The Role of Macronutrients and Gut Microbiota in Neuroinflammation Post-Traumatic Brain Injury: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Pathophysiology of Traumatic Brain Injury
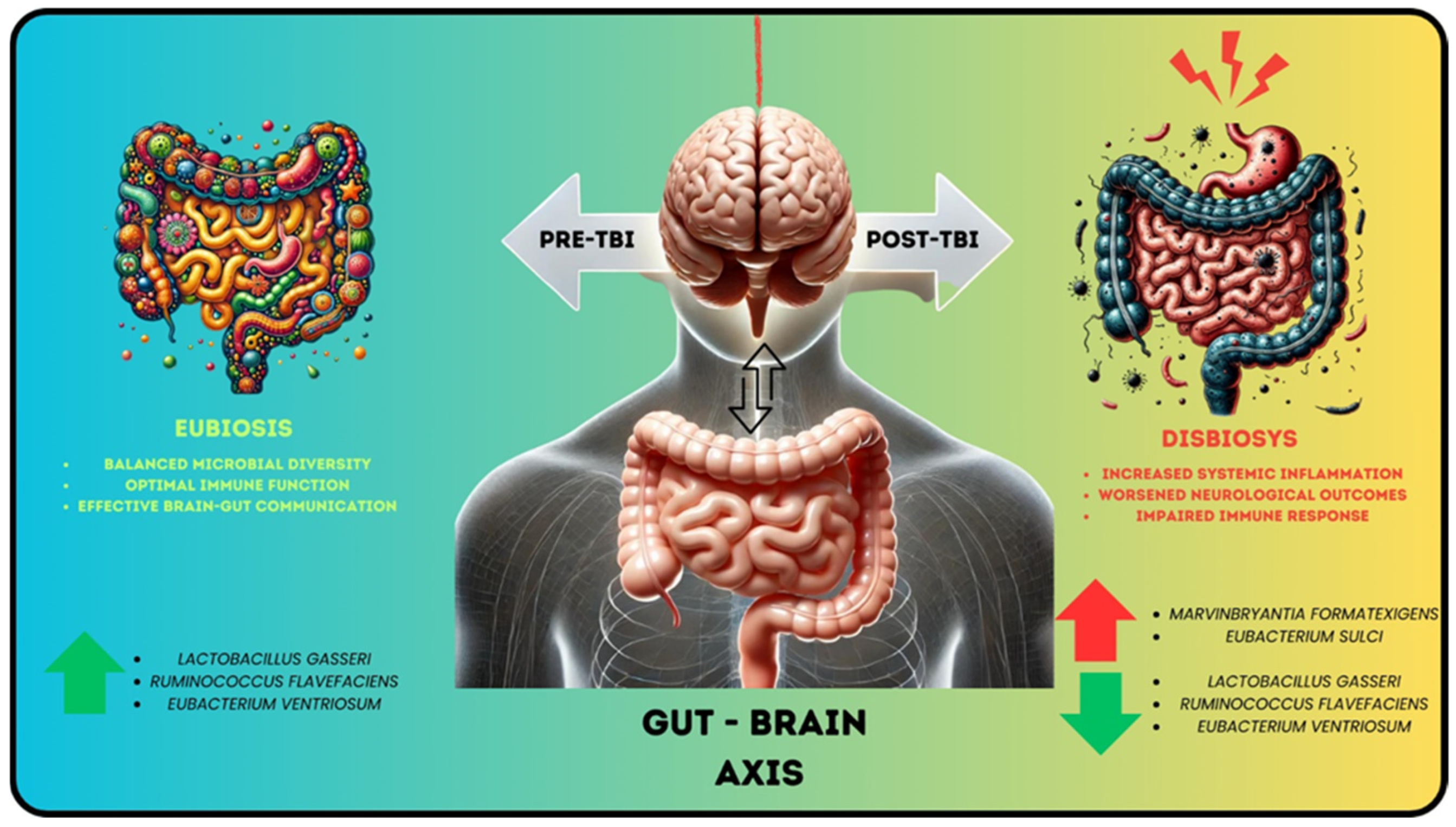
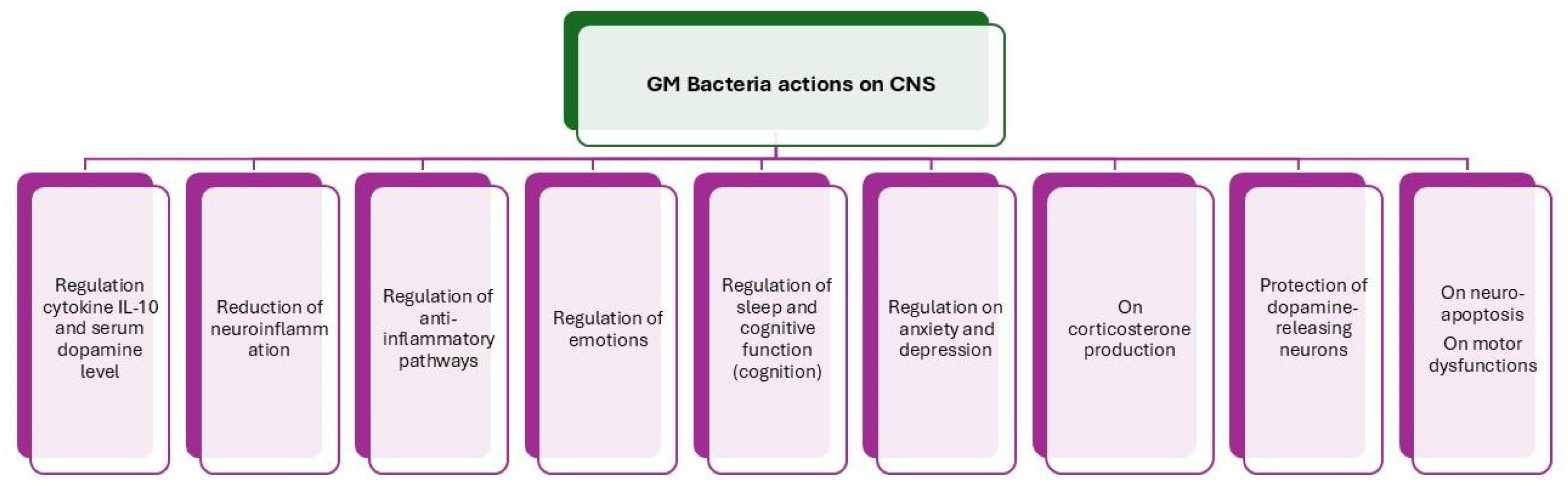
4. Gut Microbiota’s Role in Central Nervous System (CNS)
5. The Role of the Gut Microbiota’s Metabolome in Neurotransmission
6. The Role of Bioactive Lipids in Neuroprotection and Neuroplasticity Following Traumatic Brain Injury
7. The Role of Amino Acids in Neuroinflammation and Neuroprotection: Implications for Traumatic Brain Injury Recovery
8. The Role of Carbohydrates in Traumatic Brain Injury
9. Functional Foods: Symbiotics (Probiotics/Psychobiotics and Prebiotics) and Metabiotics (Postbiotics and Parabiotics)
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hill, C.S.; Coleman, M.P.; Menon, D.K. Traumatic Axonal Injury: Mechanisms and Translational Opportunities. Trends Neurosci. 2016, 39, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Nolan, S. Traumatic Brain Injury A Review. 2005. Available online: http://www.cdc.gov (accessed on 30 September 2024).
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Majdan, M.; Plancikova, D.; Brazinova, A.; Rusnak, M.; Nieboer, D.; Feigin, V.L.; Maas, A. Epidemiology of Traumatic Brain Injuries in Europe: A Cross-Sectional Analysis. 2016. Available online: www.thelancet.com/ (accessed on 20 October 2024).
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.-C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2019, 130, 1080–1097. [Google Scholar] [CrossRef] [PubMed]
- Dewan, S.; Schimmel, S.; Borlongan, C.V. Treating childhood traumatic brain injury with autologous stem cell therapy. Expert Opin. Biol. Ther. 2018, 18, 515–524. [Google Scholar] [CrossRef]
- George, A.K.; Behera, J.; Homme, R.P.; Tyagi, N.; Tyagi, S.C.; Singh, M. Rebuilding Microbiome for Mitigating Traumatic Brain Injury: Importance of Restructuring the Gut-Microbiome-Brain Axis. Mol. Neurobiol. 2021, 58, 3614–3627. [Google Scholar] [CrossRef]
- Hosang, L.; Canals, R.C.; van der Flier, F.J.; Hollensteiner, J.; Daniel, R.; Flügel, A.; Odoardi, F. The lung microbiome regulates brain autoimmunity. Nature 2022, 603, 138–144. [Google Scholar] [CrossRef]
- Cotoia, A.; Paradiso, R.; Ferrara, G.; Borriello, G.; Santoro, F.; Spina, I.; Mirabella, L.; Mariano, K.; Fusco, G.; Cinnella, G.; et al. Modifications of lung microbiota structure in traumatic brain injury ventilated patients according to time and enteral feeding formulas: A prospective randomized study. Crit. Care 2023, 27, 244. [Google Scholar] [CrossRef]
- Das, M.; Leonardo, C.C.; Rangooni, S.; Mohapatra, S.S.; Mohapatra, S.; Pennypacker, K.R. Lateral Fluid Percussion Injury of the Brain Induces CCL20 Inflammatory Chemokine Expression in Rats. 2011. Available online: http://www.jneuroinflammation.com/content/8/1/148 (accessed on 9 December 2024).
- Pilitsis, J.G.; Coplin, W.M.; O’Regan, M.H.; Wellwood, J.M.; Diaz, F.G.; Fairfax, M.R.; Michael, D.B.; Phillis, J.W. Free fatty acids in cerebrospinal fluids from patients with traumatic brain injury. Neurosci. Lett. 2003, 349, 136–138. [Google Scholar] [CrossRef]
- Hauser, N.; Kirk, L.M.; Rahbar, E. Circulating Polyunsaturated Fatty Acids (PUFAs) as Biological Indicators in Trauma. In Biomarkers in Trauma, Injury and Critical Care; Springer: Cham, Switzerland, 2022; pp. 1–27. [Google Scholar] [CrossRef]
- Rajendram, R.; Preedy, V.R.; Patel, V.B. (Eds.) Biomarkers in Trauma, Injury and Critical Care; Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Treangen, T.J.; Wagner, J.; Burns, M.P.; Villapol, S. Traumatic Brain Injury in Mice Induces Acute Bacterial Dysbiosis Within the Fecal Microbiome. Front. Immunol. 2018, 9, 2757. [Google Scholar] [CrossRef]
- Taraskina, A.; Ignatyeva, O.; Lisovaya, D.; Ivanov, M.; Ivanova, L.; Golovicheva, V.; Baydakova, G.; Silachev, D.; Popkov, V.; Ivanets, T.; et al. Effects of Traumatic Brain Injury on the Gut Microbiota Composition and Serum Amino Acid Profile in Rats. Cells 2022, 11, 1409. [Google Scholar] [CrossRef]
- Wang, Y.; Bing, H.; Jiang, C.; Wang, J.; Wang, X.; Xia, Z.; Chu, Q. Gut microbiota dysbiosis and neurological function recovery after intracerebral hemorrhage: An analysis of clinical samples. Microbiol. Spectr. 2024, 12, e0117824. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, S.; Acosta, S.; Lozano, D. Neuroinflammation in traumatic brain injury: A chronic response to an acute injury. Brain Circ. 2017, 3, 135. [Google Scholar] [CrossRef] [PubMed]
- Longo, S.; Rizza, S.; Federici, M. Microbiota-gut-brain axis: Relationships among the vagus nerve, gut microbiota, obesity, and diabetes. Acta Diabetol. 2023, 60, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B. Anti-inflammatory effects of vagal nerve stimulation with a special attention to intestinal barrier dysfunction. Neurogastroenterol. Motil. 2022, 34, e14456. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016, 594, 5781–5790. [Google Scholar] [CrossRef]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; Macri, J.; McCoy, K.D.; et al. The Intestinal Microbiota Affect Central Levels of Brain-Derived Neurotropic Factor and Behavior in Mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef]
- Dziedzic, A.; Maciak, K.; Bliźniewska-Kowalska, K.; Gałecka, M.; Kobierecka, W.; Saluk, J. The Power of Psychobiotics in Depression: A Modern Approach through the Microbiota–Gut–Brain Axis: A Literature Review. Nutrients 2024, 16, 1054. [Google Scholar] [CrossRef]
- Montagnani, M.; Bottalico, L.; Potenza, M.A.; Charitos, I.A.; Topi, S.; Colella, M.; Santacroce, L. The Crosstalk between Gut Microbiota and Nervous System: A Bidirectional Interaction between Microorganisms and Metabolome. Int. J. Mol. Sci. 2023, 24, 10322. [Google Scholar] [CrossRef]
- Rusch, J.A.; Layden, B.T.; Dugas, L.R. Signalling cognition: The gut microbiota and hypothalamic-pituitary-adrenal axis. Front. Endocrinol. 2023, 14, 1130689. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions Between Enteric Microbiota, Central and Enteric Nervous Systems. 2015. Available online: www.annalsgastro.gr (accessed on 19 November 2024).
- Meng, J.; Huang, Y.; Huang, J.; Yang, K. The role of the sensor kinase, QseC, an adrenergic receptor of Escherichia coli, in bacterial translocation during hemorrhagic shock. J. Trauma Acute Care Surg. 2016, 80, 972–976. [Google Scholar] [CrossRef]
- Hamed, A.; Pullinger, G.; Stevens, M.; Farveen, F.; Freestone, P. Characterisation of the E. coli and Salmonella qseC and qseE mutants reveals a metabolic rather than adrenergic receptor role. FEMS Microbiol. Lett. 2022, 369, fnac012. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.B.; Hughes, D.T.; Zhu, C.; Boedeker, E.C.; Sperandio, V. The QseC sensor kinase: A bacterial adrenergic receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 10420–10425. [Google Scholar] [CrossRef] [PubMed]
- Gwak, M.-G.; Chang, S.-Y. Gut-Brain Connection: Microbiome, Gut Barrier, and Environmental Sensors. Immune Netw. 2021, 21, e20. [Google Scholar] [CrossRef]
- Demaude, J. Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: Implications for delayed epithelial barrier dysfunction. Gut 2006, 55, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, P.; Wang, Y.-W.; Sha, H.-H.; Dong, H.-Q.; Qian, Y.-N. Neuroimmune connections between corticotropin-releasing hormone and mast cells: Novel strategies for the treatment of neurodegenerative diseases. Neural Regen. Res. 2021, 16, 2184. [Google Scholar] [CrossRef] [PubMed]
- Varghese, A.K.; Verdú, E.F.; Bercik, P.; Khan, W.I.; Blennerhassett, P.A.; Szechtman, H.; Collins, S.M. Antidepressants Attenuate Increased Susceptibility to Colitis in a Murine Model of Depression. Gastroenterology 2006, 130, 1743–1753. [Google Scholar] [CrossRef]
- Park, A.J.; Collins, J.; Blennerhassett, P.A.; Ghia, J.E.; Verdu, E.F.; Bercik, P.; Collins, S.M. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol. Motil. 2013, 25, 733. [Google Scholar] [CrossRef]
- Celorrio, M.; Abellanas, M.A.; Rhodes, J.; Goodwin, V.; Moritz, J.; Vadivelu, S.; Wang, L.; Rodgers, R.; Xiao, S.; Anabayan, I.; et al. Gut microbial dysbiosis after traumatic brain injury modulates the immune response and impairs neurogenesis. Acta Neuropathol. Commun. 2021, 9, 40. [Google Scholar] [CrossRef]
- DeSana, A.J.; Estus, S.; Barrett, T.A.; Saatman, K.E. Acute gastrointestinal permeability after traumatic brain injury in mice precedes a bloom in Akkermansia muciniphila supported by intestinal hypoxia. Sci. Rep. 2024, 14, 2990. [Google Scholar] [CrossRef]
- Alverdy, J.; Holbrook, C.; Rocha, F.; Seiden, L.; Licheng, R.; Wu, R.L.; Musch, M.; Chang, E.; Ohman, D.; Suh, S. Gut-Derived Sepsis Occurs When the Right Pathogen with the Right Virulence Genes Meets the Right Host. Ann. Surg. 2000, 232, 480–489. [Google Scholar] [CrossRef]
- Cogan, T.A.; Thomas, A.O.; Rees, L.E.N.; Taylor, A.H.; Jepson, M.A.; Williams, P.H.; Ketley, J.; Humphrey, T.J. Norepinephrine increases the pathogenic potential of Campylobacter jejuni. Gut 2007, 56, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xi, B.; Chen, K.; Song, R.; Qin, T.; Xie, J.; Pan, L. The stress hormone norepinephrine increases the growth and virulence of Aeromonas hydrophila. Microbiologyopen 2019, 8, e00664. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Sun, S.; Wang, P.; Sun, Y.; Hu, Q.; Wang, X. The Mechanism of Secretion and Metabolism of Gut-Derived 5-Hydroxytryptamine. Int. J. Mol. Sci. 2021, 22, 7931. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.; Farzi, A.; Zhu, W. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms 2022, 10, 1838. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Anand, S.; Kaur, H.; Mande, S.S. Comparative in silico analysis of butyrate production pathways in gut commensals and pathogens. Front. Microbiol. 2016, 7, 1945. [Google Scholar] [CrossRef] [PubMed]
- Vital, M.; Howe, A.C.; Tiedje, J.M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. mBio 2014, 5, e00889-14. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H. Carrier-Mediated Blood-Brain Barrier Transport of Short-Chain Monocarboxylic Organic Acids. 1973. Available online: www.physiology.org/journal/ajplegacy (accessed on 9 December 2024).
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef] [PubMed]
- van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain–gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Rea, K.; Dinan, T.G.; Cryan, J.F. A gut (microbiome) feeling about the brain. Curr. Opin. Gastroenterol. 2016, 32, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Fonseca, S.; Carding, S.R. Gut microbes and metabolites as modulators of blood-brain barrier integrity and brain health. Gut Microbes 2020, 11, 135–157. [Google Scholar] [CrossRef]
- Liddle, R.A. Neuropods. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 739–747. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Hoyles, L.; Snelling, T.; Umlai, U.-K.; Nicholson, J.K.; Carding, S.R.; Glen, R.C.; McArthur, S. Microbiome–host systems interactions: Protective effects of propionate upon the blood–brain barrier. Microbiome 2018, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Fock, E.; Parnova, R. Mechanisms of Blood–Brain Barrier Protection by Microbiota-Derived Short-Chain Fatty Acids. Cells 2023, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hu, H.; Ju, Y.; Liu, J.; Wang, M.; Liu, B.; Zhang, Y. Gut microbiota-derived short-chain fatty acids and depression: Deep insight into biological mechanisms and potential applications. Gen. Psychiatry 2024, 37, e10137. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Noto, D.; Hoshino, Y.; Mizuno, M.; Miyake, S. Butyrate suppresses demyelination and enhances remyelination. J. Neuroinflamm. 2019, 16, 165. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cui, C. The role of short-chain fatty acids in central nervous system diseases. Mol. Cell Biochem. 2022, 477, 2595–2607. [Google Scholar] [CrossRef]
- Swer, N.M.; Venkidesh, B.S.; Murali, T.S.; Mumbrekar, K.D. Gut microbiota-derived metabolites and their importance in neurological disorders. Mol. Biol. Rep. 2023, 50, 1663–1675. [Google Scholar] [CrossRef]
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.-H. Butyrate producers, ‘The Sentinel of Gut’: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2023, 13, 1103836. [Google Scholar] [CrossRef]
- Vargas-Caraveo, A.; Sayd, A.; Robledo-Montaña, J.; Caso, J.R.; Madrigal, J.L.M.; García-Bueno, B.; Leza, J.C. Toll-like receptor 4 agonist and antagonist lipopolysaccharides modify innate immune response in rat brain circumventricular organs. J. Neuroinflamm. 2020, 17, 6. [Google Scholar] [CrossRef]
- Muzio, L.; Viotti, A.; Martino, G. Microglia in Neuroinflammation and Neurodegeneration: From Understanding to Therapy. Front. Neurosci. 2021, 15, 742065. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-chain fatty acids (SCFAs) alone or in combination regulate select immune functions of microglia-like cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, Y.; Gong, Y.; Yang, R.; Chen, Z.; Hu, W.; Wu, Y.; Gao, M.; Xu, X.; Qin, Y.; et al. Sodium butyrate triggers a functional elongation of microglial process via Akt-small RhoGTPase activation and HDACs inhibition. Neurobiol. Dis. 2018, 111, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Rothhammer, V.; Mascanfroni, I.D.; Bunse, L.; Takenaka, M.C.; Kenison, J.E.; Mayo, L.; Chao, C.-C.; Patel, B.; Yan, R.; Blain, M.; et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 2016, 22, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774. [Google Scholar] [CrossRef]
- Shimizu, T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef]
- Anthonymuthu, T.S.; Kenny, E.M.; Lamade, A.M.; Kagan, V.E.; Bayır, H. Oxidized phospholipid signaling in traumatic brain injury. Free. Radic. Biol. Med. 2018, 124, 493–503. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 197–202. [Google Scholar] [CrossRef]
- Serhan, C.N.; Bäck, M.; Chiurchiù, V.; Hersberger, M.; Mittendorfer, B.; Calder, P.C.; Waitzberg, D.L.; Stoppe, C.; Klek, S.; Martindale, R.G.; et al. Expert consensus report on lipid mediators: Role in resolution of inflammation and muscle preservation. FASEB J. 2024, 38, e23699. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Feng, X.; Jiao, W. Omega-3 polyunsaturated fatty acids alleviate early brain injury after traumatic brain injury by inhibiting neuroinflammation and necroptosis. Transl. Neurosci. 2023, 14, 20220277. [Google Scholar] [CrossRef]
- Lin, L.; Zheng, S.; Lai, J.; Ye, D.; Huang, Q.; Wu, Z.; Chen, X.; Wang, S. Omega-3 Polyunsaturated Fatty Acids Protect Neurological Function After Traumatic Brain Injury by Suppressing Microglial Transformation to the Proinflammatory Phenotype and Activating Exosomal NGF/TrkA Signaling. Mol. Neurobiol. 2023, 60, 5592–5606. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. J. Neurotrauma 2007, 24, 1587–1595. [Google Scholar] [CrossRef]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Dietary Omega-3 Fatty Acids Normalize BDNF Levels, Reduce Oxidative Damage, and Counteract Learning Disability after Traumatic Brain Injury in Rats. J. Neurotrauma 2004, 21, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.D.; Hadley, K.; Bailes, J.E. Dietary supplementation with the Omega-3 fatty acid docosahexaenoic acid in traumatic brain injury. Neurosurgery 2011, 68, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Bailes, J.E.; Mills, J.D. Docosahexaenoic acid reduces traumatic axonal injury in a rodent head injury model. J. Neurotrauma 2010, 27, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.D.; Bailes, J.E.; Sedney, C.L.; Hutchins, H.; Sears, B. Omega-3 fatty acid supplementation and reduction of traumatic axonal injury in a rodent head injury model: Laboratory investigation. J. Neurosurg. 2011, 114, 77–84. [Google Scholar] [CrossRef]
- Shin, S.S.; Dixon, C.E. Oral fish oil restores striatal dopamine release after traumatic brain injury. Neurosci. Lett. 2011, 496, 168–171. [Google Scholar] [CrossRef]
- Zhang, E.; Wan, X.; Yang, L.; Wang, D.; Chen, Z.; Chen, Y.; Liu, M.; Zhang, G.; Wu, J.; Han, H.; et al. Omega-3 Polyunsaturated Fatty Acids Alleviate Traumatic Brain Injury by Regulating the Glymphatic Pathway in Mice. Front. Neurol. 2020, 11, 707. [Google Scholar] [CrossRef]
- Luo, C.-L.; Li, Q.-Q.; Chen, X.-P.; Zhang, X.-M.; Li, L.-L.; Li, B.-X.; Zhao, Z.-Q.; Tao, L.-Y. Lipoxin A4 attenuates brain damage and downregulates the production of pro-inflammatory cytokines and phosphorylated mitogen-activated protein kinases in a mouse model of traumatic brain injury. Brain Res. 2013, 1502, 1–10. [Google Scholar] [CrossRef]
- Chen, X.; Wu, S.; Chen, C.; Xie, B.; Fang, Z.; Hu, W.; Chen, J.; Fu, H.; He, H. Omega-3 polyunsaturated fatty acid supplementation attenuates microglial-induced inflammation by inhibiting the HMGB1/TLR4/NF-ΚB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2017, 14, 143. [Google Scholar] [CrossRef]
- Chen, X.; Chen, C.; Fan, S.; Wu, S.; Yang, F.; Fang, Z.; Fu, H.; Li, Y. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-ΚB pathway following experimental traumatic brain injury. J. Neuroinflamm. 2018, 15, 116. [Google Scholar] [CrossRef]
- Chen, X.; Pan, Z.; Fang, Z.; Lin, W.; Wu, S.; Yang, F.; Li, Y.; Fu, H.; Gao, H.; Li, S. Omega-3 polyunsaturated fatty acid attenuates traumatic brain injury-induced neuronal apoptosis by inducing autophagy through the upregulation of SIRT1-mediated deacetylation of Beclin-1. J. Neuroinflamm. 2018, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Bisicchia, E.; Sasso, V.; Catanzaro, G.; Leuti, A.; Besharat, Z.M.; Chiacchiarini, M.; Molinari, M.; Ferretti, E.; Viscomi, M.T.; Chiurchiù, V. Resolvin D1 Halts Remote Neuroinflammation and Improves Functional Recovery after Focal Brain Damage Via ALX/FPR2 Receptor-Regulated MicroRNAs. Mol. Neurobiol. 2018, 55, 6894–6905. [Google Scholar] [CrossRef]
- Roberts, L.; Bailes, J.; Dedhia, H.; Zikos, A.; Singh, A.; McDowell, D.; Failinger, C.; Biundo, R.; Petrick, J.; Carpenter, J. Surviving a Mine Explosion. J. Am. Coll. Surg. 2008, 207, 276–283. [Google Scholar] [CrossRef]
- Lewis, M.; Ghassemi, P.; Hibbeln, J. Therapeutic use of omega-3 fatty acids in severe head trauma. Am. J. Emerg. Med. 2013, 31, 273.e5–273.e8. [Google Scholar] [CrossRef]
- Bailes, J.E.; Abusuwwa, R.; Arshad, M.; Chowdhry, S.A.; Schleicher, D.; Hempeck, N.; Gandhi, Y.N.; Jaffa, Z.; Bokhari, F.; Karahalios, D.; et al. BROCA’S AREA Omega-3 fatty acid supplementation in severe brain trauma: Case for a large multicenter trial. J. Neurosurg. 2020, 133, 598–602. [Google Scholar] [CrossRef]
- Leontyev, D.; Pulliam, A.N.; Ma, X.; Gaul, D.A.; LaPlaca, M.C.; Fernández, F.M. Spatial lipidomics maps brain alterations associated with mild traumatic brain injury. Front. Chem. 2024, 12, 1394064. [Google Scholar] [CrossRef]
- Gier, E.C.; Pulliam, A.N.; Gaul, D.A.; Moore, S.G.; Laplaca, M.C.; Fernández, F.M. Lipidome Alterations Following Mild Traumatic Brain Injury in the Rat. Metabolites 2022, 12, 150. [Google Scholar] [CrossRef]
- Thomas, I.; Dickens, A.M.; Posti, J.P.; Czeiter, E.; Duberg, D.; Sinioja, T.; Krakstrom, M.; Helmrich, I.R.A.R.; Wang, K.K.W.; Maas, A.I.R.; et al. Serum metabolome associated with severity of acute traumatic brain injury. Nat. Commun. 2022, 13, 2545. [Google Scholar] [CrossRef]
- Drasar, B.S.; Crowther, J.S.; Goddard, P.; Hawksworth, G.; Hill, M.J.; Peach, S.; Williams, R.E.O.; Renwich, A. The relation between diet and the gut microflora in man. Proc. Nutr. Soc. 2007, 32, 49–52. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef] [PubMed]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. The effect of amino acids on wound healing: A systematic review and meta-analysis on arginine and glutamine. Nutrients 2021, 13, 2498. [Google Scholar] [CrossRef] [PubMed]
- Cotoia, A.; Cantatore, L.P.; Beck, R.; Tullo, L.; Fortarezza, D.; Marchese, F.; Ferrara, G.; Cinnella, G. Immunological effects of glutamine supplementation in polytrauma patients in intensive care unit. J. Anesthesia Analg. Crit. Care 2022, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xu, J. Effect of dipeptide of glutamine and alanine on severe traumatic brain injury. Chin. J. Traumatol. 2007, 10, 145–149. [Google Scholar]
- Zeng, J.; Zhao, X.; Huang, Q.; Wang, E. Effects of glutamine-enriched enteral nutrition on nutritional status and prognosis of patients with severe head injury. Zhonghua Shao Shang Za Zhi 2009, 25, 335–338. [Google Scholar]
- Falc, I.S.; Falc, F.; Arruda, D.E.; Jos, J.; De Aguilar-Nascimento, J.E. Benefits of early enteral nutrition with glutamine and probiotics in brain injury patients. Clin. Sci. 2004, 106, 287–292. [Google Scholar]
- Xiong, W.; Qian, K. Low-protein, hypocaloric nutrition with glutamine versus full-feeding in the acute phase in ICU patients with severe traumatic brain injury. Neuropsychiatr. Dis. Treat. 2021, 17, 703–710. [Google Scholar] [CrossRef]
- Heyland, D.; Muscedere, J.; Wischmeyer, P.E.; Cook, D.; Jones, G.; Albert, M.; Elke, G.; Berger, M.M.; Day, A.G. A Randomized Trial of Glutamine and Antioxidants in Critically Ill Patients. N. Engl. J. Med. 2013, 368, 1489–1497. [Google Scholar] [CrossRef]
- Martí i Líndez, A.A.; Reith, W. Arginine-dependent immune responses. Cell. Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef]
- Mader, M.M.D.; Czorlich, P. The role of L-arginine metabolism in neurocritical care patients. Neural Regen. Res. 2022, 17, 1446–1453. [Google Scholar] [CrossRef]
- Gualano, B.; Artioli, G.G.; Poortmans, J.R.; Lancha Junior, A.H. Exploring the therapeutic role of creatine supplementation. Amino Acids 2010, 38, 31–44. [Google Scholar] [CrossRef]
- Beal, M.F. Neuroprotective effects of creatine. Amino Acids 2011, 40, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.D.; Bröer, S. Creatine as a booster for human brain function. How might it work? Neurochem. Int. 2015, 89, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Bougioukas, K.I.; Kapogiannis, D. Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp. Gerontol. 2018, 108, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Dolan, E.; Gualano, B.; Rawson, E.S. Beyond muscle: The effects of creatine supplementation on brain creatine, cognitive processing, traumatic brain injury. Eur. J. Sport Sci. 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Alosco, M.L.; Tripodis, Y.; Rowland, B.; Chua, A.S.; Liao, H.; Martin, B.; Jarnagin, J.; Chaisson, C.E.; Pasternak, O.; Karmacharya, S.; et al. A magnetic resonance spectroscopy investigation in symptomatic former NFL players. Brain Imaging Behav. 2020, 14, 1419–1429. [Google Scholar] [CrossRef]
- Ipsiroglu, O.S.; Stromberger, C.; Ilas, J.; Höger, H.; Mühl, A.; Stöckler-Ipsiroglu, S. Changes of tissue creatine concentrations upon oral supplementation of creatine-monohydrate in various animal species. Life Sci. 2001, 69, 1805–1815. [Google Scholar] [CrossRef]
- Sullivan, P.G.; Geiger, J.D.; Mattson, M.P.; Scheff, S.W. Dietary supplement creatine protects against traumatic brain injury. Ann. Neurol. 2000, 48, 723–729. [Google Scholar] [CrossRef]
- Jeter, C.B.; Hergenroeder, G.W.; Ward, N.H.; Moore, A.N.; Dash, P.K. Human mild traumatic brain injury decreases circulating branched-chain amino acids and their metabolite levels. J. Neurotrauma 2013, 30, 671–679. [Google Scholar] [CrossRef]
- Elkind, J.A.; Lim, M.M.; Johnson, B.N.; Palmer, C.P.; Putnam, B.J.; Kirschen, M.P.; Cohen, A.S. Efficacy, dosage, and duration of action of branched chain amino acid therapy for traumatic brain injury. Front. Neurol. 2015, 6, 73. [Google Scholar] [CrossRef]
- Aquilani, R.; Iadarola, P.; Contardi, A.; Boselli, M.; Verri, M.; Pastoris, O.; Boschi, F.; Arcidiaco, P.; Viglio, S. Branched-chain amino acids enhance the cognitive recovery of patients with severe traumatic brain injury. Arch. Phys. Med. Rehabil. 2005, 86, 1729–1735. [Google Scholar] [CrossRef]
- Sharma, B.; Lawrence, D.W.; Hutchison, M.G. Branched Chain Amino Acids (BCAAs) and Traumatic Brain Injury: A Systematic Review. J. Head Trauma Rehabil. 2018, 33, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, C.E.; Martin, J.A.; Manriquez, F.V.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Focus on the essentials: Tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin. Pharmacol. 2019, 48, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Pautova, A.; Khesina, Z.; Getsina, M.; Sobolev, P.; Revelsky, A.; Beloborodova, N. Determination of tryptophan metabolites in serumand cerebrospinal fluid samples using microextraction by packed sorbent, silylation and GC-MS detection. Molecules 2020, 25, 3258. [Google Scholar] [CrossRef] [PubMed]
- Nwafor, D.; Goeckeritz, J.; Hasanpour, Z.; Davidson, C.; Lucke-Wold, B. Nutritional Support Following Traumatic Brain Injury: A Comprehensive Review. Explor. Res. Hypothesis Med. 2022, 8, 236–247. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, S.; Wu, C.; Cao, Y.; Gu, Q.; Zhu, Y.; Zhang, W.; Hu, W. Gut Microbiota Dysbiosis after Traumatic Brain Injury Contributes to Persistent Microglial Activation Associated with Upregulated Lyz2 and Shifted Tryptophan Metabolic Phenotype. Nutrients 2022, 14, 3467. [Google Scholar] [CrossRef]
- Holesh, J.E.; Aslam, S.; Martin, A. Physiology, Carbohydrates; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Cummings, J.H.; Stephen, A.M. Carbohydrate terminology and classification. Eur. J. Clin. Nutr. 2007, 61, S5–S18. [Google Scholar] [CrossRef] [PubMed]
- Muth, A.-K.; Park, S.Q. The impact of dietary macronutrient intake on cognitive function and the brain. Clin. Nutr. 2021, 40, 3999–4010. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Mariani, M. Simple vs. Complex Carbohydrate Dietary Patterns and the Global Overweight and Obesity Pandemic. Int. J. Environ. Res. Public Health 2017, 14, 1174. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charitos, I.A.; Aliani, M.; Tondo, P.; Venneri, M.; Castellana, G.; Scioscia, G.; Castellaneta, F.; Lacedonia, D.; Carone, M. Biomolecular Actions by Intestinal Endotoxemia in Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 2841. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donati Zeppa, S.; Gervasi, M.; Bartolacci, A.; Ferrini, F.; Patti, A.; Sestili, P.; Stocchi, V.; Agostini, D. Targeting the Gut Microbiota for Prevention and Management of Type 2 Diabetes. Nutrients 2024, 16, 3951. [Google Scholar] [CrossRef]
- Ley, E.J.; Srour, M.K.; Clond, M.A.; Barnajian, M.; Tillou, A.; Mirocha, J.; Salim, A. Diabetic patients with traumatic brain injury: Insulin deficiency is associated with increased mortality. J. Trauma Acute Care Surg. 2011, 70, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.; Czosnyka, M.; Sudhan, N.; Varsos, G.V.; Nasr, N.; Jalloh, I.; Liu, X.; Dias, C.; Sekhon, M.S.; Carpenter, K.L.H.; et al. Increased blood glucose is related to disturbed cerebrovascular pressure reactivity after traumatic brain injury. Neurocrit. Care 2015, 22, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Charitos, I.A.; Cantore, S.; Topi, S.; Bottalico, L.; Santacroce, L. About Functional Foods: The Probiotics and Prebiotics State of Art. Antibiotics 2023, 12, 635. [Google Scholar] [CrossRef]
- Rogers, G.B.; Keating, D.J.; Young, R.L.; Wong, M.L.; Licinio, J.; Wesselingh, S. From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Mol. Psychiatry 2016, 21, 738–748. [Google Scholar] [CrossRef]
- Kok, C.R.; Hutkins, R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr. Rev. 2018, 76, 4–15. [Google Scholar] [CrossRef]
- Parvez, S.; Malik, K.A.; Kang, S.A.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. 2021, 26, 135–148. [Google Scholar] [CrossRef]
- Kunze, W.A.; Mao, Y.; Wang, B.; Huizinga, J.D.; Ma, X.; Forsythe, P.; Bienenstock, J. Lactobacillus reuteri enhances excitability of colonic AH neurons by inhibiting calcium-dependent potassium channel opening. J. Cell. Mol. Med. 2009, 13, 2261–2270. [Google Scholar] [CrossRef]
- Mitra, S.; Dash, R.; Al Nishan, A.; Habiba, S.U.; Moon, I.S. Brain modulation by the gut microbiota: From disease to therapy. J. Adv. Res. 2023, 53, 153–173. [Google Scholar] [CrossRef]
- Carlman, H.M.T.E.; Rode, J.; König, J.; Repsilber, D.; Hutchinson, A.N.; Thunberg, P.; Persson, J.; Kiselev, A.; Pruessner, J.C.; Brummer, R.J. Probiotic Mixture Containing Lactobacillus helveticus, Bifidobacterium longum and Lactiplantibacillus plantarum Affects Brain Responses to an Arithmetic Stress Task in Healthy Subjects: A Randomised Clinical Trial and Proof-of-Concept Study. Nutrients 2022, 14, 1329. [Google Scholar] [CrossRef]
- Ait-Belgnaoui, A.; Colom, A.; Braniste, V.; Ramalho, L.; Marrot, A.; Cartier, C.; Houdeau, E.; Theodorou, V.; Tompkins, T. Probiotic gut effect prevents the chronic psychological stress-induced brain activity abnormality in mice. Neurogastroenterol. Motil. 2014, 26, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Hu, C.; Fang, H.; Zhu, L.; Gao, N.; Zhu, J. The Effects of lactobacillus acidophilus on the intestinal smooth muscle contraction through PKC/MLCK/MLC signaling pathway in TBI mouse model. PLoS ONE 2015, 10, e0128214. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Yin, H.H.; Zhu, J.C. Increased gut absorptive capacity in rats with severe head injury after feeding with probiotics. Nutrition 2011, 27, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, T.; Fu, J.; Fu, S.; Hu, C.; Sun, B.; Fan, X.; Zhu, J. Lactobacillus acidophilus exerts neuroprotective effects in mice with traumatic brain injury. J. Nutr. 2019, 149, 1543–1552. [Google Scholar] [CrossRef]
- Brenner, L.A.; Forster, J.E.; Stearns-Yoder, K.A.; Stamper, C.E.; Hoisington, A.J.; Brostow, D.P.; Mealer, M.; Wortzel, H.S.; Postolache, T.T.; Lowry, C.A. Evaluation of an Immunomodulatory Probiotic Intervention for Veterans with Co-occurring Mild Traumatic Brain Injury and Posttraumatic Stress Disorder: A Pilot Study. Front. Neurol. 2020, 11, 1015. [Google Scholar] [CrossRef]
- Tan, M.; Zhu, J.-C.; Du, J.; Zhang, L.-M.; Yin, H.-H. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: A prospective randomized pilot study. Crit. Care 2011, 15, R290. [Google Scholar] [CrossRef]
- Brenner, L.A.; Stearns-Yoder, K.A.; Hoffberg, A.S.; Penzenik, M.E.; Starosta, A.J.; Hernández, T.D.; Hadidi, D.A.; Lowry, C.A. Growing literature but limited evidence: A systematic review regarding prebiotic and probiotic interventions for those with traumatic brain injury and/or posttraumatic stress disorder. Brain Behav. Immun. 2017, 65, 57–67. [Google Scholar] [CrossRef]
- Pagkou, D.; Kogias, E.; Foroglou, N.; Kotzampassi, K. Probiotics in Traumatic Brain Injury: New Insights into Mechanisms and Future Perspectives. J. Clin. Med. 2024, 13, 4546. [Google Scholar] [CrossRef]
- Rice, M.W.; Pandya, J.D.; Shear, D.A. Gut Microbiota as a Therapeutic Target to Ameliorate the Biochemical, Neuroanatomical, and Behavioral Effects of Traumatic Brain Injuries. Front. Neurol. 2019, 10, 875. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Roy, S.; Dhaneshwar, S. Correction to ‘Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives’. World J. Gastroenterol. 2023, 29, 5178–5179. [Google Scholar] [CrossRef] [PubMed]
- Di Domenico, M.; Ballini, A.; Boccellino, M.; Scacco, S.; Lovero, R.; Charitos, I.A.; Santacroce, L. The Intestinal Microbiota May Be a Potential Theranostic Tool for Personalized Medicine. J. Pers. Med. 2022, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Shenderov, B.A. Metabiotics: Novel idea or natural development of probiotic conception. Microb. Ecol. Health Dis. 2013, 24, 20399. [Google Scholar] [CrossRef] [PubMed]
- Pihurov, M.; Păcularu-Burada, B.; Cotârleţ, M.; Vasile, M.A.; Bahrim, G.E. Novel Insights for Metabiotics Production by Using Artisanal Probiotic Cultures. Microorganisms 2021, 9, 2184. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Lee, N.-K.; Paik, H.-D. A Narrative Review on the Advance of Probiotics to Metabiotics. J. Microbiol. Biotechnol. 2024, 34, 487–494. [Google Scholar] [CrossRef]
- Sarubbo, F.; Cavallucci, V.; Pani, G. The Influence of Gut Microbiota on Neurogenesis: Evidence and Hopes. Cells 2022, 11, 382. [Google Scholar] [CrossRef]
- Xiao, W.; Su, J.; Gao, X.; Yang, H.; Weng, R.; Ni, W.; Gu, Y. The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids. Microbiome 2022, 10, 62. [Google Scholar] [CrossRef]
- Roth, W.; Zadeh, K.; Vekariya, R.; Ge, Y.; Mohamadzadeh, M. Tryptophan Metabolism and Gut-Brain Homeostasis. Int. J. Mol. Sci. 2021, 22, 2973. [Google Scholar] [CrossRef]


| Role of Lipids | |
|---|---|
| Cell Membrane Structure & Energy Source | Lipids are crucial for cellular membrane integrity and energy storage |
| Bioactive Lipids | Emerging focus on bioactive lipids, categorized into four families: Endocannabinoids Lysoglycerophospholipids/Sphingolipids Classical Eicosanoids (Pro-inflammatory) Specialized Pro-Resolving Mediators (SPMs) (Anti-inflammatory) |
| Benefits of Omega-3 Fatty Acids (PUFAs) | Neuroprotection & Neuroplasticity
Animal studies indicate that omega-3 supplementation before/after traumatic brain injury (TBI):
|
| Benefits of Specialized Pro-Resolving Mediators (SPMs) | Inflammation Resolution: SPMs play a critical role in transitioning from pro-inflammatory to anti-inflammatory states, potentially improving outcomes after neuroinflammatory damage |
Benefits of Short-Chain Fatty Acids (SCFAs)
| Neuroinflammation Modulation:SCFAs have significant roles in regulating central nervous system (CNS) inflammation and overall brain function:
|
| Amino Acid Role on Nervous System | |||
|---|---|---|---|
| Amino Acids | Role | Impact on Health | Potential Therapy |
| Glutamate | Primary excitatory neurotransmitter; critical for synaptic plasticity and cognitive function. | Excessive glutamate can lead to excitotoxicity, triggering neuroinflammation and contributing to neurodegenerative diseases. | Glutamate-related interventions may aid in neuroprotection against brain insults. |
| Arginine | Precursor to nitric oxide (NO), involved in inflammatory pathways. | Modulates immune responses and neuronal signaling relevant to neuroinflammation. | While its effects in TBI need further study, arginine’s role in NO production poses potential benefits. |
| Tryptophan | Precursor to serotonin and involved in gut-brain signaling through metabolites influencing neuroinflammation. | Metabolites (like kynurenine) can be linked to neuroinflammation and neurodegenerative diseases | Potential benefits warrant further investigation in clinical settings. |
| Glutamine | Supports protein synthesis and immune function; may become “conditionally essential” during hypercatabolic states. | May reduce infections and length of hospital stay in TBI patients | Recommended supplementation should not exceed 0.2–0.3 g/kg/d to avoid adverse effects. |
| Creatine | Involved in ATP synthesis and energy metabolism; supports cellular energy supply. | Demonstrated neuroprotective properties; may reduce oxidative stress and apoptosis in neurons, beneficial in post-TBI recovery. | Creatine supplementation has shown promise for improving cognitive function and recovery following TBI. |
| Branched-Chain Amino Acids (BCAAs) | Includes valine, isoleucine, and leucine; crucial for maintaining muscle mass and energy, and modulating inflammation. | Reduced BCAA levels post-TBI could impair neurotransmitter synthesis, potentially leading to cognitive and mood disorders. | Supplementation may support brain recovery and improve overall metabolic balance. |
| Metabiotic Component Bacteria Strains | ||||
|---|---|---|---|---|
| Neurotransmitters | Metabolome | |||
| Dopamine | GABA | Serotonin | Histamine | Metabolites/SCFAs * |
| Bifidobacterium bifidum Bifidobacterium longum Lacticaseibacillus rhamnosus Lacticaseibacillus rhamnosus GG Lactiplantibacillus plantarum LP28 Lactiplantibacillus plantarum PS128 Limosilactobacillus reuteri ATG-F4 Lactococcus lactis subsp. Lactis | Lacticaseibacillus rhamnosus JB-1 Lacticaseibacillus rhamnosus YS9 | Enterococcus Escherichia Lactococcus Streptococcus Lactobacillaceae family | Enterococcus Escherichia Lactococcus Streptococcus Lactobacillaceae family | Bacteroides fragilis NCTC 9343 Bifidobacterium breve A1 * Lacticaseibacillus rhamnosus * Limosilactobacillus reuteri * Bacterioides fragilis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotoia, A.; Charitos, I.A.; Corriero, A.; Tamburrano, S.; Cinnella, G. The Role of Macronutrients and Gut Microbiota in Neuroinflammation Post-Traumatic Brain Injury: A Narrative Review. Nutrients 2024, 16, 4359. https://doi.org/10.3390/nu16244359
Cotoia A, Charitos IA, Corriero A, Tamburrano S, Cinnella G. The Role of Macronutrients and Gut Microbiota in Neuroinflammation Post-Traumatic Brain Injury: A Narrative Review. Nutrients. 2024; 16(24):4359. https://doi.org/10.3390/nu16244359
Chicago/Turabian StyleCotoia, Antonella, Ioannis Alexandros Charitos, Alberto Corriero, Stefania Tamburrano, and Gilda Cinnella. 2024. "The Role of Macronutrients and Gut Microbiota in Neuroinflammation Post-Traumatic Brain Injury: A Narrative Review" Nutrients 16, no. 24: 4359. https://doi.org/10.3390/nu16244359
APA StyleCotoia, A., Charitos, I. A., Corriero, A., Tamburrano, S., & Cinnella, G. (2024). The Role of Macronutrients and Gut Microbiota in Neuroinflammation Post-Traumatic Brain Injury: A Narrative Review. Nutrients, 16(24), 4359. https://doi.org/10.3390/nu16244359







