Targeting the Calcium-Sensing Receptor in Chemically Induced Medium-Grade Colitis in Female BALB/C Mice
Highlights
- NPS 2143, a negative allosteric modulator of the CaSR, reduced the inflammation in mice with chemically induced medium-grade colitis.
- The PGE2 pathway seems to be a mediator of CaSR-related inflammation in the colon.
- The treatment was less effective in mice with medium-grade colitis compared to those with severe colitis, suggesting that its effectiveness is proportional to disease severity.
- The CaSR may be a promising target for the treatment of and/or prevention of IBD.
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design, Induction of Colitis, and Organ Collection
2.3. Clinical Assessment of Colitis
2.4. Histology Score
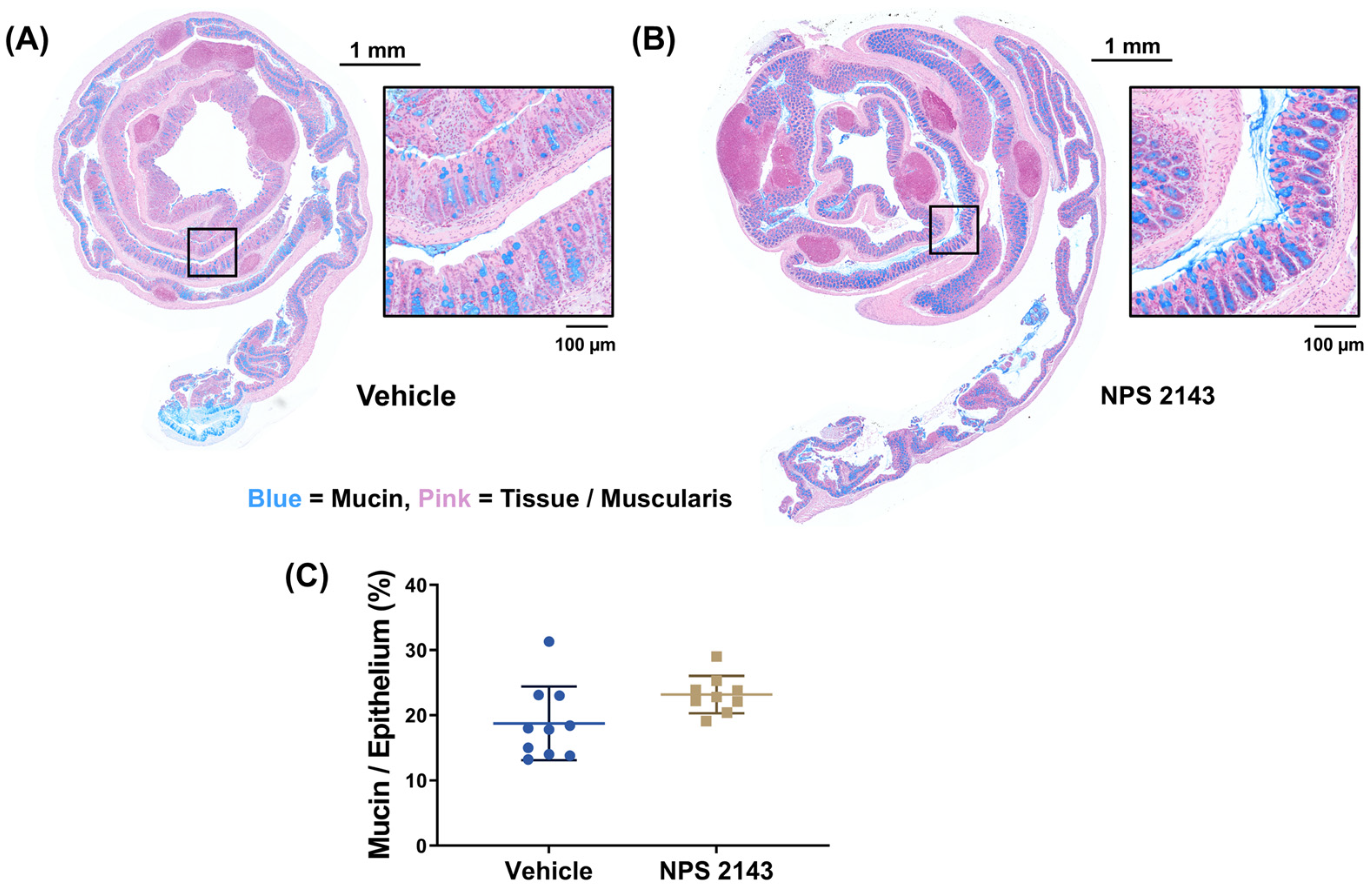
2.5. Mucin Quantification
2.6. Cytokine Multiplex Assay
2.7. Immunofluorescence Staining
2.8. Quantification of Immunofluorescence
2.9. Gene Expression
2.10. Statistical Analysis
3. Results
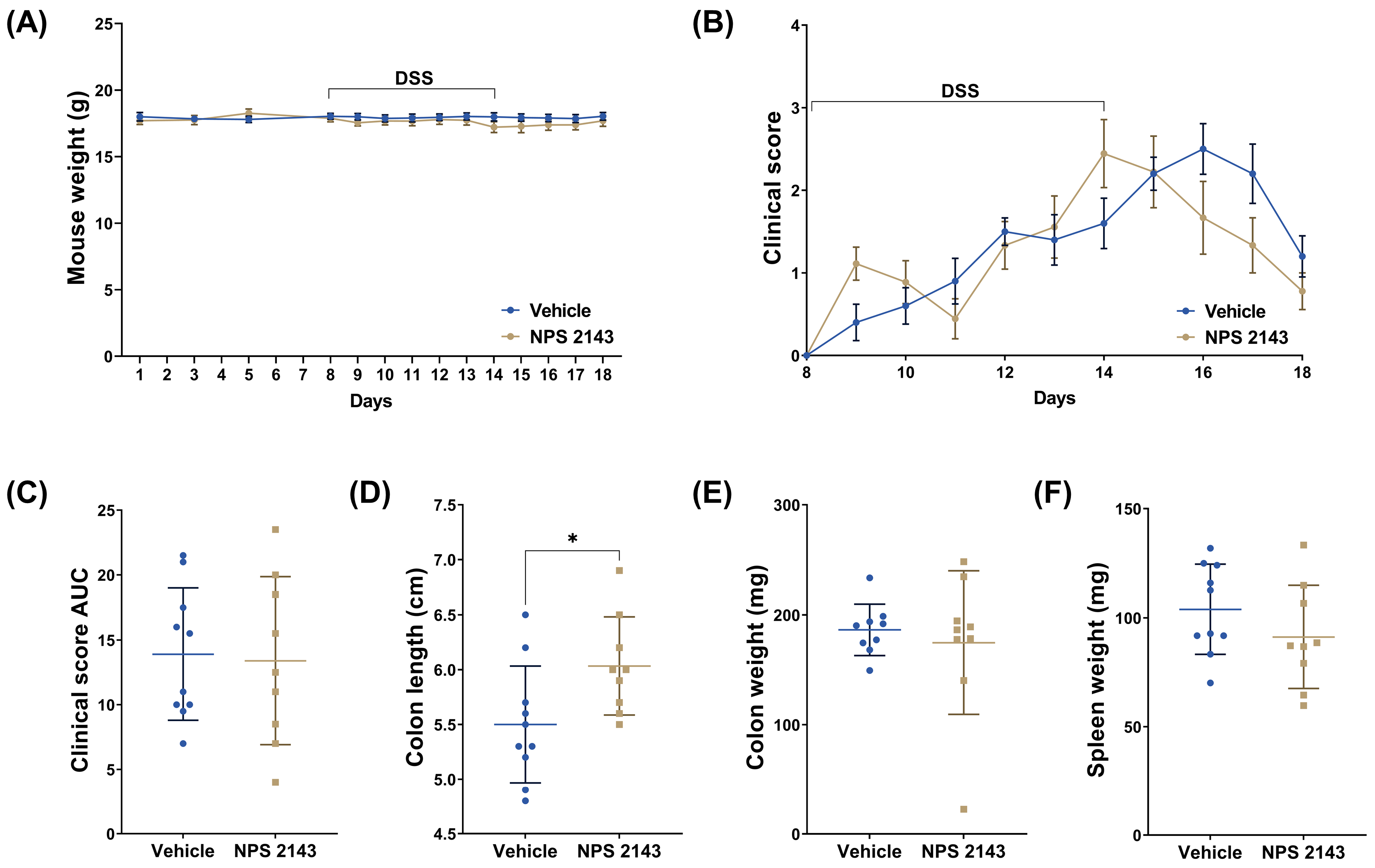
3.1. Treatment with NPS 2143 Had No Effect on the Clinical Score but Prevented Colon Shortening
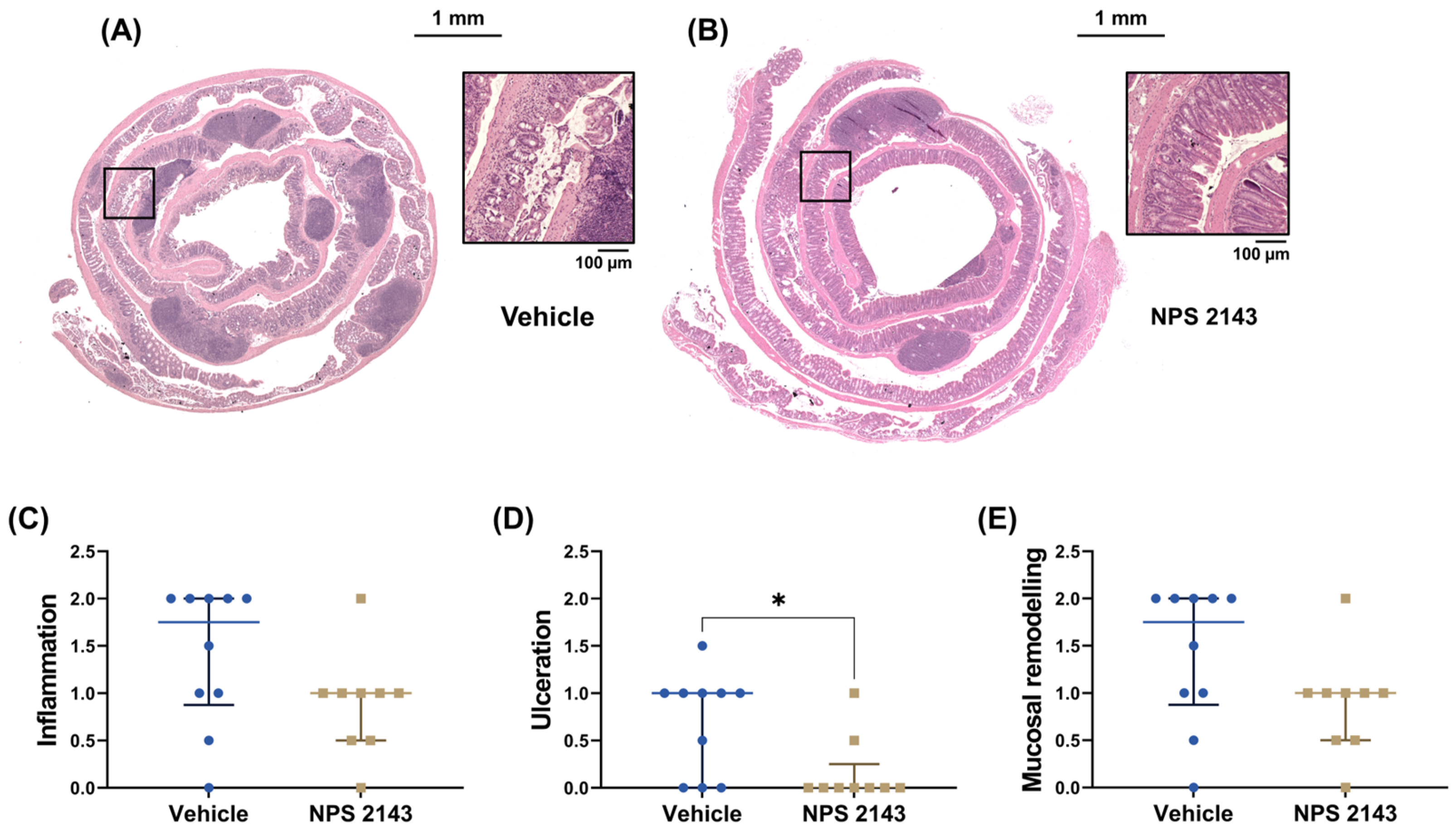
3.2. NPS 2143 Reduced Histological Inflammation
3.3. NPS 2143 Reduced Cell Proliferation in Colonic Lymph Nodes
3.4. Immune Cell Infiltration into the Colon Was Unaffected by the Treatment
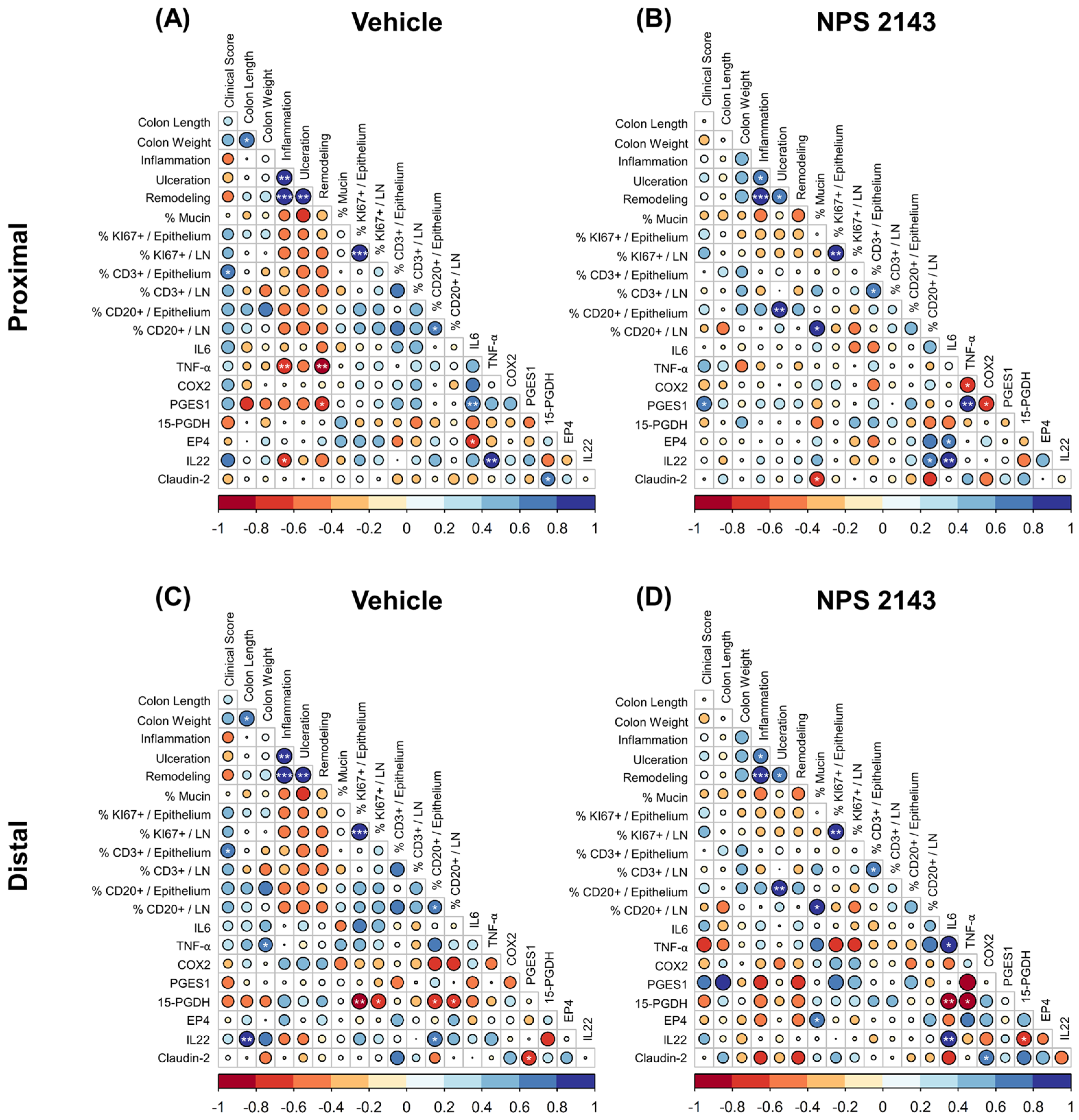
3.5. Effect of NPS 2143 Treatment on Inflammatory Markers in the Plasma and Colonic Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Gamba, G.; Riccardi, D.; Lombardi, M.; Butters, R.; Kifor, O.; Sun, A.; Hediger, M.A.; Lytton, J.; Hebert, S.C. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 1993, 366, 575–580. [Google Scholar] [CrossRef]
- Hannan, F.M.; Kallay, E.; Chang, W.; Brandi, M.L.; Thakker, R.V. The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat. Rev. Endocrinol. 2018, 15, 33–51. [Google Scholar] [CrossRef]
- Yarova, P.L.; Stewart, A.L.; Sathish, V.; Britt, R.D., Jr.; Thompson, M.A.; AP, P.L.; Freeman, M.; Aravamudan, B.; Kita, H.; Brennan, S.C.; et al. Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci. Transl. Med. 2015, 7, 284ra260. [Google Scholar] [CrossRef]
- Yarova, P.L.; Huang, P.; Schepelmann, M.W.; Bruce, R.; Ecker, R.; Nica, R.; Telezhkin, V.; Traini, D.; Gomes Dos Reis, L.; Kidd, E.J.; et al. Characterization of Negative Allosteric Modulators of the Calcium-Sensing Receptor for Repurposing as a Treatment of Asthma. J. Pharmacol. Exp. Ther. 2021, 376, 51–63. [Google Scholar] [CrossRef]
- Chiarini, A.; Armato, U.; Hu, P.; Dal Prà, I. CaSR Antagonist (Calcilytic) NPS 2143 Hinders the Release of Neuroinflammatory IL-6, Soluble ICAM-1, RANTES, and MCP-2 from Aβ-Exposed Human Cortical Astrocytes. Cells 2020, 9, 1386. [Google Scholar] [CrossRef]
- Mattar, P.; Sanhueza, S.; Yuri, G.; Briones, L.; Perez-Leighton, C.; Rudich, A.; Lavandero, S.; Cifuentes, M. Calcium-Sensing Receptor in Adipose Tissue: Possible Association with Obesity-Related Elevated Autophagy. Int. J. Mol. Sci. 2020, 21, 7617. [Google Scholar] [CrossRef]
- Sundararaman, S.S.; van der Vorst, E.P.C. Calcium-Sensing Receptor (CaSR), Its Impact on Inflammation and the Consequences on Cardiovascular Health. Int. J. Mol. Sci. 2021, 22, 2478. [Google Scholar] [CrossRef]
- Cheng, S.X.; Lightfoot, Y.L.; Yang, T.; Zadeh, M.; Tang, L.; Sahay, B.; Wang, G.P.; Owen, J.L.; Mohamadzadeh, M. Epithelial CaSR deficiency alters intestinal integrity and promotes proinflammatory immune responses. FEBS Lett. 2014, 588, 4158–4166. [Google Scholar] [CrossRef] [PubMed]
- Iamartino, L.; Elajnaf, T.; Kallay, E.; Schepelmann, M. Calcium-sensing receptor in colorectal inflammation and cancer: Current insights and future perspectives. World J. Gastroenterol. 2018, 24, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
- Iamartino, L.; Elajnaf, T.; Gall, K.; David, J.; Manhardt, T.; Heffeter, P.; Grusch, M.; Derdak, S.; Baumgartner-Parzer, S.; Schepelmann, M.; et al. Effects of pharmacological calcimimetics on colorectal cancer cells over-expressing the human calcium-sensing receptor. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118836. [Google Scholar] [CrossRef] [PubMed]
- Schepelmann, M.; Kupper, N.; Sladczyk, M.; Mansfield, B.; Manhardt, T.; Piatek, K.; Iamartino, L.; Riccardi, D.; Kariuki, B.M.; Bassetto, M.; et al. Stereo-Specific Modulation of the Extracellular Calcium-Sensing Receptor in Colon Cancer Cells. Int. J. Mol. Sci. 2021, 22, 10124. [Google Scholar] [CrossRef]
- Gushchina, V.; Kupper, N.; Schwarzkopf, M.; Frisch, G.; Piatek, K.; Aigner, C.; Michel, A.; Schueffl, H.; Iamartino, L.; Elajnaf, T.; et al. The calcium-sensing receptor modulates the prostaglandin E(2) pathway in intestinal inflammation. Front. Pharmacol. 2023, 14, 1151144. [Google Scholar] [CrossRef]
- Elajnaf, T.; Iamartino, L.; Mesteri, I.; Muller, C.; Bassetto, M.; Manhardt, T.; Baumgartner-Parzer, S.; Kallay, E.; Schepelmann, M. Nutritional and Pharmacological Targeting of the Calcium-Sensing Receptor Influences Chemically Induced Colitis in Mice. Nutrients 2019, 11, 3072. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.11–15.25.14. [Google Scholar] [CrossRef]
- Schepelmann, M.; Kupper, N.; Gushchina, V.; Mesteri, I.; Manhardt, T.; Moritsch, S.; Muller, C.; Piatek, K.; Salzmann, M.; Vlasaty, A.; et al. AOM/DSS Induced Colitis-Associated Colorectal Cancer in 14-Month-Old Female Balb/C and C57/Bl6 Mice-A Pilot Study. Int. J. Mol. Sci. 2022, 23, 5278. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xu, H.; Cai, F.; Li, P.; Wang, X.; Yao, Y.; Chang, X.; Bi, Z.; Sun, H.; Zhuang, H.; Hua, Z.C. Characterization and Analysis of the Temporal and Spatial Dynamic of Several Enteritis Modeling Methodologies. Front. Immunol. 2021, 12, 727664. [Google Scholar] [CrossRef]
- Tessner, T.G.; Cohn, S.M.; Schloemann, S.; Stenson, W.F. Prostaglandins prevent decreased epithelial cell proliferation associated with dextran sodium sulfate injury in mice. Gastroenterology 1998, 115, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Araki, Y.; Mukaisyo, K.; Sugihara, H.; Fujiyama, Y.; Hattori, T. Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium-induced colitis in mice. Oncol. Rep. 2010, 24, 869–874. [Google Scholar] [CrossRef]
- Xue, G.; Hua, L.; Zhou, N.; Li, J. Characteristics of immune cell infiltration and associated diagnostic biomarkers in ulcerative colitis: Results from bioinformatics analysis. Bioengineered 2021, 12, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Frede, A.; Czarnewski, P.; Monasterio, G.; Tripathi, K.P.; Bejarano, D.A.; Ramirez Flores, R.O.; Sorini, C.; Larsson, L.; Luo, X.; Geerlings, L.; et al. B cell expansion hinders the stroma-epithelium regenerative cross talk during mucosal healing. Immunity 2022, 55, 2336–2351.e12. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Munoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef]
- Rijnierse, A.; Koster, A.S.; Nijkamp, F.P.; Kraneveld, A.D. TNF-alpha is crucial for the development of mast cell-dependent colitis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G969–G976. [Google Scholar] [CrossRef]
- Pugliese, D.; Felice, C.; Papa, A.; Gasbarrini, A.; Rapaccini, G.L.; Guidi, L.; Armuzzi, A. Anti TNF-alpha therapy for ulcerative colitis: Current status and prospects for the future. Expert. Rev. Clin. Immunol. 2017, 13, 223–233. [Google Scholar] [CrossRef]
- Werner, L.E.; Wagner, U. Calcium-sensing receptor-mediated NLRP3 inflammasome activation in rheumatoid arthritis and autoinflammation. Front. Physiol. 2022, 13, 1078569. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, H.A.; Kwon, O.K.; Park, J.W.; Lee, G.; Lee, H.J.; Lee, S.J.; Oh, S.R.; Ahn, K.S. NPS 2143, a selective calcium-sensing receptor antagonist inhibits lipopolysaccharide-induced pulmonary inflammation. Mol. Immunol. 2017, 90, 150–157. [Google Scholar] [CrossRef]
- Cifuentes, M.; Fuentes, C.; Tobar, N.; Acevedo, I.; Villalobos, E.; Hugo, E.; Ben-Jonathan, N.; Reyes, M. Calcium sensing receptor activation elevates proinflammatory factor expression in human adipose cells and adipose tissue. Mol. Cell. Endocrinol. 2012, 361, 24–30. [Google Scholar] [CrossRef]
- Schepelmann, M.; Yarova, P.L.; Lopez-Fernandez, I.; Davies, T.S.; Brennan, S.C.; Edwards, P.J.; Aggarwal, A.; Graca, J.; Rietdorf, K.; Matchkov, V.; et al. The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure. Am. J. Physiol. Cell Physiol. 2016, 310, C193–C204. [Google Scholar] [CrossRef] [PubMed]
- Schepelmann, M.; Ranieri, M.; Lopez-Fernandez, I.; Webberley, T.S.; Brennan, S.C.; Yarova, P.L.; Graca, J.; Hanif, U.K.; Muller, C.; Manhardt, T.; et al. Impaired Mineral Ion Metabolism in a Mouse Model of Targeted Calcium-Sensing Receptor (CaSR) Deletion from Vascular Smooth Muscle Cells. J. Am. Soc. Nephrol. 2022, 33, 1323–1340. [Google Scholar] [CrossRef]
- Lee, J.J.; Liu, X.; O’Neill, D.; Beggs, M.R.; Weissgerber, P.; Flockerzi, V.; Chen, X.Z.; Dimke, H.; Alexander, R.T. Activation of the calcium sensing receptor attenuates TRPV6-dependent intestinal calcium absorption. JCI Insight 2019, 4, e128013. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Leong, I.L.; Leung, Y.M. Ca(2+)-sensing receptor-TRP channel-mediated Ca(2+) signaling: Functional diversity and pharmacological complexity. Eur. J. Pharmacol. 2024, 977, 176717. [Google Scholar] [CrossRef] [PubMed]
- Oak, A.A.; Chhetri, P.D.; Rivera, A.A.; Verkman, A.S.; Cil, O. Repurposing calcium-sensing receptor agonist cinacalcet for treatment of CFTR-mediated secretory diarrheas. JCI Insight 2021, 6, e146823. [Google Scholar] [CrossRef]
- Singer, I.I.; Kawka, D.W.; Schloemann, S.; Tessner, T.; Riehl, T.; Stenson, W.F. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology 1998, 115, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Rerko, R.M.; Platzer, P.; Dawson, D.; Willis, J.; Tong, M.; Lawrence, E.; Lutterbaugh, J.; Lu, S.; Willson, J.K.; et al. 15-Hydroxyprostaglandin dehydrogenase, a COX-2 oncogene antagonist, is a TGF-beta-induced suppressor of human gastrointestinal cancers. Proc. Natl. Acad. Sci. USA 2004, 101, 17468–17473. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.C.; Zhou, Z.Q.; Yang, D.; Chen, Z.L.; Zhou, Y.Y.; Wen, W.; Feng, C.; Zheng, L.; Peng, X.Y.; Tang, C.F. Recent advances in studies of 15-PGDH as a key enzyme for the degradation of prostaglandins. Int. Immunopharmacol. 2021, 101, 108176. [Google Scholar] [CrossRef]
- Mizoguchi, A.; Yano, A.; Himuro, H.; Ezaki, Y.; Sadanaga, T.; Mizoguchi, E. Clinical importance of IL-22 cascade in IBD. J. Gastroenterol. 2018, 53, 465–474. [Google Scholar] [CrossRef]
- Perez, L.G.; Kempski, J.; McGee, H.M.; Pelzcar, P.; Agalioti, T.; Giannou, A.; Konczalla, L.; Brockmann, L.; Wahib, R.; Xu, H.; et al. TGF-beta signaling in Th17 cells promotes IL-22 production and colitis-associated colon cancer. Nat. Commun. 2020, 11, 2608. [Google Scholar] [CrossRef]
- Rogler, G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014, 345, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Kumar, B.; Thapa, I.; Tamang, R.L.; Yadav, S.K.; Washington, M.K.; Talmon, G.A.; Yu, A.S.; Bastola, D.K.; Dhawan, P.; et al. Claudin-2 protects against colitis-associated cancer by promoting colitis-associated mucosal healing. J. Clin. Investig. 2023, 133, e170771. [Google Scholar] [CrossRef] [PubMed]
- Tang, A.; Li, N.; Li, X.; Yang, H.; Wang, W.; Zhang, L.; Li, G.; Xiong, W.; Ma, J.; Shen, S. Dynamic activation of the key pathways: Linking colitis to colorectal cancer in a mouse model. Carcinogenesis 2012, 33, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Chino, X.M.S.; Martinez, C.J.; Garzon, V.R.V.; Gonzalez, I.A.; Trevino, S.V.; Bujaidar, E.M.; Ortiz, G.D.; Hoyos, R.B. Cooked Chickpea Consumption Inhibits Colon Carcinogenesis in Mice Induced with Azoxymethane and Dextran Sulfate Sodium. J. Am. Coll. Nutr. 2017, 36, 391–398. [Google Scholar] [CrossRef]
- Damazo-Lima, M.; Rosas-Perez, G.; Reynoso-Camacho, R.; Perez-Ramirez, I.F.; Rocha-Guzman, N.E.; de Los Rios, E.A.; Ramos-Gomez, M. Chemopreventive Effect of the Germinated Oat and its Phenolic-AVA Extract in Azoxymethane/Dextran Sulfate Sodium (AOM/DSS) Model of Colon Carcinogenesis in Mice. Foods 2020, 9, 169. [Google Scholar] [CrossRef]
- Fichera, A.; Little, N.; Dougherty, U.; Mustafi, R.; Cerda, S.; Li, Y.C.; Delgado, J.; Arora, A.; Campbell, L.K.; Joseph, L.; et al. A vitamin D analogue inhibits colonic carcinogenesis in the AOM/DSS model. J. Surg. Res. 2007, 142, 239–245. [Google Scholar] [CrossRef]




| Vehicle | NPS 2143 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Target | Mean Concentration (pg/mL) | ± | SD | N | Mean Concentration (pg/mL) | ± | SD | N | p |
| ENA-78 (LIX) | 38.46 | ± | 27.47 | 8 | 42.34 | ± | 42.31 | 8 | ns |
| Eotaxin | 931.10 | ± | 246.55 | 10 | 1070.00 | ± | 142.45 | 10 | ns |
| G-CSF | 23.72 | ± | 21.33 | 9 | 103.90 | ± | 134.11 | 8 | ns |
| Gro-α | 15.77 | ± | 9.56 | 8 | 22.57 | ± | 24.95 | 10 | ns |
| II-22 | 35.80 | ± | 28.87 | 8 | 41.03 | ± | 29.66 | 6 | ns |
| IL-13 | 5.80 | ± | 2.36 | 8 | 5.20 | ± | 2.53 | 7 | ns |
| IL-18 | 132.4 | ± | 72.05 | 5 | 148.3 | ± | 165.39 | 2 | ns |
| IL-27 | 9.94 | ± | 11.00 | 9 | 14.03 | ± | 13.24 | 9 | ns |
| IL-5 | 4.11 | ± | 4.19 | 9 | 3.87 | ± | 4.77 | 5 | ns |
| IL-6 | 4.75 | ± | 2.21 | 4 | 6.11 | ± | 5.44 | 7 | ns |
| IL-9 | 64.74 | ± | 37.06 | 5 | 71.85 | ± | 42.35 | 6 | ns |
| IP-10 | 3.68 | ± | 2.95 | 10 | 2.30 | ± | 0.55 | 10 | ns |
| MCP-3 | 69.72 | ± | 36.49 | 10 | 59.82 | ± | 13.55 | 10 | ns |
| MIP-1α | 0.88 | ± | 0.77 | 9 | 0.95 | ± | 0.70 | 8 | ns |
| MIP-1β | 1.60 | ± | 0.75 | 8 | 1.14 | ± | 0.37 | 6 | ns |
| RANTES | 22.78 | ± | 7.52 | 10 | 19.87 | ± | 6.03 | 10 | ns |
| TNF-α | 2.26 | ± | 0.34 | 3 | 4.21 | ± | 0.61 | 4 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piatek, K.; Gushchina, V.; Kleinwächter, A.; Kupper, N.; Mesteri, I.; Elajnaf, T.; Iamartino, L.; Salzmann, M.; Müller, C.; Manhardt, T.; et al. Targeting the Calcium-Sensing Receptor in Chemically Induced Medium-Grade Colitis in Female BALB/C Mice. Nutrients 2024, 16, 4362. https://doi.org/10.3390/nu16244362
Piatek K, Gushchina V, Kleinwächter A, Kupper N, Mesteri I, Elajnaf T, Iamartino L, Salzmann M, Müller C, Manhardt T, et al. Targeting the Calcium-Sensing Receptor in Chemically Induced Medium-Grade Colitis in Female BALB/C Mice. Nutrients. 2024; 16(24):4362. https://doi.org/10.3390/nu16244362
Chicago/Turabian StylePiatek, Karina, Valeriya Gushchina, Ava Kleinwächter, Nadja Kupper, Ildiko Mesteri, Taha Elajnaf, Luca Iamartino, Martina Salzmann, Christian Müller, Teresa Manhardt, and et al. 2024. "Targeting the Calcium-Sensing Receptor in Chemically Induced Medium-Grade Colitis in Female BALB/C Mice" Nutrients 16, no. 24: 4362. https://doi.org/10.3390/nu16244362
APA StylePiatek, K., Gushchina, V., Kleinwächter, A., Kupper, N., Mesteri, I., Elajnaf, T., Iamartino, L., Salzmann, M., Müller, C., Manhardt, T., Vlasaty, A., Kallay, E., & Schepelmann, M. (2024). Targeting the Calcium-Sensing Receptor in Chemically Induced Medium-Grade Colitis in Female BALB/C Mice. Nutrients, 16(24), 4362. https://doi.org/10.3390/nu16244362








