Abstract
Systematic Alzheimer’s disease (AD) is a neurodegenerative disease increasingly prevalent in the aging population. AD is characterized by pathological features such as β-amyloid (Aβ) plaque accumulation, tau neurofibrillary tangles formation, oxidative stress, an impaired cholinergic system, and neuroinflammation. Many therapeutic drugs have been developed to slow the progression of AD by targeting these pathological mechanisms. However, synthetic drugs, such as donepezil and memantine, can often lead to side effects. In this context, seaweeds have been drawing attention as a nutrient source and a potential source of health-improving metabolites. Studies have shown that extracts from brown macroalgae can potentially reduce the inflammation associated with neurodegenerative diseases by inhibiting proinflammatory cytokine expression. Furthermore, their bioactive compounds exhibit antioxidant properties vital in combating oxidative stress. Antioxidants, mainly carotenoids and phenolic compounds, have been linked to improved cognitive function and a reduced risk of neurodegenerative disorders by protecting neuronal cells through their ability to scavenge free radicals. In addition, omega-3 fatty acids found in certain macroalgae have the potential to support brain health and cognitive function, further enhancing their neuroprotective effects. In conclusion, this review has comprehensively evaluated the research conducted on brown macroalgae in the last five years, covering their potential bioactive compounds, methods of obtaining these compounds, and their neuroprotective properties against AD. The limited number of clinical studies in the literature highlights the need for further research. This narrative review provides a basic framework for new approaches to neuroprotective strategies, such as those associated with brown macroalgae natural resources. Furthermore, they may play an increasingly important role in developing functional foods and nutraceuticals that can support human health in preventing and managing neurodegenerative diseases.
1. Introduction
Demographic change towards an aging population is one of the main reasons for the increase in neurodegenerative diseases, especially Alzheimer’s disease (AD). Currently, approximately 35 million people worldwide are affected by AD, and this number is estimated to increase to 65 million by 2030 [1]. AD is a complex neurodegenerative disease due to its multifactorial nature, including amyloid-beta (Aβ) accumulation, tau protein hyperphosphorylation, neuroinflammation, and oxidative stress [2]. Current treatments focus on symptomatic relief rather than addressing the underlying causes of the disease [3]. Due to the inability of this approach to completely halt the progression of the disease, there is increasing interest in alternative treatment strategies for a more effective treatment. Bioactive compounds derived from algae may offer a new generation of versatile therapeutic approaches for treating AD.
Macroalgae, commonly known as seaweed, is divided into three taxonomic groups: Rhodophyta (red), Chlorophyta (green), and Phaeophyta (brown). Of these, brown algae are the most consumed species (66.5%), followed by red (33%) and green (5%) algae [4]. Seaweeds live in various marine environments, from tropical reefs to polar waters, and show distinct adaptations to different habitats. Environmental factors, such as geographic origin, water temperature, salinity, and nutrient availability, significantly affect seaweeds’ biochemical composition and bioactive properties [5]. These environmental factors may alter the biochemical content of seaweeds, further diversifying the health benefits and potential bioactive compounds of species with the generally recognized as safe (GRAS) status. Species such as Fucus vesiculosus, Fucus serratus, Himanthalia elongata, Undaria pinnatifida, Laminaria digitata, Laminaria saccharina, and Laminaria japonica are considered safe for human consumption, and Undaria pinnatifida (wakame) and Laminaria japonica (kombu) in particular have GRAS status. These are a source of sustainable diets and require minimal resources to cultivate when compared with terrestrial crops, making macroalgae an environmentally friendly alternative for producing functional products rich in bioactive substances [6]. As they are rich in various bioactive compounds, including polyphenols [7], polysaccharides [8], carotenoids [9], and omega-3 fatty acids [10], brown algae play a role in regulating neuroinflammatory pathways, inhibiting cholinesterase, and thus protecting against the neurodegenerative processes associated with aging (Figure 1).
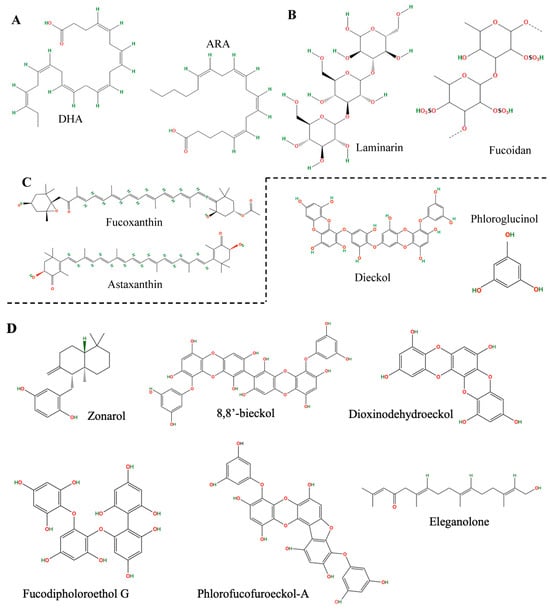
Figure 1.
Chemical structure of some neuroprotective compounds from brown macroalgae. (A) Omega-3 fatty acids: Docosahexaenoic acid (DHA) and arachidonic acid (ARA). (B) Polysaccharides: Laminarin and fucoidan. (C) Carotenoids: Fucoxanthin and astaxanthin. (D) Polyphenols and their derivatives: Dieckol, phloroglucinol, zonarol, dioxynodehydroeckol, eleganolone, fluorofucofuroeccol-A, fucodifluoroetol G and 8,8′-bieckol.
The initial focus of the thorough evaluation of the potential of these compounds was the extraction and investigation of bioactive compounds from macroalgae. Efficient extraction of bioactive compounds from macroalgae depends on the type and efficiency of the extraction methods used. In this direction, the combined evaluation of traditional methods and modern techniques is essential in improving the quality and functionality of the products obtained. In particular, advanced extraction techniques, such as enzyme-assisted extraction and supercritical fluid extraction, are used to maximize the yield and bioactivity of these products [11,12]. Efficient extraction and utilization of these bioactive products can significantly contribute to the development of new dietary supplements to prevent neurodegenerative diseases [13]. Due to the variability in the bioactive organic contents of different macroalgae species, the effects of these compounds on neurodegenerative diseases should be carefully evaluated [14]. Moreover, promising macroalgae compounds for neuroprotection should be assessed with dosage and formulation tests in order to minimize their potential risks and maximize their benefits.
Research is still ongoing to understand the deficiencies in neuroprotective mechanisms. Thanks to their rich nutrient content, macroalgae are expected to play an increasingly important role in developing functional foods and dietary supplements that support brain health. This narrative review aims to reveal the potential of bioactive compounds found in brown macroalgae as a source of neuroprotection.
2. Neuroprotective Compound Extraction from Brown Seaweed
Natural compounds with neuroprotective properties against AD are being investigated intensively because effective treatment options are limited and because such compounds generally have low side effect profile activities. Lignans, flavonoids, tannins, polyphenols, triterpenes, sterols, and alkaloids obtained from different natural sources have attracted attention as anti-AD compounds due to their antioxidant, anti-amyloidogenic, anticholinesterase, and anti-inflammatory properties [3]. Developing effective extraction methods is critical when seeking to benefit from the potential therapeutic effects of these natural compounds. Extraction techniques are decisive in obtaining these bioactive compounds efficiently, preserving their structures, and optimizing their biological activities. For instance, conventional extraction methods (solid–liquid extractions (SLEs)) are most commonly applied due to the simple configuration of the equipment and the readily available material required. However, such extractions usually employ highly toxic solvents (e.g., hexane, hydrochloric acid, chloroform, etc.) [15,16] and could be more efficient regarding extraction yield, solvent consumption, and extraction times. Therefore, emerging technologies such as ultrasound-assisted extraction (UAE), supercritical fluid extraction (SFE), pressurized liquid extraction (PLE), and subcritical water extraction (SWE), among others, have been recently described in the literature as greener alternatives to improve the efficient recovery of compounds with neuroprotective activity [17,18,19]. Details of such applications for several brown seaweed species are described in the following sections.
2.1. Conventional Solid–Liquid Extraction
Though eco-friendly ways to extract active substances from matrixes are needed, simple methods like SLE are still popular because they are ready and provide helpful information about seaweed extracts.
Brown algae species can be a rich source of carotenoids such as fucoxanthin [20] and other specific compounds that are mainly found in brown seaweed, for instance, phlorotannins [21] and fucoidans [22,23], that present potential against AD. The SLE methods mostly applied to obtain seaweed extracts are soaking (also reported as stirring) or Soxhlet extraction. Both methods rely on the solvent selection, that is, in the affinity with the solutes. Soaking involves placing the ground or powdered material in the same flask as the solvent for enough time to transfer the solutes from the solid matrix to the solvent [24]. The sample is placed into a cotton thimble in a chamber in the Soxhlet extraction. The solvent (e.g., petroleum ether, hexane, ethanol, etc.) is then boiled, thus flowing through the sample several times (facilitated by a continuous condenser), and the extractable compounds are finally collected in the bulk liquid [25].
Fucoidans from Sargassum angustifolium have been studied as potential cholinesterase inhibitors [16]. The authors used pretreatment depigmentation of the powder material before extracting fucoidans under acidic conditions using HCl 0.1 M, stirring at 250 rpm at 60 °C for 2 h. Further steps were applied to separate the fucoidans fraction. Ethanol was mixed with the extract, and the mixture was cooled for 12 h before fucoidans were resuspended in distilled water and dialyzed. Recently, another class of compounds from Sargassum macrocarpum, named meroterpenoids, has been described as a possible alternative path to decrease Aβ aggregation [26]. The extraction of the meroterpenoids was performed using SLE with ethanol, followed by purification steps using hexane, methanol, and ethyl acetate as solvents. About 16 meroterpenoids were isolated from the brown algae [26].
Shrestha et al. (2021) focused on a unique class of phenolics from Ecklonia radiata, phlorotannins, particularly dibenzodioxin-fucodiphloroethol. To obtain an enriched extract in this phlorotannin, authors applied SLE followed by purification steps [27]. The global extract was obtained with ethanol, while a subsequent refined extraction to obtain the purified compound was achieved by applying SLE with water, ethyl acetate, and butanol. High-performance counter-current chromatography (HPCCC) was also used to extract high-purity content in the target compound.
Recently, Martens et al. (2023) evaluated extracts from seven brown seaweeds (Alaria esculenta, Ascophyllum nodosum, Fucus vesiculosus, Himanthalia elongata, Saccharina latissima, Sargassum muticum, and Sargassum fusiforme) as potential neuroprotectors [28]. The extraction procedure involved soaking the dried seaweed samples in a chloroform:methanol mixture (2:1) at room temperature and under UV light exposure overnight. Then, 10 min sonication followed by paper filtering was applied to the rota-evaporated mixture to obtain the lipid extract. For the first of these, the most efficient activation was achieved using the extracts from H. elongata, which presented the highest saringosterol content. In contrast, the best activation results for the latter stood for A. esculenta, A. nosodum, F. vesiculosus, S. Latissima, and S. muticum.
2.2. Advanced Extraction Technologies for Brown Macroalgae Neuroprotection: A Greener Perspective
Traditional extraction methods use harmful solvents, but new techniques like supercritical CO2 extraction, subcritical water extraction (SWE), pressurized liquid extraction (PLE), ultrasound-assisted extraction (UAE), enzymatic-assisted extraction (EAE), and microwave-assisted extraction (MAE), use safer options and improve the process, making them better choices for extracting valuable compounds. For instance, PLE comprises a wide range of compound polarity as many solvents can be used, and it usually operates at temperatures above the boiling point of the solvent, which is one of the main advantages of this technique [29]. Similarly, in SWE, the extraction occurs at high temperatures, with water as the extraction solvent [30]. Likewise, SWE and EAE are usually associated with some hydrolysis processes and are thus applied when the target is a smaller fraction of some compound such as peptides, monosaccharides, etc. [31,32].
Non-pressurized techniques, such as UAE, usually intensify the extraction process through cavitation phenomena that create, expand, and implode the formed microbubbles in the system. These phenomena favor the extraction of compounds when they occur close enough to the solid matrix [33]. Such technologies support the release of the compounds from the matrix to the solvent. The following examples associate these new methods with compounds from brown algae with neuroprotective activity.
Hans et al. (2024) performed SWE to recover biopeptides from Padina tetrastromatica sequentially after fucoidan extraction [32]. The extraction temperatures ranged from 100 to 220 °C, and the pressure was fixed at 400 bar. The brown algae biomass to water ratio was 1:50 (w/v) and stirring of the system was kept at 250 rpm. The hydrolysis was stopped by cooling the system at the end of the reaction. The authors also performed conventional extraction using an aqueous solution (pH = 11) incubated at 30 °C for 45 min. The protein and free amino acid contents from the hydrolysates obtained from SWE was up to 80% higher than conventional extraction, and their recovery was higher as the temperature increased. These peptides, very likely obtained at 220 °C, exerted positive effects against AChE activity, achieving an IC50 value of 17.9 mg mL−1 against 65.9 mg mL−1 from conventional extraction. It is worth noting that the by-product from fucoidan production was employed to obtain biopeptides; therefore, the approach is an excellent alternative when seeking to simultaneously obtain products with different properties.
Soares et al. (2021) also used the ScW process to obtain extracts from two different brown algae: Fucus vesiculosus L. and Codium tomentosum [34]. Unlike the previously mentioned work, the pressure was 100 bar instead of 400 bar. The authors implemented the extraction in a continuous operational mode, under 10 mL min−1 water flow. They obtained four samples with temperatures ranging from room temperature to 250 °C. The activity in the enzyme inhibitions of the respective fractions were investigated for AChE, BChE, and tyrosinase, all brain enzymes that are associated with neurodegenerative diseases. Similar to the results obtained from Hans et al. (2024) [32], the result of enzyme inhibition showed positive effects when using higher extraction temperatures, as the last fraction (190–250 °C) showed the best inhibition effect for all enzymes tested.
High-pressured liquids (water and others) were also used as solvents to recover bioactives from brown algae. In the example of Ruiz-Domínguez et al. (2023), PLE was applied to Durvillaea antarctica (popularly known as cochayuyo) [19]. Firstly, the effects of different solvents (water, water/ethanol 50:0 v/v, ethanol, ethyl acetate, acetone and heptane) in the global yield and antioxidant capacities (DPPH and TEAC) obtained by conventional extraction were evaluated. As a further step, for the best solvents (water, water/ethanol), a pressurized liquid extraction system (static) was set to 10.34 bar and 30 min, with temperatures varying from 40 to 180 °C and ethanol concentration from 0 to 100% (v v−1), where the best AChE result (IC50 = 148.62 µg mL−1) was found for 110 °C and pure water. When 100% ethanol was used, AChE consistently presented the worst result (IC50 = 569.05 µg mL−1), suggesting a possible correlation of enzyme inhibition with the concentration of more hydrophilic compounds. However, considering all effects in the responses (yield, antioxidant capacity and AChE inhibition), the best extraction condition was obtained at near to 180 °C, confirming brown algae extracts’ tendency to achieve enhanced enzyme inhibition results at higher temperatures.
On the other hand, in their targeted extraction of polyphenols, Jiang et al. (2024) applied ultrasound-assisted extraction (UAE, 40 kHz, 500 W) to Sargassum pallidum [18]. The optimized condition was as follows: ethanol = 50%, ultrasonic temperature = 60 °C, and total extraction time = 40 min. The authors tested the inhibition activity of the extracts against the enzymes α-amylase, α-glucosidase, tyrosinase, and AChE. Although positive controls (acarbose, kojic acid, and donepezil) demonstrated more substantial inhibitory potential, excellent results were achieved for α-glucosidase (IC50 = 6.290 μg mL−1), suggesting a potential alternative and indirect treatment for AD by regulating blood glucose [35].
To obtain a fucoxanthin-enriched extract from Sargassum species, Hong et al. (2023) used an alternative method (acetone extraction followed by ultrasonication) to obtain a highly purified fraction of fucoxanthin for Sargassum oligocystum (from 0.4 to 3.0 mg g−1, depending on the sample collection season) [20]. The extracts resulted in promising neuroprotective effects, confirmed by the absence of cytotoxicity and protection against induced neuronal death.
Boucelkha et al. (2017) have described an EAE method by which to access the oligoguluronates from Stypocaulon scoparium using the enzyme alginate lyase [31]. Carbohydrate hydrolysis was achieved more sustainably than other conventional methods, such as acid hydrolysis, which is often described for the recovery of these compounds [36].
Regarding saccharides from brown algae, fucoidans play a key role as they present promising neuroprotective activities [37]. The fucoidans were extracted (method unknown) from Fucus vesiculosus and Undaria pinnatifida, with the latter showing the most promising results for neuroprotective activity, as its effects were most closely related to the fucoidan molecular structure, which may vary depending on the extraction/purification method. In this sense, process intensification represents an alternative to overcome such limitations. Quitain et al. (2013) present a possible alternative by applying a multi-step extraction to enhance fucoidan recovery from Undaria pinnatifida [38]. The authors applied SFE as a sample defatting method before MAE hydrolysis (600 W, 5–120 min, 110–200 °C), successfully recovering low molecular weight fucoidans at 140 °C and 30 min. Such a strategy suggests an enhancement in the bioactivity against AD when applying this extract.
3. Chemical Characterization of Extracts from Brown Macroalgae
After extracting the target compounds, detecting and identifying the analyzed molecules is essential to characterizing and quantifying the analytes. Different analytical platforms must consider the wide range of chemical structures associated with brown algae’s neuroprotective potential. Chromatographic separation, followed by a detection instrument, is one of the most widely employed techniques for determining compounds from these natural sources. Although several chromatography techniques can be used based on the physicochemical properties of the analytes, advanced techniques, such as high-performance liquid chromatography (HPLC), are usually the most utilized for bioactive compounds. To detect and provide information about the structure of bioactive compounds, numerous technologies are available, including infrared (IR) spectroscopy, fluorescence (FL) spectroscopy, ultraviolet–visible (UV–vis) spectroscopy, nuclear magnetic resonance (NMR), ion mobility spectroscopy (IMS), Raman spectroscopy, and mass spectrometry (MS). Thus, the physicochemical properties of the target compounds are determinants for the selection of the instrument. However, MS is frequently used by the scientific community, in tandem with HPLC, to characterize and to determine the composition of the chemical structure of several neuroprotective compounds from brown algae [39].
3.1. Liquid Chromatography
HPLC is a rapid and powerful separation technique based on conventional column chromatography, where a mobile phase, together with the sample, is passed through a column packed with a stationary phase. This is a versatile tool as a wide range of diverse molecules (i.e., fatty acids, polysaccharides, pigments, polyphenols) can be analyzed by modifying the gradient, the stationary and/or the mobile phase. Reversed-phase (RP) HPLC is the most commonly used technique in neuroprotective compounds of brown algae. HPLC is typically coupled to a detector, enhancing its capabilities by offering precise information about the spectroscopic properties of the analytes. Although MS is commonly used for characterizing bioactive compounds from brown algae, other instruments are also used (i.e., UV–vis, FL, or diode array detector (DAD)). Research experimental works where HPLC is coupled to several detectors have been applied to determine and characterize neuroprotective compounds extracted from brown algae and are described below and in Table 1.
Martens et al. (2023) analyzed the lipidomic extract of seven brown macroalgae (A. esculenta, A. nodosum, F. vesiculosus, H. elongata, S. latissima, S. muticum, and S. fusiform) using HPLC coupled to high-resolution mass spectrometry (HRMS) and MS/MS [28]. The use of HRMS makes it easier to determine the molecular structure of a compound by utilizing advanced software that analyzes isotope patterns and precise m/z measurements. Additionally, generating fragments from the parent molecules (MS/MS) provides a higher confirmation level for compound identification. They tentatively identified up to 323 lipids those, 47 fatty acids, 116 phospholipids, and 160 glycerolipids.
Another tool that is widely combined with liquid chromatography (LC), especially for phenolic compounds, is UV–vis spectroscopy through UV detectors and DAD (also named photodiode array detectors (PDA)). This method allows for the real-time recording of the UV–vis absorption of target molecules [40]. Nho et al. (2020) applied HPLC–DAD to quantify dieckol at 230 nm from Ecklonia cava extract, obtaining 48.08 ± 0.67 mg g−1 [41]. Another type of molecule suitable for DAD are those of the pigments. In this sense, a screening experiment where fucoxanthin was quantified in nine brown algae of the genus Sargassum was performed by Hong et al. (2023) [20]. The values quantified ranged from 3.82 to 2927.98 µg g−1 dry weight (d.w.), S. oligocystum was found to be the species with the highest accumulation capacity of fucoxanthin.
Although UV and DAD detectors have been demonstrated as useful tools for quantifying bioactive compounds, this approach presents certain limitations for compound identification. For instance, reference standards must be injected with the sample to compare retention times and UV–vis spectra with the unknown analytes. Additionally, more than chromatography may be needed to resolve and effectively separate the compounds in complex mixtures, complicating the identification process. Consequently, the use of LC coupled with UV detectors or DAD and MS, to provide complementary information when profiling bioactive compounds in complex matrices, is becoming more widespread [39].
An example of this can be observed in the work performed by Palaniveloo et al. (2023) [42], where the extract of Laurencia snackeyi was analyzed by HPLC coupled with DAD and HRMS. Thirty-seven metabolites were tentatively identified in this extract, mainly amino acids, fatty acids, terpenoids, phenolics, aromatics, and cyclic ketones (Table 1).
These analytical technologies were also used in tandem to characterize the chemical composition of the Petalonia binghamiae extract [43]. The major constituents identified were vachanic acid methyl ester (R)-ricinoleic acid, saikosaponin E, acanthoside D, and kansuinin D.
In very complex matrices with high chemical variability, it is possible that one-dimensional liquid chromatography does not have enough resolving power to separate the target molecules. In this case, two-dimensional (2D) LC is an interesting option, primarily when two different stationary phases are implemented, as it facilitates the characterization of the sample across multiple dimensions within a single analysis [44]. Montero et al. (2014) developed a 2D LC method for the separation and characterization of phlorotannins from brown algae Cystoseira abies-marina in which hydrophilic interaction liquid chromatography (HILIC) in the first dimension was combined with RP in the second dimension and coupled to DAD-MS [45]. In this work, up to 52 compounds were separated and identified, 43 of which were putatively characterized as different phlorotannins.
3.2. Other Techniques
Although LC is the most used technique to separate or quantify neuroprotection from brown algae, other techniques can also be used; for instance, gas chromatography–mass spectrometry (GC–MS) is usually used to identify thermally stable volatile chemicals. This technique sometimes requires a derivatization step where the suitability of analytes for GC analysis is enhanced, improving the sensitivity and peak separation [46]. According to Martens et al. (2023), the levels of saringosterol and fucosterol were measured using gas chromatography coupled with mass spectrometry (GC–MS) in seven species of brown algae. The concentrations of saringosterol ranged between 2 to 34 ng mg−1 (d.w.), while fucosterol concentrations ranged from 37 to 771 ng mg−1 (d. w.) along the analyzed species [28].
As an alternative to MS, Fourier transform infrared spectroscopy (FT-IR) detects compounds that can absorb infrared light. This is widely used to determine phenolics and other compounds, such as fatty acids [47]. Hang et al. (2024) used FT-IR to determine the composition of sulfated polysaccharides in the extract of Sargassum horneri. The authors putatively identified the fucoidan [47].
NMR is a valuable technique, especially for identifying pure compounds. Compared with the other detectors, one of the most significant disadvantages of NMR is that it requires several milligrams of high-purity sample. Nevertheless, NMR can give a general indication of the overall structure of the analyzed extracts [48]. Palaniveloo et al. (2023) performed an example of an NMR procedure on an extract from L. snackeyi. The author complemented LCMS with NMR and examined six metabolites, characterizing three of them as Palisadin A, Aplysistatin, and 5-Acetoxypalisadin B. The other three isolated compounds could not be identified due to the low yield [42].

Table 1.
Analytical methods for the chemical characterization of various brown algae extracts.
Table 1.
Analytical methods for the chemical characterization of various brown algae extracts.
| Family | Compounds | Brown Algae Species | Separation Technique—Detection Instrument (GC/LC Column) | Stationary Phase and Mobile Phases | Ionization Source | References |
|---|---|---|---|---|---|---|
| Lipids | Fatty acids, phospholipid, glyceroglycolipid, diglyceride and triglyceride. | Alaria esculenta, Ascophyllum nodosum, Fucus vesiculosus, Himanthalia elongata, Saccharina latissima, Sargassum muticum, and Sargassum fusiforme. | HPLC–Orbitrap–MS/MS (Poreshell 120 EC-C18) | RP. A: ACN/water 60:40 (0.1% FA and 10 mM NH4HCO2); B: IPA/ACN 90:10 (0.1% FA and 10 mM NH4HCO2). | ESI+; ESI- | [28] |
| Lipids | Sterols (saringosterol and fucosterol). | GC–MS (DB-XLB 122-1232) | - | EI | ||
| Polyphenol | Dieckol. | Ecklonia cava. | HPLC–DAD (C18 Kromasil 100-5) | RP. A: water; B: MeOH. | - | [41] |
| Amino acids, fatty acids, terpenoids, phenolics, aromatics, and cyclic ketones. | 6-aminocaproic acid, carnitine, 2-decenoic acid, docosahexaenoic acid, 5,8,11,14-eicosatetraynoic acid, 2,7,12,17-octadecanetetrol, 10,12-hexadecadienal, erucamide, 15-oxo-11,13-eicosadienoic acid, 16-hydroxypalmitic acid, 2-hydroxymyristic acid, 9,12,13-trihydroxy-15-octadecenoic acid, oleic acid, palmitic acid, 3,5-dibromo-4-hydroxybenzoic acid, 11-(2-hexyl-5-hydroxyphenoxy)-N- (2-hydroxyethyl) undecanamide, 6-gingerol, paradol, decanophenone, heptanophenone, haplamine, N-benzylformamide, 15-hydroxy-1-[2-(hydroxymethyl)-1-piperidinyl] prost-13-ene-1,9-dione, 8-[3-oxo-2-(2-penten-1-yl)-1-cyclopenten- 1-yl]octanoic acid, jasmone, laurendecumenyne A, coronaridine, ar-turmerone, retinaldehyde. | Laurencia snackeyi. | HPLC–DAD–Orbitrap MS/MS (Phenomenex C18) | RP A: water (0.1% FA); B: ACN (0.1% FA). | ESI+; ESI- | [42] |
| Palisadin A, aplysistatin and 5-acetoxypalisadin B. | NMR. | |||||
| Fatty acids, terpenoids | Vachanic acid methyl ester, (R)-ricinoleic acid, saikosaponin E, acanthoside D, and kansuinin D. | Petalonia binghamiae. | HPLC–DAD–QToF–MS (ZORBAX Eclipse Plus C18). | RP A: water (0.1% FA); B: CAN (0.1% FA). | ESI- | [43] |
| Phenolics | Total of 49 compounds: 18 phenolic acids, 22 flavonoids, 6 other polyphenols, 1 lignan and 1 stillbene. | Phyllospora comosa, Ecklonia radiata, Durvillaea sp., Sargassum sp., Cystophora sp. | LC–ESI–QTOF–MS/MS (Synergi Hydro-Reverse Phase 80°A). | RP A: water (0.1% FA); B: ACN/water (0.1% FA) (95:5). | ESI+; ESI- | [49] |
| Pigment | Fucoxanthin. | Sargassum mucclurei (1650.03 ± 7.10), S. binderi (296.07 ± 5.50), S. polycystum (3.82 ± 0.89), S. duplicatum (255.03 ± 5.71), S. denticarpum (24.25 ± 2.72), S. swartzii (217.60 ± 5.04), S. microcystum (161.52 ± 2.90), S. crassifolium (26.65 ± 2.86), S. oligocystum (2927.98 ± 8.01) *. | HPLC–DAD (Symmetry® C18). | RP ACN/MeOH (1:9, v/v). | - | [20] |
| Phenolics | Phlorotannins. | Cystoseira abies-marina. | HILIC × RP–DAD–MS/MS (lichrospher diol-5; Ascentis Express C18). | HILIC A: ACN/AcOH (98:2, v/v) B: MeOH/Water/AcOH (95:3:2, v/v/v). RP A: water (0.1% FA); B: can. | Ion trap with ESI- | [45] |
| Sulfate polysaccharide | Fucoidan. | Sargassum Horneri. | Fourier-transform infrared spectroscopy (FT-IR). | [47] | ||
| Polysaccharide | Alginate (through the measure of monosaccharides xylose, galactose, glucose, mannose, fructose, and rhamnose). | Padina pavonica, Sargassum cinereum, Turbinaria turbinata, and Dictyota dichotoma. | HPLC–UV–vis (C18). | RP A: water/ACN (90:10, v/v); B: ACN (0.045% KH2PO4–0.05% trimethylamine). | [50] | |
| Meroterpenoids | New sargasilols (9): (10′E)-10′-dehydroxy-11′,12′-dihydro-10′,11′-didehydro-12′-hydroperoxysargachromanol G; (10′E)-10′-dehydroxy-11′,12′-dihydro-10′,11′-dide-hydro-12′-hydroxysargachromanol G; 9′-deoxy-9′-oxo-11′,12′-dihydrosargachro-manol K; 9′-deoxysargachromanol K; 3′,4′-dihydro-4′,16′-didehydro-3′-oxosargachromanol E; 6′-hydroxy derivative of sargachromanol G; 15′-hydroxysargachromanol L; methylsargachro-manol E. | Sargassum siliquastrum. | NMR. | [51] |
* Concentration of target compound, measured in each specie. RP: Reversed phase; ACN: acetonitrile; MeOH: methanol; AcOH: acetic acid; NH4HCO2: ammonium formate; KH2PO4: monopotassium phosphate.
4. Neuroprotection Assays of Brown Macroalgae Extracts
AD is a complex and multifactorial disease resulting from the combination of several interacting factors (Figure 2). Due to this complexity, an ideal set of biological assays should be used to evaluate the potential of anti-AD compounds. These methods include the evaluation of enzyme inhibitors such as AChE and BChE and of biomarkers such as the inhibition of Aβ plaques. Therefore, in neuroprotection tests, to evaluate the effectiveness of anti-AD extracts, both AChE and BChE inhibitors and measurement of the inhibition of Aβ plaques play essential roles. These tests assess the effects of compound candidates on different aspects of AD, contributing to the development of treatment strategies.
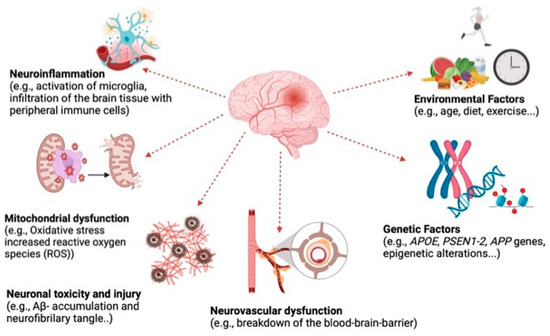
Figure 2.
Fundamental mechanisms that cause neurodegeneration (created by BioRender.com).
Table 2 presents the neuroprotective activity of various brown algae extracts in vitro and in vivo studies. It summarizes the findings evaluating the potential benefits of compounds derived from algae against AD in different biological models.
4.1. Cholinesterase Inhibitors
AChE and BChE inhibitor tests are generally in vitro assays for assessing neuroprotection. AChE is an enzyme that plays a critical role in neurotransmission and is responsible for the breakdown of a neurotransmitter called acetylcholine. Similarly, BChE is responsible for the degradation of butyrylcholine, a different substrate [52]. In diseases such as Alzheimer’s disease, excessive activity of AChE can, in particular, cause communication disorders between nerve cells, which may aggravate the symptoms of the disease. Therefore, AChE and BChE inhibitor tests play a critical role in discovering and developing potential therapeutics.
Various brown algae metabolites that inhibit cholinesterase activity are reported in Table 2. For example, Pedro et al. (2021) found that extracts of Undaria pinnatifida inhibited AChE enzyme activity by 50% at a concentration of 1 mg mL−1 [53]. In the study of Gomes et al. (2022), the neuroprotective potential of extracts obtained from Himanthalia elongata and Eisenia bicycle species was investigated. In the study, it was shown that a fraction of H. elongata and E. bicyclis (at a concentration of 2 mg mL−1) provided 40% AChE inhibition for H. elongata, 50% for E. bicyclis, and 40% BChE inhibition in both species. Although no high effect was observed regarding enzyme inhibition, it was determined that the fourth fraction obtained at the highest temperature (250 °C) showed particularly strong radical scavenging activities [54]. This was because the fractions obtained at high temperatures consisted of phenolic compounds and Maillard reaction products. This combination contributed to the antioxidant activities of the extracts and thus their neuroprotective properties. Ruiz-Domínguez et al. (2023) showed that the most effective extracts in terms of AChE inhibitory capacity (IC50 value was 148.62 μg mL−1) could be obtained at low-medium temperatures (40–110 °C) and using 100% water by pressurized liquid extraction (PLE) from Durvillaea antarctica [19].
Fucoxanthin is a naturally occurring carotenoid in brown macroalgae, and its AChE inhibition activity means that it is capable of promising neuroprotective effects. Fucoxanthin isolated from Sargassum oligocystum has also showed inhibitory activity on AChE (IC50 = 130.12 ± 6.65 µg mL−1) [20]. Hong et al. (2023) studied the biological activities of fucoxanthin obtained from the seaweed Sargassum oligocystum with acetone using sonication at room temperature. After being isolated from the seaweed S. oligocystum, fucoxanthin showed inhibitory activity on AChE (IC50 = 130.12 ± 6.65 µg mL−1) [20].
Brown macroalgae are rich natural sources of phenolic compounds and can inhibit cholinesterase activity. For instance, polyphenol-rich extract (PEEC) from Ecklonia cava was shown to have a strong inhibitory effect on AChE and BChE enzymes. In addition, the IC50 value of PEEC on AChE inhibition was determined as 68.9 μg ml−1, and the IC50 value on BChE inhibition was determined as 217.7 μg ml−1 [41]. Shrestha et al., (2021) showed that dibenzodioxin-fucodiphloroethol (DFD) from Ecklonia radiata, had moderate AChE inhibitory activity (IC50 value 41 μM) and shared similar binding residues with donepezil [27]. Park et al., (2019) showed the capacity of polyphenol extracts from Ecklonia cava to inhibit AChE, and the IC50 value was determined as 70.63 μg mL−1. In addition, the fucoidan extract in the study effectively suppressed AChE activity, increasing Ach levels in the brain tissue in the TMT-induced cholinergic system dysfunction model [8]. Although the polyphenol-containing extract showed AChE inhibition in vitro, this effect could not be sufficiently demonstrated in the in vivo model. It was determined that fucoidan from Sargassum angustifolium had moderate AChE inhibitory activity and that its IC50 value was 1.20 µg mL−1 [16]. Hans et al. (2024) determined that the hydrolysate obtained from Padina tetrastromatica inhibits AChE in vitro, with the IC50 value being determined to be 17.9 ± 0.1 mg mL−1 [32]. In the amino acid profile of the hydrolysate, 45% essential amino acids were detected, and it was revealed that the hydrolysate also contained phenolic compounds (23.9 ± 1.4 mg GAE g−1) and flavonoids (1.23 ± 0.1 mg QE g−1). It has been reported that the extracts of Durvillaea incurvate obtained by the UAE method effectively inhibited AChE (IC50: 48.55 ± 0.021 µg mL−1, 51.5% inhibition) and BChE (IC50: 87.58 ± 0.044 µg mL−1, 32.8% inhibition) enzymes [55].
Sulfated polysaccharides from brown macroalgae inhibit cholinesterase enzymes, contributing to synaptic transmission and preservation of cognitive functions. For example, sulfated polysaccharides of Sargassum horneri showed high selectivity on AChE, and AChE inhibition was IC50 = 9.77 µM. These findings indicate that sulfated polysaccharides obtained from Sargassum horneri can be evaluated as potential cholinesterase inhibitors against AD [47].
Future studies focusing on obtaining a wider range of biologically active compounds, examining their neuroprotective effects, optimizing these techniques, and demonstrating their applicability on an industrial scale may enable the more widespread use of cholinesterase inhibitors obtained from natural sources.
Furthermore, there are studies in which the inhibitory interactions and binding mechanisms of bioactive compounds from brown algae with target enzymes are evaluated in detail through enzyme kinetics and molecular docking simulations. Palaniveloo et al. (2023) have reported that their extracts from Laurencia snacke (a purplish-brown algae), showed IC50 values of 14.45 ± 0.34 μg mL−1 for AChE and 39.59 ± 0.24 μg mL−1 for BChE [42]. In addition, in their molecular docking analysis to identify potential candidates that can inhibit AChE and BChE, 5,8,11,14-eicosatetraynoic acid and 15-hydroxy-1-[2-(hydroxymethyl)-1-piperidinyl]prost-13-ene-1,9-dione were the compounds that exhibited better inhibition properties.
In another study, phlorotannins from Ecklonia cava were shown to have AChE inhibition, with IC50 values ranging from 0.9 ± 0.8 to 66.5 ± 0.4 μM, and BChE inhibition, with IC50 values ranging from 1.4 ± 3.8 to 25.2 ± 0.1 μM. In addition, enzyme kinetics and molecular docking simulations evaluated the inhibitory interaction and binding mechanism between these active compounds and the target enzyme, thus supporting their inhibitory potential [56].
A study using molecular dynamics simulation and binding energy calculations, using AChE as a target, identified diplorethohydroxycarmalol and phlorofucofuroeckol from brown seaweeds as potential lead compounds for neurodegenerative diseases [57]. When in vitro tests and simulations (dockings) are analyzed together, a more comprehensive perspective is obtained to understand the effects of potential drug compounds on biological targets. Using these two methods together offers numerous advantages when evaluating the effectiveness of potential drug candidates in biological systems. In vitro tests provide essential data to confirm the biological significance of molecular interactions and how compounds may behave in real life. By identifying the most likely interactions among millions of possible interactions, simulations can reduce the cost and time of laboratory studies and help one to understand molecular mechanisms. As a result, these simulations play a vital role in discovering potential drug candidates for treating complex diseases such as AD.
In conclusion, studies conducted in the last five years have evaluated the cholinesterase inhibitory activities of compounds derived from brown algae. These inhibitor activities are generally expressed as IC50 values, ranging from 0.0012 to 5 mg mL−1. This range varies depending on the algae species, the richness of the extracts in bioactive compounds, and the compound structure and type. AD involves many factors beyond cholinesterase inhibitors. Characteristic features of Alzheimer’s include Aβ plaque formation, tau protein aggregates, oxidative stress, and neuroinflammation. Therefore, evaluating the neuroprotective effects of extracts from brown algae requires a multifaceted approach.
4.2. Amyloid-Beta (Aβ) Inhibition
Aβ inhibitors are essential in treating AD because they aim to reduce the production or accumulation of Aβ peptides in the brain. For example, Chagas Monteiro et al. (2023) have stated that inhibiting β-secretase (BACE-1) and γ-secretases, which play a role in the degradation of amyloid precursor protein (APP), is effective in preventing the accumulation of Aβ plaques and oligomers [58].
Several metabolites from seaweeds have shown anti-amyloidogenic potentials (Table 2). For example, Kim et al. (2020) have stated that the ethanol extract of Ishige foliacea inhibits the BACE1 enzyme dose dependently (effective at the highest concentration (77.8 ± 1.9%, 1000 μg mL−1), and the IC50 value for BACE1 inhibition was 266.8 ± 34.9 μg mL−1 [59].
In one study, 8,8′-bieckol, dieckol, and eckol from Ecklonia cava were found to have lower IC50 values for BACE1 inhibition than the resveratrol (IC50, 14.89 ± 0.54 µM) used as a positive control—1.62 ± 0.14 µM for 8,8′-bieckol, 2.34 ± 0.10 µM for dieckol, and 7.67 ± 0.71 µM for eckol. This study demonstrates the effective inhibition of these compounds on BACE1 [60].
Monoamine oxidases A and B (hMAO-A and hMAO-B) are important isoenzymes that catalyze the oxidative deamination of neurotransmitters and amines and play critical roles in regulating synaptic neurotransmission. However, overexpression of hMAOs can cause oxidation reduction imbalances and mitochondrial damage, leading to neurodegenerative diseases [61]. Kwon et al. (2022) identified two new terpenoid lactones (1 and 2) from Sargassum macrocarpum and found that these compounds showed inhibitory activity on hMAO-A. These studies provide significant progress in understanding the potential mechanisms of compounds derived from seaweeds in treating AD [62].
4.3. Inhibition of Neurotoxic Effects in Cells
Excessive activation of immune cells such as microglia and astrocytes results in the release of inflammatory mediators such as pro-inflammatory cytokines (e.g., TNF-α, IL-1β, and IL-6). These mediators cause increased oxidative stress in brain cells [63]. Oxidative stress, caused by excessive accumulation of oxidizing species, causes imbalances and disruptions in antioxidant defense systems. This process damages the biomolecules of nerve cells, disrupting cell functions and causing cell death and neurotoxicity [64]. Therefore, compounds with antioxidant, anti-inflammatory, anti-amyloidogenic, and anti-aggregation activities are expected to show potential neuroprotective effects. It has been reported that some metabolites isolated from brown seaweeds provide neurological protection against various toxic stimuli thanks to their antioxidant and anti-inflammatory properties.
For instance, The Keap1-Nrf2/HO-1 pathway protects cells against oxidative damage by Keap1 releasing Nrf2 under oxidative stress and Nrf2 traveling to the nucleus and increasing the expression of antioxidant genes. In this sense, Qi et al. (2024) determined that acetoxypachydiol (APHD), a diterpene extracted from brown algae belonging to the Dictyota genus, provides activation of the Keap1-Nrf2/HO-1 signaling cascade [65].
The acetone extract of Padina gymnosperm (ACTPG) and its active component α-bisabolol showed Aβ-mediated neuroprotective effects. They reduced reactive oxygen and nitrogen species by inhibiting cholinesterase and β-secretase enzymes [66].
Compounds from P. binghamiae showed antioxidant and anti-apoptotic effects, protecting against glutamate-induced cell damage in HT-22 cells through the Nrf2/HO-1 signaling pathways [43]. In another study, extracts from Fucus vesiculosus and Pelvetia canaliculata restored cell viability against Aβ25–35-induced toxicity. The P. canaliculata extract showed more protective effects over a wider concentration range than the F. vesiculosus extract [67].
Olasehinde et al. (2019) examined the neuroprotective effects of seaweeds (Ecklonia maxima (KPM), Gracilaria gracilis (GCL), Ulva lactuca (ULT) and Gelidium pristoides (MNP)) against zinc (Zn)-induced neurotoxicity [68]. In the study on HT-22 cells, seaweed extracts pre-applied to cells treated with zinc sulfate positively affected cell viability and inhibited zinc-induced cell death. Additionally, seaweed extracts showed effects on increased apoptosis, oxidative stress markers (malondialdehyde and nitric oxide), and antioxidant enzyme activities (catalase and superoxide dismutase) after Zn treatment.
The neuroprotective effects of six different component fractions of Ecklonia radiata were examined in the study [69]. All fractions inhibited Aβ1–42-induced apoptosis and increased neurite outflow, and five fractions reduced Aβ1–42 aggregation and increased the viability of PC-12 cells by reducing hydrogen peroxide (H2O2)-induced oxidative stress.
The potential role of phlorotannins in AD pathologies has also been highlighted. For example, four phlorotannin compounds (eckol, dieckol, fluorofofuroekol-A (PFFA) and 974-A) obtained from the brown seaweed species Ecklonia were examined; PFFA and 974-A protected PC12 cells against H2O2 and t-BHP-induced oxidative stress and Aβ1–42-induced neurotoxicity. They did not inhibit Aβ1–42 aggregate morphology, indicating that their neuroprotective activity is independent of direct interactions with the Aβ1–42 protein [41,70].
Another research showed eckol, dieckol and phlorofucofuroeckol-A (PFFA) compounds obtained from brown seaweeds to prevent the development of AD pathologies and protect cognitive functions by improving the cytokine-induced intestinal epithelial barrier function and reducing reactive oxygen species [70].
Dieckol (DEK), one of the phlorotannins isolated from Ecklonia cava, has been found to strongly reduce intracellular reactive oxygen species (ROS), mitochondrial Ca2+ overload, mitochondrial membrane potential (ΔΨm) impairment, and adenosine triphosphate (ATP) depletion through activation of the Nrf2/HO-1 pathway, thus showing a neuroprotective effect against glutamate toxicity [71]. Dibenzodioxin-fucodiphloroethol (DFD) compound isolated from Ecklonia radiata prevented the aggregation of Aβ1–42. It also reduced Aβ1–42-induced oxidative stress by suppressing mitochondrial dysfunction and caspase activation and contributing to the down-regulation of pro-inflammatory enzymes through negative regulation of the NF-κB pathway [27].
Barbosa et al. (2020) showed that phlorotannin extracts obtained from Fucus species inhibited the activity of monoamine oxidase A and B, tyrosinase, and cholinesterases, enzymes associated with events contributing to the onset and progression of neurodegeneration. Moreover, phlorotannin extracts exhibited multifunctional antioxidant properties that counteract glutamate toxicity in human-derived SH-SY5Y neuron cells [72].
The main phlorotannins from E. cava, eckol, dieckol, and 8,8′-bieckol, were found to potently inhibit BACE-1 and AChE enzymes. According to in silico analyses, in the inhibition of BACE-1, eckol established hydrogen bonds with GLY34 and SER36; dieckol with TRP76, THR232, and LYS321; and 8,8′-bieckol with LYS107, GLY230, THR231 and SER325. In the inhibition of AChE, eckol established hydrogen bonds with THR83, TRP86, TYR124, and SER125; dieckol with ASN233, THR238, ARG296, and HIS405; and 8,8′-bieckol with ARG296. Kinetic studies have shown that eckol, dieckol, and 8,8′-bieckol bind to the BACE1 enzyme as a noncompetitive inhibitor and reduce the Vmax value without changing the Km value between the enzyme and the substrate. These compounds increase the Km value without changing the Vmax value by binding to the AChE enzyme as a competitive inhibitor [73].
Meshalkina et al. (2023) showed that extracts enriched in intracellular and cell wall-bound phlorotannins isolated from Fucus vesiculosus and Pelvetia canaliculata had a significant protective effect against Aβ25-35 toxicity. While this effect was effective for F. vesiculosus only at high concentrations (5 and 10 μg mL−1), P. canaliculata extracts were protected at nearly control levels at all tested concentrations [67].
Fucosterol (FST) isolated from Sargassum horneri reduced oxidative stress in TNF-α/IFN-γ-stimulated human dermal fibroblasts (HDF) and inflammatory responses in the cells by regulating Nrf2/HO-1 and NF-κB/MAPK signaling pathways [74]. Fucosterol from Padina australis showed anti-Alzheimer effects by protecting SH-SY5Y cells against Aβ-induced neurotoxicity, reducing APP mRNA levels and intracellular Aβ levels, and increasing Ngb mRNA levels [75].
Anti-inflammatory compounds have also been investigated for their protective effects on nerve cells. Eleganolone, isolated from Bifurcaria bifurcata, reverses 6-OHDA-induced neurotoxicity by approximately 20%. It exhibits neuroprotective effects through mitochondrial protection, oxidative stress reduction, inflammation and apoptosis inhibition, and NF-kB pathway inhibition [76].
Terpenoids are important compounds, naturally obtained from biodiversity-rich sources such as seaweeds. Terpenoids, especially those isolated from seaweeds, exhibit antioxidant, anti-inflammatory, and neuroprotective effects at the cellular level. Qi et al. (2023) isolated five new and 15 known xenican diterpenes from Dictyota coriacea. The study determined that all compounds provided significant neuroprotective effects against oxidative stress in PC12 cells and that 18-acetoxy-6,7-epoxy-4-hydroxydictyo-19-al had an antioxidant effect mechanism through the Nrf2/ARE signaling pathway [77].
Diterpene compounds isolated from Dictyota sp. showed strong antioxidant effects against H2O2-induced oxidative damage in neuron-like PC12 cells, and the antioxidant property of dictyol C, one of these compounds, was found to be associated with the activation of the Nrf2/ARE signaling pathway [78].
Kumagai et al. (2024) also isolated seven meroterpenoids from Dictyopteris polypodioides. Among these, yahazunol, zonarol, and isozonarol compounds were found to suppress NO production and induce nitric oxide synthase, interleukin-6 and C-C motif chemokine ligand 2 mRNA expression by inhibiting lipopolysaccharide-induced inflammation in RAW264 cells. It has also been revealed that the hydroquinone group in the compounds has a vital role in anti-inflammatory activity [79].
Nine new chroman-type meroterpenoids isolated from Sargassum siliquastrum, especially sargasilol A with a shorter carbon chain, showed anti-neuroinflammatory effects by inhibiting LPS-induced NO production. By targeting the IKK/IκB/NF-κB signaling pathway, the compounds blocked the transport of p65 from the cytosol to the nucleus, which reduced the expression of anti-inflammatory cytokines (IL-1β, IL-6, TNF-α) [51].
Studies on the effects of fucoidan species on neurological diseases have revealed their protective properties against damage caused by Aβ1–42 and oxidative stress. Xing et al. (2023) found that Type II fucoidan (FvF) obtained from Fucus vesiculosus reduced MPP+-induced mitochondrial damage and ROS accumulation and prevented neuronal apoptosis [80]. Five different fucoidans with different chemical compositions were obtained from the species Fucus vesiculosus (FE, FF, and S) and Undaria pinnatifida (UE and UF). Of these, fucoidan S, UE, and UF showed anti-aggregation effects against Aβ1–42, and all fucoidan samples reduced Aβ1–42 and hydrogen peroxide-induced cytotoxicity and inhibited Aβ1–42-induced apoptosis [22].
Subermaniam et al. (2023) have reported that compounds obtained from Padina australis (methyl α-eleostearate, ethyl α-eleostearate, niacinamide, stearamide, and linoleic acid) inhibit the excessive production of reactive oxygen species (ROS) and proinflammatory cytokines, suppressing neuroinflammation and microglial activation in central nervous system disorders [81]. Fucoxanthin from Sargassum oligocystum Montagne counteracts the neurotoxicity caused by amyloid β-protein fragment 25-35 in a C6 neuron cell line by regulating the gene expression of antioxidant enzymes (CAT and GPx) and the PI3K/Akt signaling pathway (GSK-3β) and protects against it by increasing the expression of genes involved in autophagy (p62 and ATG5) and acetylcholine biosynthesis (VAChT and ChAT) [20].
In summary, the resulting bioactive compounds can show neuroprotective effects based on their antioxidant, anti-inflammatory, anti-amyloidogenic, and anti-aggregation activities by targeting processes that play an essential role in the pathophysiology of AD. In short, they can reduce oxidative stress and control inflammation by regulating signaling pathways such as NF-κB/MAPK and Nrf2/HO-1. These mechanisms support neuronal health by contributing to the protection of biomolecules in nerve cells. Furthermore, phlorotannin compounds, such as eckol, dieckol, and phlorofucofuroeckol-A, can prevent neurotoxicity and protect cognitive functions in the early stages of AD, primarily by reducing Aβ1–42-induced toxicity and inhibiting apoptosis.
4.4. In Vivo Evaluation of Neuroprotective Effects
The neuroprotective properties of some brown algae extracts and compounds obtained from seaweeds are supported by in vivo studies in which various biological activities have been reported (Table 2). For example, Fei et al. (2023) showed that, after oral and intravenous administration of zonarol (20–600 ng mL−1) obtained from Dictyopteris undulata, this marine hydroquinone is found at a higher level in the brain tissue of mice than in other tissues [82].
Jo et al. (2023) observed that E. cava reduced neuroinflammation in mice in the chronic inflammation model caused by lipopolysaccharide (LPS). Mice were pretreated with E. cava extract (10–100 mg kg−1) orally for 19 days and then exposed to LPS for 1 week. E. cava treatment reduced pro-inflammatory cytokine levels in the blood and brains of mice. The study also examined the activation of inflammation-related proteins such as NF-κB and STAT3. E. cava treatment was found to regulate brain inflammatory response by reducing NF-κB and STAT3 phosphorylation [83].
Oral administration of ethanol extract of Ishige foliacea (250–500 mg kg−1) increased the expression of brain-derived neurotrophic factor (BDNF) and tropomyosin receptor kinase B (TrkB)-phosphorylated extracellular signal-regulated kinase protein expression, which is associated with synaptic plasticity in the hippocampus. In vitro tests supported the neuroprotective effects, suggesting that the extract may prevent neurodegenerative disorders [59].
Yende et al. (2021) demonstrated the cognitive-improving effects of the oral administration (200–600 mg kg−1) of Sargassum ilicifolium (SI) and Padina tetrastromatica (PT) extracts containing scopolamine on amnesic mice. The extracts significantly ameliorated scopolamine-induced memory impairments, protecting against antioxidant enzymes and inhibiting the AChE enzyme [84].
In the study of Wang et al. (2022), it was determined that fucoidan (Fucus vesiculosis) administered intraperitoneally at 10 mg kg−1 showed neuroprotective effects, improving neuroinflammation, promoting neurogenesis, and reducing blood–brain barrier and intestinal barrier permeability in mice with LPS-induced cognitive impairment [85]. A different study evaluated the protective effect of fucoidan isolated from Sargassum wightii Greville on streptozotocin-induced cognitive impairments, oxidative stress, and amyloidosis. Streptozotocin-treated animals performed poorly in behavioral assays and exhibited marked cognitive impairments. A significant increase in hippocampal MDA, nitrite, AGE, AChE activity and a decrease in GSH and SOD levels were observed in the disease group. Moreover, behavioral disorders, oxidative stress, hyperphosphorylated tau protein, and amyloidosis symptoms were significantly improved in groups treated with 100 and 200 mg kg−1 doses of fucoidan. Histochemical studies further supported the neuroprotective role of fucoidan in the cerebral cortex and hippocampus [86].
Consequently, these bioactive compounds from brown algae are potential candidates for treating neurodegenerative disorders such as AD. Research in this field should continue and be examined in more depth. This study describes the basic steps of in vivo testing and summarizes the potential effects of these compounds based on current research findings.

Table 2.
Neuroprotective effects of various brown algae extracts studied in vitro and in vivo.
Table 2.
Neuroprotective effects of various brown algae extracts studied in vitro and in vivo.
| In vitro | |||||
|---|---|---|---|---|---|
| Species | Extraction Approach | Possible Bioactive Compound | Pharmacological Markers/Biological Tests | Neuroprotective Effect | References |
| Sargassum Horneri | Enzyme-assisted extraction | Sulphated fucooligosaccharide | AChE and BChE | AChE (IC50: 4.0–14.4 µM) and BChE (IC50: 18.5–25.3 µM) inhibition | [47] |
| Ecklonia cava | Ethanol reflux extraction | Polyphenol and fucoidan | Mitochondrial reactive oxygen species (ROS) content amyloid-β production; tau hyperphosphorylation-mediated proteins; mitochondrial membrane potential (MMP, ΔΨm); adenosine triphosphate (ATP) content; mitochondria-mediated protein analysis (BAX, cytochrome C) | Reduced AChE activity; reduced mitochondrial ROS; ATP production and MMP restored down-regulating amyloid-β production (by JNK/IRS-1/IDE pathway); reduced tau hyperphosphorylation (by PI3K/Akt/GSK-3 pathway) | [8] |
| Ecklonia cava | Ethanol extraction | Phlorotannins | AChE and BChE | AChE (IC50: 0.9 ± 0.8 to 66.5 ± 0.4 μM) and BChE (IC50: 1.4 ± 3.8 to 25.2 ± 0.1 μM) inhibition | [56] |
| Ecklonia cava | Ethanol extraction | Eckol, dieckol, phlorofucofuroeckol-A (PFFA) and 974-A | H2O2, t-BHP, Aβ1–42 | Effective in ROS scavenging but not in protecting against oxidative stress-evoked neurotoxicity; PFFA and 974-Al; provided broad neuroprotective activity, including protection against oxidative stress and Aβ1–42. | [70] |
| Ecklonia cava | Ethanol extraction | Phlorotanninn and dieckol | AChE and BChE, AAPH and H2O2-induced oxidative stress in PC-12 and SH-SY5Y cells | AChE Inhibition (95.4%), BChE inhibition (74.7%), reduction in oxidative stress (26.3 to 51.1%) | [41] |
| Durvillaea incurvata | Ultrasound-assisted and conventional extraction | Crude ethanol extract | AChE and BChE | AChE (IC50: 48.55 ± 0.021 µg mL−1, 51.5% inhibition), BChE (IC50: 87.58 ± 0.044 µg mL−1, 32.8% inhibition) | [55] |
| Petalonia binghamiae | Sequential maceration | Achanic acid methyl ester, (R)-ricinoleic acid, saikosaponin E, acanthoside D and cansunin D | Glutamate-induced excitotoxicity, ROS scavenging, cell viability | Protection HT-22 cells from glutamate-induced excitotoxicity, increased cell viability and preserved cell morphology, reduced intracellular ROS production and increased HO-1 expression via Nrf2 activation. | [43] |
| Sargassum oligocystum Montagne | Maceration | Fucoxanthin | AChE, cytotoxic effect on C6 cells, neuroprotective effects against H2O2-induced oxidative stress and Aβ25–35-induced neurotoxicity, gene expression related to antioxidant enzymes (SOD, CAT, GPx), gene expression related to PI3K/Akt signaling (GSK-3β), ER stress and apoptosis-related gene expression (CHOP, Bax, caspase-3), gene expression related to ACh biosynthesis (ChAT, VAChT), protein translation (S6K1), autophagy regulation (p62, ATG5) | AChE (IC50: 130.12 ± 6.65 μg mL−1), protected C6 cells, viability increased to 91.23% at 100 μg mL−1 after H2O2 exposure, Increased cell survival rate significantly from 59.01% to 80.98% at 100 μg mL−1 after Aβ25–35 exposure; increased GPx activity by 105.81% at 100 μg mL−1 and CAT activity by 31.98% at 50 μg mL−1 after H2O2 exposure; increased mRNA expression of CAT and GPx; reversed the decrease in GSK3β induced by Aβ25–35; increased mRNA levels of ChAT and VAChT after Aβ25–35 treatment; inverted effect on S6K1 compared with galantamine; increased ATG5 mRNA levels and reduced p62 mRNA levels | [20] |
| Himanthalia elongata (L.) | Subcritical water extraction | Crude extract | •NO and O2•− scavenging activity, AChE and BChE | 40% AChE and 40% BChE inhibition, protects against oxidative and nitrosative stresses | [54] |
| Eisenia bicyclis (Kjellman) | Subcritical water extraction | Crude extract | •NO and O2•− scavenging activity, AChE and BChE | 50% AChE and 40% BChE inhibition, protects against oxidative and nitrosative stresses | [54] |
| Sargassum angustifolium | Extraction with acidic solution | Fucoidan | AChE, cytotoxic effects on NB4 cell line, Alterations in cell proliferation and cell cycle-related gene expression, Bcl-2 gene | AChE (IC50:1.20 µg mL−1); induction of p53, p21, and pro-apoptotic genes; inhibition of anti-apoptotic Bcl-2 gene | [16] |
| Padina tetrastromatica | Subcritical water hydrolysis | Low molecular weight peptides, flavonoids and phenolic compounds | AChE and α-amylase | AChE inhibition (IC50: 17.9 ± 0.1 to 65.9 ± 0.1 mg mL−1); α-amylase inhibition (2.4 ± 0.1 to 16.0 ± 0.5%) | [32] |
| Ishige foliacea | Maceration | Crude ethanol extract | AChE, BACE1, ROS scavenging | AChE inhibition (IC50: 205.1 μg mL−1), BACE1 (IC50: 266.8 μg mL−1), reduces H2O2 and Aβ-induced cell death in SH-SY5Y cells | [59] |
| Sargassum macrocarpum | Maceration | Terpenoid lactones | Human monoamine oxidases A and B | hMAO-A inhibition (42.18 ± 2.68% at 200 μM), any activity against hMAO-B | [62] |
| Dictyota coriacea | Maceration | Acetoxypachydiol | Keap1-Nrf2/HO-1, ROS scavenging | Reduces OGD/R and H2O2-induced cell death in SH-SY5Y cells, increased the mRNA and protein levels of Nrf2 and HO-1, decreased the protein level of Keap1, promoted the transport of Nrf2 to the cell nucleus | [65] |
| Padina gymnospora | Maceration | Crude extract and α-bisabolol | AChE, BACE1, ROS and RNS, apoptotic gene expression | Inhibition of cholinesterase (ChE) and β-secretase (BACE1) activity (specific inhibition values not stated); Reduction of ROS and RNS production; Attenuation of lipid and protein oxidation; Restoration of mitochondrial; Reduced Caspase-3 activation, increased Bcl-2 expression membrane potential | [66] |
| Fucus vesiculosus | Maceration | Intracellular and cell wall-bound phlorotannins | Aβ25–35-induced AD model (SH-SY5Y cells) | Protection at 5 μg mL−1 and 10 μg mL−1 | [67] |
| Pelvetia canaliculata | Maceration | Intracellular and cell wall-bound phlorotannins | Aβ25–35-induced AD model (SH-SY5Y cells) | Protection at all concentrations tested (1–10 µg mL−1) | [67] |
| Ecklonia maxima | Maceration | Phloroglucinol, catechin, epicatechin, biochanin A, vulgaxanthin and 7,2,4—trihydoxyisoflavanol | Cell viability; apoptosis (AO/EB staining); SOD, CAT, GSH, MDA, NO, AChE | Increased cell viability in HT-22 cells treated with ZnSO4; reduced apoptosis in Zn-treated cells; increased SOD and CAT activities; increased GSH levels; reduced MDA levels; decreased NO levels; reduced AChE activity | [68] |
| Gelidium pristoides | Maceration | Phloroglucinol, catechin, epicatechin, biochanin A, vulgaxanthin and 7,2,4—trihydoxyisoflavanol | Cell viability; apoptosis (AO/EB staining); SOD, CAT, GSH, MDA, NO, AChE | Increased cell viability in HT-22 cells treated with ZnSO4; reduced apoptosis in Zn-treated cells; increased SOD and CAT activities; increased GSH levels; reduced MDA levels; decreased NO levels; reduced AChE activity | [68] |
| Ecklonia radiata | Enzyme-assisted extraction | Crude extract (CE), phlorotannin (PT), poly-saccharide (PS), free sugar (FS) | Aβ1–42 aggregation, H2O2-induced cytotoxicity, cell viability | High neuroprotective activity (viability >92% at 3.125–100 μg mL−1); inhibition of Aβ1–42 aggregation; antioxidant activity at 12.5–50 μg mL−1; enhanced neurite outgrowth (More than 19%) | [69] |
| Ecklonia cava | Maceration | Dieckol | Cell viability, LDH, morphological assessment, ROS scavenging, mitochondrial function, ATP, mitochondrial membrane potential (ΔΨm), mitochondrial Ca2+ and ROS, Nrf2/HO-1 | Increased cell viability in primary cortical neurons and HT22 neurons; decreased LDH release indicating reduced cytotoxicity; improved neuronal morphology post-glutamate exposure; decreased intracellular ROS levels in both primary cortical neurons and HT22 cells; protected against glutamate-induced mitochondrial dysfunction; rescued ATP depletion in HT22 neurons; prevented ΔΨm disruption in HT22 neurons; attenuated mitochondrial Ca2+ overload in HT22 neurons; reduced mitochondrial ROS levels in HT22 neurons; increased HO-1 expression and Nrf2 nuclear translocation | [71] |
| Ecklonia radiata | Maceration | Dibenzodioxin-fucodiphloroethol | Cell viability, Aβ1–42 toxicity and aggregation, molecular docking, AChE, ROS scavenging | DFD (50 µM) does not induce toxicity in PC-12 cells; Rescued PC-12 cell viability at 1.0 and 1.5 µM Aβ1–42; Reduced Aβ1–42 aggregation; Attenuated ROS levels in PC-12 cells; AChE inhibition (IC50 = 41.09 µM); Binded to Aβ1–42 with a docking score of −43.28, forming hydrogen bonds with HIS14 and GLU11; Interacted with AChE with a docking score of −43.48, forming hydrogen bonds and π-π stacking; Binded to Aβ1–42 with a docking score of −43.28, forming hydrogen bonds with HIS14 and GLU11; Binded to Aβ1–42 pentamer with a docking score of −64.01, interacting with multiple residues | [28] |
| Sargassum horneri | Maceration | Fucosterol | Cell viability, ROS scavenging, Nrf2/HO-1, TNF-α/IFN-γ, NF-κB/MAPK | Biocompatible with HDF cells up to 120 μM; decreased in a dose-dependent manner the intracellular ROS production in HDFs; upregulated Nrf2 and HO-1 expression in HDF cells; down-regulated inflammatory mediators in TNF-α/IFN-γ-stimulated HDF cells; reduced phosphorylation of NF-κB and MAPK mediators in a dose-dependent manner; decreased molecules related to connective tissue degradation | [70] |
| Bifurcaria bifurcata | Maceration | Eleganolone | Cell viability, ROS scavenging, mitochondrial function, caspase-3, NF-κB, TNF-α, IL-6, IL-10 | Increased cell viability after 6-OHDA treatment; reduced ROS levels and H2O2 production in SH-SY5Y cells exposed to 6-OHDA; preserved mitochondrial membrane potential (MMP) and ATP levels in SH-SY5Y cells; reduced caspase-3 activity in SH-SY5Y cells exposed to 6-OHDA; inhibited NF-κB p65 translocation in SH-SY5Y cells after 6-OHDA exposure; reduced LPS-induced NO production; decreased TNF-α and IL-6 production in LPS-stimulated RAW 264.7 cells | [76] |
| Fucus guiryi | Maceration | Phlorotannins | AChE, BChE, MAO-A, MAO-B | AChE inhibition (IC50 μg DE mL−1): 969.51 ± 76.99; BChE inhibition (IC50 μg DE mL−1): 1065.29 ± 35.35; MAO-A inhibition (IC50 μg DE mL−1): 168.24 ± 5.40; MAO-B inhibition (IC50 μg DE mL−1): >500; tyrosinaz inhibition (IC50 μg DE mL−1): 47.99 ± 0.59 | [72] |
| Fucus serratus | Maceration | Phlorotannins | AChE, BChE, MAO-A, MAO-B | AChE inhibition (IC50 μg DE mL−1): 2709.58 ± 55.25; BChE inhibition (IC50 μg DE mL−1): 3539.79 ± 109.43; MAO-A inhibition (IC50 μg DE mL−1): 173.80 ± 25.52; MAO-B inhibition (IC50 μg DE mL−1): >500; tyrosinaz Inhibition (IC50 μg DE mL−1): 47.66 ± 2.84 | [72] |
| Fucus spiralis L. | Maceration | Phlorotannins | AChE, BChE, MAO-A, MAO-B | AChE inhibition (IC50 μg DE mL−1): >5000; BChE inhibition (IC50 μg DE mL−1): >5000; MAO-A inhibition (IC50 μg DE mL−1): 1929.65 ± 100.44; MAO-B inhibition (IC50 μg DE mL−1): >500; tyrosinaz inhibition (IC50 μg DE mL−1): 861.73 ± 7.37 | [72] |
| Fucus vesiculosus L. | Maceration | Phlorotannins | AChE, BChE, MAO-A, MAO-B | AChE inhibition (IC50 μg DE mL−1): >5000; BChE inhibition (IC50 μg DE mL−1): >5000; MAO-A inhibition (IC50 μg DE mL−1): >3000; MAO-B inhibition (IC50 μg DE mL−1): >500; tyrosinaz inhibition (IC50 μg DE mL−1): 2546.82 ± 98.00 | [72] |
| Padina australis | Maceration | Fucosterol | Cell viability, apoptosis, APP mRNA, intracellular Aβ, BACE1, ROS scavenging | Increased cell viability in SH-SY5Y cells treated with 2 μM Aβ; reduced apoptosis (30.93%); reduced APP mRNA levels; reduced intracellular Aβ levels; noncompetitive inhibitor on β-secretase (BACE1); reduced oxidative stress by increasing anti-oxidant enzyme activities | [75] |
| Dictyota coriacea | Maceration | Xenicane diterpenes | Cell viability, LDH, Nrf2/ARE | Increased cell viability in PC12 cells damaged by H2O2; reduced LDH levels released from PC12 cells; increased Nrf2 and HO-1 expression in PC12 cells; promoted nuclear translocation of Nrf2 | [77] |
| Dictyota spp. | Maceration | Hydroazulene diterpenes | Cell viability, Nrf2/ARE | Increased cell viability in H2O2-damaged PC12 cells at a concentration of 2 μM; antioxidant effect by activating the Nrf2/ARE signaling pathway | [78] |
| Dictyopteris polypodioides | Maceration | Meroterpenoids: yahazunol (1), zonarol (2), isozonarol (3), and four other meroterpenoids | NO production, iNOS, IL-6, and CCL2 mRNA expression, structure activity of compounds | Inhibited NO production in lipopolysaccharide (LPS)-stimulated RAW264 cells; inhibited iNOS, IL-6, and CCL2 mRNA expression. The hydroquinone unit is important in the anti-inflammatory activity of these sesquiterpenoids | [79] |
| Sargassum siliquastrum | Maceration | Meroterpenoids | BV-2 microglial cells, anti-inflammatory cytokines | Reduced the expression of anti-inflammatory cytokines (IL-1β, IL-6, TNF-α); inhibited LPS-induced NO production in BV-2 microglial cells by targeting IKK/IκB/NF-κB pathways | [51] |
| Fucus vesiculosus | Maceration | Fucoidan | CCK-8 (cell viability), LDH, Hoechst staining, MAP2 immunostaining, MitoSOX staining (for ROS), protein target identification (ATP5F1a) | Enhanced cell viability at concentrations of 5, 10, and 25 μM in MPP+-induced SH-SY5Y cells; reduced LDH release in MPP+-induced SH-SY5Y cells and primary neurons, indicating reduced cell damage. Decreased the proportion of apoptotic cells in MPP+-treated primary neurons; protected neurons from MPP+-induced axon loss and damage. Reduced mitochondrial ROS production in MPP+-treated SH-SY5Y cells. ATP5F1a knockdown reversed the neuroprotective effects of FvF, confirming its role in mitigating mitochondrial dysfunction and apoptosis | [80] |
| Fucus vesiculosus | Maceration | Fucoidan | Aβ1–42 aggregation and cytotoxicity, ROS Scavenging, neurite outgrowth | Protected against Aβ1–42-induced cytotoxicity; inhibited Aβ1–42 aggregation; slight protection against hydrogen peroxide-induced cytotoxicity; inhibited apoptosis induced by Aβ1–42 | [22] |
| Padina australis | Maceration | Crude ethanol extract | Cell viability, NO, prostaglandin E2, ROS scavenging, iNOS and COX-2 expression, TNF-α and IL-6 secretion | No cytotoxicity at concentrations of 0.25–2.0 mg mL−1, reduced at higher concentrations (4.0–8.0 mg mL−1); reduced NO production compared with LPS-stimulated levels dose-dependently (0.5–2.0 mg mL−1); suppressed PGE2 production at concentrations of 0.5–1.0 mg mL−1, increased PGE2 levels at higher concentration (2.0 mg mL−1); reduced intracellular ROS generation compared with LPS-stimulated levels dose-dependently; down-regulated iNOS and COX-2 expression induced by LPS; inhibited TNF-α and IL-6 secretion compared with LPS-stimulated levels | [81] |
| In vivo | |||||
| Species | Possible Bioactive Compound | Pharmacological Markers/Biological Tests | Neuroprotective Effect | References | |
| Dictyopteris undulata | Zonarol | Brain tissue distribution after administration | Higher level in brain tissue than in other tissues | [82] | |
| Ecklonia cava | Crude water extracts | Reduction of pro-inflammatory cytokines, NF-κB, STAT3 phosphorylation | Reduced neuroinflammation in LPS-induced model | [83] | |
| Ishige foliacea | Crude ethanol extract | BDNF and TrkB expression, synaptic plasticity | Increased BDNF, TrkB-phosphorylated ERK expression | [59] | |
| Sargassum fusiformis | Fucoxanthin (Fx) | Reduction of NO, ROS, cell death | Reduced inflammatory responses | [9] | |
| Sargassum fusiformis | Phlorotannin and fucoidan mixture | Protection against Aβ-induced learning and memory impairments | Anti-amnesiac effect | [9] | |
| Sargassum ilicifolium and Padina tetrastromatica | Crude chloroform and ethanol extracts | Improvement of memory impairments, oxidative enzyme protection, AChE inhibition | Cognitive improvement | [84] | |
| Fucus vesiculosus | Fucoidan | Improvement of neuroinflammation, promotion of neurogenesis, reduced blood–brain and intestinal barrier permeability | Neuroprotective effects | [85] | |
| Sargassum wightii Greville | Fucoidan | Behavioral assays, oxidative stress markers, hyperphosphorylated tau protein, amyloidosis symptoms | Improvement of cognitive impairments and oxidative stress | [86] | |
| Padina gymnospora | Crude extract and α-bisabolol | Lifespan in CL2006 and CL4176 mutants | Lowering the expression of AD-related genes (ace-1, hsp-4) in C. Elegans | [66] | |
| Dictyota coriacea | Xenicane diterpenes | CIRI Model | Reduced brain infarct size and neurological deficit score in the transient middle cerebral artery occlusion (MCAO) rat model | [83] | |
| Dictyota spp. | Hydroazulene diterpenes | CIRI Model | Reduction in both brain infarct size and neurological deficit score in transient middle cerebral artery occlusion (MCAO) rat model | [78] | |
| Fucus vesiculosus | Fucoidan (FvF) | MPTP mouse model | Improved motor coordination and balance; slowed loss of TH markers; mitigated dopamine neuron loss in the substantia nigra pars compacta (SNpc). | [80] | |
| Laminaria japonica | Fucoidan | Adenine-induced CKD mice | Reduced signs associated with cognitive behavior; improvement in genes related to Alzheimer’s disease and memory; regulated of inflammatory response via microglia/macrophage polarization; ameliorated oxidative stress via Nrf2-HO-1 signaling pathway; improved cognitive impairments (new object recognition, object location, passive avoidance tests) | [87] | |
| Fucus vesiculosus | Fucoidan | Mongolian gerbils (Meriones unguiculatus) | Reduced tGCI-induced hyperactivity; reduced the loss of NeuN+ pyramidal cells in the CA1 region after tGCI; reduced the number of F-J B+ cells in the CA1 region; reduced the activation of GFAP+ astroglia and the production of reactive oxygen species; reduced the activation of Iba-1+ microglia and the production of reactive oxygen species; reduced HNE immunoreactivity and lipid peroxidation in CA1 pyramidal cells; reduced superoxide levels in CA1 pyramidal cells; increased SOD1 and SOD2 expression in CA1 pyramidal cells | [88] | |
| Laminaria digitata | Laminarin | Mongolian gerbils | Decreased superoxide anions; decreased IL-1β and TNF-α; increased SOD expression; increased IL-4 and IL-13 expression | [89] | |
| Petalonia binghamiae | Water extract | MCAO/R mice model | Decreased neuronal death in ischemic lesion; attenuated sensorimotor deficits; reduced ROS levels; decreased apoptosis in ischemic area; enhanced Nrf2 nuclear translocation; increased HO-1 expression | [43] | |
| Sargassum boveanum | Crude ethanolic extract | Sprague–Dawley rats | Increased antioxidant enzyme activities (SOD, GPx) and gene expressions (SOD, GPx, Nrf2, HO-1); decreased expression of antioxidant genes (SOD, GPx, Nrf2, HO-1); increased expression of inflammatory markers (TNF-α, NF-κB) | [90] | |
| Sargassum angustifolium | Hydroalcoholic, methanolic, and hexane extract | Reversal of memory impairment, passive avoidance test, Morris water maze test | Improvement of cognitive functions | [91] | |
5. Conclusions
This narrative review systematically examined the breadth of the existing literature on bioactive compounds from brown macroalgae and identified knowledge gaps in this area. Furthermore, the potential of brown macroalgae for bioactive compounds with neuroprotective properties was evaluated. In conclusion, compounds obtained from brown algae, especially polysaccharides, phenolic compounds, and omega-3 fatty acids, have antioxidant, anti-inflammatory, and neuroprotective effects. Advanced extraction methods, especially supercritical fluid extraction (SFE) and ultrasound-assisted extraction (UAE) offer significant advantages in increasing the isolation efficiency of these valuable compounds. Furthermore, the wide range of neuroprotective compounds requires the development of advanced analytical methods due to the structural diversity and complexity of the extracts. In this context, HPLC is the most frequently used technique. Nevertheless, HPLC and GC should be used in tandem with off-target (Q-TOF) and on-target (triple quadrupole) or NMR to obtain an in-depth chemical characterization of these natural compounds. This will allow a comprehensive evaluation of the efficiency and the biological activities of the obtained bioactive compounds. To fully evaluate the true potential of the brown algae extracts, in vitro studies are needed to understand the mechanisms, and in vivo experiments are required to better realize their predictable effects on humans. In this way, it will be possible to understand the mechanisms of action of existing compounds on neuroprotection, particularly against AD, before their potential use as functional foods and dietary supplements. However, more research is needed to explore brown algae’s potential in nutrition and health applications.
Author Contributions
M.C., L.C.d.S., C.J.d.A. and N.R.C.-R. wrote the initial draft of the paper reviewed and drafted revisions for the final version. H.K., E.I. and A.C. reviewed and edited the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grant PID2020-113050RB-I00, funded by MCIN/AEI (Spain).
Acknowledgments
Melis Cokdinleyen thanks the Ministry of National Education (Turkey) for her doctoral scholarship, YLSY-2018. Luana C. Dos Santos would like to thank her grant JDC2022-049001-I, funded by MCIN/AEI/10.13039/501100011033 and by “European Union NextGenerationEU/PRTR.” Nieves R. Colás-Ruiz thanks her grant PTA2022-022137-I, funded by the Spanish State Research Agency. Cristiano J, Andrade wishes to offer thanks for her grant Coordenação de Aperfeicoamento de Pessoal de Nível Superior (CAPES/PRINT) 88887.884281/2023-00.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lamisa, A.B.; Ahammad, I.; Bhattacharjee, A.; Hossain, M.U.; Ishtiaque, A.; Chowdhury, Z.M.; Das, K.C.; Salimullah, M.; Keya, C.A. A Meta-Analysis of Bulk RNA-Seq Datasets Identifies Potential Biomarkers and Repurposable Therapeutics against Alzheimer’s Disease. Sci. Rep. 2024, 14, 24717. [Google Scholar] [CrossRef] [PubMed]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s Disease: Inhibition of Amyloid Beta and Tau Tangle Formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Alhazmi, H.A.; Albratty, M. An Update on the Novel and Approved Drugs for Alzheimer Disease. Saudi Pharm. J. 2022, 30, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D. How Many Species of Algae Are There? A Reprise. Four Kingdoms, 14 Phyla, 63 Classes and Still Growing. J. Phycol. 2024, 60, 214–228. [Google Scholar] [CrossRef]
- El-Manaway, I.M.; Rashedy, S.H. The Ecology and Physiology of Seaweeds: An Overview. In Sustainable Global Resources of Seaweeds Volume 1: Bioresources, Cultivation, Trade and Multifarious Applications; Ranga Rao, A., Ravishankar, G.A., Eds.; Springer: Cham, Switzerland, 2022; Volume 1, pp. 3–16. [Google Scholar]
- Wan, A.H.L.; Davies, S.J.; Soler-Vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a Sustainable Aquafeed Ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, H.; Wang, Y.; Peng, Z.; Guo, Z.; Ma, Y.; Zhang, R.; Zhang, M.; Wu, Q.; Xiao, J.; et al. Effects of Different Extraction Methods on Contents, Profiles, and Antioxidant Abilities of Free and Bound Phenolics of Sargassum polycystum from the South China Sea. J. Food Sci. 2022, 87, 968–981. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.O.; Kim, G.H.; Heo, H.J. Fucoidan-Rich Substances from Ecklonia cava Improve Trimethyltin-Induced Cognitive Dysfunction via Down-Regulation of Amyloid β Production/Tau Hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef]
- Dai, Y.L.; Jiang, Y.F.; Lu, Y.A.; Yu, J.B.; Kang, M.C.; Jeon, Y.J. Fucoxanthin-Rich Fraction from Sargassum fusiformis Alleviates Particulate Matter-Induced Inflammation in Vitro and in Vivo. Toxicol. Rep. 2021, 8, 349–358. [Google Scholar] [CrossRef]
- Martić, A.; Čižmek, L.; Ul’yanovskii, N.V.; Paradžik, T.; Perković, L.; Matijević, G.; Vujović, T.; Baković, M.; Babić, S.; Kosyakov, D.S.; et al. Intra-Species Variations of Bioactive Compounds of Two Dictyota Species from the Adriatic Sea: Antioxidant, Antimicrobial, Dermatological, Dietary, and Neuroprotective Potential. Antioxidants 2023, 12, 857. [Google Scholar] [CrossRef]
- Ciko, A.M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Fernandes, M.; Lima, M.; Gomes, J.P.; Silva, F.; Castro, S.; Sampaio, F.; Gomes, A.C. Nanotechnology to the Rescue: Therapeutic Strategies Based on Brown Algae for Neurodegenerative Diseases. Appl. Sci. 2023, 13, 1883. [Google Scholar] [CrossRef]
- Aakre, I.; Solli, D.D.; Markhus, M.W.; Mæhre, H.K.; Dahl, L.; Henjum, S.; Alexander, J.; Korneliussen, P.A.; Madsen, L.; Kjellevold, M. Commercially Available Kelp and Seaweed Products—Valuable Iodine Source or Risk of Excess Intake? Food Nutr. Res. 2021, 65, 7584. [Google Scholar] [CrossRef] [PubMed]
- Byoung, W.C.; Ryu, G.; Soo, H.P.; Eun, S.K.; Shin, J.; Seok, S.R.; Hyeon, C.S.; Bong, H.L. Anticholinesterase Activity of Plastoquinones from Sargassum sagamianum: Lead Compounds for Alzheimer’s Disease Therapy. Phytother. Res. 2007, 21, 423–426. [Google Scholar] [CrossRef]
- Hosseinpouri, A.; Mohammadi, M.; Ehsandoost, E.; Sharafi-Badr, P.; Obeidi, N. Chemical Identification, Antioxidant, Cholinesterase Inhibitory, and Cytotoxic Properties of Fucoidan Extracted from Persian Gulf Sargassum angustifolium. Acta Oceanol. Sin. 2022, 41, 133–141. [Google Scholar] [CrossRef]
- Ktari, L.; Mdallel, C.; Aoun, B.; Chebil Ajjabi, L.; Sadok, S. Fucoxanthin and Phenolic Contents of Six Dictyotales from the Tunisian Coasts with an Emphasis for a Green Extraction Using a Supercritical CO2 Method. Front. Mar. Sci. 2021, 8, 647159. [Google Scholar] [CrossRef]
- Jiang, H.; Kong, L.; Tang, H.; Wang, Z.; Liu, C.; Zhang, J.; Chen, Y.; Shen, J.; Zhou, Y. Study on the Preparation and Enzyme Inhibitory Activity of Polyphenols from Sargassum pallidum. PLoS ONE 2024, 19, e0297434. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Mendiola, J.A.; Sánchez-Martínez, J.D.; Bueno, M.; Cerezal-Mezquita, P.; Ibáñez, E. Evaluation of the Antioxidant and Neuroprotective Activity of the Seaweed Durvillaea antarctica (Cochayuyo) Extracts Using Pressurized Liquids. J. Appl. Phycol. 2023, 35, 835–847. [Google Scholar] [CrossRef]
- Hong, D.D.; Thom, L.T.; Ha, N.C.; Thu, N.T.H.; Hien, H.T.M.; Tam, L.T.; Dat, N.M.; Duc, T.M.; Van Tru, N.; Hang, N.T.M.; et al. Isolation of Fucoxanthin from Sargassum oligocystum Montagne, 1845 Seaweed in Vietnam and Its Neuroprotective Activity. Biomedicines 2023, 11, 2310. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.P.; Montero, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Application of Hansen Solubility Approach for the Subcritical and Supercritical Selective Extraction of Phlorotannins from Cystoseira abies-marina. RSC Adv. 2016, 6, 94884–94895. [Google Scholar] [CrossRef]
- Alghazwi, M.; Smid, S.; Karpiniec, S.; Zhang, W. Comparative Study on Neuroprotective Activities of Fucoidans from Fucus vesiculosus and Undaria pinnatifida. Int. J. Biol. Macromol. 2019, 122, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Ehrig, K.; Alban, S. Sulfated Galactofucan from the Brown Alga Saccharina latissima-Variability of Yield, Structural Composition and Bioactivity. Mar. Drugs 2015, 13, 76–101. [Google Scholar] [CrossRef] [PubMed]
- Palma, M.; Barbero, G.F.; PiñEiro, Z.; Liazid, A.; Barroso, C.G.; Rostagno, M.A.; Prado, J.M.; Meireles, M.A.A. Chapter 2: Extraction of Natural Products: Principles and Fundamental Aspects. In Natural Product Extraction: Principles and Applications; Green Chemistry Series; Royal Society of Chemistry: London, UK, 2013; pp. 58–88. [Google Scholar] [CrossRef]
- Dertli, H.; Saloglu, D. A Valorization Approach of Food Industry Wastewater Using Microwave-Assisted Extraction. In Advanced Technologies in Wastewater Treatment: Food Processing Industry; Elsevier: Amsterdam, The Netherlands, 2023; Chapter 6; pp. 155–178. [Google Scholar] [CrossRef]
- Shinoda, S.; Tozawa, Y.; Kurimoto, S.; Shigemori, H.; Sekiguchi, M. Three New Meroterpenoids from Sargassum macrocarpum and Their Inhibitory Activity against Amyloid β Aggregation. J. Nat. Med. 2023, 77, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Johnston, M.R.; Zhang, W.; Smid, S.D. A Phlorotannin Isolated from Ecklonia radiata, Dibenzodioxin-Fucodiphloroethol, Inhibits Neurotoxicity and Aggregation of β-Amyloid. Phytomedicine Plus 2021, 1, 100125. [Google Scholar] [CrossRef]
- Martens, N.; Zhan, N.; Voortman, G.; Leijten, F.P.J.; van Rheenen, C.; van Leerdam, S.; Geng, X.; Huybrechts, M.; Liu, H.; Jonker, J.W.; et al. Activation of Liver X Receptors and Peroxisome Proliferator-Activated Receptors by Lipid Extracts of Brown Seaweeds: A Potential Application in Alzheimer’s Disease? Nutrients 2023, 15, 3004. [Google Scholar] [CrossRef]
- Priego-Capote, F.; De La Torre, M.D.P.D. Chapter 5: Accelerated Liquid Extraction. In Natural Product Extraction: Principles and Applications; Green Chemistry Series; Royal Society of Chemistry: London, UK, 2013; pp. 157–195. [Google Scholar] [CrossRef]
- Zohar, M.; Matzrafi, M.; Abu-Nassar, J.; Khoury, O.; Gaur, R.Z.; Posmanik, R. Subcritical Water Extraction as a Circular Economy Approach to Recover Energy and Agrochemicals from Sewage Sludge. J. Environ. Manag. 2021, 285, 112111. [Google Scholar] [CrossRef]
- Boucelkha, A.; Petit, E.; Elboutachfaiti, R.; Molinié, R.; Amari, S.; Yahaoui, R.Z. Production of Guluronate Oligosaccharide of Alginate from Brown Algae Stypocaulon scoparium Using an Alginate Lyase. J. Appl. Phycol. 2017, 29, 509–519. [Google Scholar] [CrossRef]
- Hans, N.; Solanki, D.; Nagpal, T.; Amir, H.; Naik, S.; Malik, A. Process Optimization and Characterization of Hydrolysate from Underutilized Brown Macroalgae (Padina tetrastromatica) after Fucoidan Extraction through Subcritical Water Hydrolysis. J. Environ. Manag. 2024, 349, 119497. [Google Scholar] [CrossRef]
- Pingret, D.; Fabiano-Tixier, A.S.; Chemat, F. Chapter 3: Ultrasound-Assisted Extraction. In Natural Product Extraction: Principles and Applications; Green Chemistry Series; Royal Society of Chemistry: London, UK, 2013; pp. 89–112. [Google Scholar] [CrossRef]
- Soares, C.; Paíga, P.; Marques, M.; Neto, T.; Carvalho, A.P.; Paiva, A.; Simões, P.; Costa, L.; Bernardo, A.; Fernández, N.; et al. Multi-Step Subcritical Water Extracts of Fucus vesiculosus L. and Codium tomentosum Stackhouse: Composition, Health-Benefits and Safety. Processes 2021, 9, 893. [Google Scholar] [CrossRef]
- Wee, A.S.; Nhu, T.D.; Khaw, K.Y.; Tang, K.S.; Yeong, K.Y. Linking Diabetes to Alzheimer’s Disease: Potential Roles of Glucose Metabolism and Alpha-Glucosidase. Curr. Neuropharmacol. 2022, 21, 2036–2048. [Google Scholar] [CrossRef]
- Cebrián-Lloret, V.; Cartan-Moya, S.; Martínez-Sanz, M.; Gómez-Cortés, P.; Calvo, M.V.; López-Rubio, A.; Martínez-Abad, A. Characterization of the Invasive Macroalgae Rugulopteryx Okamurae for Potential Biomass Valorisation. Food Chem. 2024, 440, 138241. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a Valuable Source of Naturally Occurring Bioactive Compounds for the Treatment of Alzheimer’s Disease. Mar. Drugs 2019, 17, 609. [Google Scholar] [CrossRef] [PubMed]
- Quitain, A.T.; Kai, T.; Sasaki, M.; Goto, M. Microwave-Hydrothermal Extraction and Degradation of Fucoidan from Supercritical Carbon Dioxide Deoiled Undaria pinnatifida. Ind. Eng. Chem. Res. 2013, 52, 7940–7946. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.M. Extraction Systems and Analytical Techniques for Food Phenolic Compounds: A Review. Foods 2022, 11, 3671. [Google Scholar] [CrossRef]
- Anjana, K.; Arunkumar, K. Brown Algae Biomass for Fucoxanthin, Fucoidan and Alginate; Update Review on Structure, Biosynthesis, Biological Activities and Extraction Valorisation. Int. J. Biol. Macromol. 2024, 280, 135632. [Google Scholar] [CrossRef]
- Nho, J.A.; Shin, Y.S.; Jeong, H.R.; Cho, S.; Heo, H.J.; Kim, G.H.; Kim, D.O. Neuroprotective Effects of Phlorotannin-Rich Extract from Brown Seaweed Ecklonia cava on Neuronal PC-12 and SH-SY5Y Cells with Oxidative Stress. J. Microbiol. Biotechnol. 2020, 30, 359–367. [Google Scholar] [CrossRef]
- Palaniveloo, K.; Ong, K.H.; Satriawan, H.; Abdul Razak, S.; Suciati, S.; Hung, H.Y.; Hirayama, S.; Rizman-Idid, M.; Tan, J.K.; Yong, Y.S.; et al. In Vitro and in Silico Cholinesterase Inhibitory Potential of Metabolites from Laurencia snackeyi (Weber-van Bosse) M. Masuda. 3 Biotech 2023, 13, 337. [Google Scholar] [CrossRef]
- Eom, S.H.; Hong, G.L.; Kang, H.B.; Lee, N.S.; Kim, D.K.; Jeong, Y.G.; Kim, C.S.; Yoo, Y.C.; Lee, B.H.; Jung, J.Y.; et al. Neuroprotective Effects of Water Extract from Brown Algae Petalonia Binghamiae in an Experimental Model of Focal Cerebral Ischemia In Vitro and In Vivo. Curr. Issues Mol. Biol. 2023, 45, 8427–8443. [Google Scholar] [CrossRef]
- van den Hurk, R.S.; Pursch, M.; Stoll, D.R.; Pirok, B.W.J. Recent Trends in Two-Dimensional Liquid Chromatography. TrAC Trends Anal. Chem. 2023, 166, 117166. [Google Scholar] [CrossRef]
- Montero, L.; Herrero, M.; Ibáñez, E.; Cifuentes, A. Separation and Characterization of Phlorotannins from Brown Algae Cystoseira Abies-Marina by Comprehensive Two-Dimensional Liquid Chromatography. Electrophoresis 2014, 35, 1644–1651. [Google Scholar] [CrossRef]
- Li, S.; Hu, M.; Tong, Y.; Xia, Z.; Tong, Y.; Sun, Y.; Cao, J.; Zhang, J.; Liu, J.; Zhao, S.; et al. A Review of Volatile Compounds in Edible Macroalgae. Food Res. Int. 2023, 165, 112559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Zhang, H.Z.; Liu, Y.W.; Tang, M.; Jiang, Y.J.; Li, F.N.; Guan, L.P.; Jin, Q.H. Sulphated Fucooligosaccharide from Sargassum horneri: Structural Analysis and Anti-Alzheimer Activity. Neurochem. Res. 2024, 49, 1592–1602. [Google Scholar] [CrossRef] [PubMed]
- Montero, L.; Sánchez-Camargo, A.P.; García-Cañas, V.; Tanniou, A.; Stiger-Pouvreau, V.; Russo, M.; Rastrelli, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Anti-Proliferative Activity and Chemical Characterization by Comprehensive Two-Dimensional Liquid Chromatography Coupled to Mass Spectrometry of Phlorotannins from the Brown Macroalga Sargassum muticum Collected on North-Atlantic Coasts. J. Chromatogr. A 2016, 1428, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Duan, X.; Agar, O.T.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A.R. Comparative Study on the Effect of Different Drying Techniques on Phenolic Compounds in Australian Beach-Cast Brown Seaweeds. Algal Res. 2023, 72, 103140. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Kassem, W.M.A.; Alwaleed, E.A.; Saber, H. Optimization and Characterization of Brown Seaweed Alginate for Antioxidant, Anticancer, Antimicrobial, and Antiviral Properties. Int. J. Biol. Macromol. 2024, 278, 134715. [Google Scholar] [CrossRef]
- Qi, Y.; Wang, Z.; Zhang, J.; Tang, S.; Zhu, H.; Jiang, B.; Li, X.; Wang, J.; Sun, Z.; Zhao, M.; et al. Anti-Neuroinflammatory Meroterpenoids from a Chinese Collection of the Brown Alga Sargassum siliquastrum. J. Nat. Prod. 2023, 86, 1284–1293. [Google Scholar] [CrossRef]
- Koul, B.; Farooq, U.; Yadav, D.; Song, M. Phytochemicals: A Promising Alternative for the Prevention of Alzheimer’s Disease. Life 2023, 13, 999. [Google Scholar] [CrossRef]
- Pedro, B.; Guedes, L.; André, R.; Gaspar, H.; Vaz, P.; Ascensão, L.; Melo, R.; Luísa Serralheiro, M. Undaria pinnatifida (U. pinnatifida) Bioactivity: Antioxidant, Gastro-Intestinal Motility, Cholesterol Biosynthesis and Liver Cell Lines Proteome. J. Funct. Foods 2021, 83, 104567. [Google Scholar] [CrossRef]
- Gomes, I.; Rodrigues, H.; Rodrigues, C.; Marques, M.; Paíga, P.; Paiva, A.; Simões, P.; Fernandes, V.C.; Vieira, M.; Delerue-Matos, C.; et al. Evaluation of the Biological Potential of Himanthalia Elongata (L.) S.F.Gray and Eisenia Bicyclis (Kjellman) Setchell Subcritical Water Extracts. Foods 2022, 11, 746. [Google Scholar] [CrossRef]
- Muñoz-Molina, N.; Parada, J.; Simirgiotis, M.; Montecinos-González, R. The Potential of Using Cochayuyo (Durvillaea incurvata) Extract Obtained by Ultrasound-Assisted Extraction to Fight against Aging-Related Diseases. Foods 2024, 13, 269. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, Y.H.; Yang, S.Y. Enzyme Kinetics and Molecular Docking Investigation of Acetylcholinesterase and Butyrylcholinesterase Inhibitors from the Marine Alga Ecklonia cava. Nat. Product. Sci. 2023, 29, 182–192. [Google Scholar] [CrossRef]
- Dhanabalan, A.K.; Kumar, P.; Vasudevan, S.; Chworos, A.; Velmurugan, D. Identification of a Novel Drug Molecule for Neurodegenerative Disease from Marine Algae through in-silico Analysis. J. Biomol. Struct. Dyn. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chagas Monteiro, K.L.; dos Santos Alcântara, M.G.; Lins Freire, N.M.; Brandão, E.M.; do Nascimento, V.L.; dos Santos Viana, L.M.; de Aquino, T.M.; da Silva-Júnior, E.F. BACE-1 Inhibitors Targeting Alzheimer’s Disease. Curr. Alzheimer Res. 2023, 20, 131–148. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.E.; Son, H.J.; Lim, D.W.; Yoon, M.; Lee, J.; Kim, Y.T.; Han, D.; Lee, C.; Um, M.Y. Memory-Enhancing Effects of Ishige foliacea Extract: In Vitro and in Vivo Study. J. Food Biochem. 2020, 44, e13162. [Google Scholar] [CrossRef] [PubMed]
- Dhanabalan, A.K.; Vasudevan, S.; Velmurugan, D.; Khan, M.S. Identification of Potential Marine Bioactive Compounds from Brown Seaweeds towards BACE1 Inhibitors: Molecular Docking and Molecular Dynamics Simulations Approach. In Silico Pharmacol. 2024, 12, 40. [Google Scholar] [CrossRef]
- Dezsi, L.; Vecsei, L. Monoamine Oxidase B Inhibitors in Parkinson’s Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 425–439. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, K.; Hwang, H.; Kim, S.H.; Park, S.E.; Durai, P.; Park, K.; Kim, H.S.; Jang, D.S.; Choi, J.S.; et al. New Monocyclic Terpenoid Lactones from a Brown Algae Sargassum macrocarpum as Monoamine Oxidase Inhibitors. Plants 2022, 11, 1998. [Google Scholar] [CrossRef]
- Tuppo, E.E.; Arias, H.R. The Role of Inflammation in Alzheimer’s Disease. Int. J. Biochem. Cell Biol. 2005, 37, 289–305. [Google Scholar] [CrossRef]
- Dhapola, R.; Beura, S.K.; Sharma, P.; Singh, S.K.; HariKrishnaReddy, D. Oxidative Stress in Alzheimer’s Disease: Current Knowledge of Signaling Pathways and Therapeutics. Mol. Biol. Rep. 2024, 51, 48. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, G.; Jin, S.; Jian, R.; Zou, Z.; Wang, C.; Zhang, Y.; Zhao, M.; Zhu, H.; Yan, P. Neuroprotective Effect of Acetoxypachydiol Against Oxidative Stress Through Activation of the Keap1-Nrf2/HO-1 Pathway. BMC Complement. Med. Ther. 2024, 24, 175. [Google Scholar] [CrossRef]
- Shanmuganathan, B.; Sathya, S.; Balasubramaniam, B.; Balamurugan, K.; Devi, K.P. Amyloid-β Induced Neuropathological Actions Are Suppressed by Padina gymnospora (Phaeophyceae) and Its Active Constituent α-Bisabolol in Neuro2a Cells and Transgenic Caenorhabditis elegans Alzheimer’s Model. Nitric Oxide 2019, 91, 52–66. [Google Scholar] [CrossRef]
- Meshalkina, D.; Tsvetkova, E.; Orlova, A.; Islamova, R.; Grashina, M.; Gorbach, D.; Babakov, V.; Francioso, A.; Birkemeyer, C.; Mosca, L.; et al. First Insight into the Neuroprotective and Antibacterial Effects of Phlorotannins Isolated from the Cell Walls of Brown Algae Fucus vesiculosus and Pelvetia canaliculata. Antioxidants 2023, 12, 696. [Google Scholar] [CrossRef] [PubMed]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Neuroprotective Effects of Some Seaweeds against Zn—Induced Neuronal Damage in HT-22 Cells via Modulation of Redox Imbalance, Inhibition of Apoptosis and Acetylcholinesterase Activity. Metab. Brain Dis. 2019, 34, 1615–1627. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.; Charoensiddhi, S.; Smid, S.; Zhang, W. Impact of Ecklonia radiata Extracts on the Neuroprotective Activities against Amyloid Beta (Aβ1–42) Toxicity and Aggregation. J. Funct. Foods 2020, 68, 103893. [Google Scholar] [CrossRef]
- Shrestha, S.; Choi, J.S.; Zhang, W.; Smid, S.D. Neuroprotective Activity of Macroalgal Fucofuroeckols against Amyloid β Peptide-Induced Cell Death and Oxidative Stress. Int. J. Food Sci. Technol. 2022, 57, 4286–4295. [Google Scholar] [CrossRef]
- Cui, Y.; Amarsanaa, K.; Lee, J.H.; Rhim, J.K.; Kwon, J.M.; Kim, S.H.; Park, J.M.; Jung, S.C.; Eun, S.Y. Neuroprotective Mechanisms of Dieckol against Glutamate Toxicity through Reactive Oxygen Species Scavenging and Nuclear Factor-like 2/Heme Oxygenase-1 Pathway. Korean J. Physiol. Pharmacol. 2019, 23, 121–130. [Google Scholar] [CrossRef]
- Barbosa, M.; Valentão, P.; Ferreres, F.; Gil-Izquierdo, Á.; Andrade, P.B. In Vitro Multifunctionality of Phlorotannin Extracts from Edible Fucus species on Targets Underpinning Neurodegeneration. Food Chem. 2020, 333, 127456. [Google Scholar] [CrossRef]
- Lee, J.; Jun, M. Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava—An In Vitro and in Silico Study. Mar. Drugs 2019, 17, 91. [Google Scholar] [CrossRef]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.J.; Jee, Y.; Kim, H.J.; Do, S.G.; Fernando, I.P.S.; Ahn, G. Fucosterol Isolated from Dietary Brown Alga Sargassum horneri Protects TNF-α/IFN-γ-Stimulated Human Dermal Fibroblasts Via Regulating Nrf2/HO-1 and NF-ΚB/MAPK Pathways. Antioxidants 2022, 11, 1429. [Google Scholar] [CrossRef]
- Gan, S.Y.; Wong, L.Z.; Wong, J.W.; Tan, E.L. Fucosterol Exerts Protection against Amyloid β-Induced Neurotoxicity, Reduces Intracellular Levels of Amyloid β and Enhances the MRNA Expression of Neuroglobin in Amyloid β-Induced SH-SY5Y Cells. Int. J. Biol. Macromol. 2019, 121, 207–213. [Google Scholar] [CrossRef]
- Silva, J.; Alves, C.; Pinteus, S.; Susano, P.; Simões, M.; Guedes, M.; Martins, A.; Rehfeldt, S.; Gaspar, H.; Goettert, M.; et al. Disclosing the Potential of Eleganolone for Parkinson’s Disease Therapeutics: Neuroprotective and Anti-Inflammatory Activities. Pharmacol. Res. 2021, 168, 105589. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, G.; Fang, C.; Jing, C.; Tang, S.; Li, G.; Wang, C.; Zhu, H.; Zhao, M.; Sun, Z.; et al. Antioxidant and Neuroprotective Xenicane Diterpenes from the Brown Alga Dictyota coriacea. ACS Omega 2023, 8, 8034–8044. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xi, Y.; Li, G.; Zheng, Y.; Wang, Z.; Wang, J.; Fang, C.; Sun, Z.; Hu, L.; Jiang, W.; et al. Hydroazulene Diterpenes from A Dictyota Brown Alga and Their Antioxidant and Neuroprotective Effects against Cerebral Ischemia-Reperfusion Injury. J. Nat. Prod. 2021, 84, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, M.; Matsuda, A.; Shiiba, N.; Tsuruta, T.; Endo, H.; Nishikawa, K.; Morimoto, Y. Structure-Activity Relationship of Anti-Inflammatory Meroterpenoids Isolated from Dictyopteris polypodioides in RAW264 Cells. Biosci. Biotechnol. Biochem. 2024, 88, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Li, G.; Liu, Y.; Yang, L.; Zhang, Y.; Zhang, Y.; Ding, J.; Lu, M.; Yu, G.; Hu, G. Fucoidan from Fucus vesiculosus Prevents the Loss of Dopaminergic Neurons by Alleviating Mitochondrial Dysfunction through Targeting ATP5F1a. Carbohydr. Polym. 2023, 303, 120470. [Google Scholar] [CrossRef]
- Subermaniam, K.; Lew, S.Y.; Yow, Y.Y.; Lim, S.H.; Yu, W.S.; Lim, L.W.; Wong, K.H. Malaysian Brown Macroalga Padina australis Mitigates Lipopolysaccharide-Stimulated Neuroinflammation in BV2 Microglial Cells. Iran. J. Basic. Med. Sci. 2023, 26, 669. [Google Scholar] [CrossRef]
- Fei, J.; Yamada, S.; Satoh, T.; Koyama, T. Pharmacokinetic Analysis of Zonarol, a Marine Algal Hydroquinone, in Mice Using HPLC with Fluorescence Detection. Antibiotics 2023, 12, 1013. [Google Scholar] [CrossRef]
- Jo, S.L.; Yang, H.; Jeong, K.J.; Lee, H.W.; Hong, E.J. Neuroprotective Effects of Ecklonia cava in a Chronic Neuroinflammatory Disease Model. Nutrients 2023, 15, 2007. [Google Scholar] [CrossRef]
- Yende, S.R.; Arora, S.K.; Ittadwar, A.M. Antioxidant and Cognitive Enhancing Activities of Sargassum ilicifolium and Padina tetrastromatica in Scopolamine Treated Mice. J. Biol. Act. Prod. Nat. 2021, 11, 11–21. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Duan, L.; Li, X.; Yang, W.; Huang, T.; Kong, M.; Guan, F.; Ma, S. Fucoidan Ameliorates LPS-Induced Neuronal Cell Damage and Cognitive Impairment in Mice. Int. J. Biol. Macromol. 2022, 222, 759–771. [Google Scholar] [CrossRef]
- Ramu, S.; Anbu, J.; Ammunje, D.N.; Krishnaraj, K. Fucoidan Isolated from Sargassum wightii Greville Ameliorates Intracerebro-Ventricular Streptozotocin Induced Cognitive Deficits, Oxidative Stress and Amyloidosis in Wistar Rats. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100309. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Z.; Feng, X.; Deng, J.; He, C.; Li, R.; Zhao, Y.; Ge, Y.; Zhang, Y.; Song, C.; et al. The Emerging Evidence for a Protective Role of Fucoidan from Laminaria Japonica in Chronic Kidney Disease-Triggered Cognitive Dysfunction. Mar. Drugs 2022, 20, 258. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Ahn, J.H.; Song, M.; Kim, D.W.; Lee, T.K.; Lee, J.C.; Kim, Y.M.; Kim, J.D.; Cho, J.H.; Hwang, I.K.; et al. Pretreated Fucoidan Confers Neuroprotection against Transient Global Cerebral Ischemic Injury in the Gerbil Hippocampal CA1 Area via Reducing of Glial Cell Activation and Oxidative Stress. Biomed. Pharmacother. 2019, 109, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Ahn, J.H.; Park, C.W.; Kim, B.; Park, Y.E.; Lee, J.C.; Park, J.H.; Yang, G.E.; Shin, M.C.; Cho, J.H.; et al. Pre-Treatment with Laminarin Protects Hippocampal CA1 Pyramidal Neurons and Attenuates Reactive Gliosis Following Transient Forebrain Ischemia in Gerbils. Mar. Drugs 2020, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Janahmadi, Z.; Jaberie, H.; Esmaili, A.; Nabipour, I. Hepatoprotective Effects of Brown Algae Sargassum Boveanum on Bile Duct-Ligated Cholestasis in Rats Are Mediated by Modulating NF-ΚB/TNF-α and Nrf2/HO-1 Gene Expression. Avicenna J. Phytomed. 2023, 13, 513–530. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Yegdaneh, A.; Rabbani, M. Effects of Hydroalcoholic, Methanolic, and Hexane Extracts of Brown Algae Sargassum Angustifolium on Scopolamine-Induced Memory Impairment and Learning Deficit in Rodents. Res Pharm Sci 2023, 18, 292–302. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).