The Mediating Role of Body Mass Index in the Association Between Dietary Index for Gut Microbiota and Biological Age: A Study Based on NHANES 2007–2018
Abstract
1. Introduction
2. Method
2.1. Study Design and Participants
2.2. Outcome Variable
2.3. Predictive Variables
2.4. Covariance
2.5. Statistical Analysis Methods
3. Results
3.1. Basic Information
3.2. Association of the DI-GM and Biological Age Indicators
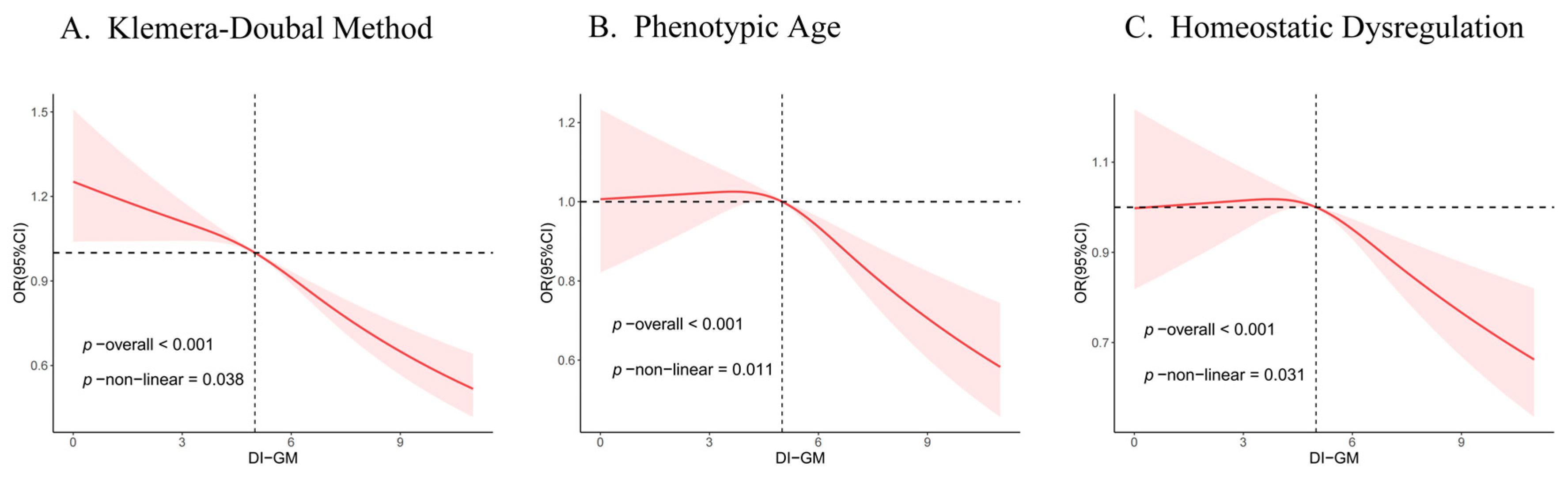
3.3. Non-Linear Trends of the DI-GM and Biological Age Indicators
3.4. Association of the BMI and DI-GM
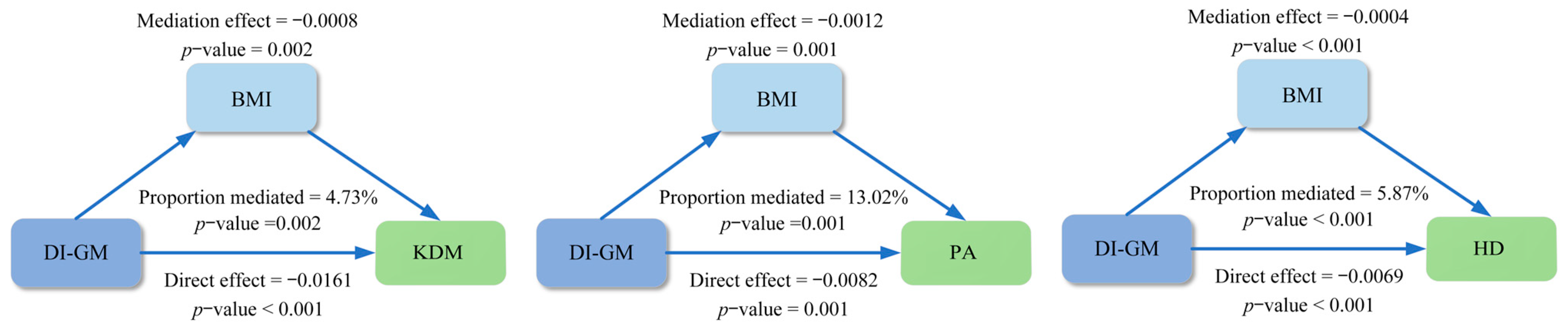
3.5. Mediation Analyses
3.6. Subgroup Analyses
3.7. Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, L.; Jia, W.; Miao, W.; Huimin, H.; Xin, D.; Lingzhi, M.; Ming, L. Oxidative Stress Factors Mediate the Association Between Life’s Essential 8 and Accelerated Phenotypic Aging: NHANES 2005–2018. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2024, 79, glad240. [Google Scholar] [CrossRef]
- Wang, X.; Sarker, S.K.; Cheng, L.; Dang, K.; Hu, J.; Pan, S.; Zhang, J.; Xu, X.; Li, Y. Association of dietary inflammatory potential, dietary oxidative balance score and biological aging. Clin. Nutr. 2024, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, X.; Wang, Y.; Liu, C.; Shi, J.; Liu, T.; Lin, S.; Xie, H.; Zhang, H.; Liu, X.; et al. Association between dietary quality and accelerated aging: A cross-sectional study of two cohorts. Food Funct. 2024, 15, 7837–7848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, M.; Zhang, W.; Liu, X.; Pu, J.; Wei, T.; Zhu, Z.; Tang, Z.; Wei, N.; Liu, B.; et al. Association between life’s essential 8 and biological ageing among US adults. J. Transl. Med. 2023, 21, 622. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, S.E.; Gladyshev, V.N.; Aryee, M.J.; Bernstein, B.E. Epigenetic clocks, aging, and cancer. Science. 2022, 378, 1276–1277. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, X.; Zhang, J.; Fu, J.; Mohedaner, M.; Danzengzhuoga; Sun, X.; Yang, G.; Yang, Z.; Kuo, C.L.; et al. Accelerated aging mediates the associations of unhealthy lifestyles with cardiovascular disease, cancer, and mortality. J. Am. Geriatr. Soc. 2023, 72, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.K.; Gustafson, B.; Kirkland, J.L.; Smith, U. Cellular senescence: At the nexus between ageing and diabetes. Diabetologia 2019, 62, 1835–1841. [Google Scholar] [CrossRef]
- Ma, J.; Li, P.; Jiang, Y.; Yang, X.; Luo, Y.; Tao, L.; Guo, X.; Gao, B. The Association between Dietary Nutrient Intake and Acceleration of Aging: Evidence from NHANES. Nutrients 2024, 16, 1635. [Google Scholar] [CrossRef]
- Zhu, X.; Xue, J.; Maimaitituerxun, R.; Xu, H.; Zhou, Q.; Zhou, Q.; Dai, W.; Chen, W. Relationship between dietary macronutrients intake and biological aging: A cross-sectional analysis of NHANES data. Eur. J. Nutr. 2023, 63, 243–251. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Z.; Colucci, M.; Deng, L.; Yang, M.; Huang, X.; Zhou, X.; Jin, Y.; Lazzarini, E.; Balbi, C.; et al. The mixed effect of Endocrine-Disrupting chemicals on biological age Acceleration: Unveiling the mechanism and potential intervention target. Environ. Int. 2024, 184, 108447. [Google Scholar] [CrossRef]
- Xingwei, H.; Shanshan, J.; Lirong, S.; Yuanyuan, Y.; Hang, L.; Xiaoping, C. Association of dietary live microbe intake with frailty in US adults evidence from NHANES. J. Nutr. Health Aging 2024, 28, 100171. [Google Scholar] [CrossRef]
- Ling, Z.; Liu, X.; Cheng, Y.; Yan, X.; Wu, S. Gut microbiota and aging. Crit. Rev. Food Sci. Nutr. 2020, 62, 3509–3534. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.-H.; Chou, P.-Y.; Hou, A.-T.; Huang, C.-L.; Shiu, W.-L.; Wang, S. Lactobacillus paracasei PS23 improves cognitive deficits via modulating the hippocampal gene expression and the gut microbiota in d-galactose-induced aging mice. Food Funct. 2022, 13, 5240–5251. [Google Scholar] [CrossRef]
- Wang, J.; Qie, J.; Zhu, D.; Zhang, X.; Zhang, Q.; Xu, Y.; Wang, Y.; Mi, K.; Pei, Y.; Liu, Y.; et al. The landscape in the gut microbiome of long-lived families reveals new insights on longevity and aging—Relevant neural and immune function. Gut Microbes 2022, 14, 2107288. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Chen, Y.; Wei, M.; Tang, M.; Lu, Y.; Zhang, Q.; Cao, Q. Mediation Role of Recreational Physical Activity in the Relationship between the Dietary Intake of Live Microbes and the Systemic Immune-Inflammation Index: A Real-World Cross-Sectional Study. Nutrients 2024, 16, 777. [Google Scholar] [CrossRef] [PubMed]
- Kase, B.E.; Liese, A.D.; Zhang, J.; Murphy, E.A.; Zhao, L.; Steck, S.E. The Development and Evaluation of a Literature-Based Dietary Index for Gut Microbiota. Nutrients 2024, 16, 1045. [Google Scholar] [CrossRef]
- He, Q.; Zheng, Q.; Diao, H.; Li, M.; Zhu, Q.; Fang, F.; Cui, W. The role of body mass index on the association between the energy-adjusted dietary inflammatory index and hyperuricemia: A mediation analysis based on NHANES (2007–2016). Int. J. Obes. 2023, 48, 339–345. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.-K.; Tang, M.; Lei, L.; Li, J.-R.; Sun, H.; Jiang, J.; Dong, B.; Li, H.-Y.; Jiang, J.-D.; et al. Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes 2024, 16, 2304159. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, L.; Zhao, Y.; Wang, C.; Duan, C.; Yang, G.; Niu, C.; Li, S. Lactobacillus plantarum NA136 ameliorates nonalcoholic fatty liver disease by modulating gut microbiota, improving intestinal barrier integrity, and attenuating inflammation. Appl. Microbiol. Biotechnol. 2020, 104, 5273–5282. [Google Scholar] [CrossRef]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszon-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National Health and Nutrition Examination Survey: Analytic Guidelines,1999–2010. Vital Health Stat. Ser. 2 Data Eval. Methods Res. 2013, 161, 1–24. [Google Scholar]
- Kwon, D.; Belsky, D.W. A toolkit for quantification of biological age from blood chemistry and organ function test data: BioAge. GeroScience 2021, 43, 2795–2808. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Y.; Li, X.; Mansuri, A.; McCall, W.V.; Liu, Y.; Su, S. Day-to-day deviations in sleep parameters and biological aging: Findings from the NHANES 2011-2014. Sleep Health 2023, 9, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zheng, J.; Li, J.; Shen, Y.; Zhu, L.; Jin, Y.; Zhang, M.; Yang, S.; Du, J.; Wang, H.; et al. Phthalate exposure and markers of biological aging: The mediating role of inflammation and moderating role of dietary nutrient intake. Ecotoxicol. Environ. Saf. 2024, 281, 116649. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, W.; Xue, J.; Dai, W.; BS, R.M.; Liu, Y.; Xu, H.; Zhou, Q.; Zhou, Q.; Chen, C.; et al. Dietary Live Microbes Intake Associated with Biological Aging and Mortality. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2024, 79, glae202. [Google Scholar] [CrossRef]
- Tian, X.; Xue, B.; Wang, B.; Lei, R.; Shan, X.; Niu, J.; Luo, B. Physical activity reduces the role of blood cadmium on depression: A cross-sectional analysis with NHANES data. Environ. Pollut. 2022, 304, 119211. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, Y.; Nie, J.; Xu, H.; Yu, C.; Wang, S. Higher HEI-2015 Score Is Associated with Reduced Risk of Depression: Result from NHANES 2005–2016. Nutrients 2021, 13, 348. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef]
- Gagnon, E.; Mitchell, P.L.; Manikpurage, H.D.; Abner, E.; Taba, N.; Esko, T.; Ghodsian, N.; Thériault, S.; Mathieu, P.; Arsenault, B.J. Impact of the gut microbiota and associated metabolites on cardiometabolic traits, chronic diseases and human longevity: A Mendelian randomization study. J. Transl. Med. 2023, 21, 60. [Google Scholar] [CrossRef]
- Yang, X.; Lu, D.; Zhuo, J.; Lin, Z.; Yang, M.; Xu, X. The Gut-liver Axis in Immune Remodeling: New insight into Liver Diseases. Int. J. Biol. Sci. 2020, 16, 2357–2366. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2013, 505, 559–563. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Rampelli, S.; Jeffery, I.B.; Santoro, A.; Neto, M.; Capri, M.; Giampieri, E.; Jennings, A.; Candela, M.; Turroni, S.; et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: The NU-AGE 1-year dietary intervention across five European countries. Gut 2020, 69, 1218–1228. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zheng, X.; Ma, X.; Jiang, R.; Zhou, W.; Zhou, S.; Zhang, Y.; Lei, S.; Wang, S.; Kuang, J.; et al. Theabrownin from Pu-erh tea attenuates hypercholesterolemia via modulation of gut microbiota and bile acid metabolism. Nat. Commun. 2019, 10, 4971. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, X.; Wang, R.; Liu, R.; Lv, X.; Ma, Y.; Li, Q. Lacticaseibacillus rhamnosus HF01 fermented yogurt alleviated high-fat diet-induced obesity and hepatic steatosis via the gut microbiota–butyric acid–hepatic lipid metabolism axis. Food Funct. 2024, 15, 4475–4489. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Romano, S.; Ansorge, R.; Aboelnour, A.; Le Gall, G.; Savva, G.M.; Pontifex, M.G.; Telatin, A.; Baker, D.; Jones, E.; et al. Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain. Microbiome 2022, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, Z.; Gao, X.; Bao, Y.; Hong, Y.; He, X.; Zhu, W.; Li, Y.; Huang, W.; Zheng, N.; et al. Gut microbiota remodeling improves natural aging-related disorders through Akkermansia muciniphila and its derived acetic acid. Pharmacol. Res. 2023, 189, 106687. [Google Scholar] [CrossRef]
- Cao, X.; Yang, G.; Li, X.; Fu, J.; Mohedaner, M.; Danzengzhuoga; Høj Jørgensen, T.S.; Agogo, G.O.; Wang, L.; Zhang, X.; et al. Weight change across adulthood and accelerated biological aging in middle-aged and older adults. Am. J. Clin. Nutr. 2023, 117, 1–11. [Google Scholar] [CrossRef]
- Chen, S.; Kong, Y.; Wang, N.; Kang, N.; Chen, H.; Zhang, Z.; Liu, L.; Chen, L. Association between weight change and serum anti-aging protein α-Klotho: A cross-sectional study in middle-aged and older adults. Sci. Rep. 2024, 14, 18624. [Google Scholar] [CrossRef]
- Jayanama, K.; Theou, O.; Godin, J.; Mayo, A.; Cahill, L.; Rockwood, K. Relationship of body mass index with frailty and all-cause mortality among middle-aged and older adults. BMC Med. 2022, 20, 404. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, W.; Zeng, L.-Q.; Bai, H.; Li, J.; Zhou, J.; Zhou, G.-Y.; Fang, C.-W.; Wang, F.; Qin, X.-J. Exercise and dietary intervention ameliorate high-fat diet-induced NAFLD and liver aging by inducing lipophagy. Redox Biol. 2020, 36, 101635. [Google Scholar] [CrossRef] [PubMed]
- Fathi, Y.; Ghodrati, N.; Zibaeenezhad, M.-J.; Faghih, S. Kefir drink causes a significant yet similar improvement in serum lipid profile, compared with low-fat milk, in a dairy-rich diet in overweight or obese premenopausal women: A randomized controlled trial. J. Clin. Lipidol. 2017, 11, 136–146. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, R.; Mai, Z.; Chen, H.; Zhang, J.; Zhao, L.; Yang, Z.; Yu, H.; Kong, D.; Ding, Y. Association between systemic immune-inflammatory index and diabetes mellitus: Mediation analysis involving obesity indicators in the NHANES. Front. Public Health 2024, 11, 1331159. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Basisty, N.; Wilson, D.M.; Ferrucci, L. Connecting aging biology and inflammation in the omics era. J. Clin. Invest. 2022, 132, e158448. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.-W.; Chen, Y.-C.; Yen, C.-C.; Chen, H.-L.; Tung, M.-C.; Fan, H.-C.; Chen, C.-M. Kefir peptides mitigate bleomycin-induced pulmonary fibrosis in mice through modulating oxidative stress, inflammation and gut microbiota. Biomed. Pharmacother. 2024, 174, 116431. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-m.; Zhou, Y.; Zuo, L.; Nie, D.; Li, X.-a. Dietary fiber regulates intestinal flora and suppresses liver and systemic inflammation to alleviate liver fibrosis in mice. Nutrition 2021, 81, 110959. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Nephew, L.D.; Vuppalanchi, R.; Gawrieh, S.; Mladenovic, A.; Pike, F.; Samala, N.; Chalasani, N. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology 2021, 75, 1491–1506. [Google Scholar] [CrossRef]
- Frame, L.A.; Costa, E.; Jackson, S.A. Current explorations of nutrition and the gut microbiome: A comprehensive evaluation of the review literature. Nutr. Rev. 2020, 78, 798–812. [Google Scholar] [CrossRef]
- Mohammad, M.R.; Ismail, L. Structural and functional alteration of blood vessels caused by cigarette smoking: An overview of molecular mechanisms. Curr. Vasc. Pharmacol. 2007, 5, 276–292. [Google Scholar] [CrossRef]
- Wilson, R.; Willis, J.; Gearry, R.; Skidmore, P.; Fleming, E.; Frampton, C.; Carr, A. Inadequate Vitamin C Status in Prediabetes and Type 2 Diabetes Mellitus: Associations with Glycaemic Control, Obesity, and Smoking. Nutrients 2017, 9, 997. [Google Scholar] [CrossRef]
- Yoshihara, A.; Nakashima, K.; Suwama, K.; Odajima, A.; Yamaga, T.; Ogawa, H. Interaction between serum vitamin C levels and smoking on the periodontal condition in older adults. J. Periodontal Res. 2022, 57, 587–593. [Google Scholar] [CrossRef]
- Chiara, H.; Allison, J.; Iona, E.; Janhavi R, R.; Michal, Z.; David, C.; Andrew, W.; Hermann, B.; Rebecca C, R.; Martin, W. Cigarette smoking and e-cigarette use induce shared DNA methylation changes linked to carcinogenesis. Cancer Res. Treat. 2024, 84, 1898–1914. [Google Scholar] [CrossRef]



| Variable | Total | Q1 [0, 4] | Q2 [5] | Q3 [6] | Q4 [7, 14] | p |
|---|---|---|---|---|---|---|
| Age (years) | 48.15 (0.27) | 46.89 (0.30) | 47.42 (0.36) | 48.62 (0.40) | 50.70 (0.45) | <0.001 |
| Sex, n (%) | <0.001 | |||||
| Female | 10,482 (51.09) | 3897 (47.46) | 2455 (52.57) | 1930 (50.45) | 2200 (56.38) | |
| Male | 10,189 (48.91) | 4227 (52.54) | 2324 (47.43) | 1851 (49.55) | 1787 (43.62) | |
| Race, n (%) | <0.001 | |||||
| Non-Hispanic White | 9406 (70.68) | 3410 (66.60) | 2116 (69.15) | 1824 (73.49) | 2056 (76.85) | |
| Non-Hispanic Black | 4154 (9.90) | 1967 (12.87) | 975 (10.15) | 660 (8.23) | 552 (6.02) | |
| Mexican | 2929 (7.60) | 1203 (8.67) | 738 (8.54) | 539 (7.17) | 449 (5.14) | |
| Others | 4182 (11.82) | 1544 (11.87) | 950 (12.17) | 758 (11.11) | 930 (11.99) | |
| Education, n (%) | <0.001 | |||||
| Less than high school | 4443 (13.66) | 2082 (17.19) | 1073 (14.43) | 730 (11.17) | 558 (8.99) | |
| High school | 4720 (22.73) | 2130 (27.43) | 1133 (23.64) | 768 (19.87) | 689 (16.24) | |
| Collage or higher | 11,508 (63.60) | 3912 (55.38) | 2573 (61.93) | 2283 (68.96) | 2740 (74.77) | |
| Marital status | <0.001 | |||||
| Married/Partner | 12,439 (64.41) | 4680 (61.70) | 2905 (65.17) | 2298 (64.88) | 2556 (67.84) | |
| Never married | 3666 (17.35) | 1611 (19.35) | 834 (17.69) | 648 (17.11) | 573 (13.76) | |
| Widowed/Divorced/Separated | 4566 (18.24) | 1833 (18.95) | 1040 (17.14) | 835 (18.01) | 858 (18.40) | |
| PIR, n (%) | <0.001 | |||||
| Low income | 4098 (12.92) | 1908 (16.03) | 1033 (14.43) | 654 (10.87) | 503 (7.78) | |
| Middle income | 8672 (35.18) | 3692 (39.34) | 1953 (34.64) | 1551 (33.93) | 1476 (29.70) | |
| High income | 7901 (51.90) | 2524 (44.63) | 1793 (50.93) | 1576 (55.20) | 2008 (62.52) | |
| HEI, n (%) | <0.001 | |||||
| Low | 10,041 (49.05) | 5504 (68.67) | 2395 (52.94) | 1340 (38.12) | 802 (20.88) | |
| Medium | 8599 (41.07) | 2477 (29.61) | 2078 (41.18) | 1933 (49.58) | 2111 (53.02) | |
| High | 2031 (9.88) | 143 (1.73) | 306 (5.88) | 508 (12.30) | 1074 (26.09) | |
| BMI (kg/m2) | <0.001 | |||||
| <25 | 5679 (28.52) | 2068 (25.16) | 1260 (27.00) | 1056 (29.53) | 1295 (35.03) | |
| 25~30 | 6796 (32.99) | 2577 (32.04) | 1558 (33.39) | 1288 (33.31) | 1373 (33.94) | |
| ≥30 | 8196 (38.49) | 3479 (42.80) | 1961 (39.61) | 1437 (37.16) | 1319 (31.03) | |
| MET | 3765.92 (74.52) | 4125.44 (111.75) | 3812.26 (123.75) | 3643.40 (118.59) | 3205.58 (105.68) | <0.001 |
| Triglycerides (mg/dL) | 152.86 (1.38) | 156.66 (1.86) | 154.61 (2.70) | 152.66 (2.61) | 144.58 (2.83) | 0.003 |
| Globulin (g/dL) | 2.82 (0.01) | 2.85 (0.01) | 2.83 (0.01) | 2.80 (0.01) | 2.76 (0.01) | <0.001 |
| Energy intake (kcal/day) | 2106.66 (8.78) | 2064.32 (11.56) | 2095.00 (14.52) | 2137.51 (21.77) | 2164.40 (14.07) | <0.001 |
| Smoking, n (%) | <0.001 | |||||
| Current | 4057 (18.50) | 1861 (21.65) | 970 (19.20) | 682 (17.86) | 544 (12.88) | |
| Former | 5199 (25.65) | 1971 (24.15) | 1149 (24.99) | 951 (24.82) | 1128 (29.74) | |
| Never | 11,415 (55.85) | 4292 (54.21) | 2660 (55.82) | 2148 (57.32) | 2315 (57.38) | |
| Drinking, n (%) | 0.001 | |||||
| Current | 14,640 (77.02) | 5678 (75.71) | 3338 (75.86) | 2690 (77.96) | 2934 (79.67) | |
| Former | 3269 (12.78) | 1354 (13.33) | 762 (13.29) | 587 (12.35) | 566 (11.70) | |
| Never | 2762 (10.20) | 1092 (10.97) | 679 (10.85) | 504 (9.69) | 487 (8.63) | |
| CVD, n (%) | 0.210 | |||||
| No | 18,369 (91.29) | 7133 (90.77) | 4257 (91.70) | 3385 (91.04) | 3594 (91.96) | |
| Yes | 2302 (8.71) | 991 (9.23) | 522 (8.30) | 396 (8.96) | 393 (8.04) | |
| Hypertension, n (%) | < 0.001 | |||||
| No | 11,719 (61.74) | 4496 (59.41) | 2695 (61.98) | 2196 (63.74) | 2332 (63.67) | |
| Yes | 8952 (38.26) | 3628 (40.59) | 2084 (38.02) | 1585 (36.26) | 1655 (36.33) | |
| Diabetes, n (%) | <0.001 | |||||
| No | 16,726 (85.62) | 6404 (83.52) | 3894 (85.89) | 3109 (87.34) | 3319 (87.39) | |
| Yes | 3945 (14.38) | 1720 (16.48) | 885 (14.11) | 672 (12.66) | 668 (12.61) | |
| Cancer, n (%) | < 0.001 | |||||
| No | 18,595 (89.38) | 7387 (90.63) | 4318 (89.46) | 3381 (88.94) | 3509 (87.55) | |
| Yes | 2076 (10.62) | 737 (9.37) | 461 (10.54) | 400 (11.06) | 478 (12.45) | |
| PA, n (%) | <0.001 | |||||
| No | 13,734 (71.07) | 5006 (65.89) | 3133 (70.16) | 2618 (74.16) | 2977 (78.20) | |
| Yes | 6937 (28.93) | 3118 (34.11) | 1646 (29.84) | 1163 (25.84) | 1010 (21.80) | |
| KDM, n (%) | <0.001 | |||||
| No | 13,342 (65.64) | 4869 (60.45) | 3044 (64.60) | 2522 (67.80) | 2907 (73.76) | |
| Yes | 7329 (34.36) | 3255 (39.55) | 1735 (35.40) | 1259 (32.20) | 1080 (26.24) | |
| HD, n (%) | <0.001 | |||||
| No | 10,336 (56.92) | 3822 (53.96) | 2373 (57.10) | 1948 (57.92) | 2193 (60.94) | |
| Yes | 10,335 (43.08) | 4302 (46.04) | 2406 (42.90) | 1833 (42.08) | 1794 (39.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, S.; Qin, J.; Gong, X.; Li, S.; Ding, H.; Zhao, X.; He, H.; Zhou, L.; Deng, X.; Chu, X. The Mediating Role of Body Mass Index in the Association Between Dietary Index for Gut Microbiota and Biological Age: A Study Based on NHANES 2007–2018. Nutrients 2024, 16, 4164. https://doi.org/10.3390/nu16234164
An S, Qin J, Gong X, Li S, Ding H, Zhao X, He H, Zhou L, Deng X, Chu X. The Mediating Role of Body Mass Index in the Association Between Dietary Index for Gut Microbiota and Biological Age: A Study Based on NHANES 2007–2018. Nutrients. 2024; 16(23):4164. https://doi.org/10.3390/nu16234164
Chicago/Turabian StyleAn, Shuli, Jian Qin, Xinjie Gong, Shuangshuang Li, Haiyan Ding, Xue Zhao, Hongqi He, Linwei Zhou, Xinrui Deng, and Xia Chu. 2024. "The Mediating Role of Body Mass Index in the Association Between Dietary Index for Gut Microbiota and Biological Age: A Study Based on NHANES 2007–2018" Nutrients 16, no. 23: 4164. https://doi.org/10.3390/nu16234164
APA StyleAn, S., Qin, J., Gong, X., Li, S., Ding, H., Zhao, X., He, H., Zhou, L., Deng, X., & Chu, X. (2024). The Mediating Role of Body Mass Index in the Association Between Dietary Index for Gut Microbiota and Biological Age: A Study Based on NHANES 2007–2018. Nutrients, 16(23), 4164. https://doi.org/10.3390/nu16234164






