Evaluating the Clinical Impact of a Polyphenol-Rich Extract from Salicornia ramosissima on Patients with Transient Ischemic Attack and Minor Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Statement
2.2. Investigational Product and Blinding
2.3. Participant Recruitment
2.4. Safety Outcome Collection
2.5. Lifestyle Questionnaires
2.6. Cognitive and Gait Assessment
2.7. Blood Analysis
2.8. Statistical Analysis
3. Results
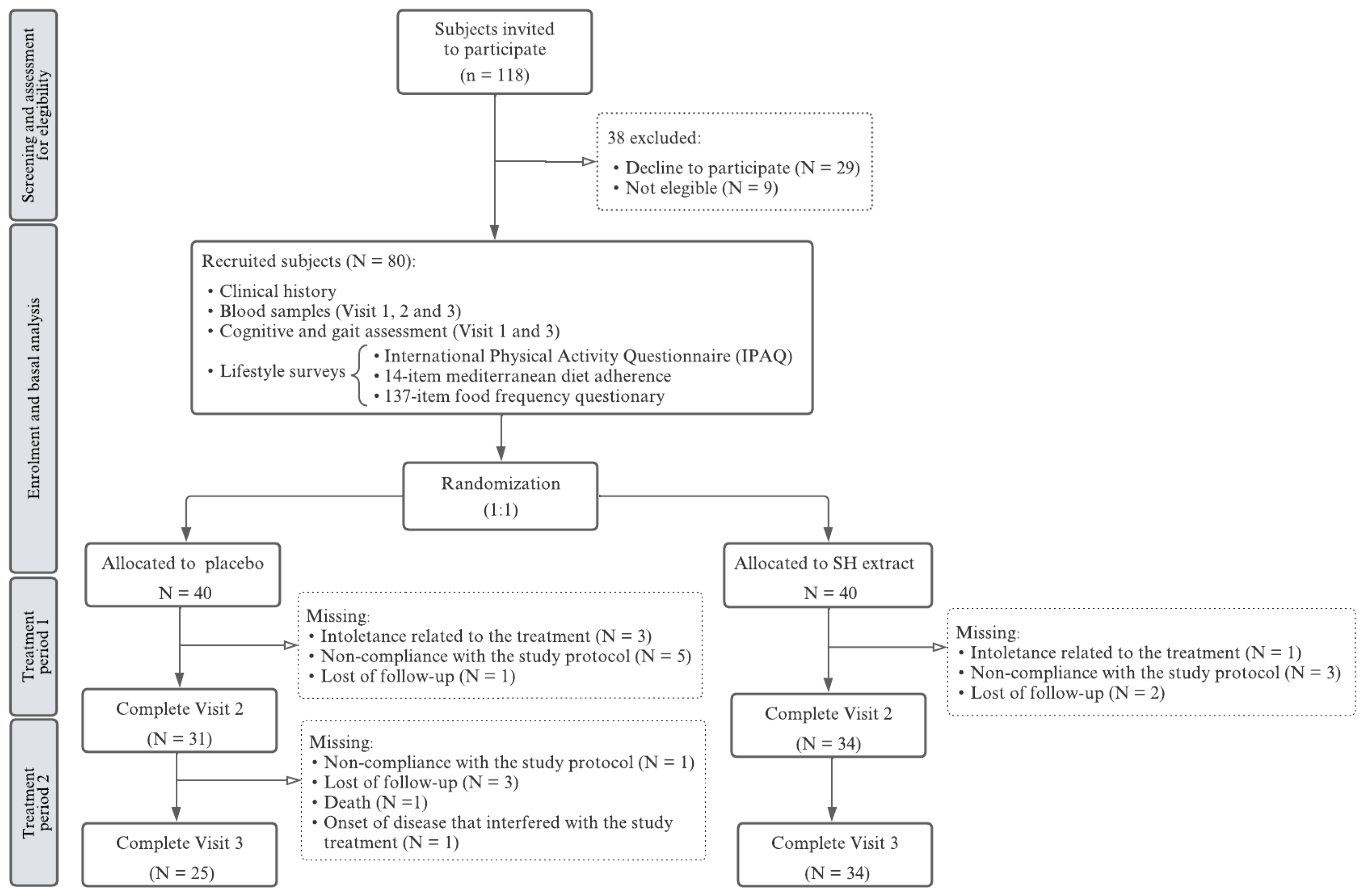
3.1. Participant Characteristics, Group Allocation, and Study Attrition
3.2. Safety Outcomes
3.3. Assessment of Eating Habits and Physical Activity
3.4. Gait Performance
3.5. Cognitive Changes
3.6. Blood Parameters
3.7. Sex-Specific Analysis of Key Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collaborators, G.S. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Johnson, W.; Onuma, O.; Owolabi, M.; Sachdev, S. Stroke: A global response is needed. Bull. World Health Organ. 2016, 94, 634–634A. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.J.; Werring, D.J. Stroke: Causes and clinical features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Amin, H.P.; Madsen, T.E.; Bravata, D.M.; Wira, C.R.; Johnston, S.C.; Ashcraft, S.; Burrus, T.M.; Panagos, P.D.; Wintermark, M.; Esenwa, C.; et al. Diagnosis, Workup, Risk Reduction of Transient Ischemic Attack in the Emergency Department Setting: A Scientific Statement From the American Heart Association. Stroke 2023, 54, e109–e121. [Google Scholar] [CrossRef]
- Hankey, G.J. Secondary stroke prevention. Lancet Neurol. 2014, 13, 178–194. [Google Scholar] [CrossRef]
- Panuganti, K.K.; Tadi, P.; Lui, F. Transient Ischemic Attack; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chiuve, S.E.; Rexrode, K.M.; Spiegelman, D.; Logroscino, G.; Manson, J.E.; Rimm, E.B. Primary prevention of stroke by healthy lifestyle. Circulation 2008, 118, 947–954. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Chin, S.L.; Rangarajan, S.; Xavier, D.; Liu, L.; Zhang, H.; Rao-Melacini, P.; Zhang, X.; Pais, P.; Agapay, S.; et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): A case-control study. Lancet 2016, 388, 761–775. [Google Scholar] [CrossRef]
- Patnode, C.D.; Redmond, N.; Iacocca, M.O.; Henninger, M. Behavioral Counseling Interventions to Promote a Healthy Diet and Physical Activity for Cardiovascular Disease Prevention in Adults Without Known Cardiovascular Disease Risk Factors: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2022, 328, 375–388. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Pacifici, F.; Rovella, V.; Pastore, D.; Bellia, A.; Abete, P.; Donadel, G.; Santini, S.; Beck, H.; Ricordi, C.; Daniele, N.D.; et al. Polyphenols and Ischemic Stroke: Insight into One of the Best Strategies for Prevention and Treatment. Nutrients 2021, 13, 1967. [Google Scholar] [CrossRef]
- Pinto, D.; Reis, J.; Silva, A.M.; Salazar, M.; Dall’Acqua, S.; Delerue-Matos, C.; Rodrigues, F. Valorisation of Salicornia ramosissima biowaste by a green approach—An optimizing study using response surface methodology. Sustain. Chem. Pharm. 2021, 24, 100548. [Google Scholar] [CrossRef]
- Lopes, M.; Roque, M.J.; Cavaleiro, C.; Ramos, F. Nutrient value of Salicornia ramosissima—A green extraction process for mineral analysis. J. Food Compos. Anal. 2021, 104, 104135. [Google Scholar] [CrossRef]
- Nájar, A.M.; Romero-Bernal, M.; Del Río, C.; Montaner, J. A Review on Polyphenols in Salicornia ramosissima with Special Emphasis on Their Beneficial Effects on Brain Ischemia. Nutrients 2023, 15, 793. [Google Scholar] [CrossRef] [PubMed]
- García-Rodríguez, P.; Ma, F.; Río, C.D.; Romero-Bernal, M.; Najar, A.M.; Cádiz-Gurrea, M.L.; Leyva-Jimenez, F.J.; Ramiro, L.; Menéndez-Valladares, P.; Pérez-Sánchez, S.; et al. Diet Supplementation with Polyphenol-Rich Salicornia ramosissima Extracts Protects against Tissue Damage in Experimental Models of Cerebral Ischemia. Nutrients 2022, 14, 5077. [Google Scholar] [CrossRef]
- Karthivashan, G.; Park, S.Y.; Kweon, M.H.; Kim, J.; Haque, M.E.; Cho, D.Y.; Kim, I.S.; Cho, E.A.; Ganesan, P.; Choi, D.K. Ameliorative potential of desalted Salicornia europaea L. extract in multifaceted Alzheimer’s-like scopolamine-induced amnesic mice model. Sci. Rep. 2018, 8, 7174. [Google Scholar] [CrossRef]
- Kim, J.; Karthivashan, G.; Kweon, M.H.; Kim, D.H.; Choi, D.K. The Ameliorative Effects of the Ethyl Acetate Extract of. Oxid. Med. Cell Longev. 2019, 2019, 6764756. [Google Scholar] [CrossRef]
- On, J.Y.; Kim, S.H.; Kim, J.M.; Park, S.; Kim, K.H.; Lee, C.H.; Kim, S.K. Effects of Fermented Artemisia annua L. and Salicornia herbacea L. on Inhibition of Obesity In Vitro and In Mice. Nutrients 2023, 15, 2022. [Google Scholar] [CrossRef]
- Chrigui, S.; Hadj Taieb, S.; Jemai, H.; Mbarek, S.; Benlarbi, M.; Feki, M.; Haouas, Z.; Zemmel, A.; Chaouacha-Chekir, R.B.; Boudhrioua, N. Anti-Obesity and Anti-Dyslipidemic Effects of Salicornia arabica Decocted Extract in Tunisian Psammomys obesus Fed a High-Calorie Diet. Foods 2023, 12, 1185. [Google Scholar] [CrossRef]
- Park, S.H.; Ko, S.K.; Choi, J.G.; Chung, S.H. Salicornia herbacea prevents high fat diet-induced hyperglycemia and hyperlipidemia in ICR mice. Arch. Pharm. Res. 2006, 29, 256–264. [Google Scholar] [CrossRef]
- Won, K.J.; Lee, K.P.; Baek, S.; Cui, L.; Kweon, M.H.; Jung, S.H.; Ryu, Y.K.; Hong, J.M.; Cho, E.A.; Shin, H.S.; et al. Desalted Salicornia europaea extract attenuated vascular neointima formation by inhibiting the MAPK pathway-mediated migration and proliferation in vascular smooth muscle cells. Biomed. Pharmacother. 2017, 94, 430–438. [Google Scholar] [CrossRef]
- Nájar, A.M.; Pérez-Sánchez, S.; del Río, C.; Domínguez, C.; Azcárate, C.L.; de Torres, R.; Lamana-Vallverdú, M.; Romero-Bernal, M.; González-Díaz, Á.; de la Luz Cádiz-Gurrea, M.; et al. Dietary supplementation with polyphenol-rich Salicornia ramosissima extracts: Assessing safety, efficacy, and impact on cardiovascular health biomarkers in healthy volunteers. J. Funct. Foods 2024, 122, 106539. [Google Scholar] [CrossRef]
- English, C.; MacDonald-Wicks, L.; Patterson, A.; Attia, J.; Hankey, G.J. The role of diet in secondary stroke prevention. Lancet Neurol. 2021, 20, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Hankey, G.J. Primary and Secondary Prevention of Ischemic Stroke and Cerebral Hemorrhage: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 1804–1818. [Google Scholar] [CrossRef] [PubMed]
- Bellone, J.A.; Murray, J.R.; Jorge, P.; Fogel, T.G.; Kim, M.; Wallace, D.R.; Hartman, R.E. Pomegranate supplementation improves cognitive and functional recovery following ischemic stroke: A randomized trial. Nutr. Neurosci. 2019, 22, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Oskouei, D.S.; Rikhtegar, R.; Hashemilar, M.; Sadeghi-Bazargani, H.; Sharifi-Bonab, M.; Sadeghi-Hokmabadi, E.; Zarrintan, S.; Sharifipour, E. The effect of Ginkgo biloba on functional outcome of patients with acute ischemic stroke: A double-blind, placebo-controlled, randomized clinical trial. J. Stroke Cerebrovasc. Dis. 2013, 22, e557–e563. [Google Scholar] [CrossRef]
- Grabež, M.; Škrbić, R.; Stojiljković, M.P.; Vučić, V.; Rudić Grujić, V.; Jakovljević, V.; Djuric, D.M.; Suručić, R.; Šavikin, K.; Bigović, D.; et al. A prospective, randomized, double-blind, placebo-controlled trial of polyphenols on the outcomes of inflammatory factors and oxidative stress in patients with type 2 diabetes mellitus. Rev. Cardiovasc. Med. 2022, 23, 57. [Google Scholar] [CrossRef]
- Fenercioglu, A.K.; Saler, T.; Genc, E.; Sabuncu, H.; Altuntas, Y. The effects of polyphenol-containing antioxidants on oxidative stress and lipid peroxidation in Type 2 diabetes mellitus without complications. J. Endocrinol. Investig. 2010, 33, 118–124. [Google Scholar] [CrossRef]
- Zhao, L.; Pu, L.; Wei, J.; Li, J.; Wu, J.; Xin, Z.; Gao, W.; Guo, C. Brazilian Green Propolis Improves Antioxidant Function in Patients with Type 2 Diabetes Mellitus. Int. J. Environ. Res. Public. Health 2016, 13, 498. [Google Scholar] [CrossRef]
- Curtis, P.J.; van der Velpen, V.; Berends, L.; Jennings, A.; Feelisch, M.; Umpleby, A.M.; Evans, M.; Fernandez, B.O.; Meiss, M.S.; Minnion, M.; et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syndrome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019, 109, 1535–1545. [Google Scholar] [CrossRef]
- Espinosa-Moncada, J.; Marín-Echeverri, C.; Galvis-Pérez, Y.; Ciro-Gómez, G.; Aristizábal, J.C.; Blesso, C.N.; Fernandez, M.L.; Barona-Acevedo, J. Evaluation of Agraz Consumption on Adipocytokines, Inflammation, and Oxidative Stress Markers in Women with Metabolic Syndrome. Nutrients 2018, 10, 1639. [Google Scholar] [CrossRef]
- Marhuenda, J.; Pérez-Piñero, S.; Arcusa, R.; Victoria-Montesinos, D.; Cánovas, F.; Sánchez-Macarro, M.; García-Muñoz, A.M.; Querol-Calderón, M.; López-Román, F.J. A Randomized, Double-Blind, Placebo-Controlled Trial to Determine the Effectiveness of a Polyphenolic Extract (Hibiscus sabdariffa and Lippia citriodora) for Reducing Blood Pressure in Prehypertensive and Type 1 Hypertensive Subjects. Molecules 2021, 26, 1783. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Shin, Y.W.; Kim, D.E.; Kweon, M.H.; Kim, M. Effect of desalted Salicornia europaea L. ethanol extract (PM-EE) on the subjects complaining memory dysfunction without dementia: A 12 week, randomized, double-blind, placebo-controlled clinical trial. Sci. Rep. 2020, 10, 19914. [Google Scholar] [CrossRef] [PubMed]
- Association, W.M. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- European Medicines Agency. ICH E6 (R2) Good Clinical Practice. 2002. Available online: https://www.ema.europa.eu/en/ich-e6-r2-good-clinical-practice-scientific-guideline (accessed on 1 September 2024).
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Macchiavelli, A.; Giffone, A.; Ferrarello, F.; Paci, M. Reliability of the six-minute walk test in individuals with stroke: Systematic review and meta-analysis. Neurol. Sci. 2021, 42, 81–87. [Google Scholar] [CrossRef]
- Miyata, K.; Tamura, S.; Kobayashi, S.; Takeda, R.; Iwamoto, H. Berg Balance Scale is a Valid Measure for Plan Interventions and for Assessing Changes in Postural Balance in Patients with Stroke. J. Rehabil. Med. 2022, 54, jrm00359. [Google Scholar] [CrossRef]
- Martínez-González, M.A.; García-Arellano, A.; Toledo, E.; Salas-Salvadó, J.; Buil-Cosiales, P.; Corella, D.; Covas, M.I.; Schröder, H.; Arós, F.; Gómez-Gracia, E.; et al. A 14-item Mediterranean diet assessment tool and obesity indexes among high-risk subjects: The PREDIMED trial. PLoS ONE 2012, 7, e43134. [Google Scholar] [CrossRef]
- García-Conesa, M.T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef]
- Ganesh, A.; Barber, P.A. The Cognitive Sequelae of Transient Ischemic Attacks-Recent Insights and Future Directions. J. Clin. Med. 2022, 11, 2637. [Google Scholar] [CrossRef]
- Ramírez-Moreno, J.M.; Bartolomé Alberca, S.; Muñoz Vega, P.; Guerrero Barona, E.J. Screening for cognitive impairment with the Montreal Cognitive Assessment in Spanish patients with minor stroke or transient ischaemic attack. Neurologia (Engl. Ed.) 2022, 37, 38–44. [Google Scholar] [CrossRef]
- Lamport, D.J.; Williams, C.M. Polyphenols and Cognition In Humans: An Overview of Current Evidence from Recent Systematic Reviews and Meta-Analyses. Brain Plast. 2021, 6, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shen, F.; Wang, X.; Qian, H.; Liu, Y. Chlorogenic acid improves the cognitive deficits of sleep-deprived mice via regulation of immunity function and intestinal flora. Phytomedicine 2024, 123, 155194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xue, R.; Hu, R. The neuroprotective effect and action mechanism of polyphenols in diabetes mellitus-related cognitive dysfunction. Eur. J. Nutr. 2020, 59, 1295–1311. [Google Scholar] [CrossRef]
- Suganthy, N.; Devi, K.P.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Bioactive effects of quercetin in the central nervous system: Focusing on the mechanisms of actions. Biomed. Pharmacother. 2016, 84, 892–908. [Google Scholar] [CrossRef]
- Mulati, A.; Zhang, X.; Zhao, T.; Ren, B.; Wang, L.; Liu, X.; Lan, Y.; Liu, X. Isorhamnetin attenuates high-fat and high-fructose diet induced cognitive impairments and neuroinflammation by mediating MAPK and NFkappaB signaling pathways. Food Funct. 2021, 12, 9261–9272. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Pouchieu, C.; Pourtau, L.; Gaudout, D.; Pallet, V.; Drummond, P.D. Effects of a polyphenol-rich grape and blueberry extract (Memophenol™) on cognitive function in older adults with mild cognitive impairment: A randomized, double-blind, placebo-controlled study. Front. Psychol. 2023, 14, 1144231. [Google Scholar] [CrossRef]
- Saitou, K.; Ochiai, R.; Kozuma, K.; Sato, H.; Koikeda, T.; Osaki, N.; Katsuragi, Y. Effect of Chlorogenic Acids on Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 1337. [Google Scholar] [CrossRef]
- Zhang, Z.; Gu, X.; Fang, X.; Tang, Z.; Guan, S.; Liu, H.; Wu, X.; Wang, C.; Zhao, Y. Homocysteine and the Risk of Cardiovascular Events and All-Cause Death in Elderly Population: A Community-Based Prospective Cohort Study. Ther. Clin. Risk Manag. 2020, 16, 471–481. [Google Scholar] [CrossRef]
- Lauriola, M.; D’Onofrio, G.; Ciccone, F.; Germano, C.; Cascavilla, L.; Paris, F.; Greco, A. Relationship of Homocysteine Plasma Levels with Mild Cognitive Impairment, Alzheimer’s Disease, Vascular Dementia, Psychobehavioral, and Functional Complications. J. Alzheimers Dis. 2021, 82, 235–248. [Google Scholar] [CrossRef]
- Rabelo, N.N.; Telles, J.P.M.; Pipek, L.Z.; Farias Vidigal Nascimento, R.; Gusmão, R.C.; Teixeira, M.J.; Figueiredo, E.G. Homocysteine is associated with higher risks of ischemic stroke: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0276087. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Zhou, R.; Xu, W.; Wang, X.; Chao, W.; Xue, S. Correlation Between Cognitive Impairment and Homocysteine and S100B Protein in Patients with Progressive Ischemic Stroke. Neuropsychiatr. Dis. Treat. 2023, 19, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Çelik, N.; Vurmaz, A.; Kahraman, A. Protective effect of quercetin on homocysteine-induced oxidative stress. Nutrition 2017, 33, 291–296. [Google Scholar] [CrossRef]
- Schroecksnadel, K.; Winkler, C.; Wirleitner, B.; Schennach, H.; Weiss, G.; Fuchs, D. Anti-inflammatory compound resveratrol suppresses homocysteine formation in stimulated human peripheral blood mononuclear cells in vitro. Clin. Chem. Lab. Med. 2005, 43, 1084–1088. [Google Scholar] [CrossRef]
- Zhao, H.P.; Feng, J.; Sun, K.; Liu, Y.Y.; Wei, X.H.; Fan, J.Y.; Huang, P.; Mao, X.W.; Zhou, Z.; Wang, C.S.; et al. Caffeic acid inhibits acute hyperhomocysteinemia-induced leukocyte rolling and adhesion in mouse cerebral venules. Microcirculation 2012, 19, 233–244. [Google Scholar] [CrossRef]
- Li, H.Z.; Liu, K.G.; Zeng, N.X.; Wu, X.F.; Lu, W.J.; Xu, H.F.; Yan, C.; Wu, L.L. Luteolin Enhances Choroid Plexus 5-MTHF Brain Transport to Promote Hippocampal Neurogenesis in LOD Rats. Front. Pharmacol. 2022, 13, 826568. [Google Scholar] [CrossRef]
- Meng, B.; Gao, W.; Wei, J.; Pu, L.; Tang, Z.; Guo, C. Quercetin Increases Hepatic Homocysteine Remethylation and Transsulfuration in Rats Fed a Methionine-Enriched Diet. Biomed. Res. Int. 2015, 2015, 815210. [Google Scholar] [CrossRef]
- van Guldener, C.; Nanayakkara, P.W.; Stehouwer, C.D. Homocysteine and blood pressure. Curr. Hypertens. Rep. 2003, 5, 26–31. [Google Scholar] [CrossRef]
- Panth, N.; Park, S.H.; Kim, H.J.; Kim, D.H.; Oak, M.H. Protective Effect of Salicornia europaea Extracts on High Salt Intake-Induced Vascular Dysfunction and Hypertension. Int. J. Mol. Sci. 2016, 17, 1176. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M.M. Marine Macroalgae Polyphenols as Potential Neuroprotective Antioxidants in Neurodegenerative Diseases. Mar. Drugs 2023, 21, 261. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Chen, B.; Xu, M.; Liu, S.; Su, Y.; Qiao, K.; Liu, Z. Advances in Research on Marine-Derived Lipid-Lowering Active Substances and Their Molecular Mechanisms. Nutrients 2023, 15, 5118. [Google Scholar] [CrossRef]
- Sun, P.; Zhao, L.; Zhang, N.; Zhou, J.; Zhang, L.; Wu, W.; Ji, B.; Zhou, F. Bioactivity of Dietary Polyphenols: The Role in LDL-C Lowering. Foods 2021, 10, 2666. [Google Scholar] [CrossRef] [PubMed]
- Bobot, M.; Suissa, L.; Hak, J.F.; Burtey, S.; Guillet, B.; Hache, G. Kidney disease and stroke: Epidemiology and potential mechanisms of susceptibility. Nephrol. Dial. Transplant. 2023, 38, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Shi, J.; Wang, Z.; Liang, Y.; Yu, J.; Wang, H.; Song, Z.; Tang, Z.; Zhang, D.; et al. Isorhamnetin Alleviates Renal Fibrosis by Inducing Endogenous Hydrogen Sulfide and Regulating Thiol-Based Redox State in Obstructed Kidneys. Biomolecules 2024, 14, 1233. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Zhang, M.; Xu, W.; Pan, T.; Luan, J.; Zhao, Y.; Zhang, Z. Chlorogenic acid alleviate kidney fibrosis through regulating TLR4/NF-κB mediated oxidative stress and inflammation. J. Ethnopharmacol. 2024, 335, 118693. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Li, J.; Zha, D.; Zhang, L.; Gao, P.; Yao, T.; Wu, X. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-kB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Cheng, H.-L.; Liao, C.-K.; Kuan, Y.-H.; Liang, T.-J.; Tseng, T.-J.; Lin, H.-C. Luteolin improves nephropathy in hyperglycemic rats through anti-oxidant, anti-inflammatory, and anti-apoptotic mechanisms. J. Funct. Foods 2023, 102, 105461. [Google Scholar] [CrossRef]
- Monteiro, E.B.; Borges, N.A.; Monteiro, M.; de Castro Resende, A.; Daleprane, J.B.; Soulage, C.O. Polyphenol-rich acai seed extract exhibits reno-protective and anti-fibrotic activities in renal tubular cells and mice with kidney failure. Sci. Rep. 2022, 12, 20855. [Google Scholar] [CrossRef]
- Cho, S.H.; Park, J.M.; Kwon, O.Y. Gender differences in three dimensional gait analysis data from 98 healthy Korean adults. Clin. Biomech. 2004, 19, 145–152. [Google Scholar] [CrossRef]
- Elbaz, A.; Artaud, F.; Dugravot, A.; Tzourio, C.; Singh-Manoux, A. The gait speed advantage of taller stature is lost with age. Sci. Rep. 2018, 8, 1485. [Google Scholar] [CrossRef]
- Schimpl, M.; Moore, C.; Lederer, C.; Neuhaus, A.; Sambrook, J.; Danesh, J.; Ouwehand, W.; Daumer, M. Association between walking speed and age in healthy, free-living individuals using mobile accelerometry—A cross-sectional study. PLoS ONE 2011, 6, e23299. [Google Scholar] [CrossRef]
- Shahjouei, S.; Sadighi, A.; Chaudhary, D.; Li, J.; Abedi, V.; Holland, N.; Phipps, M.; Zand, R. A 5-Decade Analysis of Incidence Trends of Ischemic Stroke After Transient Ischemic Attack: A Systematic Review and Meta-analysis. JAMA Neurol. 2021, 78, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Lavallee, P.C.; Charles, H.; Albers, G.W.; Caplan, L.R.; Donnan, G.A.; Ferro, J.M.; Hennerici, M.G.; Labreuche, J.; Molina, C.; Rothwell, P.M.; et al. Underlying Causes of TIA and Minor Ischemic Stroke and Risk of Major Vascular Events. JAMA Neurol. 2023, 80, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
| Placebo | SH Extract | p | |
|---|---|---|---|
| 40 | 40 | ||
| Age (median [min; max]) | 70 (40–87) | 69.5 (43–85) | 0.242 1 |
| Male/female (n) | 30/10 | 19/21 | 0.012 2 |
| Tobacco use (n) | 0.806 2 | ||
| No | 23 | 21 | |
| Yes | 4 | 6 | |
| Ex | 13 | 13 | |
| Alcohol consumption (n) | 0.380 2 | ||
| No | 32 | 36 | |
| Yes | 6 | 2 | |
| Ex | 2 | 2 | |
| Medical history (n) | |||
| Allergies | 9 | 13 | 0.317 2 |
| Intolerances | 2 | 2 | 1 2 |
| Hypertension | 25 | 27 | 0.639 2 |
| Diabetes | 12 | 10 | 0.616 2 |
| Dyslipidemia | 21 | 23 | 0.653 2 |
| Obstructive sleep apnea syndrome | 3 | 7 | 0.176 2 |
| Transient ischemic attack | 6 | 6 | 1 2 |
| Peripheral vascular disease | 3 | 3 | 1 2 |
| Coronary disease | 8 | 10 | 0.592 2 |
| Atrial fibrillation | 8 | 8 | 1 2 |
| Renal insufficiency | 1 | 1 | 1 2 |
| Placebo | SH Extract | p | |
|---|---|---|---|
| N | N | ||
| Global | 9 | 3 | 0.060 |
| Gastrointestinal disorders | |||
| Epigastralgia | 1 | 0 | 0.314 |
| Gastroesophageal reflux | 1 | 1 | 1 |
| Diarrhea | 1 | 0 | 0.314 |
| Constipation | 1 | 0 | 0.314 |
| Gas symptoms | 1 | 1 | 1 |
| Nervous system disorders | |||
| Headache | 0 | 1 | 0.314 |
| Renal and urinary disorders | |||
| Polyuria | 3 | 0 | 0.077 |
| Vascular disorders | |||
| Elevated blood pressure | 1 | 1 | 1 |
| Endocrine disorders | |||
| Blood glucose elevation | 1 | 0 | 0.314 |
| Metabolism and nutrition disorders | |||
| Polydipsia | 2 | 0 | 0.152 |
| Placebo 25 | SH Extract 34 | p | |
|---|---|---|---|
| Physical activity level 1 (n) | 0.718 1 | ||
| Low | 5 | 4 | |
| Moderate | 5 | 5 | |
| High | 15 | 21 | |
| Adherence to Mediterranean diet 1 (n) | 0.064 1 | ||
| Low adherence (≤5) | 2 | 3 | |
| Medium adherence (6–9) | 10 | 21 | |
| High adherence (≥10) | 12 | 6 | |
| Daily food intake 2 (Median (IQR)) | |||
| Sum of vegetables (g/day) | 268.2 (180–448.8) | 252 (191–352.8) | 0.468 2 |
| Sum of fruits (g/day) | 254.8 (155.9–349.2) | 274.5 (196.2–380.2) | 0.651 3 |
| Sum of pulses (g/day) | 24.8 (16.4–33.6) | 16 (13–19.2) | <0.001 3 |
| Sum of cereals (g/day) | 234.1 (140.8–257.5) | 206.5 (132–238.8) | 0.312 2 |
| Sum of whole grains (g/day) | 0 (0–0) | 0 (0–0) | 0.975 3 |
| Sum of dairy products (g/day) | 270 (215.4–447.5) | 278.5 (203.5–546.2) | 0.957 3 |
| Sum of meat and meat products (g/day) | 114.2 (75.1–166.4) | 103 (72.2–129.5) | 0.412 3 |
| Total olive oil (g/day) | 25 (25–50) | 25 (25–33.5) | 0.419 3 |
| Sum of fish (g/day) | 77.4 (50.1–105.6) | 64.5 (44.8–91.8) | 0.247 3 |
| Sum of biscuits, cakes and sweets (g/day) | 19.1 (13–29.7) | 14.5 (9.2–37.2) | 0.575 3 |
| Sum of industrial bakery products (g/day) | 13 (3.4–21) | 7 (3–30.8) | 0.385 3 |
| Visit 1 | Visit 3 | |||||
|---|---|---|---|---|---|---|
| Placebo | SH Extract | p | Placebo | SH Extract | p | |
| 22 | 24 | 22 | 24 | |||
| Height (cm) | 169.5 (164–173.5) | 160.5 (155.5–169) | 0.028 2 | – | – | - |
| BMI | 28.3 (26.3–30.7) | 29.1 (26.4–32.3) | 0.375 2 | 28.3 (26.6–29.8) | 28.7 (25.7–32.7) | 0.506 2 |
| SBP (mm Hg)-pre | 137 (129.5–152.2) | 136 (114.2–152) | 0.756 2 | 143.5 (127–150) | 124 (112–136.2) * | 0.020 1 |
| DBP (mm Hg)-pre | 79 (70.2–86.5) | 72 (68.5–77.5) | 0.090 1 | 77 (67.5–82.5) | 70 (67.8–73.2) | 0.031 2 |
| HR (bpm)-pre | 68 (62.2–82.2) | 73 (65.5–80.2) | 0.538 1 | 78 (67.5–81) | 75 (65–80) | 0.281 2 |
| Speed (m/s)-pre | 116.8 (104.9–125.2) | 91.3 (81.4–110) | 0.016 1 | 121.7 (106–128.9) | 103.6 (83.2–124.9) ** | 0.2392 |
| FAP-pre | 99 (93–100) | 96 (90–98.2) | 0.137 1 | 100 (96.5–100) | 95 (88–98.5) | 0.016 1 |
| 6MWT (m) | 481.5 (392.8–525.5) | 403.5 (314.2–463) | 0.066 1 | 494 (345–524.5) | 409 (359.5–489) | 0.271 2 |
| Speed (m/s)-post | 137.4 (118.3–146.4) | 105 (84.2–123.7) | 0.011 2 | 128.7 (113.6–142) | 107 (91.4–133.6) | 0.065 2 |
| FAP-post | 95.5 (87.8–98.8) | 92.5 (89.8–97.5) | 0.566 1 | 96.5 (95–98.8) | 95 (91.5–97.2) | 0.212 1 |
| SBP (mm Hg)-post | 168 (151.2–175) | 148.5 (131.8–172.8) | 0.098 2 | 157.5 (139–169.8) | 134.5 (126–158) | 0.029 1 |
| DBP (mm Hg)-post | 76 (73.2–79.8) | 72 (66.8–78.8) | 0.071 2 | 74.5 (71.2–79.5) | 73 (68–79.2) | 0.260 2 |
| HR (bpm)-post | 89.5 (80.5–104.2) | 81.5 (77.5–103.8) | 0.381 2 | 85 (76.2–93.5) | 83 (78.8–96) | 0.412 2 |
| SBP (mm Hg)-post 1′ | 157.5 (135.2–166.8) | 139.5 (122.8–163.5) | 0.197 2 | 143.5 (128.2–153.5) | 125 (117.2–133.2) * | 0.010 1 |
| DBP (mm Hg)-post 1′ | 76 (69.5–80.8) | 71 (65–76) | 0.034 2 | 78.5 (72–82.8) | 72.5 (68.8–77.2) | 0.022 2 |
| HR (bpm)-post 1′ | 79 (72–90.2) | 81.5 (71–96) | 0.859 2 | 80.5 (72.2–84.8) | 78 (70–85.5) * | 0.523 2 |
| SBP (mm Hg)-post 5′ | 135 (127.5–142.5) | 132 (115–147.2) | 0.695 2 | 131.5 (120.8–146.5) | 119 (108.8–124.2) | 0.009 1 |
| DBP (mm Hg)-post 5′ | 78 (70.5–82.8) | 71 (67.8–76) | 0.012 2 | 74.5 (70.2–80) | 70.5 (65.2–75) | 0.026 1 |
| HR (bpm)-post 5′ | 74.5 (68.5–81.5) | 76 (67.5–89.2) | 0.892 2 | 74 (71.5–80.8) | 75 (66.2–83.2) | 0.758 1 |
| Berg’s score | 56 (55–56) | 54 (51–55.2) | 0.009 1 | 55.5 (54.2–56) | 55 (53.8–56) * | 0.582 1 |
| Left Dynamometry | 30 (23.2–33) | 16.5 (12.8–24.2) | <0.001 2 | 26 (23–30) | 17 (15–28.5) * | 0.019 1 |
| Right Dynamometry | 29 (21.8–31) | 16.5 (13–20.5) | <0.001 1 | 29 (25.5–32.8) | 20 (15–30.2) ** | 0.026 1 |
| Visit 1 | Visit 3 | |||||
|---|---|---|---|---|---|---|
| Placebo | SH Extract | p | Placebo | SH Extract | p | |
| 22 | 23 | 22 | 23 | |||
| MOCA | ||||||
| Direct Score | 22 (20.2–24.5) | 20 (17.5–24) | 0.355 2 | 23.5 (21.2–26) | 23 (20.5–26) ** | 0.793 1 |
| Adjusted SS (Age and Education) | 9 (7–11) | 8 (6.5–10.5) | 0.486 1 | 10 (8–12) | 10 (7–11.5) * | 0.577 2 |
| Placebo | SH Extract | p | ||
|---|---|---|---|---|
| Glucose (mg/dL) | Visit 1 | 96 (86–112) | 101 (92–113.8) | 0.293 1 |
| Visit 2 | 103 (91–112) | 95 (89.2–107) | 0.272 1 | |
| Visit 3 | 110 (93–118) | 93 (86.2–108) | 0.084 1 | |
| Albumin (g/dL) | Visit 1 | 4.4 (4.3–4.6) | 4.4 (4.3–4.6) | 0.500 2 |
| Visit 2 | 4.4 (4.3–4.6) | 4.4 (4.1–4.5) ** | 0.238 2 | |
| Visit 3 | 4.3 (4.3–4.5) | 4.4 (4.2–4.5) * | 0.673 2 | |
| Total protein (g/dL) | Visit 1 | 7.2 (7–7.4) | 7 (6.7–7.2) | 0.010 2 |
| Visit 2 | 7 (6.8–7.2) ** | 6.9 (6.5–7.1) | 0.113 1 | |
| Visit 3 | 6.9 (6.6–7.1) ** | 6.8 (6.6–7.1) ** | 0.601 2 | |
| Urea (mg/dL) | Visit 1 | 35 (28–43) | 38.5 (32.2–44.5) | 0.094 2 |
| Visit 2 | 36 (32.5–42) | 36 (30.2–44) | 0.776 1 | |
| Visit 3 | 37 (30–41) | 36 (32–46.2) | 0.630 2 | |
| Creatinine (mg/dL) | Visit 1 | 0.9 (0.7–1.1) | 0.9 (0.7–1) | 0.866 1 |
| Visit 2 | 0.9 (0.7–1) | 0.8 (0.7–1) | 0.307 1 | |
| Visit 3 | 0.9 (0.8–1) | 0.8 (0.7–1) ** | 0.163 1 | |
| GFR (ml/min) | Visit 1 | 86 (71–95) | 86.5 (70.8–94.8) | 0.890 1 |
| Visit 2 | 87 (65–91) | 85.5 (70–98.8) | 0.645 1 | |
| Visit 3 | 82 (72–92) | 86.5 (75.5–96.8) * | 0.630 2 | |
| Cholesterol (mg/dL) | Visit 1 | 149 (112–180) | 133 (117.2–159.5) | 0.304 1 |
| Visit 2 | 151 (115–173) | 137.5 (115.2–169.5) | 0.804 2 | |
| Visit 3 | 161 (115–184) | 126.5 (112.2–167.5) | 0.304 1 | |
| HDL (mg/dL) | Visit 1 | 52 (42–57) | 49.5 (44.2–59.8) | 0.989 2 |
| Visit 2 | 52 (41–57.8) | 49 (43–53.8) | 0.579 1 | |
| Visit 3 | 46 (41–56) | 43.5 (38–54) ** | 0.390 1 | |
| LDL (mg/dL) | Visit 1 | 76.5 (53–94.5) | 66 (54.8–74.8) | 0.294 1 |
| Visit 2 | 74.5 (60–88.5) | 70.5 (55.8–91.2) | 0.890 2 | |
| Visit 3 | 82.5 (55–101) | 65 (54–97) * | 0.477 1 | |
| TGs (mg/dL) | Visit 1 | 98 (73–117) | 84.5 (69.5–110.8) | 0.421 1 |
| Visit 2 | 84 (76.5–121) | 85.5 (73–108.5) | 0.660 1 | |
| Visit 3 | 83 (70–143) | 85.5 (62.8–111.8) | 0.452 1 | |
| GGT (U/L) | Visit 1 | 25 (19–38) | 23 (16–33.8) | 0.200 1 |
| Visit 2 | 23 (18.5–33) | 23.5 (17.2–37.5) | 0.858 1 | |
| Visit 3 | 27 (21–37) | 21 (18–36) | 0.222 1 | |
| AST (U/L) | Visit 1 | 24.5 (19–27) | 22 (18–24) | 0.278 1 |
| Visit 2 | 21 (17–22) * | 24 (19–29.5) | 0.035 1 | |
| Visit 3 | 24 (18–30) | 25 (21.2–33.8) ** | 0.089 1 | |
| ALT (U/L) | Visit 1 | 22 (15–26) | 18 (14.2–25.8) | 0.673 1 |
| Visit 2 | 17 (15–22) * | 21.5 (16–29.2) | 0.033 1 | |
| Visit 3 | 18 (15–30) | 23.5 (17.2–27.8) * | 0.177 1 | |
| Sodium (mEq/L) | Visit 1 | 142 (140–143) | 141.5 (140.2–143) | 0.840 1 |
| Visit 2 | 141 (139–143) | 141 (140–142) | 0.560 2 | |
| Visit 3 | 142 (141–142) | 140 (139.2–142) | 0.103 2 | |
| Potassium (mEq/L) | Visit 1 | 4.6 (4.5–4.8) | 4.5 (4.3–4.7) | 0.123 2 |
| Visit 2 | 4.6 (4.3–4.7) | 4.4 (4.1–4.7) | 0.207 1 | |
| Visit 3 | 4.3 (4.2–4.7) ** | 4.4 (4.1–4.7) | 0.831 2 | |
| Vitamin B12 | Visit 1 | 321 (262–429) | 343 (247.5–413) | 0.860 1 |
| Visit 2 | 318 (238.2–377.8) * | 346.5 (246.2–449.8) | 0.279 1 | |
| Visit 3 | 306 (208–373) ** | 361 (260–478) | 0.118 1 | |
| Folic acid | Visit 1 | 5.1 (3.8–7.4) | 6.4 (5.1–7.4) | 0.135 1 |
| Visit 2 | 6.2 (4.3–9.5) ** | 6.2 (4.7–8) | 0.699 1 | |
| Visit 3 | 5.2 (3.9–7.3) | 6 (4.9–9) | 0.102 1 | |
| HCys (µmol/L) | Visit 1 | 16.6 (12.8–20) | 14.4 (12.5–17.1) | 0.172 1 |
| Visit 2 | 15.4 (12.3–18.6) | 11.5 (9.6–16.8) ** | 0.019 1 | |
| Visit 3 | 14.6 (12.1–17.7) ** | 10.6 (8.7–13.7) ** | 0.016 1 | |
| HbA1c (%) | Visit 1 | 6 (5.6–6.4) | 5.8 (5.6–6.2) * | 0.360 1 |
| Visit 2 | 6 (5.5–6.4) | 5.8 (5.5–6.4) | 0.619 1 | |
| Visit 3 | 5.9 (5.7–6.5) | 5.8 (5.6–6.3) * | 0.564 1 | |
| TSH (U/mL) | Visit 1 | 1.4 (1–2.6) | 1.6 (1.2–2) | 0.629 1 |
| Visit 2 | 1.7 (1.1–2.6) | 1.5 (1.2–2.3) | 0.439 1 | |
| Visit 3 | 1.5 (1.2–2.5) | 1.5 (1.1–1.9) | 0.495 1 |
| Men | Women | ||||||
|---|---|---|---|---|---|---|---|
| Placebo | Extract | p | Placebo | Extract | p | ||
| Median (IQR) | Median (IQR) | Median (IQR) | Median (IQR) | ||||
| Gait performance (n) | 18 | 11 | 4 | 13 | |||
| Height (cm) | 171.5 (166.2–174.8) | 169 (165–174) | 0.731 2 | 156.5 (153–160.2) | 158 (152–160) | 0.824 2 | |
| SBP (mm Hg)-pre | Visit 1 | 137 (128.2–152.2) | 144 (127–152) | 0.828 2 | 143 (134.8–154.8) | 133 (112–151) | 0.545 2 |
| Visit 3 | 145 (126.2–151) | 117 (112–136) * | 0.562 2 | 140 (135–144) | 124 (112–136) | 0.004 1 | |
| DBP (mm Hg)-pre | Visit 1 | 76 (70–86) | 73 (71–76) | 0.928 1 | 82 (78.2–87.2) | 70 (65–79) | 0.015 2 |
| Visit 3 | 73 (66.2–80.5) | 72 (68–74.5) | 0.048 1 | 81 (78.2–83.5) | 70 (67–70) | 0.444 2 | |
| Speed (m/s)-pre | Visit 1 | 116.8 (105.8–123.2) | 104.3 (88.6–127.2) | 0.550 1 | 122.2 (104.6–146.9) | 87.5 (76.2–98.8) | 0.013 2 |
| Visit 3 | 121.7 (106–128.2) | 113.8 (92.6–151.9) * | 0.751 2 | 124.4 (108.7–136.8) | 97.1 (76.3–105.2) | 0.096 2 | |
| FAP-pre | Visit 1 | 99 (93–100) | 96 (94.5–98) | 0.421 1 | 97 (87.5–100) | 91 (89–99) | 0.608 1 |
| Visit 3 | 100 (98–100) | 92 (88–96.5) | 0.014 1 | 97.5 (95.2–99.2) | 96 (89–100)* | 0.646 1 | |
| Speed (m/s)-post | Visit 1 | 137.4 (117.5–144.3) | 112.8 (101.2–140.4) | 0.356 2 | 136.6 (125.6–149.9) | 99.6 (81.6–115.7) | 0.021 2 |
| Visit 3 | 126.7 (108.4–142) | 97.6 (48.4–143.9) | 0.056 2 | 130.7 (124.4–139.8) | 103.5 (88–125.1) | 0.079 1 | |
| SBP (mm Hg)-post | Visit 1 | 168 (148.2–176.5) | 156 (144.5–175.5) | 0.598 2 | 168 (161.2–172) | 136 (126–170) | 0.204 2 |
| Visit 3 | 162 (137.8–175.5) | 135 (124–167.5) | 0.230 2 | 150.5 (143–156.8)* | 134 (130–145) | 0.271 2 | |
| SBP (mm Hg)-post | Visit 1 | 158.5 (132.5–166.8) | 146 (133–166) | 0.805 1 | 153.5 (148.5–160) | 127 (118–163) | 0.157 1 |
| Visit 3 | 148 (127.2–156.2) | 124 (116–135.5) | 0.062 1 | 142 (138–143.2) | 126 (120–133) | 0.100 1 | |
| DBP (mm Hg)-post 1′ | Visit 1 | 75 (69–79.8) | 76 (69.5–79.5) | 0.883 2 | 82 (79.8–85.5) | 65 (64–73) | 0.010 1 |
| Visit 3 | 75.5 (71.2–80) | 77 (70.5–78) | 0.374 2 | 83 (80.5–84.5) | 72 (68–74) | 0.005 2 | |
| SBP (mm Hg)-post 5′ | Visit 1 | 135 (126.2–141) | 137 (119–158) | 0.402 2 | 144 (134–155) | 121 (108–146) | 0.229 2 |
| Visit 3 | 131.5 (118.5–147) | 117 (103–123) * | 0.034 2 | 130.5 (126.8–135) | 120 (109–125) | 0.089 1 | |
| DBP (mm Hg)-post 5′ | Visit 1 | 76.5 (70–81.8) | 74 (70–80.5) | 0.599 2 | 81 (78.5–86.2) | 70 (63–73) | 0.001 2 |
| Visit 3 | 72.5 (70–79.2) | 71 (64.5–75) | 0.191 1 | 81.5 (77.8–85) | 70 (68–73) | 0.013 2 | |
| Berg’s score | Visit 1 | 56 (54.2–56) | 55 (53.5–55.5) | 0.126 1 | 56 (55.8–56) | 54 (49–55) | 0.042 1 |
| Visit 3 | 55 (54–56) | 55 (54.5–56) | 0.962 1 | 56 (55.8–56) | 55 (53–56)* | 0.219 1 | |
| Left Dynamometry | Visit 1 | 30.5 (27–33.5) | 25 (17–28) | 0.021 1 | 24.5 (23–26.8) | 13 (11–17) | <0.001 2 |
| Visit 3 | 26.5 (24.2–30.8) | 30 (21–40) * | 0.370 2 | 21.5 (19.2–24.8) * | 15 (14–16) | 0.001 2 | |
| Right Dynamometry | Visit 1 | 29.5 (24.2–34.5) | 22 (19–30.5) | 0.072 2 | 21.5 (20.8–24) | 14 (12–16) | <0.001 2 |
| Visit 3 | 30 (27.2–34.5) | 31 (29.5–40.5) ** | 0.443 1 | 23.5 (21.5–25.5) | 16 (15–17)* | 0.002 2 | |
| MOCA test (n) | 19 | 12 | 3 | 11 | |||
| Direct Score | Visit 1 | 22 (19.5–22) | 24 (19.8–27) | 0.460 2 | 25 (24–25) | 20 (16–21.5) | 0.012 1 |
| Visit 3 | 23 (21–26) | 24.5 (22.5–27) | 0.882 2 | 26 (25–26) | 22 (19.5–24) * | 0.481 1 | |
| Adjusted SS (Age and Education) | Visit 1 | 9 (7–11) | 9.5 (5.8–11) | 0.500 2 | 11 (8–12) | 8 (7–9.5) | 0.143 2 |
| Visit 3 | 10 (8–13) | 9.5 (7–12.2) | 0.853 2 | 10 (9–10.5) | 10 (7–11) | 0.902 2 | |
| Biochemical analysis (n) | 21 | 18 | 4 | 16 | |||
| Creatinine (mg/dL) | Visit 1 | 0.9 (0.9–1.1) | 0.9 (0.8–1) | 0.693 1 | 0.7 (0.6–0.7) | 0.7 (0.7–0.9) | 0.320 1 |
| Visit 2 | 0.9 (0.8–1.1) | 0.9 (0.8–1) | 0.455 1 | 0.7 (0.6–0.7) | 0.8 (0.6–0.8) | 0.237 1 | |
| Visit 3 | 0.9 (0.8–1) | 0.9 (0.7–1) ** | 0.375 1 | 0.8 (0.7–0.8) | 0.7 (0.6–0.9) | 0.705 1 | |
| GFR (ml/min) | Visit 1 | 86 (67–95) | 89.5 (74–100) | 0.481 1 | 86.5 (84–97.2) | 84 (67.2–91.2) | 0.368 1 |
| Visit 2 | 85 (65–91) | 92.5 (76.8–100.8) | 0.163 1 | 88 (87–97) | 79.5 (70–92) | 0.201 1 | |
| Visit 3 | 83 (71–92) | 91 (77.8–103.8) * | 0.162 2 | 77.5 (74.2–89) | 83 (69.8–94.2) | 0.524 2 | |
| HDL (mg/dL) | Visit 1 | 48 (40–54) | 47 (44–50.8) | 0.761 2 | 55.5 (53–67) | 56.5 (48.5–62) | 0.670 1 |
| Visit 2 | 47.5 (39.5–52.8) | 48 (43–50) | 0.751 1 | 60 (57.8–69.5) | 52 (42.5–60.8) | 0.122 1 | |
| Visit 3 | 43 (41–50) | 41 (38–45.5) * | 0.162 1 | 63 (58–68.5) | 54 (42.2–59.8) * | 0.127 2 | |
| LDL (mg/dL) | Visit 1 | 71 (53–90) | 64.5 (53–72.8) | 0.422 1 | 96 (85–105) | 67 (56.2–93) | 0.121 2 |
| Visit 2 | 71 (57.5–86.8) | 72 (58.8–89.8) | 0.861 2 | 88 (76–103) | 69.5 (55.8–91.5) | 0.309 2 | |
| Visit 3 | 68 (54.2–99.2) | 63 (53.2–75.5) | 0.539 1 | 106.5 (99.8–109.2) | 76 (58.5–105.5) * | 0.245 2 | |
| Homocysteine (µmol/L) | Visit 1 | 17 (15.6–20.1) | 16.1 (13.8–19.2) | 0.390 1 | 12.6 (10.8–14.8) | 13.4 (11.2–15) | 0.813 1 |
| Visit 2 | 15.6 (13.7–20.1) | 11.4 (9.2–15.2) ** | 0.010 1 | 10.8 (9–13.3) * | 11.8 (9.6–17.6) | 0.537 2 | |
| Visit 3 | 14.7 (12.2–18) ** | 11.5 (10.3–14.6) ** | 0.111 1 | 11.6 (10.6–13.5) | 9.6 (8.5–12.9) ** | 0.298 1 | |
| HbA1c (%) | Visit 1 | 6.1 (5.7–6.4) | 5.7 (5.4–6) | 0.101 1 | 5.5 (5.4–5.9) | 5.8 (5.6–6.2) | 0.235 1 |
| Visit 2 | 6 (5.6–6.4) | 5.8 (5.4–5.9) | 0.085 1 | 5.4 (5.4–5.8) | 5.8 (5.7–6.5) * | 0.218 1 | |
| Visit 3 | 5.9 (5.7–6.5) | 5.8 (5.6–6) | 0.153 1 | 5.6 (5.5–5.9) | 5.8 (5.7–6.6) * | 0.256 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nájar, A.M.; López Azcárate, C.; Domínguez Ruiz, C.; Núñez-Jurado, D.; de Torres, R.; López, R.; Camino-Moya, M.; Magni, E.; Montero-Ramirez, E.; Bocero, A.; et al. Evaluating the Clinical Impact of a Polyphenol-Rich Extract from Salicornia ramosissima on Patients with Transient Ischemic Attack and Minor Stroke. Nutrients 2024, 16, 4307. https://doi.org/10.3390/nu16244307
Nájar AM, López Azcárate C, Domínguez Ruiz C, Núñez-Jurado D, de Torres R, López R, Camino-Moya M, Magni E, Montero-Ramirez E, Bocero A, et al. Evaluating the Clinical Impact of a Polyphenol-Rich Extract from Salicornia ramosissima on Patients with Transient Ischemic Attack and Minor Stroke. Nutrients. 2024; 16(24):4307. https://doi.org/10.3390/nu16244307
Chicago/Turabian StyleNájar, Ana M., Cristina López Azcárate, Carmen Domínguez Ruiz, David Núñez-Jurado, Reyes de Torres, Reyes López, Miriam Camino-Moya, Eleonora Magni, Emilio Montero-Ramirez, Antonio Bocero, and et al. 2024. "Evaluating the Clinical Impact of a Polyphenol-Rich Extract from Salicornia ramosissima on Patients with Transient Ischemic Attack and Minor Stroke" Nutrients 16, no. 24: 4307. https://doi.org/10.3390/nu16244307
APA StyleNájar, A. M., López Azcárate, C., Domínguez Ruiz, C., Núñez-Jurado, D., de Torres, R., López, R., Camino-Moya, M., Magni, E., Montero-Ramirez, E., Bocero, A., Laviana, Á., Busquier Cerdán, T., León, A., del Rio, C., Montaner, J., & Pérez-Sánchez, S. (2024). Evaluating the Clinical Impact of a Polyphenol-Rich Extract from Salicornia ramosissima on Patients with Transient Ischemic Attack and Minor Stroke. Nutrients, 16(24), 4307. https://doi.org/10.3390/nu16244307





