Caffeine-Induced Upregulation of pas-1 and pas-3 Enhances Intestinal Integrity by Reducing Vitellogenin in Aged Caenorhabditis elegans Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Caenorhabditis elegans Strains and Caffeine Treatment
2.2. RNA Sequencing and Data Visualization
2.3. RNA Extraction and Quantitative Reverse Transcriptase-PCR (qRT-PCR)
2.4. RNA Interference (RNAi)
2.5. Intestinal Integrity Assays
2.6. Motility and Life Span Assays
2.7. Statistical Analysis
3. Results
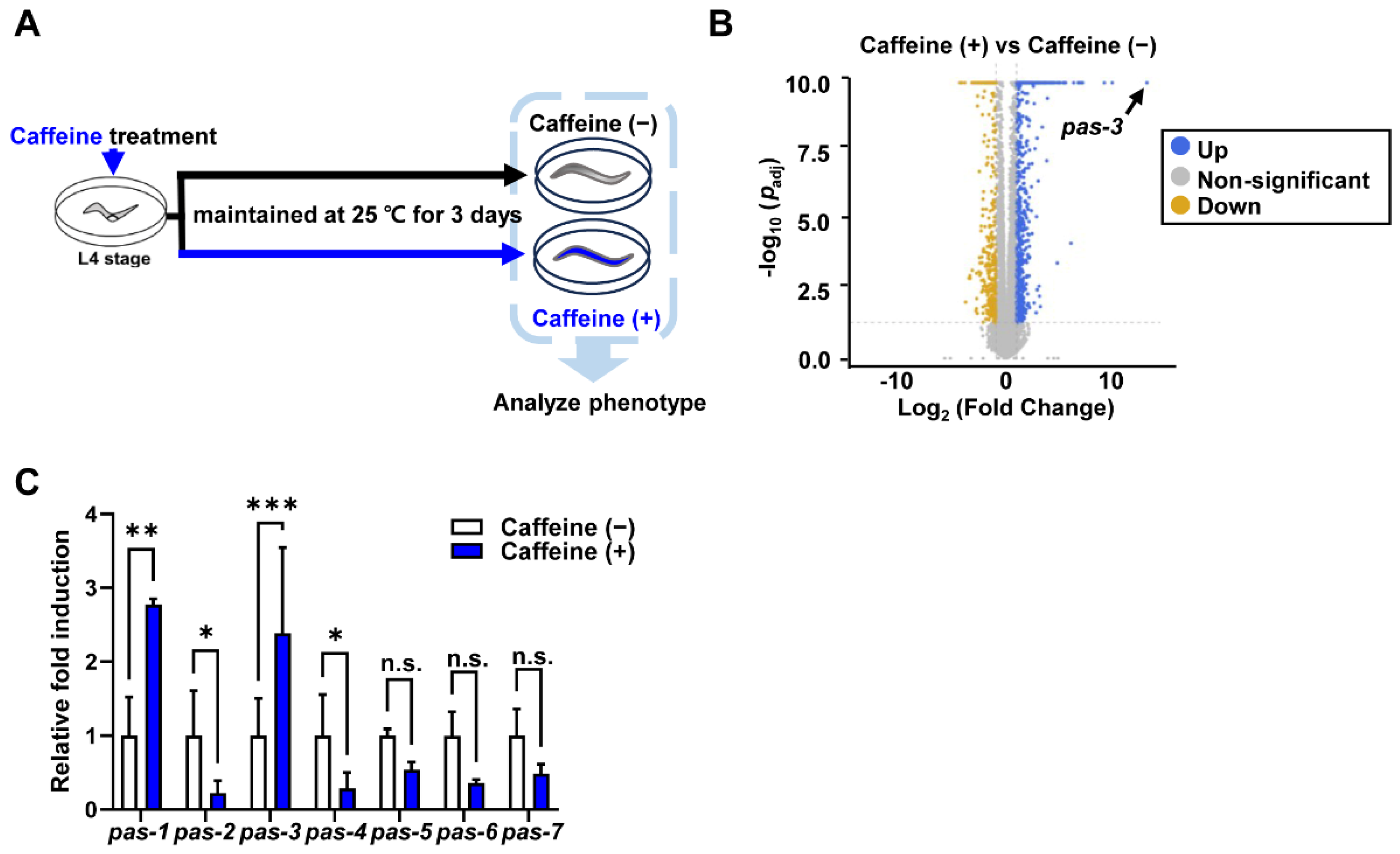
3.1. Caffeine Intake Significantly Increases Expression of Proteasome α-Subunit Genes, pas-1 and pas-3 in aged C. elegans
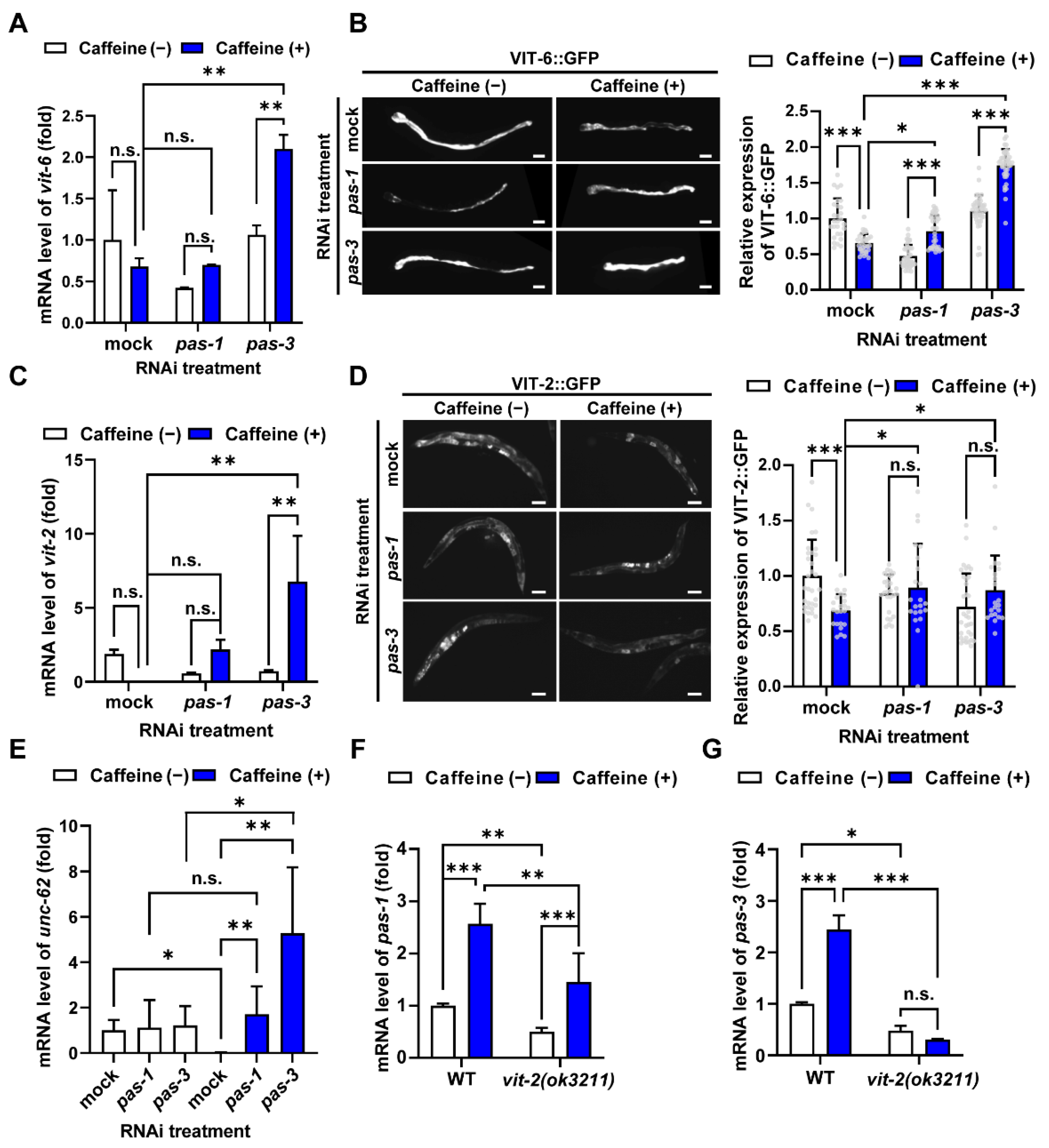
3.2. Caffeine Intake Reduces Vitellogenin Production by Modulating pas-1 and pas-3 During Aging in C. elegans
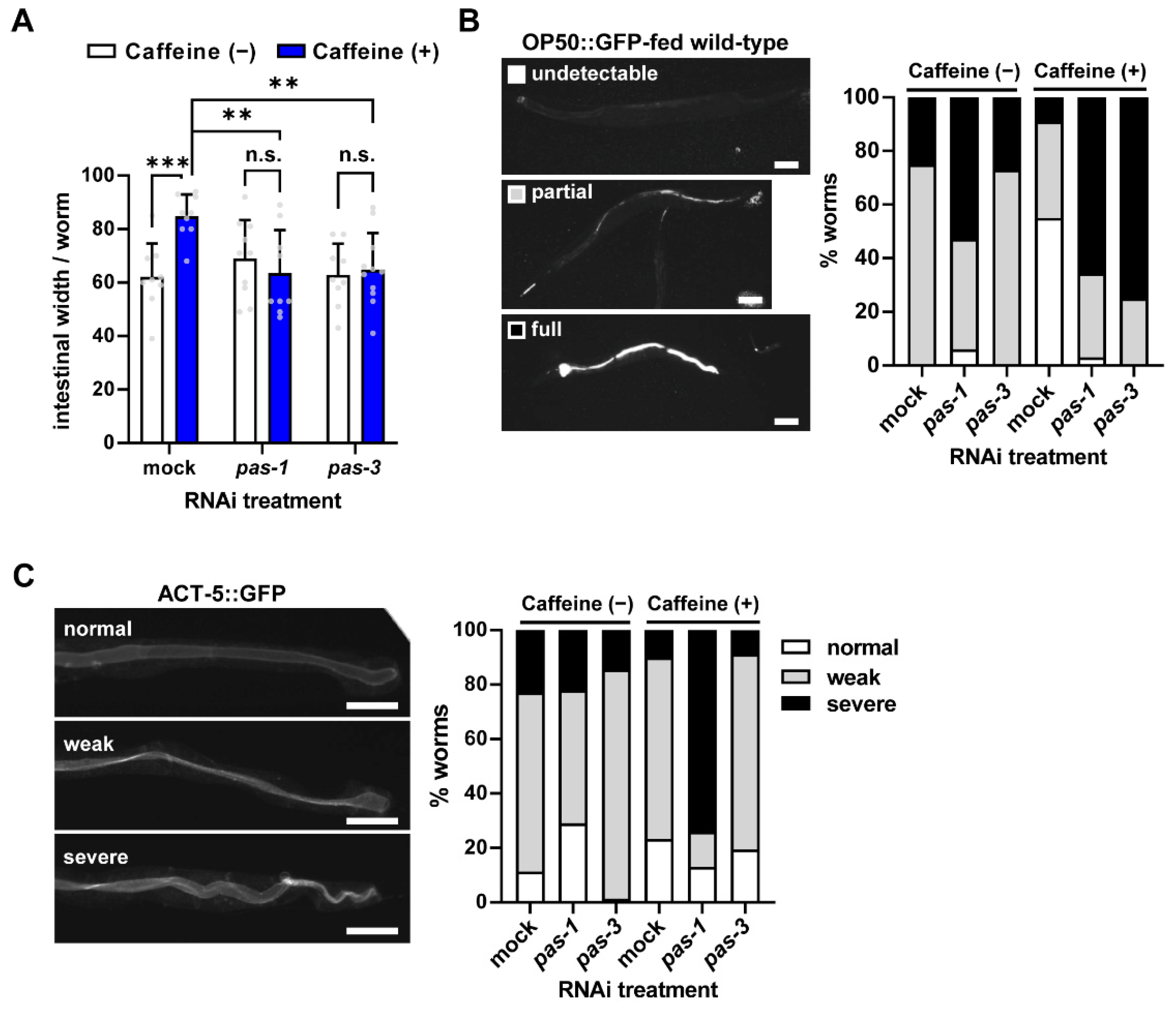
3.3. Caffeine Intake Improves Intestinal Integrity via pas-1 and pas-3 in Aged Adults
3.4. pas-1 Extends SKN-1-Mediated Lifespan During Aging in Caffeine-Fed Aged C. elegans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grubben, M.J.; Van Den Braak, C.C.; Broekhuizen, R.; De Jong, R.; Van Rijt, L.; De Ruijter, E.; Peters, W.H.; Katan, M.B.; Nagengast, F.M. The Effect of Unfiltered Coffee on Potential Biomarkers for Colonic Cancer Risk in Healthy Volunteers: A Randomized Trial. Aliment. Pharmacol. Ther. 2000, 14, 1181–1190. [Google Scholar]
- Arnaud, M.J. The Pharmacology of Caffeine. Prog. Drug Res. 1987, 31, 273–313. [Google Scholar]
- Reyes, C.M.; Cornelis, M.C. Caffeine in the Diet: Country-Level Consumption and Guidelines. Nutrients 2018, 10, 1772. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Jacinto, T.A.; Gonçalves, A.C.; Gaspar, D.; Silva, L.R. Overview of Caffeine Effects on Human Health and Emerging Delivery Strategies. Pharmaceuticals 2023, 16, 1067. [Google Scholar] [CrossRef]
- Min, H.; Kawasaki, I.; Gong, J.; Shim, Y.H. Caffeine Induces High Expression of cyp-35A Family Genes and Inhibits the Early Larval Development in Caenorhabditis elegans. Mol. Cells 2015, 38, 236–242. [Google Scholar]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of Caffeine on Human Health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar]
- Gavrieli, A.; Yannakoulia, M.; Fragopoulou, E.; Margaritopoulos, D.; Chamberland, J.P.; Kaisari, P.; Kavouras, S.A.; Mantzoros, C.S. Caffeinated Coffee Does Not Acutely Affect Energy Intake, Appetite, or Inflammation but Prevents Serum Cortisol Concentrations from Falling in Healthy Men. J. Nutr. 2011, 141, 703–707. [Google Scholar]
- Min, H.; Youn, E.; Shim, Y.H. Maternal Caffeine Intake Disrupts Eggshell Integrity and Retards Larval Development by Reducing Yolk Production in a Caenorhabditis elegans Model. Nutrients 2020, 12, 1334. [Google Scholar] [CrossRef]
- Takahashi, K.; Yanai, S.; Shimokado, K.; Ishigami, A. Coffee Consumption in Aged Mice Increases Energy Production and Decreases Hepatic MTOR Levels. Nutrition 2017, 38, 1–8. [Google Scholar]
- Min, H.; Youn, E.; Shim, Y.H. Long-Term Caffeine Intake Exerts Protective Effects on Intestinal Aging by Regulating Vitellogenesis and Mitochondrial Function in an Aged Caenorhabditis elegans Model. Nutrients 2021, 13, 2517. [Google Scholar] [CrossRef]
- Min, H.; Lee, M.; Kang, S.; Shim, Y.H. Vitamin B12 Supplementation Improves Oocyte Development by Modulating Mitochondria and Yolk Protein in a Caffeine-Ingested Caenorhabditis elegans Model. Antioxidants 2024, 13, 53. [Google Scholar]
- Sheng, X.; Zhu, Y.; Zhou, J.; Yan, L.; Du, G.; Liu, Z.; Chen, H. Antioxidant Effects of Caffeic Acid Lead to Protection of Drosophila Intestinal Stem Cell Aging. Front. Cell Dev. Biol. 2021, 9, 735483. [Google Scholar]
- González, S.; Salazar, N.; Ruiz-Saavedra, S.; Gómez-Martín, M.; de los Reyes-Gavilán, C.G.; Gueimonde, M. Long-Term Coffee Consumption Is Associated with Fecal Microbial Composition in Humans. Nutrients 2020, 12, 1287. [Google Scholar] [CrossRef]
- Baspinar, B.; Eskici, G.; Ozcelik, A.O. How Coffee Affects Metabolic Syndrome and Its Components. Food Funct. 2017, 8, 2089–2101. [Google Scholar]
- O’Keefe, J.H.; DiNicolantonio, J.J.; Lavie, C.J. Coffee for Cardioprotection and Longevity. Prog. Cardiovasc. Dis. 2018, 61, 38–42. [Google Scholar]
- Gkegkes, I.D.; Minis, E.E.; Iavazzo, C. Effect of Caffeine Intake on Postoperative Ileus: A Systematic Review and Meta-Analysis. Dig. Surg. 2020, 37, 22–31. [Google Scholar]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of Diet on the Gut Microbiome and Implications for Human Health. J. Transl. Med. 2017, 15, 73. [Google Scholar]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and Age-related Diseases: From Mechanisms to Therapeutic Strategies. Biogerontology 2021, 22, 165–187. [Google Scholar]
- Lee, K.X.; Quek, K.F.; Ramadas, A. Dietary and Lifestyle Risk Factors of Obesity Among Young Adults: A Scoping Review of Observational Studies. Curr. Nutr. Rep. 2023, 12, 733–743. [Google Scholar]
- Zhang, K.; Ma, Y.; Luo, Y.; Song, Y.; Xiong, G.; Ma, Y.; Sun, X.; Kan, C. Metabolic Diseases and Healthy Aging: Identifying Environmental and Behavioral Risk Factors and Promoting Public Health. Front. Public Health 2023, 11, 1253506. [Google Scholar]
- Surugiu, R.; Iancu, M.A.; Vintilescu, Ș.B.; Stepan, M.D.; Burdusel, D.; Genunche-Dumitrescu, A.V.; Dogaru, C.A.; Dumitra, G.G. Molecular Mechanisms of Healthy Aging: The Role of Caloric Restriction, Intermittent Fasting, Mediterranean Diet, and Ketogenic Diet—A Scoping Review. Nutrients 2024, 16, 2878. [Google Scholar] [CrossRef]
- Drozdowski, L.; Thomson, A.B. Aging and the Intestine. World J. Gastroenterol. 2006, 12, 7578–7584. [Google Scholar]
- McGee, M.D.; Weber, D.; Day, N.; Vitelli, C.; Crippen, D.; Herndon, L.A.; Hall, D.H.; Melov, S. Loss of Intestinal Nuclei and Intestinal Integrity in Aging C. elegans. Aging Cell 2011, 10, 699–710. [Google Scholar]
- Perez, M.F.; Lehner, B. Vitellogenins—Yolk Gene Function and Regulation in Caenorhabditis elegans. Front. Physiol. 2019, 10, 1067. [Google Scholar]
- Baker, M.E. Is Vitellogenin an Ancestor of Apolipoprotein B-100 of Human Low-Density Lipoprotein and Human Lipoprotein Lipase? Biochem. J. 1988, 255, 1057–1060. [Google Scholar]
- Kimbleand, J.; Sharrock, W.J. Tissue-Specific Synthesis of Yolk Proteins in Caenorhabditis elegans. Dev. Biol. 1983, 96, 189–196. [Google Scholar]
- Ezcurra, M.; Benedetto, A.; Sornda, T.; Gilliat, A.F.; Au, C.; Zhang, Q.; van Schelt, S.; Petrache, A.L.; Wang, H.; de la Guardia, Y.; et al. C. elegans Eats Its Own Intestine to Make Yolk Leading to Multiple Senescent Pathologies. Curr. Biol. 2018, 28, 2544–2556.e5. [Google Scholar]
- Sornda, T.; Ezcurra, M.; Kern, C.; Galimov, E.R.; Au, C.; De La Guardia, Y.; Gems, D. Production of YP170 Vitellogenins Promotes Intestinal Senescence in Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 1180–1188. [Google Scholar] [CrossRef]
- Song, R.; Hu, M.; Qin, X.; Qiu, L.; Wang, P.; Zhang, X.; Liu, R.; Wang, X. The Roles of Lipid Metabolism in the Pathogenesis of Chronic Diseases in the Elderly. Nutrients 2023, 15, 3433. [Google Scholar] [CrossRef]
- Libina, N.; Berman, J.R.; Kenyon, C. Tissue-Specific Activities of C. elegans DAF-16 in the Regulation of Lifespan. Cell 2003, 115, 489–502. [Google Scholar]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Hoogewijs, D.; Houthoofd, K.; Matthijssens, F.; Vandesompele, J.; Vanfleteren, J.R. Selection and Validation of a Set of Reliable Reference Genes for Quantitative Sod Gene Expression Analysis in C. elegans. BMC Mol. Biol. 2008, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Maeda, I.; Kohara, Y.; Yamamoto, M.; Sugimoto, A. Large-Scale Analysis of Gene Function in Caenorhabditis elegans by High-Throughput RNAi. Curr. Biol. 2001, 11, 171–176. [Google Scholar] [CrossRef]
- Takahashi, M.; Iwasaki, H.; Inoue, H.; Takahashi, K. Reverse Genetic Analysis of the Caenorhabditis elegans 26S Proteasome Subunits by RNA Interference. Biol. Chem. 2002, 383, 1263–1266. [Google Scholar] [CrossRef]
- Walker, A.C.; Bhargava, R.; Vaziriyan-Sani, A.S.; Pourciau, C.; Donahue, E.T.; Dove, A.S.; Gebhardt, M.J.; Ellward, G.L.; Romeo, T.; Czyż, D.M. Colonization of the Caenorhabditis elegans gut with human enteric bacterial pathogens leads to proteostasis disruption that is rescued by butyrate. PLoS Pathog. 2021, 17, e1009510. [Google Scholar] [CrossRef]
- Davy, A.; Bello, P.; Thierry-Mieg, N.; Vaglio, P.; Hitti, J.; Doucette-Stamm, L.; Thierry-Mieg, D.; Reboul, J.; Boulton, S.; Walhout, A.J.; et al. A Protein-Protein Interaction Map of the Caenorhabditis elegans 26S Proteasome. EMBO Rep. 2001, 2, 821–828. [Google Scholar] [CrossRef]
- Papaevgeniou, N.; Chondrogianni, N. The Ubiquitin Proteasome System in Caenorhabditis elegans and Its Regulation. Redox Biol. 2014, 2, 333–347. [Google Scholar] [CrossRef]
- Vilchez, D.; Saez, I.; Dillin, A. The Role of Protein Clearance Mechanisms in Organismal Ageing and Age-Related Diseases. Nat. Commun. 2014, 5, 5659. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Proteostasis and Aging. Nat. Med. 2015, 21, 1406–1415. [Google Scholar] [CrossRef]
- Dowen, R.H. CEH-60/PBX and UNC-62/MEIS Coordinate a Metabolic Switch That Supports Reproduction in C. elegans. Dev. Cell 2019, 49, 235–250.e7. [Google Scholar] [CrossRef]
- Macqueen, A.J.; Baggett, J.J.; Perumov, N.; Bauer, R.A.; Januszewski, T.; Schriefer, L.; Waddle, J.A. ACT-5 Is an Essential Caenorhabditis elegans Actin Required for Intestinal Microvilli Formation. Mol. Biol. Cell 2005, 16, 3247–3259. [Google Scholar] [PubMed]
- Anderson, R.T.; Bradley, T.A.; Smith, D.M. Hyperactivation of the Proteasome in Caenorhabditis elegans Protects against Proteotoxic Stress and Extends Lifespan. J. Biol. Chem. 2022, 298, 102415. [Google Scholar] [CrossRef] [PubMed]
- Tavirani, M.R.; Farahani, M.; Tavirani, M.R.; Razzaghi, Z.; Arjmand, B.; Khodadoost, M. Introducing Coffee as a Complementary Agent Beside Metformin Against Type 2 Diabetes. Res. J. Pharmacogn. 2024, 11, 31–40. [Google Scholar]
- Lim, H.H.; Kim, O.Y. Association of Serum Apolipoprotein B with the Increased Risk of Diabetes in Korean Men. Clin. Nutr. Res. 2016, 5, 204–212. [Google Scholar] [CrossRef]
- Hashmi, S.; Wang, Y.; Parhar, R.S.; Collison, K.S.; Conca, W.; Al-Mohanna, F. A C. elegans Model to Study Human Metabolic Regulation. Nutr. Metab. 2013, 10, 31. [Google Scholar]
- Mikkonen, E.; Haglund, C.; Holmberg, C.I. Immunohistochemical Analysis Reveals Variations in Proteasome Tissue Expression in C. elegans. PLoS ONE 2017, 12, e0183403. [Google Scholar] [CrossRef]
- Li, X.; Matilainen, O.; Jin, C.; Glover-Cutter, K.M.; Holmberg, C.I.; Blackwell, T.K. Specific SKN-1/NrF Stress Responses to Perturbations in Translation Elongation and Proteasome Activity. PLoS Genet. 2011, 7, e1002119. [Google Scholar]
- Lynn, D.A.; Dalton, H.M.; Sowa, J.N.; Wang, M.C.; Soukas, A.A.; Curran, S.P.; Ruvkun, G. Omega-3 and -6 Fatty Acids Allocate Somatic and Germline Lipids to Ensure Fitness during Nutrient and Oxidative Stress in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2015, 112, 15378–15383. [Google Scholar]
- Blackwell, T.K.; Steinbaugh, M.J.; Hourihan, J.M.; Ewald, C.Y.; Isik, M. SKN-1/Nrf, Stress Responses, and Aging in Caenorhabditis elegans. Free Radic. Biol. Med. 2015, 88, 290–301. [Google Scholar]
- Balklava, Z.; Pant, S.; Fares, H.; Grant, B.D. Genome-Wide Analysis Identifies a General Requirement for Polarity Proteins in Endocytic Traffic. Nat. Cell Biol. 2007, 9, 1066–1073. [Google Scholar]
- Saez, I.; Vilchez, D. The Mechanistic Links Between Proteasome Activity, Aging and Age-Related Diseases. Curr. Genom. 2014, 15, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Tomaru, U.; Takahashi, S.; Ishizu, A.; Miyatake, Y.; Gohda, A.; Suzuki, S.; Ono, A.; Ohara, J.; Baba, T.; Murata, S.; et al. Decreased Proteasomal Activity Causes Age-Related Phenotypes and Promotes the Development of Metabolic Abnormalities. Am. J. Pathol. 2012, 180, 963–972. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Lee, J.; Kim, D.; Min, H.; Shim, Y.-H. Caffeine-Induced Upregulation of pas-1 and pas-3 Enhances Intestinal Integrity by Reducing Vitellogenin in Aged Caenorhabditis elegans Model. Nutrients 2024, 16, 4298. https://doi.org/10.3390/nu16244298
Lee M, Lee J, Kim D, Min H, Shim Y-H. Caffeine-Induced Upregulation of pas-1 and pas-3 Enhances Intestinal Integrity by Reducing Vitellogenin in Aged Caenorhabditis elegans Model. Nutrients. 2024; 16(24):4298. https://doi.org/10.3390/nu16244298
Chicago/Turabian StyleLee, Mijin, Jea Lee, Dongyeon Kim, Hyemin Min, and Yhong-Hee Shim. 2024. "Caffeine-Induced Upregulation of pas-1 and pas-3 Enhances Intestinal Integrity by Reducing Vitellogenin in Aged Caenorhabditis elegans Model" Nutrients 16, no. 24: 4298. https://doi.org/10.3390/nu16244298
APA StyleLee, M., Lee, J., Kim, D., Min, H., & Shim, Y.-H. (2024). Caffeine-Induced Upregulation of pas-1 and pas-3 Enhances Intestinal Integrity by Reducing Vitellogenin in Aged Caenorhabditis elegans Model. Nutrients, 16(24), 4298. https://doi.org/10.3390/nu16244298





