Commonly Used Dose of Montmorency Tart Cherry Powder Does Not Improve Sleep or Inflammation Outcomes in Individuals with Overweight or Obesity
Abstract
1. Introduction
2. Materials and Methods
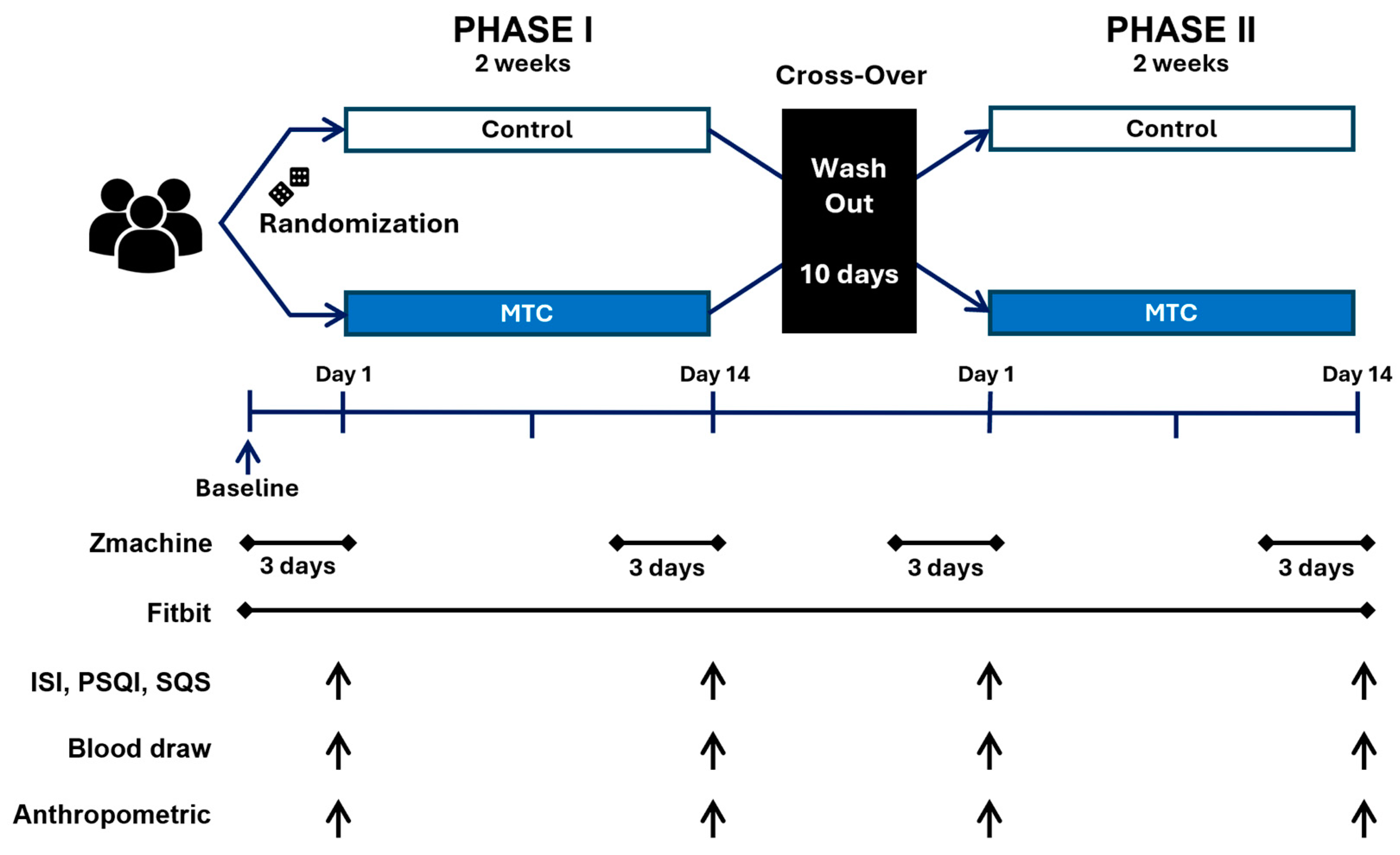
2.1. Study Design
2.2. Anthropometrics
2.3. Sleep Quality
2.4. Insomnia Symptoms
2.5. Objective Sleep Measures
2.6. Markers of Inflammation
2.7. Intervention
2.8. Statistical Analysis
3. Results
3.1. Sleep Outcomes
3.2. Inflammation Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cappuccio, F.P.; Cooper, D.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep Duration Predicts Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Studies. Eur. Heart J. 2011, 32, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Hawton, K.; i Comabella, C.C.; Haw, C.; Saunders, K. Risk Factors for Suicide in Individuals with Depression: A Systematic Review. J. Affect. Disord. 2013, 147, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Lemke, M.K.; Apostolopoulos, Y.; Hege, A.; Sönmez, S.; Wideman, L. Understanding the Role of Sleep Quality and Sleep Duration in Commercial Driving Safety. Accid. Anal. Prev. 2016, 97, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Opp, M.R.; Krueger, J.M. Sleep and Immunity: A Growing Field with Clinical Impact. Brain. Behav. Immun. 2015, 47, 1. [Google Scholar] [CrossRef] [PubMed]
- Tworoger, S.S.; Lee, S.; Schernhammer, E.S.; Grodstein, F. The Association of Self-Reported Sleep Duration, Difficulty Sleeping, and Snoring with Cognitive Function in Older Women. Alzheimer Dis. Assoc. Disord. 2006, 20, 41–48. [Google Scholar] [CrossRef]
- Yaggi, H.K.; Araujo, A.B.; McKinlay, J.B. Sleep Duration as a Risk Factor for the Development of Type 2 Diabetes. Diabetes Care 2006, 29, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Zhang, H.; Zhang, D. Sleep Duration and Depression among Adults: A Meta-Analysis of Prospective Studies. Depress. Anxiety 2015, 32, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yin, J.-Y.; Yang, W.-S.; Qin, Q.; Li, T.-T.; Shi, Y.; Deng, Q.; Wei, S.; Liu, L.; Wang, X. Sleep Duration and Cancer Risk: A Systematic Review and Meta-Analysis of Prospective Studies. Asian Pac. J. Cancer Prev. 2013, 14, 7509–7515. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.; Garzarella, J.; Jandeleit-Dahm, K.A.; Jha, J.C. Oxidative Stress and Inflammation in Renal and Cardiovascular Complications of Diabetes. Biology 2020, 10, 18. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in Obesity, Diabetes, and Related Disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- de Oliveira, J.; Kucharska, E.; Garcez, M.L.; Rodrigues, M.S.; Quevedo, J.; Moreno-Gonzalez, I.; Budni, J. Inflammatory Cascade in Alzheimer’s Disease Pathogenesis: A Review of Experimental Findings. Cells 2021, 10, 2581. [Google Scholar] [CrossRef] [PubMed]
- Meier-Ewert, H.K.; Ridker, P.M.; Rifai, N.; Regan, M.M.; Price, N.J.; Dinges, D.F.; Mullington, J.M. Effect of Sleep Loss on C-Reactive Protein, an Inflammatory Marker of Cardiovascular Risk. J. Am. Coll. Cardiol. 2004, 43, 678–683. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, X.; Yang, G.; Liu, X.; Xu, M. Effects of Chronic Intractable Insomnia on Inflammatory Cytokines, Blood Pressure Characteristics, and Antihypertensive Efficacy in Hypertensive Patients. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 9259–9264. [Google Scholar] [CrossRef] [PubMed]
- Tilinca, M.C.; Barabas-Hajdu, E.C.; Ferencz, G.T.; Nemes-Nagy, E. Involvement of Inflammatory Cytokines in Obesity and Its Complications. Rev. Romana Med. Lab. 2018, 26, 359–371. [Google Scholar] [CrossRef]
- Forsythe, L.K.; Wallace, J.M.W.; Livingstone, M.B.E. Obesity and Inflammation: The Effects of Weight Loss. Nutr. Res. Rev. 2008, 21, 117–133. [Google Scholar] [CrossRef]
- Mulyamin, W.; Kurniawan, L.B.; Adnan, E.; Widaningsih, Y.; Idris, I.; Santoso, A.; Yustisia, I. Body Mass Index as the Most Influential Factor of High-Sensitivity C-Reactive Protein in Non-Diabetic Adults. Universa Med. 2021, 40, 22–28. [Google Scholar] [CrossRef]
- Palm, A.; Janson, C.; Lindberg, E. The Impact of Obesity and Weight Gain on Development of Sleep Problems in a Population-Based Sample. Sleep Med. 2015, 16, 593–597. [Google Scholar] [CrossRef]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef]
- Sheehan, C.M.; Frochen, S.E.; Walsemann, K.M.; Ailshire, J.A. Are U.S. Adults Reporting Less Sleep?: Findings from Sleep Duration Trends in the National Health Interview Survey, 2004–2017. Sleep 2019, 42, zsy221. [Google Scholar] [CrossRef]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Hazen, N.; Herman, J.; Hillard, P.J.A.; Katz, E.S. National Sleep Foundation’s Updated Sleep Duration Recommendations: Final Report. Sleep Health 2015, 1, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ortuño, M.M.; Bélanger, L.; Ivers, H.; LeBlanc, M.; Morin, C.M. The Use of Natural Products for Sleep: A Common Practice? Sleep Med. 2009, 10, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Somers, V.K.; Xu, H.; Lopez-Jimenez, F.; Covassin, N. Trends in Use of Melatonin Supplements among US Adults, 1999-2018. JAMA 2022, 327, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Losso, J.N.; Finley, J.W.; Karki, N.; Liu, A.G.; Prudente, A.; Tipton, R.; Yu, Y.; Greenway, F.L. Pilot Study of the Tart Cherry Juice for the Treatment of Insomnia and Investigation of Mechanisms. Am. J. Ther. 2018, 25, e194. [Google Scholar] [CrossRef]
- Howatson, G.; Bell, P.G.; Tallent, J.; Middleton, B.; McHugh, M.P.; Ellis, J. Effect of Tart Cherry Juice (Prunus Cerasus) on Melatonin Levels and Enhanced Sleep Quality. Eur. J. Nutr. 2012, 51, 909–916. [Google Scholar] [CrossRef]
- Pigeon, W.R.; Carr, M.; Gorman, C.; Perlis, M.L. Effects of a Tart Cherry Juice Beverage on the Sleep of Older Adults with Insomnia: A Pilot Study. J. Med. Food 2010, 13, 579–583. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Seymour, E.M.; Llanes, D.E.U.; Kaufman, P.B.; Bolling, S.F. Chemical Profile and Antioxidant Capacities of Tart Cherry Products. Food Chem. 2009, 115, 20–25. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of Melatonin in Plants and Its Effects on Plasma Melatonin Levels and Binding to Melatonin Receptors in Vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Yeom, J.W.; Cho, C.-H. Herbal and Natural Supplements for Improving Sleep: A Literature Review. Psychiatry Investig. 2024, 21, 810. [Google Scholar] [CrossRef]
- Burkhardt, S.; Tan, D.X.; Manchester, L.C.; Hardeland, R.; Reiter, R.J. Detection and Quantification of the Antioxidant Melatonin in Montmorency and Balaton Tart Cherries (Prunus Cerasus). J. Agric. Food Chem. 2001, 49, 4898–4902. [Google Scholar] [CrossRef] [PubMed]
- Yurcheshen, M.; Seehuus, M.; Pigeon, W. Updates on Nutraceutical Sleep Therapeutics and Investigational Research. Evid. Based Complement. Alternat. Med. 2015, 2015, 105256. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Bastien, C.H.; Vallières, A.; Morin, C.M. Validation of the Insomnia Severity Index as an Outcome Measure for Insomnia Research. Sleep Med. 2001, 2, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Czank, C.; Cassidy, A.; Zhang, Q.; Morrison, D.J.; Preston, T.; Kroon, P.A.; Botting, N.P.; Kay, C.D. Human Metabolism and Elimination of the Anthocyanin, Cyanidin-3-Glucoside: A (13)C-Tracer Study. Am. J. Clin. Nutr. 2013, 97, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N.; McNair, P.; Harrison, L.C. Diurnal Rhythms of Pro-Inflammatory Cytokines: Regulation by Plasma Cortisol and Therapeutic Implications. Cytokine 1998, 10, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Zoumakis, M.; Papanicolaou, D.A.; Bixler, E.O.; Prolo, P.; Lin, H.-M.; Vela-Bueno, A.; Kales, A.; Chrousos, G.P. Chronic Insomnia Is Associated with a Shift of Interleukin-6 and Tumor Necrosis Factor Secretion from Nighttime to Daytime. Metab.-Clin. Exp. 2002, 51, 887–892. [Google Scholar] [CrossRef]
- Hurtado-Barroso, S.; Quifer-Rada, P.; Rinaldi de Alvarenga, J.F.; Pérez-Fernández, S.; Tresserra-Rimbau, A.; Lamuela-Raventos, R.M. Changing to a Low-Polyphenol Diet Alters Vascular Biomarkers in Healthy Men after Only Two Weeks. Nutrients 2018, 10, 1766. [Google Scholar] [CrossRef]
- Backhaus, J.; Junghanns, K.; Broocks, A.; Riemann, D.; Hohagen, F. Test–Retest Reliability and Validity of the Pittsburgh Sleep Quality Index in Primary Insomnia. J. Psychosom. Res. 2002, 53, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Snyder, E.; Cai, B.; DeMuro, C.; Morrison, M.F.; Ball, W. A New Single-Item Sleep Quality Scale: Results of Psychometric Evaluation in Patients with Chronic Primary Insomnia and Depression. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2018, 14, 1849–1857. [Google Scholar] [CrossRef]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The Insomnia Severity Index: Psychometric Indicators to Detect Insomnia Cases and Evaluate Treatment Response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef]
- de Zambotti, M.; Goldstone, A.; Claudatos, S.; Colrain, I.M.; Baker, F.C. A Validation Study of Fitbit Charge 2TM Compared with Polysomnography in Adults. Chronobiol. Int. 2018, 35, 465–476. [Google Scholar] [CrossRef]
- Kaplan, R.F.; Wang, Y.; Loparo, K.A.; Kelly, M.R.; Bootzin, R.R. Performance Evaluation of an Automated Single-Channel Sleep–Wake Detection Algorithm. Nat. Sci. Sleep 2014, 6, 113–122. [Google Scholar] [CrossRef]
- Haghayegh, S.; Khoshnevis, S.; Smolensky, M.H.; Diller, K.R.; Castriotta, R.J. Performance Assessment of New-Generation Fitbit Technology in Deriving Sleep Parameters and Stages. Chronobiol. Int. 2020, 37, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, C.M.; Flanigan, M.J. ABC of Sleep Disorders. Function of Sleep. BMJ 1993, 306, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Haghayegh, S.; Khoshnevis, S.; Smolensky, M.H.; Diller, K.R.; Castriotta, R.J. Accuracy of Wristband Fitbit Models in Assessing Sleep: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2019, 21, e16273. [Google Scholar] [CrossRef] [PubMed]
- Ferrie, J.E.; Kivimäki, M.; Akbaraly, T.N.; Singh-Manoux, A.; Miller, M.A.; Gimeno, D.; Kumari, M.; Davey Smith, G.; Shipley, M.J. Associations between Change in Sleep Duration and Inflammation: Findings on c-Reactive Protein and Interleukin 6 in the Whitehall II Study. Am. J. Epidemiol. 2013, 178, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Amirkalali, B.; Baradaran, H.R.; Hariri, M. The Beneficial Effect of Tart Cherry on Plasma Levels of Inflammatory Mediators (Not Recovery after Exercise): A Systematic Review and Meta-Analysis on Randomized Clinical Trials. Complement. Ther. Med. 2022, 68, 102842. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jiang, Y.; Zhu, M. The Relationship between Gglobal Sleep Score and Inflammatory Markers in Obese Adults from the United States. Nat. Sci. Sleep 2019, 11, 317–324. [Google Scholar] [CrossRef]
- van Leeuwen, W.M.A.; Lehto, M.; Karisola, P.; Lindholm, H.; Luukkonen, R.; Sallinen, M.; Härmä, M.; Porkka-Heiskanen, T.; Alenius, H. Sleep Restriction Increases the Risk of Developing Cardiovascular Diseases by Augmenting Proinflammatory Responses through IL-17 and CRP. PLoS ONE 2009, 4, e4589. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Zoumakis, E.; Bixler, E.O.; Lin, H.-M.; Follett, H.; Kales, A.; Chrousos, G.P. Adverse Effects of Modest Sleep Restriction on Sleepiness, Performance, and Inflammatory Cytokines. J. Clin. Endocrinol. Metab. 2004, 89, 2119–2126. [Google Scholar] [CrossRef]
- Martin, K.R.; Burrell, L.; Bopp, J. Authentic Tart Cherry Juice Reduces Markers of Inflammation in Overweight and Obese Subjects: A Randomized, Crossover Pilot Study. Food Funct. 2018, 9, 5290–5300. [Google Scholar] [CrossRef]
- Hillman, A.R.; Chrismas, B.C.R. Thirty Days of Montmorency Tart Cherry Supplementation Has No Effect on Gut Microbiome Composition, Inflammation, or Glycemic Control in Healthy Adults. Front. Nutr. 2021, 8, 733057. [Google Scholar] [CrossRef] [PubMed]
- Waldhauser, F.; Saletu, B.; Trinchard-Lugan, I. Sleep Laboratory Investigations on Hypnotic Properties of Melatonin. Psychopharmacology 1990, 100, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Landis, J.R.; Koch, G.G. An Application of Hierarchical Kappa-Type Statistics in the Assessment of Majority Agreement among Multiple Observers. Biometrics 1977, 33, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. The Potential of Phytomelatonin as a Nutraceutical. Molecules 2018, 23, 238. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Srinivasan, V.; Spence, D.W.; Cardinali, D.P. Role of the Melatonin System in the Control of Sleep. CNS Drugs 2007, 21, 995–1018. [Google Scholar] [CrossRef]
- Ferracioli-Oda, E.; Qawasmi, A.; Bloch, M.H. Meta-Analysis: Melatonin for the Treatment of Primary Sleep Disorders. FOCUS 2018, 16, 113–118. [Google Scholar] [CrossRef]
- Opp, M.R. Cytokines and Sleep: The First Hundred Years. Brain. Behav. Immun. 2004, 18, 295–297. [Google Scholar] [CrossRef]
- Kapsimalis, F.; Basta, M.; Varouchakis, G.; Gourgoulianis, K.; Vgontzas, A.; Kryger, M. Cytokines and Pathological Sleep. Sleep Med. 2008, 9, 603–614. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Lotsikas, A.; Zachman, K.; Kales, A.; Prolo, P.; Wong, M.-L.; Licinio, J.; Gold, P.W.; et al. Circadian Interleukin-6 Secretion and Quantity and Depth of Sleep. J. Clin. Endocrinol. Metab. 1999, 84, 2603–2607. [Google Scholar] [CrossRef]
- Irwin, M.R. Sleep and Inflammation: Partners in Sickness and in Health. Nat. Rev. Immunol. 2019, 19, 702–715. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the Anti-Inflammatory and Antioxidant Activities of Luteolin, Kaempferol, Apigenin and Quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Zarezadeh, M.; Barzegari, M.; Aghapour, B.; Adeli, S.; Khademi, F.; Musazadeh, V.; Jamilian, P.; Jamilian, P.; Fakhr, L.; Chehregosha, F.; et al. Melatonin Effectiveness in Amelioration of Oxidative Stress and Strengthening of Antioxidant Defense System: Findings from a Systematic Review and Dose–Response Meta-Analysis of Controlled Clinical Trials. Clin. Nutr. ESPEN 2022, 48, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Boutin, J.A.; Hamon de Almeida, V.; Coussay, N.; Legros, C.; Ferry, G.; Reybier, K. Melatonin Facts: Melatonin Lacks Immuno-Inflammation Boosting Capacities at the Molecular and Cellular Levels. Biochimie 2024, 222, 195–202. [Google Scholar] [CrossRef]
- Said, E.A.; Al-Reesi, I.; Al-Shizawi, N.; Jaju, S.; Al-Balushi, M.S.; Koh, C.Y.; Al-Jabri, A.A.; Jeyaseelan, L. Defining IL-6 Levels in Healthy Individuals: A Meta-Analysis. J. Med. Virol. 2021, 93, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.M.; Weschenfelder, J.; Sander, C.; Minkwitz, J.; Thormann, J.; Chittka, T.; Mergl, R.; Kirkby, K.C.; Faßhauer, M.; Stumvoll, M.; et al. Inflammatory Cytokines in General and Central Obesity and Modulating Effects of Physical Activity. PLoS ONE 2015, 10, e0121971. [Google Scholar] [CrossRef] [PubMed]
- Manigrasso, M.R.; Ferroni, P.; Santilli, F.; Taraborelli, T.; Guagnano, M.T.; Michetti, N.; Davì, G. Association between Circulating Adiponectin and Interleukin-10 Levels in Android Obesity: Effects of Weight Loss. J. Clin. Endocrinol. Metab. 2005, 90, 5876–5879. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Pontillo, A.; Giugliano, F.; Giugliano, G.; Marfella, R.; Nicoletti, G.; Giugliano, D. Association of Low Interleukin-10 Levels with the Metabolic Syndrome in Obese Women. J. Clin. Endocrinol. Metab. 2003, 88, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Formoso, G.; Taraborrelli, M.; Guagnano, M.T.; D’Adamo, M.; Pietro, N.D.; Tartaro, A.; Consoli, A. Magnetic Resonance Imaging Determined Visceral Fat Reduction Associates with Enhanced Il-10 Plasma Levels in Calorie Restricted Obese Subjects. PLoS ONE 2012, 7, e52774. [Google Scholar] [CrossRef]
- Straczkowski, M.; Dzienis-Straczkowska, S.; Stêpieñ, A.; Kowalska, I.; Szelachowska, M.; Kinalska, I. Plasma Interleukin-8 Concentrations Are Increased in Obese Subjects and Related to Fat Mass and Tumor Necrosis Factor-α System. J. Clin. Endocrinol. Metab. 2002, 87, 4602–4606. [Google Scholar] [CrossRef] [PubMed]
- Toujani, S.; Kaabachi, W.; Mjid, M.; Hamzaoui, K.; Cherif, J.; Beji, M. Vitamin D Deficiency and Interleukin-17 Relationship in Severe Obstructive Sleep Apnea-Hypopnea Syndrome. Ann. Thorac. Med. 2017, 12, 107–113. [Google Scholar] [CrossRef]
- Aronson, D.; Bartha, P.; Zinder, O.; Kerner, A.; Markiewicz, W.; Avizohar, O.; Brook, G.J.; Levy, Y. Obesity Is the Major Determinant of Elevated C-Reactive Protein in Subjects with the Metabolic Syndrome. Int. J. Obes. 2004, 28, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, J.; Śledzińska, E.; Baturo, A.; Jończyk, I.; Maleszko, A.; Samborski, P.; Begier-Krasińska, B.; Dobrowolska, A. Obesity and Inflammation. Eur. Cytokine Netw. 2018, 29, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.R.; Wang, M.; Campomayor, C.O.; Collado-Hidalgo, A.; Cole, S. Sleep Deprivation and Activation of Morning Levels of Cellular and Genomic Markers of Inflammation. Arch. Intern. Med. 2006, 166, 1756–1762. [Google Scholar] [CrossRef] [PubMed]
- Simper, T.; Gilmartin, M.; Allwood, D.; Taylor, L.; Chappell, A. The Effects of a Sleep/Recovery Supplement: ‘Night Time Recharge’ on Sleep Parameters in Young Adults. Nutr. Health 2019, 25, 265–274. [Google Scholar] [CrossRef]
- Garrido, M.; Gonzalez-Gomez, D.; Lozano, M.; Barriga, C.; Paredes, S.D.; Moratinos, A.B.R. A Jerte Valley Cherry Product Provides Beneficial Effects on Sleep Quality. Influence on Aging. J. Nutr. Health Aging 2013, 17, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Garrido, M.; Espino, J.; González-Gómez, D.; Lozano, M.; Cubero, J.; Toribio-Delgado, A.F.; Maynar-Mariño, J.I.; Terrón, M.P.; Muñoz, J.L.; Pariente, J.A.; et al. A Nutraceutical Product Based on Jerte Valley Cherries Improves Sleep and Augments the Antioxidant Status in Humans. E-SPEN Eur. E-J. Clin. Nutr. Metab. 2009, 4, e321–e323. [Google Scholar] [CrossRef]
- Garrido, M.; Paredes, S.D.; Cubero, J.; Lozano, M.; Toribio-Delgado, A.F.; Muñoz, J.L.; Reiter, R.J.; Barriga, C.; Rodríguez, A.B. Jerte Valley Cherry-Enriched Diets Improve Nocturnal Rest and Increase 6-Sulfatoxymelatonin and Total Antioxidant Capacity in the Urine of Middle-Aged and Elderly Human. J. Gerontol. Ser. A 2010, 65A, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Lear, R.; O’Leary, M.; O’Brien Andersen, L.; Holt, C.C.; Stensvold, C.R.; van der Giezen, M.; Bowtell, J.L. Tart Cherry Concentrate Does Not Alter the Gut Microbiome, Glycaemic Control or Systemic Inflammation in a Middle-Aged Population. Nutrients 2019, 11, 1063. [Google Scholar] [CrossRef]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Cheung, W.; Haskell-Ramsay, C.F.; Howatson, G. Polyphenol-Rich Tart Cherries (Prunus Cerasus, Cv Montmorency) Improve Sustained Attention, Feelings of Alertness and Mental Fatigue and Influence the Plasma Metabolome in Middle-Aged Adults: A Randomised, Placebo-Controlled Trial. Br. J. Nutr. 2022, 128, 2409–2420. [Google Scholar] [CrossRef]

| Characteristics | Total N = 34 (9 M, 25 F) | 1st MTC, 2nd Placebo N = 16 (5 M, 11 F) | 1st Placebo, 2nd MTC N = 18 (4 M, 14 F) | p Value |
|---|---|---|---|---|
| Age (y) | 32.6 ± 10.7 | 32.9 ± 11.4 | 32.3 ± 10.5 | 0.735 |
| Weight (kg) | 91.7 ± 23.5 | 98.8 ± 29.5 | 85.4 ± 14.6 | 0.192 |
| Height (cm) | 168.3 ± 10.4 | 168.2 ± 10.8 | 168.4 ± 10.4 | 0.748 |
| BMI (kg/m2) | 32.1 ± 7.0 | 34.5 ± 8.9 | 30.0 ± 3.8 | 0.120 |
| % Body fat | 37.8 ± 9.0 | 38.8 ± 10.5 | 36.9 ± 7.5 | 0.799 |
| TST (min/day) | 383.8 ± 56.5 | 369.8 ± 65.0 | 396.3 ± 46.2 | 0.545 |
| Deep sleep (min/day) | 86.5 ± 25.3 | 81.3 ± 81.3 | 91.1 ± 23.8 | 0.381 |
| REM sleep (min/day) | 96.2 ± 29.7 | 95.1 ± 35.5 | 97.2 ± 24.5 | 0.795 |
| TNF-α (pg/mL) | 1.8 ± 8.5 | 3.1 ± 12.1 | 0.7 ± 2.1 | 0.506 |
| IL-6 (pg/mL) | 3.0 ± 10.1 | 4.9 ± 13.9 | 1.2 ± 4.2 | 0.409 |
| IL-8 (pg/mL) | 0.7 ± 2.3 | 0.4 ± 1.1 | 0.9 ± 3.0 | 0.160 |
| IL-10 (pg/mL) | 5.0 ± 15.8 | 9.0 ± 22.3 | 1.5 ± 4.0 | 0.246 |
| IL-17A (pg/mL) | 0.3 ± 1.2 | 0.1 ± 0.4 | 0.6 ± 1.7 | 0.235 |
| CRP (mg/L) | 4.4 ± 10.0 | 4.8 ± 6.7 | 4.1 ± 12.5 | 0.976 |
| Variables | Time | Treatment | Estimate | SE | 95% CI | df | t | p | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | MTC | Lower | Upper | ||||||||
| Zmachine | |||||||||||
| TST | Pre | 375.4 ± 59.2 | 366.8 ± 60.7 | Time | −10.11 | 7.43 | −24.80 | 4.58 | 66.0 | −1.36 | 0.18 |
| Post | 366.2 ± 59.4 | 355.8 ± 59.0 | treatment | −9.52 | 12.25 | −33.80 | 14.72 | 66.0 | −0.78 | 0.44 | |
| Time × treatment | −1.75 | 14.85 | −31.10 | 27.63 | 66.0 | −0.12 | 0.91 | ||||
| Deep sleep | Pre | 82.1 ± 27.6 | 78.5 ± 26.4 | Time | −1.38 | 2.79 | −6.91 | 4.15 | 66.0 | −0.49 | 0.62 |
| Post | 77.7 ± 27.7 | 80.2 ± 29.7 | treatment | −0.57 | 6.16 | −12.76 | 11.62 | 66.0 | −0.09 | 0.93 | |
| Time × treatment | 6.18 | 5.59 | −4.87 | 17.23 | 66.0 | 1.11 | 0.27 | ||||
| REM sleep | Pre | 95.9 ± 28.2 | 89.88 ± 36.8 | Time | 0.43 | 0.80 | −1.16 | 2.02 | 66.0 | 0.54 | 0.59 |
| Post | 98.0 ± 41.2 | 87.13 ± 32.5 | treatment | 0.51 | 1.68 | −2.81 | 3.82 | 66.0 | 0.30 | 0.76 | |
| Time × treatment | 2.12 | 1.61 | −1.06 | 5.29 | 66.0 | 1.32 | 0.19 | ||||
| Fitbit | |||||||||||
| Nocturnal | Pre | 391.0 ± 98.4 | 385.1 ± 89.9 | Time | 4.56 | 23.2 | −41.3 | 50.4 | 60.2 | 0.20 | 0.85 |
| Post | 394.9 ± 98.7 | 390.3 ± 56.0 | treatment | −31.64 | 42.1 | −114.9 | 51.6 | 61.8 | −0.75 | 0.46 | |
| Time × treatment | −40.81 | 46.3 | −132.5 | 50.9 | 60.2 | −0.88 | 0.38 | ||||
| Nap | Pre | 6.5 ± 14.4 | 12.1 ± 23.0 | Time | 3.68 | 10.95 | −18 | 25.34 | 65.4 | 0.34 | 0.74 |
| Post | 13.3 ± 25.4 | 8.0 ± 21.7 | treatment | 0.07 | 11.62 | −22.9 | 23.06 | 65.6 | 0.01 | 0.99 | |
| Time × treatment | −34.47 | 21.89 | −77.8 | 8.85 | 65.4 | −1.57 | 0.12 | ||||
| Total Sleep | Pre | 397.6 ± 98.5 | 397.2 ± 92.7 | Time | 7.37 | 23.3 | −38.8 | 53.6 | 59.7 | 0.32 | 0.75 |
| Post | 408.2 ± 99.4 | 398.2 ± 51.4 | treatment | −30.65 | 41.0 | −111.7 | 50.4 | 61.2 | −0.75 | 0.46 | |
| Time × treatment | −76.37 | 46.7 | −168.7 | 16 | 59.7 | −1.63 | 0.11 | ||||
| Variables | Time | Treatment | Estimate | SE | 95% CI | df | t | p | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | MTC | Lower | Upper | ||||||||
| Survey | |||||||||||
| ISI | Pre | 11.85 ± 5.27 | 12.21 ± 5.39 | Time | −1.23 | 0.41 | −2.05 | −0.41 | 64.3 | −2.98 | <0.05 * |
| Post | 10.84 ± 4.62 | 10.82 ± 5.06 | treatment | 0.13 | 1.18 | −2.20 | 2.45 | 65.1 | 0.11 | 0.92 | |
| Time × treatment | −0.46 | 0.83 | −2.10 | 1.18 | 64.3 | −0.56 | 0.58 | ||||
| PSQI | Pre | 8.58 ± 3.35 | 8.88 ± 3.25 | Time | −1.09 | 0.31 | −1.69 | −0.48 | 63.9 | −3.55 | <0.001 * |
| Post | 7.33 ± 3.05 | 8.03 ± 2.79 | treatment | 0.46 | 0.70 | −0.92 | 1.85 | 65.3 | 0.66 | 0.51 | |
| Time × treatment | 0.31 | 0.61 | −0.90 | 1.52 | 63.9 | 0.51 | 0.61 | ||||
| SQS | Pre | 4.94 ± 1.58 | 5.21 ± 1.63 | Time | 0.15 | 0.19 | −0.23 | 0.53 | 62.0 | 0.78 | 0.44 |
| Post | 5.12 ± 1.93 | 5.21 ± 1.65 | treatment | 0.14 | 0.38 | −0.61 | 0.88 | 65.9 | 0.36 | 0.72 | |
| Time × treatment | −0.10 | 0.39 | −0.86 | 0.67 | 62.0 | −0.25 | 0.81 | ||||
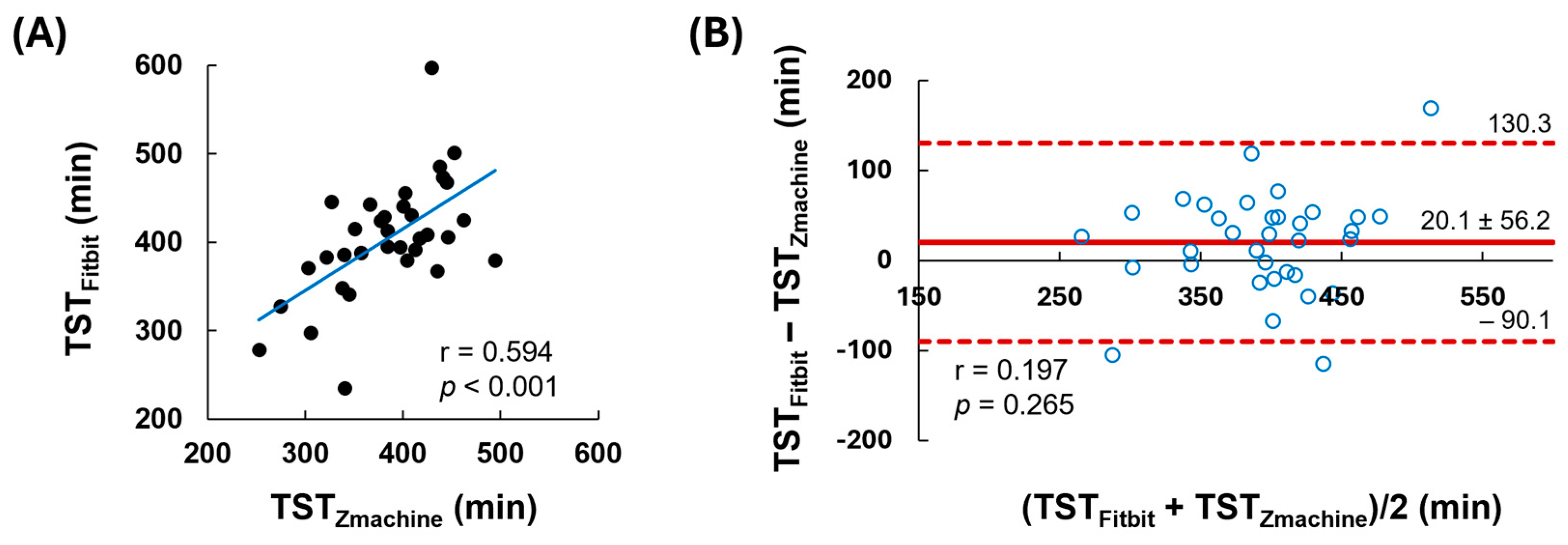
| TST (Mean ± SD) | Difference (95% CI) | Trend in Bias [p Value] | Agreement (ICC, 95% CI) | |
|---|---|---|---|---|
| Zmachine | 383.8 ± 56.5 | |||
| Fitbit | 403.9 ± 66.5 | 20.1 (0.525–39.7) | −59.6 + 0.2 M [0.265] | 0.738 (0.475–0.869) |
| Variables | Time | Treatment | Estimate | SE | Odds Ratio | Odds Ratio 95% CI | z | p | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | MTC | Lower | Upper | ||||||||
| Inflammation biomarkers | |||||||||||
| TNF-ɑ (pg/mL) | Pre | 1.80 ± 7.40 | 2.01 ± 8.45 | Time | −0.01 | 0.06 | 0.99 | 0.88 | 1.12 | −0.16 | 0.87 |
| Post | 1.85 ± 8.47 | 2.53 ± 9.02 | treatment | 0.03 | 0.06 | 1.03 | 0.91 | 1.16 | 0.41 | 0.68 | |
| Time × treatment | 0.03 | 0.12 | 1.03 | 0.81 | 1.31 | 0.22 | 0.83 | ||||
| IL-6 (pg/mL) | Pre | 2.92 ± 8.57 | 3.24 ± 10.29 | Time | −0.02 | 0.07 | 0.98 | 0.85 | 1.14 | −0.26 | 0.80 |
| Post | 3.05 ± 9.87 | 2.58 ± 7.45 | treatment | 0.00 | 0.07 | 1.00 | 0.86 | 1.15 | −0.05 | 0.96 | |
| Time × treatment | 0.02 | 0.15 | 1.02 | 0.76 | 1.37 | 0.16 | 0.87 | ||||
| IL-8 (pg/mL) | Pre | 0.78 ± 2.35 | 0.67 ± 1.58 | Time | 0.00 | 0.04 | 1.00 | 0.85 | 1.14 | −0.26 | 0.80 |
| Post | 0.42 ± 1.18 | 0.96 ± 1.97 | treatment | 0.03 | 0.04 | 1.03 | 0.86 | 1.15 | −0.05 | 0.96 | |
| Time × treatment | 0.07 | 0.08 | 1.08 | 0.76 | 1.37 | 0.16 | 0.87 | ||||
| IL-10 (pg/mL) | Pre | 5.08 ± 16.92 | 4.49 ± 15.65 | Time | 0.00 | 0.09 | 1.00 | 0.85 | 1.19 | 0.04 | 0.97 |
| Post | 4.94 ± 15.48 | 3.54 ± 9.98 | treatment | −0.04 | 0.09 | 0.96 | 0.81 | 1.14 | −0.45 | 0.65 | |
| Time × treatment | 0.07 | 0.17 | 1.07 | 0.76 | 1.50 | 0.38 | 0.70 | ||||
| IL-17A (pg/mL) | Pre | 0.38 ± 1.24 | 0.07 ± 0.28 | Time | 0.01 | 0.02 | 1.01 | 0.97 | 1.05 | 0.32 | 0.75 |
| Post | 0.36 ± 0.90 | 0.15 ± 0.38 | treatment | −0.03 | 0.02 | 0.97 | 0.93 | 1.01 | −1.59 | 0.11 | |
| Time × treatment | 0.00 | 0.04 | 1.00 | 0.92 | 1.08 | 0.01 | 1.00 | ||||
| CRP (mg/L) | Pre | 4.29 ± 9.90 | 3.18 ± 5.24 | Time | −0.02 | 0.07 | 0.98 | 0.85 | 1.13 | −0.25 | 0.80 |
| Post | 3.23 ± 6.00 | 3.12 ± 5.68 | treatment | −0.01 | 0.07 | 0.99 | 0.86 | 1.14 | −0.13 | 0.90 | |
| Time × treatment | −0.02 | 0.15 | 0.98 | 0.73 | 1.31 | −0.15 | 0.88 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucker, R.M.; Kim, N.; Gurzell, E.; Mathi, S.; Chavva, S.; Senthilkumar, D.; Bartunek, O.; Fenton, K.C.; Herndon-Fenton, S.J.; Cardino, V.N.; et al. Commonly Used Dose of Montmorency Tart Cherry Powder Does Not Improve Sleep or Inflammation Outcomes in Individuals with Overweight or Obesity. Nutrients 2024, 16, 4125. https://doi.org/10.3390/nu16234125
Tucker RM, Kim N, Gurzell E, Mathi S, Chavva S, Senthilkumar D, Bartunek O, Fenton KC, Herndon-Fenton SJ, Cardino VN, et al. Commonly Used Dose of Montmorency Tart Cherry Powder Does Not Improve Sleep or Inflammation Outcomes in Individuals with Overweight or Obesity. Nutrients. 2024; 16(23):4125. https://doi.org/10.3390/nu16234125
Chicago/Turabian StyleTucker, Robin M., Nahyun Kim, Eric Gurzell, Sruti Mathi, Shreya Chavva, Dharshini Senthilkumar, Olivia Bartunek, Kayla C. Fenton, Sidney J. Herndon-Fenton, Vanessa N. Cardino, and et al. 2024. "Commonly Used Dose of Montmorency Tart Cherry Powder Does Not Improve Sleep or Inflammation Outcomes in Individuals with Overweight or Obesity" Nutrients 16, no. 23: 4125. https://doi.org/10.3390/nu16234125
APA StyleTucker, R. M., Kim, N., Gurzell, E., Mathi, S., Chavva, S., Senthilkumar, D., Bartunek, O., Fenton, K. C., Herndon-Fenton, S. J., Cardino, V. N., Cooney, G. M., Young, S., & Fenton, J. I. (2024). Commonly Used Dose of Montmorency Tart Cherry Powder Does Not Improve Sleep or Inflammation Outcomes in Individuals with Overweight or Obesity. Nutrients, 16(23), 4125. https://doi.org/10.3390/nu16234125







