Genome-Wide Approach of Gene–Nutrient Intake Interaction Study for Essential Hypertension in a Large Korean Cohort (KoGES)
Abstract
1. Introduction
2. Methods
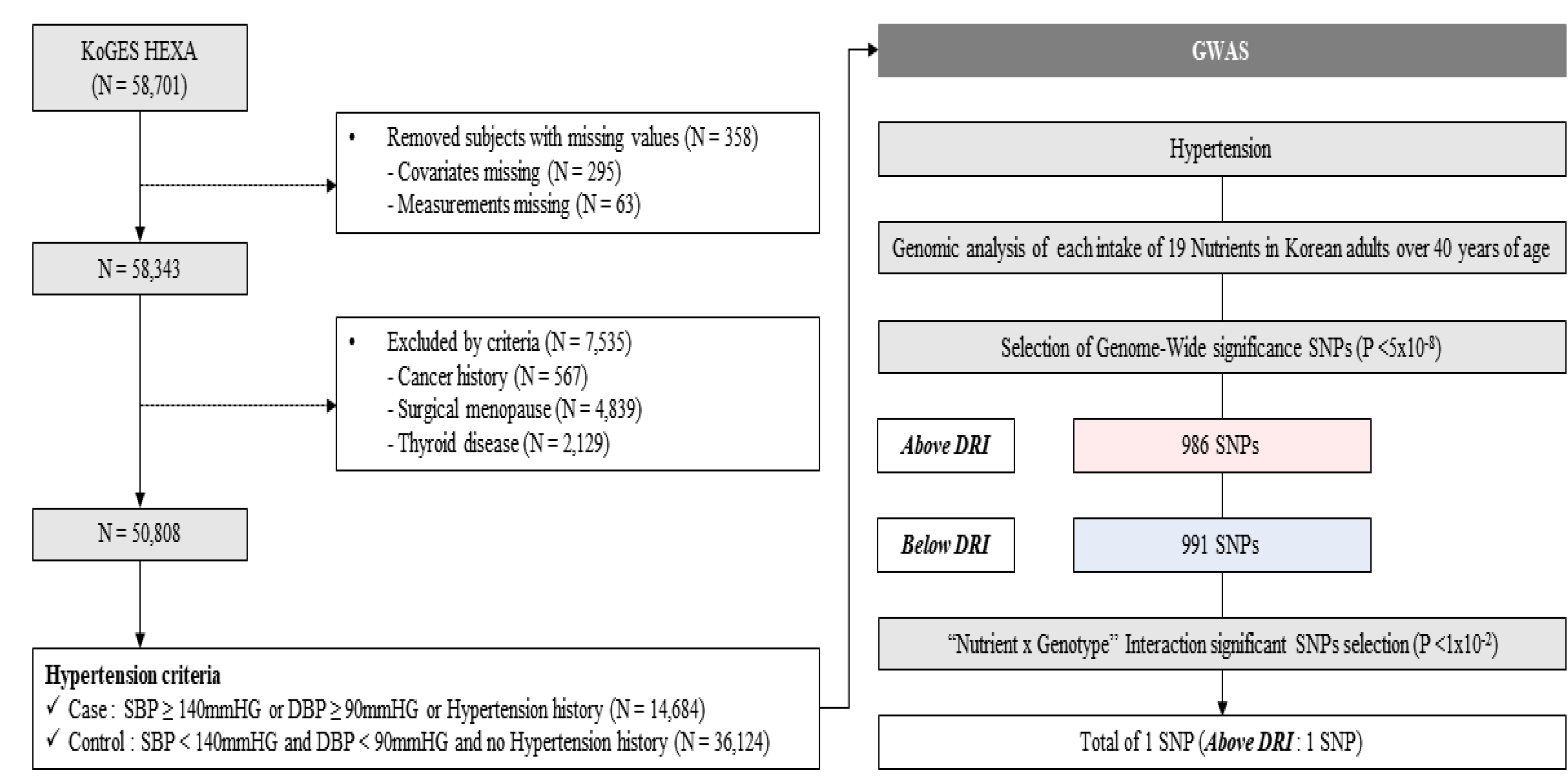
2.1. Study Population and Design
2.2. Genotyping and Covariates
2.3. Determination of Nutrition Intake Reference
2.4. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Nutritional Intake
3.3. HTN, Iron and Vit.B6 Intake, and Genotype
3.4. Nutrient-by-Gene Interaction Analysis Associated with HTN
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K.; et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Katsuya, T.; Morishita, R.; Rakugi, H.; Ogihrara, T. Genetic basis of hypertension for the development of tailored medicine. Hypertens. Res. 2009, 32, 643–648. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, e13–e115. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, Y.; Iwashima, Y. Higher blood pressure as a risk factor for diseases other than stroke and ischemic heart disease. Hypertension 2015, 66, 254–259. [Google Scholar] [CrossRef]
- Kim, H.C.; Lee, H.; Lee, H.H.; Son, D.; Cho, M.; Shin, S.; Seo, Y.; Kim, E.J.; Korean Society of Hypertension-Hypertension Epidemiology Research Working Group. Korea Hypertension Fact Sheet 2023: Analysis of nationwide population-based data with a particular focus on hypertension in special populations. Clin. Hypertens. 2024, 30, 7. [Google Scholar] [CrossRef]
- Hottenga, J.J.; Boomsma, D.I.; Kupper, N.; Posthuma, D.; Snieder, H.; Willemsen, G.; de Geus, E.J. Heritability and stability of resting blood pressure. Twin Res. Hum. Genet. 2005, 8, 499–508. [Google Scholar] [CrossRef]
- Ehret, G.B.; Ferreira, T.; Chasman, D.I.; Jackson, A.U.; Schmidt, E.M.; Johnson, T.; Thorleifsson, G.; Luan, J.; Donnelly, L.A.; Kanoni, S.; et al. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat. Genet. 2016, 48, 1171–1184. [Google Scholar] [CrossRef]
- Evangelou, E.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Ntritsos, G.; Dimou, N.; Cabrera, C.P.; Karaman, I.; et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 2018, 50, 1412–1425. [Google Scholar] [CrossRef]
- Kokubo, Y.; Padmanabhan, S.; Iwashima, Y.; Yamagishi, K.; Goto, A. Gene and environmental interactions according to the components of lifestyle modifications in hypertension guidelines. Environ. Health Prev. Med. 2019, 24, 19. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Norris, S.L.; Zhang, X.; Avenell, A.; Gregg, E.; Schmid, C.H.; Lau, J. Long-term non-pharmacological weight loss interventions for adults with prediabetes. Cochrane Database Syst. Rev. 2005, 2010, Cd005270. [Google Scholar] [CrossRef] [PubMed]
- Harsha, D.W.; Bray, G.A. Weight loss and blood pressure control (Pro). Hypertension 2008, 51, 1420–1425; discussion 1425. [Google Scholar] [CrossRef] [PubMed]
- Appel, L.J.; Sacks, F.M.; Carey, V.J.; Obarzanek, E.; Swain, J.F.; Miller, E.R., 3rd; Conlin, P.R.; Erlinger, T.P.; Rosner, B.A.; Laranjo, N.M.; et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the OmniHeart randomized trial. JAMA 2005, 294, 2455–2464. [Google Scholar] [CrossRef]
- Wang, Y.F.; Yancy, W.S., Jr.; Yu, D.; Champagne, C.; Appel, L.J.; Lin, P.H. The relationship between dietary protein intake and blood pressure: Results from the PREMIER study. J. Hum. Hypertens. 2008, 22, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, B.; Hou, L.; Han, W.; Xue, F.; Wang, Y.; Tang, Y.; Liang, S.; Wang, W.; Asaiti, K.; et al. Interaction of ACE genotype and salt intake on hypertension among Chinese Kazakhs: Results from a population-based cross-sectional study. BMJ Open 2017, 7, e014246. [Google Scholar] [CrossRef]
- Kim, Y.; Han, B.G.; KoGES Group. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- Moon, S.; Kim, Y.J.; Han, S.; Hwang, M.Y.; Shin, D.M.; Park, M.Y.; Lu, Y.; Yoon, K.; Jang, H.M.; Kim, Y.K.; et al. The Korea Biobank Array: Design and Identification of Coding Variants Associated with Blood Biochemical Traits. Sci. Rep. 2019, 9, 1382. [Google Scholar] [CrossRef]
- Lim, N.-K.; Son, K.-H.; Lee, K.-S.; Park, H.-Y.; Cho, M.-C. Predicting the Risk of Incident Hypertension in a Korean Middle-Aged Population: Korean Genome and Epidemiology Study. J. Clin. Hypertens. 2013, 15, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Ahn, Y.; Kwon, E.; Shim, J.E.; Park, M.K.; Joo, Y.; Kimm, K.; Park, C.; Kim, D.H. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Kwon, O.; Kim, H.; Kim, J.; Hwang, J.-Y.; Lee, J.; Yoon, M.O. The development of the 2020 Dietary Reference Intakes for Korean population: Lessons and challenges. J. Nutr. Health 2021, 54, 425–434. [Google Scholar] [CrossRef]
- Yetley, E.A.; MacFarlane, A.J.; Greene-Finestone, L.S.; Garza, C.; Ard, J.D.; Atkinson, S.A.; Bier, D.M.; Carriquiry, A.L.; Harlan, W.R.; Hattis, D.; et al. Options for basing Dietary Reference Intakes (DRIs) on chronic disease endpoints: Report from a joint US-/Canadian-sponsored working group. Am. J. Clin. Nutr. 2017, 105, 249S–285S. [Google Scholar] [CrossRef] [PubMed]
- Nutrition Board. Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Subcommittee on Interpretation and Uses of Dietary Reference Intakes. In DRI Dietary Reference Intakes: Applications in Dietary Assessment; National Academies Press: Washington, DC, USA, 2000. [Google Scholar] [CrossRef]
- Stanton, A.M.; Heydarpour, M.; Williams, J.S.; Williams, G.H.; Adler, G.K. CACNA1D Gene Polymorphisms Associate with Increased Blood Pressure and Salt Sensitivity of Blood Pressure in White Individuals. Hypertension 2023, 80, 2665–2673. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Qi, H.; Liu, B.; Liu, K.; Wu, J.; Cao, H.; Zhang, J.; Yan, Y.; He, Y.; Zhang, L. Genetic susceptibility to salt-sensitive hypertension in a Han Chinese population: A validation study of candidate genes. Hypertens. Res. 2017, 40, 876–884. [Google Scholar] [CrossRef]
- Jia, H.; Bao, P.; Yao, S.; Zhang, X.; Mu, J.J.; Hu, G.L.; Du, M.F.; Chu, C.; Zhang, X.Y.; Wang, L.; et al. Associations of SGLT2 genetic polymorphisms with salt sensitivity, blood pressure changes and hypertension incidence in Chinese adults. Hypertens. Res. 2023, 46, 1795–1803. [Google Scholar] [CrossRef]
- Imaizumi, T.; Ando, M.; Nakatochi, M.; Maruyama, S.; Yasuda, Y.; Honda, H.; Kuwatsuka, Y.; Kato, S.; Kondo, T.; Iwata, M.; et al. Association of interactions between dietary salt consumption and hypertension-susceptibility genetic polymorphisms with blood pressure among Japanese male workers. Clin. Exp. Nephrol. 2017, 21, 457–464. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kim, J.O.; Park, J.M.; Choi, J.E.; Park, D.H.; Song, Y.; Kim, S.J.; Lee, J.W.; Hong, K.W. Identification of Genetic Factors Underlying the Association between Sodium Intake Habits and Hypertension Risk. Nutrients 2020, 12, 2580. [Google Scholar] [CrossRef]
- Li, C.; He, J.; Chen, J.; Zhao, J.; Gu, D.; Hixson, J.E.; Rao, D.C.; Jaquish, C.E.; Gu, C.C.; Chen, J.; et al. Genome-Wide Gene-Sodium Interaction Analyses on Blood Pressure: The Genetic Epidemiology Network of Salt-Sensitivity Study. Hypertension 2016, 68, 348–355. [Google Scholar] [CrossRef]
- Guirette, M.; Lan, J.; McKeown, N.M.; Brown, M.R.; Chen, H.; de Vries, P.S.; Kim, H.; Rebholz, C.M.; Morrison, A.C.; Bartz, T.M.; et al. Genome-Wide Interaction Analysis with DASH Diet Score Identified Novel Loci for Systolic Blood Pressure. Hypertension 2024, 81, 552–560. [Google Scholar] [CrossRef]
- Zafarmand, M.H.; Spanjer, M.; Nicolaou, M.; Wijnhoven, H.A.H.; van Schaik, B.D.C.; Uitterlinden, A.G.; Snieder, H.; Vrijkotte, T.G.M. Influence of Dietary Approaches to Stop Hypertension-Type Diet, Known Genetic Variants and Their Interplay on Blood Pressure in Early Childhood: ABCD Study. Hypertension 2020, 75, 59–70. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, Q.; Liang, J.; Hu, F.B.; Sacks, F.M.; Qi, L. Neuropeptide Y promoter polymorphism modifies effects of a weight-loss diet on 2-year changes of blood pressure: The preventing overweight using novel dietary strategies trial. Hypertension 2012, 60, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Goni, L.; Cuervo, M.; Milagro, F.I.; Martinez, J.A. Influence of fat intake and BMI on the association of rs1799983 NOS3 polymorphism with blood pressure levels in an Iberian population. Eur. J. Nutr. 2017, 56, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Tagetti, A.; Ericson, U.; Montagnana, M.; Danese, E.; Almgren, P.; Nilsson, P.; Engström, G.; Hedblad, B.; Minuz, P.; Orho-Melander, M.; et al. Intakes of omega-3 polyunsaturated fatty acids and blood pressure change over time: Possible interaction with genes involved in 20-HETE and EETs metabolism. Prostaglandins Other Lipid Mediat. 2015, 120, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhou, T.; Li, X.; Heianza, Y.; Liang, Z.; Bray, G.A.; Sacks, F.M.; Qi, L. Genetic Susceptibility, Dietary Protein Intake, and Changes of Blood Pressure: The POUNDS Lost Trial. Hypertension 2019, 74, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Htun, N.C.; Miyaki, K.; Song, Y.; Ikeda, S.; Shimbo, T.; Muramatsu, M. Association of the catechol-O-methyl transferase gene Val158Met polymorphism with blood pressure and prevalence of hypertension: Interaction with dietary energy intake. Am. J. Hypertens. 2011, 24, 1022–1026. [Google Scholar] [CrossRef]
- Lau, W.L.; Scholnick, S.B. Identification of two new members of the CSMD gene family. Genomics 2003, 82, 412–415. [Google Scholar] [CrossRef]
- Kristiansen, M.; Kozyraki, R.; Jacobsen, C.; Nexø, E.; Verroust, P.J.; Moestrup, S.K. Molecular dissection of the intrinsic factor-vitamin B12 receptor, cubilin, discloses regions important for membrane association and ligand binding. J. Biol. Chem. 1999, 274, 20540–20544. [Google Scholar] [CrossRef]
- Hong, K.W.; Go, M.J.; Jin, H.S.; Lim, J.E.; Lee, J.Y.; Han, B.G.; Hwang, S.Y.; Lee, S.H.; Park, H.K.; Cho, Y.S.; et al. Genetic variations in ATP2B1, CSK, ARSG and CSMD1 loci are related to blood pressure and/or hypertension in two Korean cohorts. J. Hum. Hypertens. 2010, 24, 367–372. [Google Scholar] [CrossRef]
- Masilela, C.; Pearce, B.; Ongole, J.J.; Adeniyi, O.V.; Benjeddou, M. Genomic Association of Single Nucleotide Polymorphisms with Blood Pressure Response to Hydrochlorothiazide among South African Adults with Hypertension. J. Pers. Med. 2020, 10, 267. [Google Scholar] [CrossRef]
- Chittani, M.; Zaninello, R.; Lanzani, C.; Frau, F.; Ortu, M.F.; Salvi, E.; Fresu, G.; Citterio, L.; Braga, D.; Piras, D.A.; et al. TET2 and CSMD1 genes affect SBP response to hydrochlorothiazide in never-treated essential hypertensives. J. Hypertens. 2015, 33, 1301–1309. [Google Scholar] [CrossRef]
- Cunningham, P.N.; Wang, Z.; Grove, M.L.; Cooper-DeHoff, R.M.; Beitelshees, A.L.; Gong, Y.; Gums, J.G.; Johnson, J.A.; Turner, S.T.; Boerwinkle, E.; et al. Hypertensive APOL1 risk allele carriers demonstrate greater blood pressure reduction with angiotensin receptor blockade compared to low risk carriers. PLoS ONE 2019, 14, e0221957. [Google Scholar] [CrossRef] [PubMed]
- Nock, N.L.; Wang, X.; Thompson, C.L.; Song, Y.; Baechle, D.; Raska, P.; Stein, C.M.; Gray-McGuire, C. Defining genetic determinants of the Metabolic Syndrome in the Framingham Heart Study using association and structural equation modeling methods. BMC Proc. 2009, 3 (Suppl. 7), S50. [Google Scholar] [CrossRef] [PubMed]
- Koriyama, H.; Nakagami, H.; Katsuya, T.; Sugimoto, K.; Yamashita, H.; Takami, Y.; Maeda, S.; Kubo, M.; Takahashi, A.; Nakamura, Y.; et al. Identification of evidence suggestive of an association with peripheral arterial disease at the OSBPL10 locus by genome-wide investigation in the Japanese population. J. Atheroscler. Thromb. 2010, 17, 1054–1062. [Google Scholar] [CrossRef]
- Oliveira, F.; Rocha, S.; Fernandes, R. Iron metabolism: From health to disease. J. Clin. Lab. Anal. 2014, 28, 210–218. [Google Scholar] [CrossRef]
- Stach, K.; Stach, W.; Augoff, K. Vitamin B6 in Health and Disease. Nutrients 2021, 13, 3229. [Google Scholar] [CrossRef] [PubMed]
- Tzoulaki, I.; Brown, I.J.; Chan, Q.; Van Horn, L.; Ueshima, H.; Zhao, L.; Stamler, J.; Elliott, P.; International Collaborative Research Group on Macro-/Micronutrients and Blood Pressure. Relation of iron and red meat intake to blood pressure: Cross sectional epidemiological study. BMJ 2008, 337, a258. [Google Scholar] [CrossRef]
- Galan, P.; Vergnaud, A.C.; Tzoulaki, I.; Buyck, J.F.; Blacher, J.; Czernichow, S.; Hercberg, S. Low total and nonheme iron intakes are associated with a greater risk of hypertension. J. Nutr. 2010, 140, 75–80. [Google Scholar] [CrossRef]
- Wu, S.; Chen, P.; He, J.; Liu, Z.; Sui, Y.; Li, K.; Fang, A. Dietary intakes of total, nonheme, and heme iron and hypertension risk: A longitudinal study from the China Health and Nutrition Survey. Eur. J. Nutr. 2023, 62, 3251–3262. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, C.; Liu, M.; Zhou, C.; Li, Q.; He, P.; Zhang, Y.; Li, H.; Qin, X. Dietary Iron Intake and New-Onset Hypertension: A Nationwide Cohort Study from China. J. Nutr. Health Aging 2022, 26, 1016–1024. [Google Scholar] [CrossRef]
- Dos Santos Vieira, D.A.; Hermes Sales, C.; Galvão Cesar, C.L.; Marchioni, D.M.; Fisberg, R.M. Influence of Haem, Non-Haem, and Total Iron Intake on Metabolic Syndrome and Its Components: A Population-Based Study. Nutrients 2018, 10, 314. [Google Scholar] [CrossRef]
- Von Haehling, S.; Ebner, N.; Evertz, R.; Ponikowski, P.; Anker, S.D. Iron Deficiency in Heart Failure: An Overview. JACC Heart Fail. 2019, 7, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Lu, Y.; Chen, Y.; Cheng, J. The role of Nrf2 in oxidative stress-induced endothelial injuries. J. Endocrinol. 2015, 225, R83–R99. [Google Scholar] [CrossRef] [PubMed]
- Lakhal-Littleton, S.; Crosby, A.; Frise, M.C.; Mohammad, G.; Carr, C.A.; Loick, P.A.M.; Robbins, P.A. Intracellular iron deficiency in pulmonary arterial smooth muscle cells induces pulmonary arterial hypertension in mice. Proc. Natl. Acad. Sci. USA 2019, 116, 13122–13130. [Google Scholar] [CrossRef]
- Cleland, J.G.F. Defining iron deficiency in patients with heart failure. Nat. Rev. Cardiol. 2024, 21, 1–2. [Google Scholar] [CrossRef]
- Savarese, G.; von Haehling, S.; Butler, J.; Cleland, J.G.F.; Ponikowski, P.; Anker, S.D. Iron deficiency and cardiovascular disease. Eur. Heart J. 2022, 44, 14–27. [Google Scholar] [CrossRef]
- Zhang, P.; Tsuchiya, K.; Kinoshita, T.; Kushiyama, H.; Suidasari, S.; Hatakeyama, M.; Imura, H.; Kato, N.; Suda, T. Vitamin B6 Prevents IL-1β Protein Production by Inhibiting NLRP3 Inflammasome Activation. J. Biol. Chem. 2016, 291, 24517–24527. [Google Scholar] [CrossRef]
- Friso, S.; Jacques, P.F.; Wilson, P.W.; Rosenberg, I.H.; Selhub, J. Low circulating vitamin B(6) is associated with elevation of the inflammation marker C-reactive protein independently of plasma homocysteine levels. Circulation 2001, 103, 2788–2791. [Google Scholar] [CrossRef]
- Houston, M.C. Treatment of Hypertension with Nutrition and Nutraceutical Supplements: Part 2. Altern. Complement. Ther. 2019, 25, 23–36. [Google Scholar] [CrossRef]
- Otsuka, R.; Imai, T.; Kato, Y.; Ando, F.; Shimokata, H. Relationship between number of metabolic syndrome components and dietary factors in middle-aged and elderly Japanese subjects. Hypertens. Res. 2010, 33, 548–554. [Google Scholar] [CrossRef]
- Rimm, E.B.; Willett, W.C.; Hu, F.B.; Sampson, L.; Colditz, G.A.; Manson, J.E.; Hennekens, C.; Stampfer, M.J. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA 1998, 279, 359–364. [Google Scholar] [CrossRef]
- Liu, R.; Mi, B.; Zhao, Y.; Li, Q.; Yan, H.; Dang, S. Effect of B Vitamins from Diet on Hypertension. Arch. Med. Res. 2017, 48, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, R.A.J.M.; Rauwerda, J.A.; Steyn, M.; Twisk, J.W.R.; Stehouwer, C.D.A. Long-Term Homocysteine-Lowering Treatment with Folic Acid Plus Pyridoxine Is Associated with Decreased Blood Pressure but Not with Improved Brachial Artery Endothelium-Dependent Vasodilation or Carotid Artery Stiffness. Arter. Thromb. Vasc. Biol. 2001, 21, 2072–2079. [Google Scholar] [CrossRef] [PubMed]
- Aybak, M.; Sermet, A.; Ayyildiz, M.O.; Karakilçik, A.Z. Effect of oral pyridoxine hydrochloride supplementation on arterial blood pressure in patients with essential hypertension. Arzneimittelforschung 1995, 45, 1271–1273. [Google Scholar] [PubMed]
- Stanger, O.; Herrmann, W.; Pietrzik, K.; Fowler, B.; Geisel, J.; Dierkes, J.; Weger, M. DACH-LIGA Homocystein (German, Austrian and Swiss homocysteine society): Consensus paper on the rational clinical use of homocysteine, folic acid and B-vitamins in cardiovascular and thrombotic diseases: Guidelines and recommendations. Clin. Chem. Lab. Med. 2003, 41, 1392–1403. [Google Scholar] [CrossRef]
- Chiu, H.F.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Impact of Micronutrients on Hypertension: Evidence from Clinical Trials with a Special Focus on Meta-Analysis. Nutrients 2021, 13, 588. [Google Scholar] [CrossRef]

| Total | HTN | non-HTN | |
|---|---|---|---|
| N | 50,808 | 14,684 | 36,124 |
| Age, years | 53.59 ± 8.12 | 57.27 ± 7.57 | 52.09 ± 7.86 |
| Sex (Female), n (%) | 31,213(61.43) | 7494(51.04) | 23,719(65.66) |
| Lifestyle | |||
| Alcohol intake, g/day | 57.21 ± 176.44 | 74.98 ± 202.81 | 49.99 ± 163.97 |
| Smoking status: Never/Quit/Current (n, %) | 35,796(70.45)/8806(17.33)/6170(12.14) | 9368(63.84)/3507(23.90/1799(12.26) | 26,428(73.21)/5299(14.68)/4371(12.11) |
| Exercise status: No/Yes (n, %) | 23,136(45.54)/27,672(54.38) | 6349(43.24)/8335(56.76) | 16,787(17.46)/19,337(82.54) |
| Disease Prevalence | |||
| Type 2 Diabetes: Yes/No (n, %) | 45,887(90.31)/4921(9.69) | 12,114(82.5)/2570(17.5) | 33,773(93.49)/2351(6.51) |
| Dyslipidemia: Yes/No (n, %) | 27,268(53.69)/23,518(46.31) | 6570(44.78)/8103(55.22) | 20,698(57.31)/15,415(42.69) |
| Cardiovascular disease: Yes/No (n, %) | 49,322(97.14)/1452(2.86) | 13,945(95.06)/724(4.94) | 35,377(97.98)/728(2.02) |
| Cerebrovascular disease: Yes/No (n, %) | 50,160(98.78)/622(1.22) | 14,292(97.4)/382(2.60) | 35,868(99.34)/240(0.66) |
| Anthropometric traits | |||
| BMI, kg/m2 | 23.90 ± 2.87 | 25.03 ± 2.93 | 23.44 ± 2.71 |
| Waist Circumference, cm | 80.91 ± 9.03 | 84.52 ± 8.87 | 79.45 ± 8.68 |
| SBP, mmHg | 122.47 ± 15.02 | 135.2 ± 14.71 | 117.3 ± 11.70 |
| DBP, mmHg | 75.82 ± 9.92 | 83.33 ± 10.0 | 72.76 ± 8.10 |
| Pulse, count/min | 69.21 ± 9.17 | 70.04 ± 9.85 | 68.88 ± 8.86 |
| Biochemical traits | |||
| Serum glucose, mg/dL | 95.27 ± 19.87 | 100.56 ± 23.6 | 93.12 ± 17.69 |
| HbA1c, % | 5.71 ± 0.72 | 5.91 ± 0.88 | 5.63 ± 0.63 |
| Total cholesterol, mg/dL | 197.0 ± 35.62 | 195.99 ± 36.78 | 197.41 ± 35.12 |
| Triglycerides, mg/dL | 125.96 ± 86.65 | 145.07 ± 97.3 | 118.19 ± 80.64 |
| HDL-C, mg/dL | 53.53 ± 13.14 | 51.37 ± 12.65 | 54.41 ± 13.24 |
| Hemoglobin, g/dL | 13.94 ± 1.49 | 14.26 ± 1.44 | 13.81 ± 1.50 |
| MCV, fL | 91.39 ± 4.74 | 91.61 ± 4.32 | 91.3 ± 4.90 |
| MCH, pg | 30.47 ± 1.98 | 30.68 ± 1.75 | 30.39 ± 2.07 |
| Blood Urea Nitrogen, mg/dL | 14.48 ± 3.96 | 15.21 ± 4.37 | 14.19 ± 3.74 |
| Creatinine, mg/dL | 0.82 ± 0.22 | 0.86 ± 0.27 | 0.8 ± 0.19 |
| Nutrient | |||
| Total energy, kcal/day | 1692.05(1415.28–2017.06) | 1669.38(1408.44–1988.18) | 1700.20(1418.53–2029.29) |
| CHO (%) | 72.44(67.64–76.52) | 73.18(68.41–77.05) | 72.13(67.37–76.27) |
| Protein (%) | 13.044(11.63–14.76) | 12.91(11.52–14.67) | 13.10(11.68–14.80) |
| Fat (%) | 13.32(10.12–17.10) | 12.61(9.59–16.28) | 13.63(10.35–17.39) |
| Ca, mg/day | 394.03(272.55–551.20) | 381.35(264.15–536.90) | 399.00(276.30–555.94) |
| P, mg/day | 835.77(664.46–1046.30) | 821.23(652.52–1025.18) | 841.40(668.93–1052.81) |
| Iron, mg/day | 9.06(6.96–11.83) | 8.86(6.80–11.62) | 9.14(7.03–11.92) |
| K, mg/day | 2058.35(1553.73–2693.69) | 2010.56(1513.78–2649.49) | 2079.79(1569.70–2710.63) |
| Vitamin A (R.E) | 397.26(271.16–580.82) | 389.89(262.86–574.95) | 400.40(274.65–582.99) |
| Na, mg/day | 2246.36(1498.13–3082.89) | 2253.01(1487.17–3086.66) | 2243.31(1502.91–3080.85) |
| Vitamin B1, mg/day | 0.93(0.73–1.19) | 0.90(0.71–1.16) | 0.93(0.74–1.20) |
| Vitamin B2, mg/day | 0.83(0.61–1.09) | 0.80(0.59–1.06) | 0.84(0.62–1.10) |
| Niacin, mg/day | 13.48(10.65–17.11) | 13.19(10.39–16.71) | 13.60(10.75–17.27) |
| Vitamin C, mg/day | 91.52(61.04–133.48) | 88.15(58.64–128.55) | 92.89(61.97–135.30) |
| Zinc, mg/day | 7.35(5.89–9.26) | 7.26(5.80–9.14) | 7.38(5.92–9.31) |
| Vitamin B6, mg/day | 1.46(1.15–1.86) | 1.44(1.13–1.84) | 1.47(1.16–1.87) |
| Folate, mcg/day | 192.63(140.68–259.45) | 189.43(137.66–255.77) | 193.85(141.96–261.13) |
| Fiber, g/day | 5.22(3.86–6.87) | 5.19(3.81–6.78) | 5.24(3.88–6.90) |
| Vitamin E, mg/day | 7.23(5.30–9.81) | 7.01(5.13–9.49) | 7.33(5.38–9.95) |
| Cholesterol, mg/day | 141.72(88.83–219.39) | 132.39(81.42–207.22) | 145.89(92.20–224.50) |
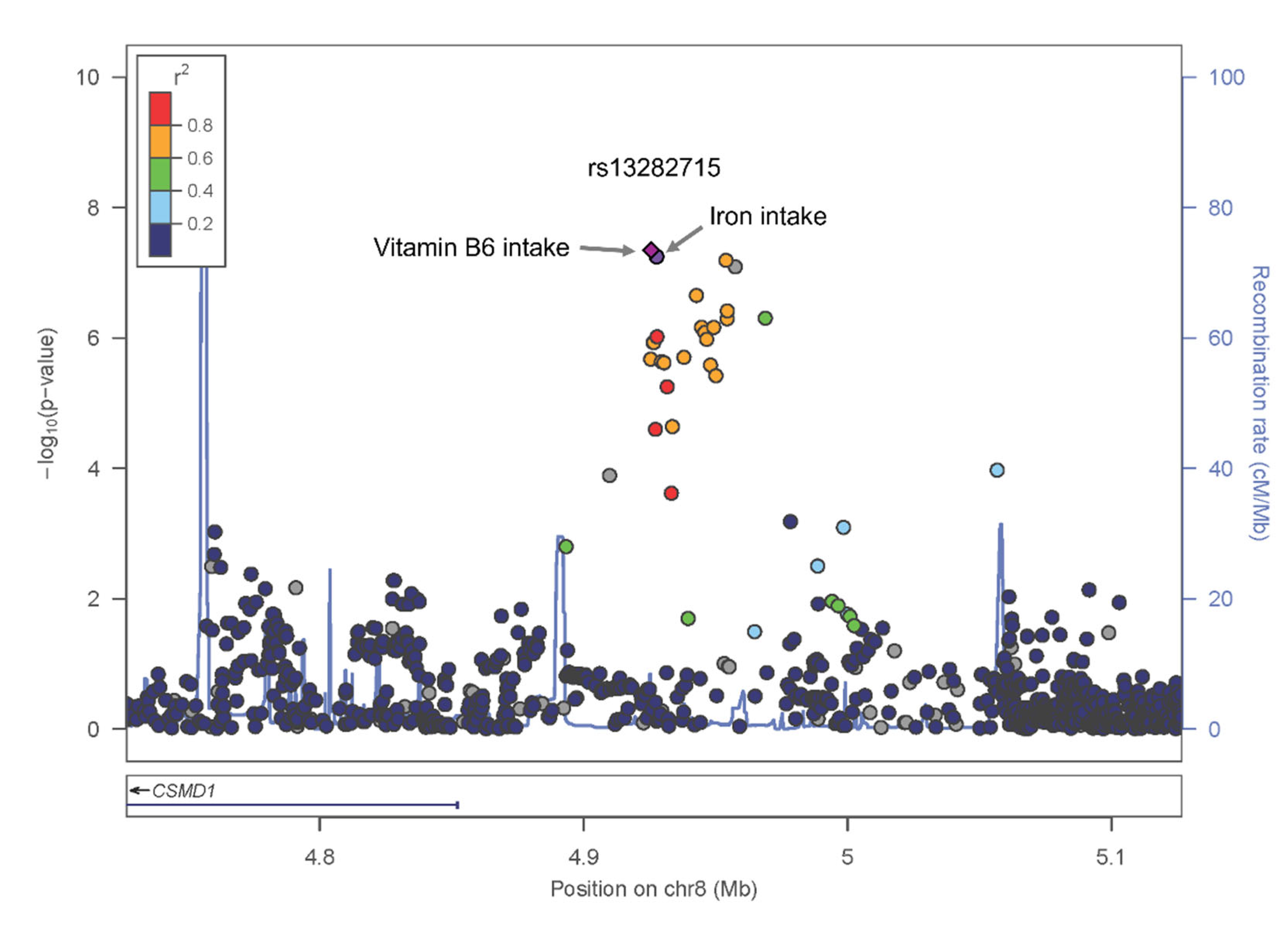
| SNP | Chr: bp | Alleles | Positional Candidate Gene | Minor Allele Frequency | Coding Allele | Coding Allele Frequency | Gene and Nutrient Association Results | Gene Association Result to Hypertension | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REF | ALT | Korean | EAS | EUR | AMR | Above Iron | Above Vit.B6 | OR (95% CI) | p-Value | |||||||
| OR(95% CI) (p-Value) | Interaction p-Value | OR(95% CI) (p-Value) | Interaction p-Value | |||||||||||||
| rs13282715 | 8:4926756 | T | A | CSMD1 | 0.065 | 0.0615 | 0.2018 | 0.117 | A | 0.040 | Model 1 | |||||
| 0.72 (0.64–0.81) (4.86 × 10−8) | 1.34 × 10−3 | 0.73 (0.66–0.82) (4.12 × 10−8) | 7.18 × 10−4 | 0.88 (0.82–0.95) | 9.21 × 10−4 | |||||||||||
| Model 2 | ||||||||||||||||
| 0.74 (0.64–0.87) (1.49 × 10−4) | 1.26 × 10−1 | 0.74 (0.64–0.86) (6.22 × 10−5) | 1.38 × 10−1 | 0.89 (0.81–0.98) | 1.83 × 10−2 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Choi, J.-E.; Park, J.-M.; Kwon, Y.-J.; Hong, K.-W.; Lee, J.-W. Genome-Wide Approach of Gene–Nutrient Intake Interaction Study for Essential Hypertension in a Large Korean Cohort (KoGES). Nutrients 2024, 16, 4147. https://doi.org/10.3390/nu16234147
Song Y, Choi J-E, Park J-M, Kwon Y-J, Hong K-W, Lee J-W. Genome-Wide Approach of Gene–Nutrient Intake Interaction Study for Essential Hypertension in a Large Korean Cohort (KoGES). Nutrients. 2024; 16(23):4147. https://doi.org/10.3390/nu16234147
Chicago/Turabian StyleSong, Youhyun, Ja-Eun Choi, Jae-Min Park, Yu-Jin Kwon, Kyung-Won Hong, and Ji-Won Lee. 2024. "Genome-Wide Approach of Gene–Nutrient Intake Interaction Study for Essential Hypertension in a Large Korean Cohort (KoGES)" Nutrients 16, no. 23: 4147. https://doi.org/10.3390/nu16234147
APA StyleSong, Y., Choi, J.-E., Park, J.-M., Kwon, Y.-J., Hong, K.-W., & Lee, J.-W. (2024). Genome-Wide Approach of Gene–Nutrient Intake Interaction Study for Essential Hypertension in a Large Korean Cohort (KoGES). Nutrients, 16(23), 4147. https://doi.org/10.3390/nu16234147






