The Improvement in Sleep Quality by Zizyphi Semen in Rodent Models Through GABAergic Transmission Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Cell Culture
2.4. Pentobarbital-Induced Sleeping Test
2.5. Caffeine-Induced Insomnia Model
2.6. Electroencephalography (EEG) and Electromyogram (EMG) Recording Schedule
2.7. Implantation Surgery for EEG and EMG
2.8. EEG and EMG Recording
2.9. Automated Sleep–Wake State Scored Algorithm Using MATLAB
2.10. Time-Dependent Power Spectrum Heatmaps
2.11. Western Blot
2.12. Intracellular Chloride Ion Measurement Assay
2.13. Statistical Analysis
3. Results
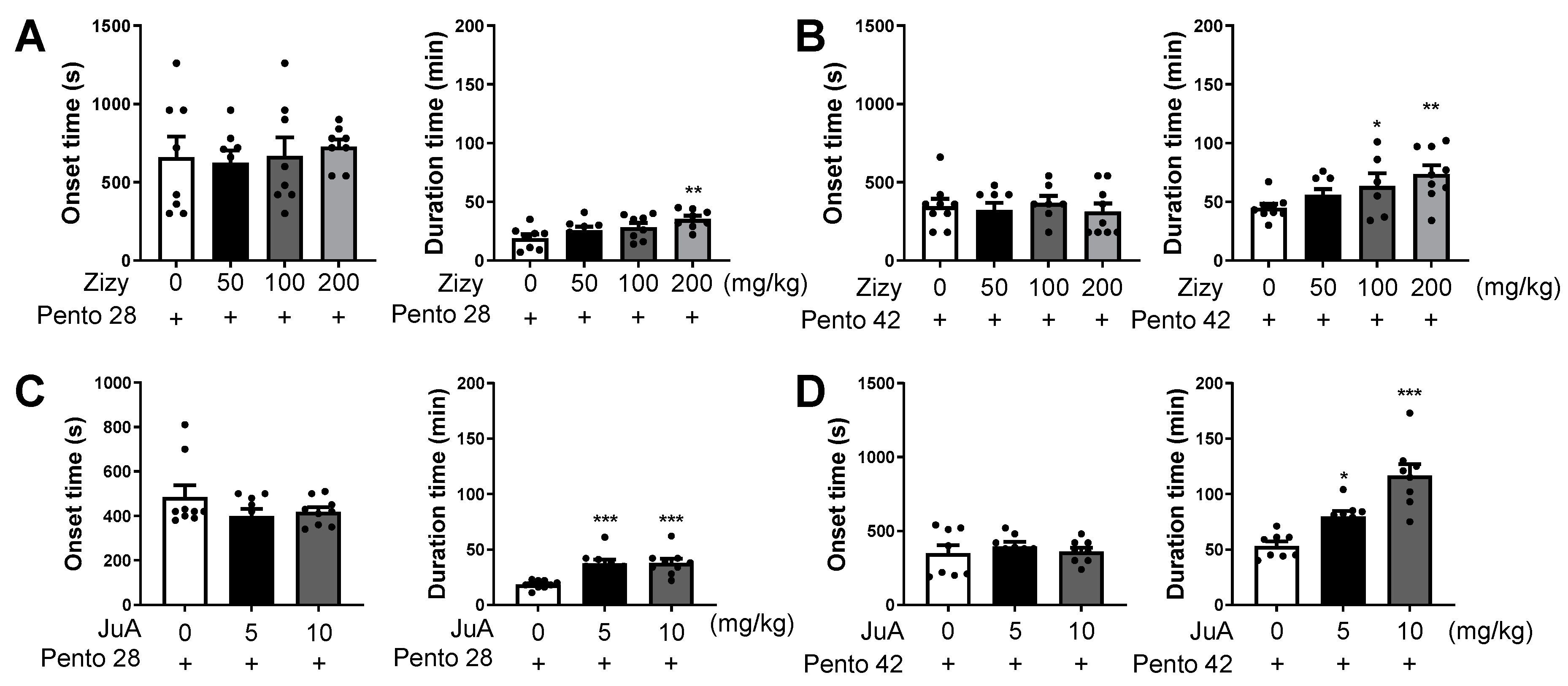
3.1. Administration of Zizyphi Semen Extract and Jujuboside A Prolongs Sleep Duration in Pentobarbital-Induced Sleep in Mice
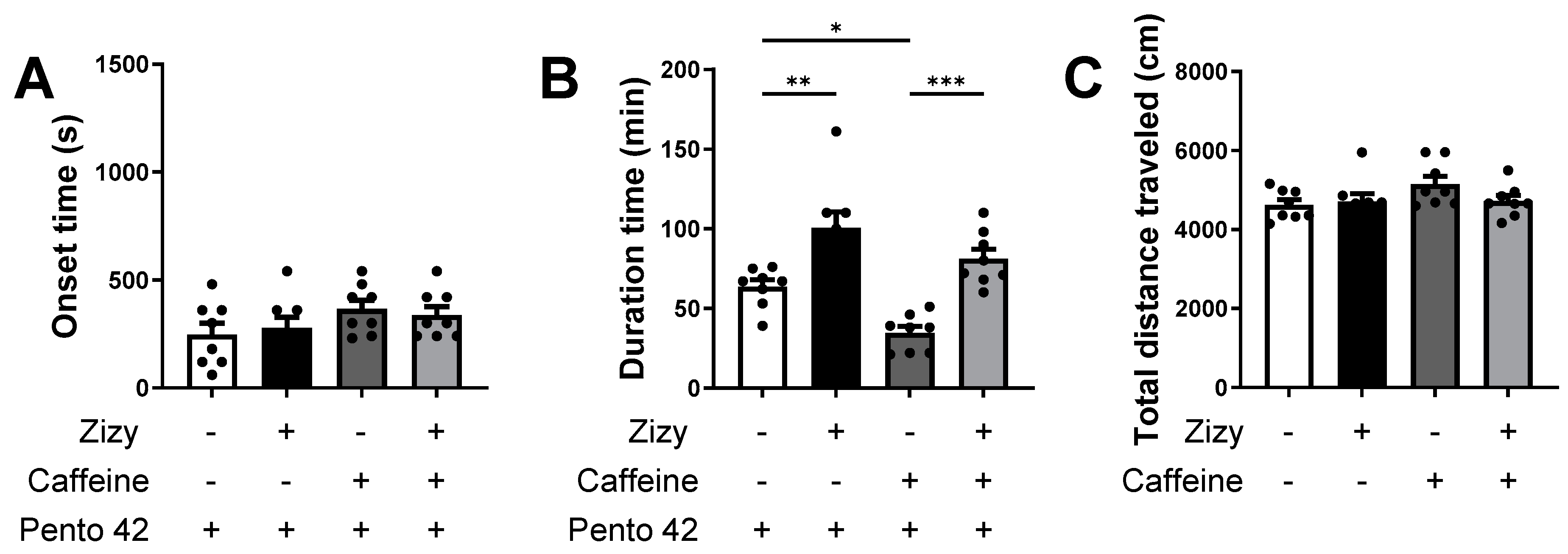
3.2. Administration of Zizyphi Semen Extract Relieves Caffeine-Induced Insomnia in Mice
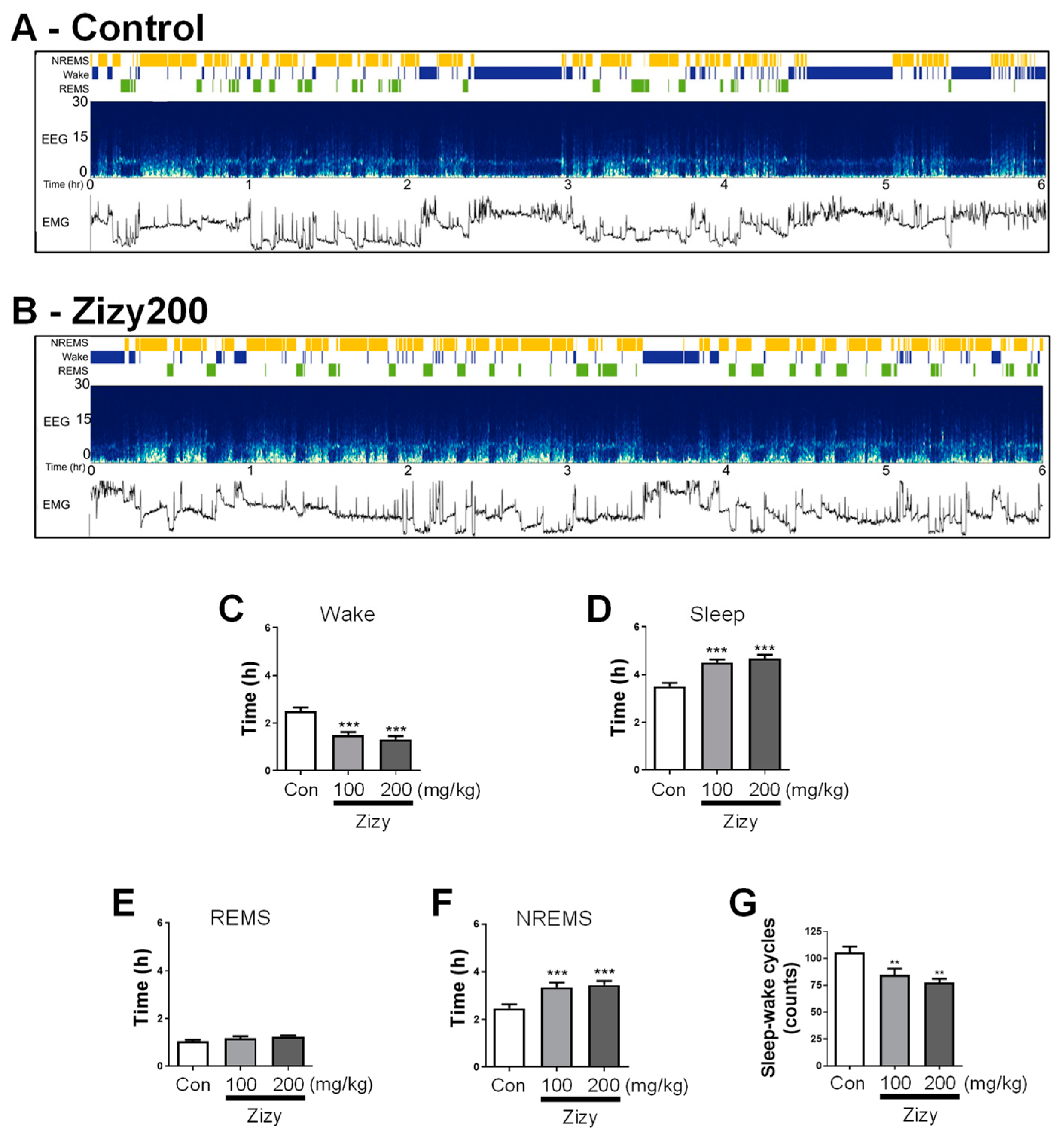
3.3. Administration of Zizyphi Semen Extract Modulates Sleep Architecture in Rats
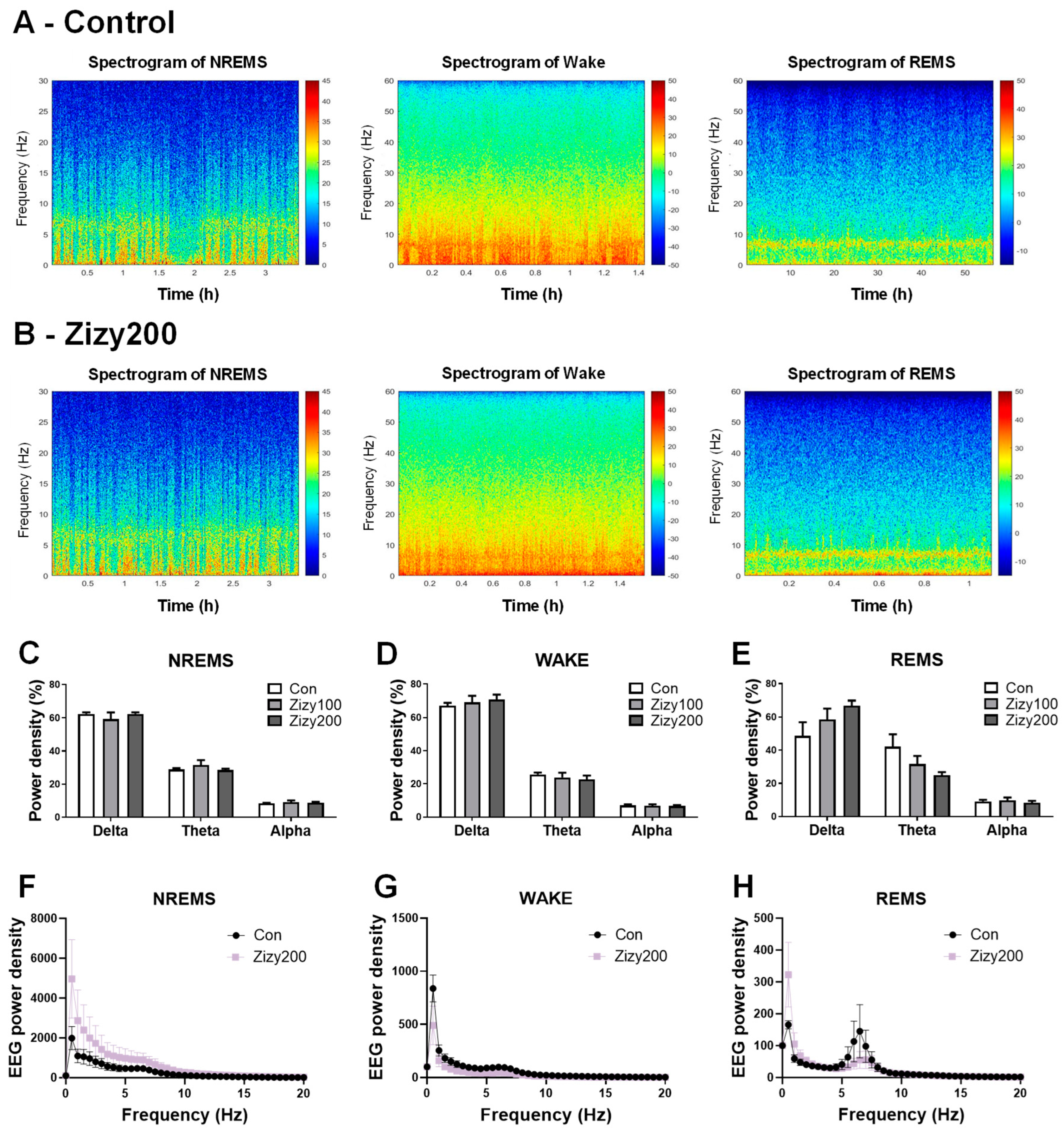
3.4. Administration of Zizyphi Semen Extract Tends to Modulate the Power Density of EEG Waves During REMS in Rats
3.5. Administration of Zizyphi Semen Extract Modulates the Expression of GABAA Receptor Subunits and GAD65/67 in the Rat Brain
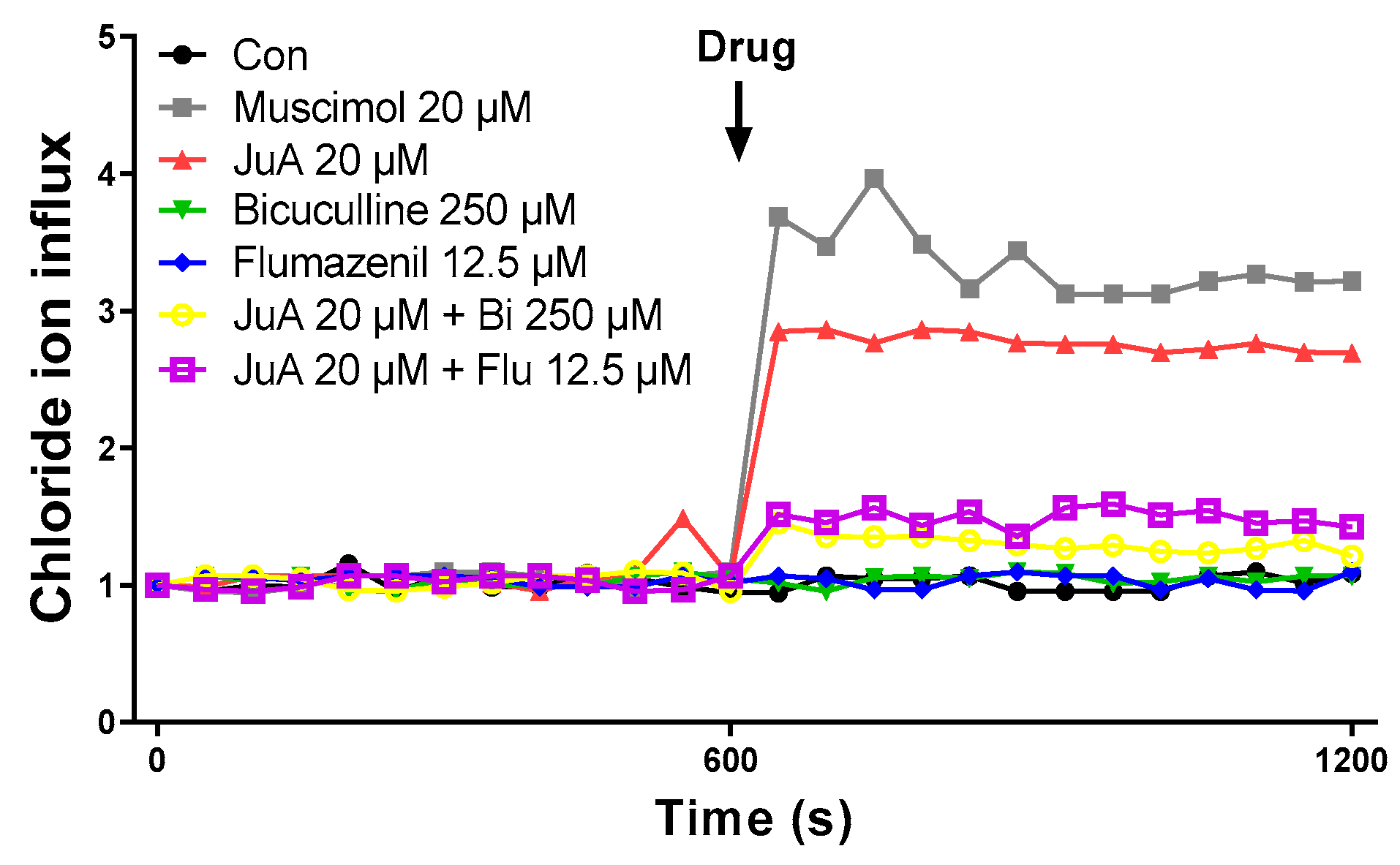
3.6. Jujuboside A Induces Intracellular Chloride Influx in SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Tsujino, N.; Kuramoto, E.; Koyama, Y.; Susaki, E.A.; Chikahisa, S.; Funato, H. Sleep as a biological problem: An overview of frontiers in sleep research. J. Physiol. Sci. 2016, 66, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Krause, A.J.; Simon, E.B.; Mander, B.A.; Greer, S.M.; Saletin, J.M.; Goldstein-Piekarski, A.N.; Walker, M.P. The sleep-deprived human brain. Nat. Rev. Neurosci. 2017, 18, 404–418. [Google Scholar] [CrossRef]
- Holingue, C.; Wennberg, A.; Berger, S.; Polotsky, V.Y.; Spira, A.P. Disturbed sleep and diabetes: A potential nexus of dementia risk. Metabolism 2018, 84, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.E.; Veasey, S.C. Impact of sleep disturbances on neurodegeneration: Insight from studies in animal models. Neurobiol. Dis. 2020, 139, 104820. [Google Scholar] [CrossRef]
- Bollu, P.C.; Kaur, H. Sleep Medicine: Insomnia and Sleep. Mo Med. 2019, 116, 68–75. [Google Scholar]
- Uccella, S.; Cordani, R.; Salfi, F.; Gorgoni, M.; Scarpelli, S.; Gemignani, A.; Geoffroy, P.A.; De Gennaro, L.; Palagini, L.; Ferrara, M.; et al. Sleep Deprivation and Insomnia in Adolescence: Implications for Mental Health. Brain Sci. 2023, 13, 569. [Google Scholar] [CrossRef]
- Zhu, B.; Vincent, C.; Kapella, M.C.; Quinn, L.; Collins, E.G.; Ruggiero, L.; Park, C.; Fritschi, C. Sleep disturbance in people with diabetes: A concept analysis. J. Clin. Nurs. 2018, 27, e50–e60. [Google Scholar] [CrossRef]
- Mehta, K.J. Effect of sleep and mood on academic performance—At interface of physiology, psychology, and education. Humanit. Soc. Sci. Commun. 2022, 9, 16. [Google Scholar] [CrossRef]
- Madari, S.; Golebiowski, R.; Mansukhani, M.P.; Kolla, B.P. Pharmacological Management of Insomnia. Neurotherapeutics 2021, 18, 44–52. [Google Scholar] [CrossRef]
- Pandey, J.; Bastola, T.; Dhakal, B.; Poudel, A.; Devkota, H.P. Chrysanthemum morifolium Ramat: A Medicinal Plant with Diverse Traditional Uses, Bioactive Constituents, and Pharmacological Activities. In Medicinal Plants of the Asteraceae Family: Traditional Uses, Phytochemistry and Pharmacological Activities; Devkota, H.P., Aftab, T., Eds.; Springer Nature: Singapore, 2022; pp. 125–143. [Google Scholar]
- Kandimalla, R.; Dash, S.; Kalita, S.; Choudhury, B.; Malampati, S.; Kalita, K.; Kalita, B.; Devi, R.; Kotoky, J. Protective Effect of Bioactivity Guided Fractions of Ziziphus jujuba Mill. Root Bark against Hepatic Injury and Chronic Inflammation via Inhibiting Inflammatory Markers and Oxidative Stress. Front. Pharmacol. 2016, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, X.; Liu, Y.; Xin, N.; Deng, Y.; Li, Y. Semen Ziziphi Spinosae attenuates blood–brain barrier dysfunction induced by lipopolysaccharide by targeting the FAK-DOCK180-Rac1-WAVE2-Arp3 signaling pathway. Npj Sci. Food 2022, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-X.; Zhang, Q.-Y.; Cui, S.-Y.; Cui, X.-Y.; Zhang, J.; Zhang, Y.-H.; Bai, Y.-J.; Zhao, Y.-Y. Hypnotic effect of jujubosides from Semen Ziziphi Spinosae. J. Ethnopharmacol. 2010, 130, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Hossain, M.A.; Jany, R.; Bari, M.A.; Uddin, M.; Kamal, A.R.M.; Ku, Y.; Kim, J.-S. Quantitative Evaluation of EEG-Biomarkers for Prediction of Sleep Stages. Sensors 2022, 22, 3079. [Google Scholar] [CrossRef] [PubMed]
- Topchiy, I.; Fink, A.M.; Maki, K.A.; Calik, M.W. Validation of PiezoSleep Scoring Against EEG/EMG Sleep Scoring in Rats. Nat. Sci. Sleep 2022, 14, 1877–1886. [Google Scholar] [CrossRef]
- Park, I.; Díaz, J.; Matsumoto, S.; Iwayama, K.; Nabekura, Y.; Ogata, H.; Kayaba, M.; Aoyagi, A.; Yajima, K.; Satoh, M.; et al. Exercise improves the quality of slow-wave sleep by increasing slow-wave stability. Sci. Rep. 2021, 11, 4410. [Google Scholar] [CrossRef]
- Le Bon, O. Relationships between REM and NREM in the NREM-REM sleep cycle: A review on competing concepts. Sleep. Med. 2020, 70, 6–16. [Google Scholar] [CrossRef]
- De Andrés, I.; Garzón, M.; Reinoso-Suárez, F. Functional Anatomy of Non-REM Sleep. Front. Neurol. 2011, 2, 70. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Kim, S.; Yoon, M.; Um, M.; Kim, D.; Kwon, S.; Cho, S. Arousal-Inducing Effect of Garcinia cambogia Peel Extract in Pentobarbital-Induced Sleep Test and Electroencephalographic Analysis. Nutrients 2021, 13, 2845. [Google Scholar] [CrossRef]
- Kim, Y.R.; Lee, S.Y.; Lee, S.M.; Shim, I.; Lee, M.Y. Effect of Hibiscus syriacus Linnaeus extract and its active constituent, saponarin, in animal models of stress-induced sleep disturbances and pentobarbital-induced sleep. Biomed Pharmacother. 2022, 146, 112301. [Google Scholar] [CrossRef]
- Hu, S.; Kao, H.-Y.; Yang, T.; Wang, Y. Early and Bi-hemispheric seizure onset in a rat glioblastoma Multiforme model. Neurosci. Lett. 2022, 766, 136351. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.A.; Gallego, R.; Ramos, M.; Lopez, J.M.; de Arcas, G.; Gonzalez-Nieto, D. Sleep-Wake Cycle and EEG-Based Biomarkers during Late Neonate to Adult Transition. Brain. Sci. 2021, 11, 298. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.; Lee, H.W.; Jung, J.-C.; Oh, S. Chrysanthemum morifolium and Its Bioactive Substance Enhanced the Sleep Quality in Rodent Models via Cl− Channel Activation. Nutrients 2023, 15, 1309. [Google Scholar] [CrossRef] [PubMed]
- Barger, Z.; Frye, C.G.; Liu, D.; Dan, Y.; Bouchard, K.E. Robust, automated sleep scoring by a compact neural network with distributional shift correction. PLoS ONE 2019, 14, e0224642. [Google Scholar] [CrossRef] [PubMed]
- Palagini, L.; Bianchini, C. Pharmacotherapeutic management of insomnia and effects on sleep processes, neural plasticity, and brain systems modulating stress: A narrative review. Front. Neurosci. 2022, 16, 893015. [Google Scholar] [CrossRef]
- Stephenson, F.A. The GABAA receptors. Biochem. J. 1995, 310, 1. [Google Scholar] [CrossRef]
- Pace-Schott, E.F.; Germain, A.; Milad, M.R. Effects of sleep on memory for conditioned fear and fear extinction. Psychol. Bull. 2015, 141, 835–857. [Google Scholar] [CrossRef]
- Lin, A.; Shih, C.-T.; Huang, C.-L.; Wu, C.-C.; Lin, C.-T.; Tsai, Y.-C. Hypnotic Effects of Lactobacillus fermentum PS150TM on Pentobarbital-Induced Sleep in Mice. Nutrients 2019, 11, 2409. [Google Scholar] [CrossRef]
- Woo, J.; Yang, H.; Yoon, M.; Gadhe, C.G.; Pae, A.N.; Cho, S.; Lee, C.J. 3-Carene, a Phytoncide from Pine Tree Has a Sleep-enhancing Effect by Targeting the GABA(A)-benzodiazepine Receptors. Exp. Neurobiol. 2019, 28, 593–601. [Google Scholar] [CrossRef]
- Xiong, X.; Ren, Y.; Gao, S.; Luo, J.; Liao, J.; Wang, C.; Yi, S.; Liu, R.; Xiang, Y.; He, J. EEG microstate in obstructive sleep apnea patients. Sci. Rep. 2021, 11, 17178. [Google Scholar] [CrossRef]
- Lucey, B.P.; Mcleland, J.S.; Toedebusch, C.D.; Boyd, J.; Morris, J.C.; Landsness, E.C.; Yamada, K.; Holtzman, D.M. Comparison of a single-channel EEG sleep study to polysomnography. J. Sleep Res. 2016, 25, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Holst, S.C.; Landolt, H.P. Sleep-Wake Neurochemistry. Sleep Med. Clin. 2022, 17, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Petersen, C.; Walsh, C.M.; Bittencourt, J.C.; Neylan, T.C.; Grinberg, L.T. The role of co-neurotransmitters in sleep and wake regulation. Mol. Psychiatry 2019, 24, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Gharpure, A.; Teng, J.; Zhuang, Y.; Howard, R.J.; Zhu, S.; Noviello, C.M.; Walsh, R.M.; Lindahl, E.; Hibbs, R.E. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature 2020, 585, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, L.J., Jr. Molecular mechanisms of antiseizure drug activity at GABAA receptors. Seizure 2013, 22, 589–600. [Google Scholar] [CrossRef]
- Saper, C.B.; Fuller, P.M.; Pedersen, N.P.; Lu, J.; Scammell, T.E. Sleep State Switching. Neuron 2010, 68, 1023–1042. [Google Scholar] [CrossRef]
- Zhu, S.; Noviello, C.M.; Teng, J.; Walsh, R.M.; Kim, J.J.; Hibbs, R.E. Structure of a human synaptic GABAA receptor. Nature 2018, 559, 67–72. [Google Scholar] [CrossRef]
- Johnston, G.A. Muscimol as an ionotropic GABA receptor agonist. Neurochem. Res. 2014, 39, 1942–1947. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Kim, Y.; Lee, H.W.; Kim, K.-M.; Kim, S.; Oh, S. The Improvement in Sleep Quality by Zizyphi Semen in Rodent Models Through GABAergic Transmission Regulation. Nutrients 2024, 16, 4266. https://doi.org/10.3390/nu16244266
Kim M, Kim Y, Lee HW, Kim K-M, Kim S, Oh S. The Improvement in Sleep Quality by Zizyphi Semen in Rodent Models Through GABAergic Transmission Regulation. Nutrients. 2024; 16(24):4266. https://doi.org/10.3390/nu16244266
Chicago/Turabian StyleKim, Mijin, YuJaung Kim, Hyang Woon Lee, Kyung-Mi Kim, Singeun Kim, and Seikwan Oh. 2024. "The Improvement in Sleep Quality by Zizyphi Semen in Rodent Models Through GABAergic Transmission Regulation" Nutrients 16, no. 24: 4266. https://doi.org/10.3390/nu16244266
APA StyleKim, M., Kim, Y., Lee, H. W., Kim, K.-M., Kim, S., & Oh, S. (2024). The Improvement in Sleep Quality by Zizyphi Semen in Rodent Models Through GABAergic Transmission Regulation. Nutrients, 16(24), 4266. https://doi.org/10.3390/nu16244266





