
APA StyleGu, S., Mo, Z., Chen, Z., Li, X., Jiang, Y., Liu, C., Guo, F., Li, Y., Mao, G., Huang, X., & Wang, X. (2024). Assessment of Individual and Mixed Effects of Six Minerals on Thyroid Hormones in Chinese Pregnant Women. Nutrients, 16(3), 450. https://doi.org/10.3390/nu16030450






