Resveratrol and Its Derivatives Diminish Lipid Accumulation in Adipocytes In Vitro—Mechanism of Action and Structure–Activity Relationship
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Differentiation
2.3. Oil Red O and Coomassie Blue Staining for Detection of Lipid and Protein Content
2.4. Measurement of Mitochondrial Activity
2.5. Assessment of Glucose Uptake
2.6. Investigating Molecular Pathways of Resveratrol
2.7. Statistical Analysis
3. Results
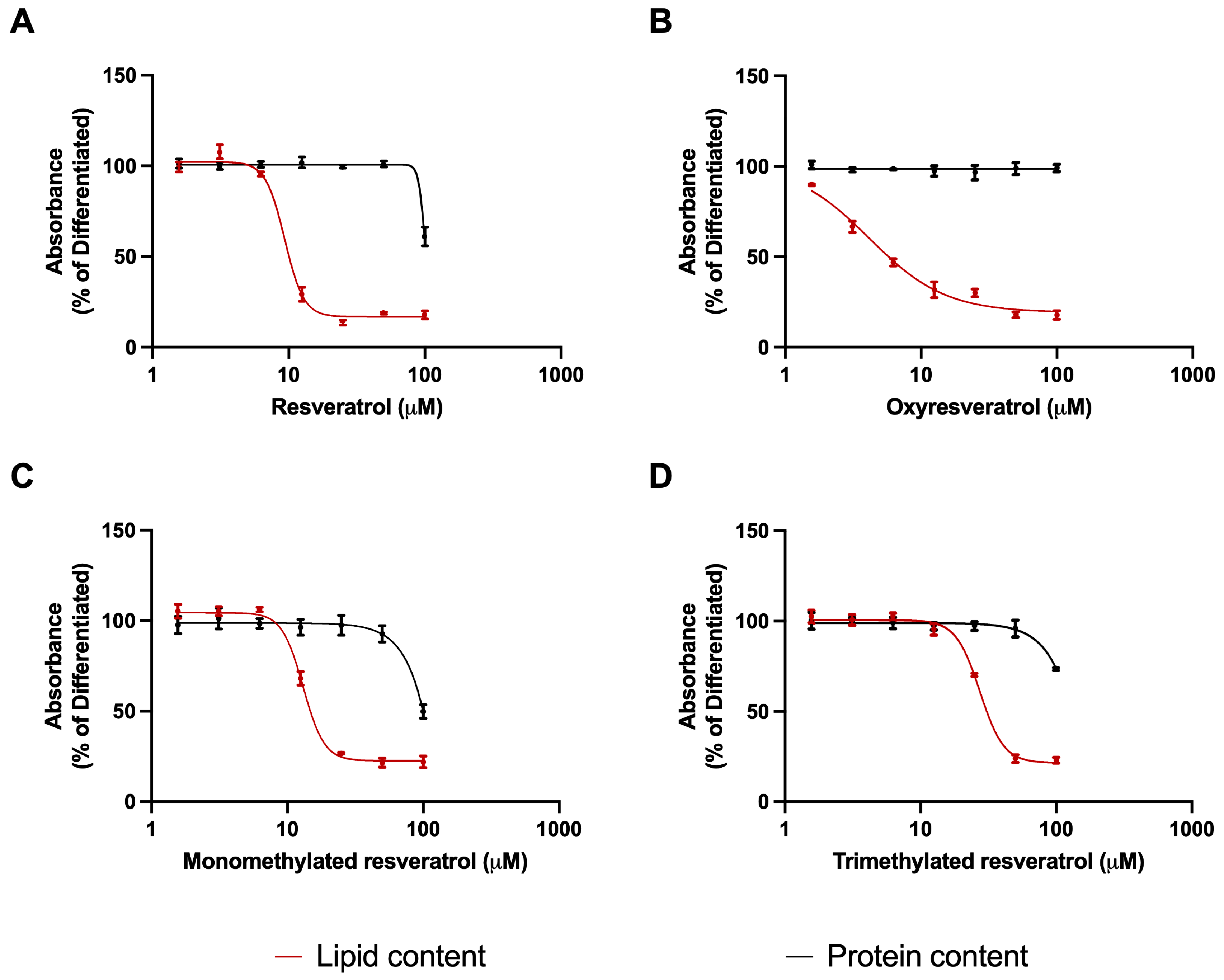
3.1. Effects of Resveratrol Analogues on Lipid Accumulation and Protein Levels of Adipocytes
3.2. Effects of Resveratrol Analogues on Mitochondrial Activity
3.3. Effects of Resveratrol Analogues on Glucose Uptake in the Presence and Absence of Insulin
3.4. Effects of Resveratrol Analogues on Lipid Accumulation and Protein Content in the Presence of Inhibitors of Molecular Pathways
4. Discussion
4.1. Involvement of Kinase Pathways in the Antiadipogenic Effect of Resveratrol Derivatives
4.2. Involvement of Insulin Signaling in the Antiadipogenic Effect of Resveratrol Derivatives
4.3. Involvement of Mitochondria and Caloric Restriction Pathways in the Antiadipogenic Effect of Resveratrol Derivatives
4.4. Involvement of Autophagy in the Antiadipogenic Effect of Resveratrol Derivatives
4.5. Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Prevalence of Overweight Among Adults. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 14 October 2024).
- White, U. Adipose tissue expansion in obesity, health, and disease. Front. Cell Dev. Biol. 2023, 11, 1188844. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, C.E.; Spalding, K.L. Spalding, White adipocyte dysfunction and obesity-associated pathologies in humans. Nat. Rev. Mol. Cell. Biol. 2024, 25, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Sarma, S.; Sockalingam, S.; Dash, S. Obesity as a multisystem disease: Trends in obesity rates and obesity-related complications. Diabetes Obes. Metab. 2021, 23 (Suppl. S1), 3–16. [Google Scholar] [CrossRef]
- Pulipati, V.P.; Pannain, S. Pharmacotherapy of obesity in complex diseases. Clin. Obes. 2022, 12, e12497. [Google Scholar] [CrossRef]
- Tiwari, A.; Balasundaram, P. Public Health Considerations Regarding Obesity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Valli, V.; Heilmann, K.; Danesi, F.; Bordoni, A.; Gerhäuser, C. Modulation of Adipocyte Differentiation and Proadipogenic Gene Expression by Sulforaphane, Genistein, and Docosahexaenoic Acid as a First Step to Counteract Obesity. Oxidative Med. Cell. Longev. 2018, 2018, 1617202. [Google Scholar] [CrossRef]
- Horwitz, A.; Birk, R. Adipose Tissue Hyperplasia and Hypertrophy in Common and Syndromic Obesity—The Case of BBS Obesity. Nutrients 2023, 15, 3445. [Google Scholar] [CrossRef]
- Savova, M.S.; Mihaylova, L.V.; Tews, D.; Wabitsch, M.; Georgiev, M.I. Targeting PI3K/AKT signaling pathway in obesity. Biomed. Pharmacother. 2023, 159, 114244. [Google Scholar] [CrossRef] [PubMed]
- Bost, F.; Aouadi, M.; Caron, L.; Binétruy, B. The role of MAPKs in adipocyte differentiation and obesity. Biochimie 2005, 87, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, F.; Wilkinson, M.; Baxter, E.; Brennan, D.J. Mitogen-Activated Protein Kinase (MAPK) and Obesity-Related Cancer. Int. J. Mol. Sci. 2020, 21, 1241. [Google Scholar] [CrossRef] [PubMed]
- Leiva, M.; Matesanz, N.; Pulgarín-Alfaro, M.; Nikolic, I.; Sabio, G. Uncovering the Role of p38 Family Members in Adipose Tissue Physiology. Front. Endocrinol. 2020, 11, 572089. [Google Scholar] [CrossRef]
- Carlson, C.J.; Koterski, S.; Sciotti, R.J.; Poccard, G.B.; Rondinone, C.M. Enhanced basal activation of mitogen-activated protein kinases in adipocytes from type 2 diabetes: Potential role of p38 in the downregulation of GLUT4 expression. Diabetes 2003, 52, 634–641. [Google Scholar] [CrossRef]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Görgün, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for JNK in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef]
- Surugiu, R.; Iancu, M.A.; Vintilescu, B.; Stepan, M.D.; Burdusel, D.; Genunche-Dumitrescu, A.V.; Dogaru, C.-A.; Dumitra, G.G. Molecular Mechanisms of Healthy Aging: The Role of Caloric Restriction, Intermittent Fasting, Mediterranean Diet, and Ketogenic Diet-A Scoping Review. Nutrients 2024, 16, 2878. [Google Scholar] [CrossRef]
- McCay, C.M.; Crowell, M.F.; Maynard, L.A. The effect of retarded growth upon the length of life span and upon the ultimate body size. J. Nutr. 1935, 5, 155–171, discussion 172. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. Calorie restriction: Is AMPK a key sensor and effector? Physiology 2011, 26, 214–224. [Google Scholar] [CrossRef]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. Front. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Duszka, K.; Gregor, A.; Guillou, H.; König, J.; Wahli, W. Peroxisome Proliferator-Activated Receptors and Caloric Restriction-Common Pathways Affecting Metabolism, Health, and Longevity. Cells 2020, 9, 1708. [Google Scholar] [CrossRef] [PubMed]
- Yessenkyzy, A.; Saliev, T.; Zhanaliyeva, M.; Masoud, A.-R.; Umbayev, B.; Sergazy, S.; Krivykh, E.; Gulyayev, A.; Nurgozhin, T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients 2020, 12, 1344. [Google Scholar] [CrossRef] [PubMed]
- Abo-Kadoum, M.A.; Abouelela, M.E.; Al Mousa, A.A.; Abo-Dahab, N.F.; Mosa, M.A.; Helmy, Y.A.; Hassane, A.M.A. Resveratrol biosynthesis, optimization, induction, bio-transformation and bio-degradation in mycoendophytes. Front. Microbiol. 2022, 13, 1010332. [Google Scholar] [CrossRef] [PubMed]
- Kopp, P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ‘French paradox’? Eur. J. Endocrinol. 1998, 138, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Bejenaru, L.E.; Biţă, A.; Belu, I.; Segneanu, A.-E.; Bejenaru, L.E.; Radu, A.; Dumitru, A.; Ciocîlteu, M.V.; Mogoşanu, G.D.; Bejenaru, C. Resveratrol: A Review on the Biological Activity and Applications. Appl. Sci. 2024, 14, 4534. [Google Scholar] [CrossRef]
- Hidalgo-Lozada, G.M.; Villarruel-López, A.; Nuño, K.; García-García, A.; Sánchez-Nuño, Y.A.; Ramos-García, C.O. Clinically Effective Molecules of Natural Origin for Obesity Prevention or Treatment. Int. J. Mol. Sci. 2024, 25, 2671. [Google Scholar] [CrossRef]
- Szkudelska, K.; Szkudelski, T. Resveratrol, obesity and diabetes. Eur. J. Pharmacol. 2010, 635, 1–8. [Google Scholar] [CrossRef]
- Chung, J.H.; VManganiello Dyck, J.R. Resveratrol as a calorie restriction mimetic: Therapeutic implications. Trends. Cell. Biol. 2012, 22, 546–554. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Sergides, C.; Chirilă, M.; Silvestro, L.; Pitta, D.; Pittas, A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016, 11, 164–170. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.S.; Ho, P.C. Preclinical pharmacokinetic evaluation of resveratrol trimethyl ether in sprague-dawley rats: The impacts of aqueous solubility, dose escalation, food and repeated dosing on oral bioavailability. J. Pharm. Sci. 2011, 100, 4491–4500. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Gertsenstein, M.; Vintersten, K.; Behringer, R. Preparing mouse embryo fibroblasts. CSH Protoc. 2006, 2006, pdb-prot4398. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol.-Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Varghese, D.; Ishida, C.; Patel, P.; Koya, H.H. Polypharmacy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Molani-Gol, R.; Rafraf, M. The anti-obesity effects of resveratrol on the 3T3-L1 adipocytes. Int. J. Vitam. Nutr. Res. 2024, 94, 252–263. [Google Scholar] [CrossRef]
- Chen, S.; Li, Z.; Li, W.; Shan, Z.; Zhu, W. Resveratrol inhibits cell differentiation in 3T3-L1 adipocytes via activation of AMPK. Can. J. Physiol. Pharmacol. 2011, 89, 793–799. [Google Scholar]
- Rayalam, S.; Yang, J.; Ambati, S.; Della-Fera, M.A.; Baile, C.A. Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother. Res. 2008, 22, 1367–1371. [Google Scholar] [CrossRef]
- Chung, Y.C.; Hyun, C.G. Inhibitory Effects of Pinostilbene on Adipogenesis in 3T3-L1 Adipocytes: A Study of Possible Mechanisms. Int. J. Mol. Sci. 2021, 22, 13446. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.W. High-dose Resveratrol Inhibits Insulin Signaling Pathway in 3T3-L1 Adipocytes. J. Lifestyle Med. 2013, 3, 41–47. [Google Scholar]
- Chen, S.; Zhao, Z.; Ke, L.; Li, Z.; Li, W.; Zhang, Z.; Zhou, Y.; Feng, X.; Zhu, W. Resveratrol improves glucose uptake in insulin-resistant adipocytes via Sirt1. J. Nutr. Biochem. 2018, 55, 209–218. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Ke, L.; Sun, Y.; Li, W.; Feng, X.; Zhu, W.; Chen, S. Resveratrol promotes white adipocytes browning and improves metabolic disorders in Sirt1-dependent manner in mice. FASEB J. 2020, 34, 4527–4539. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Song, N.-J.; Lee, A.R.; Lee, D.H.; Seo, M.-J.; Kim, S.; Chang, S.-H.; Yang, D.K.; Hwang, Y.-J.; Hwang, K.-A.; et al. Oxyresveratrol Increases Energy Expenditure through Foxo3a-Mediated Ucp1 Induction in High-Fat-Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 20, 26. [Google Scholar] [CrossRef] [PubMed]
- Imamura, H.; Nagayama, D.; Ishihara, N.; Tanaka, S.; Watanabe, R.; Watanabe, Y.; Sato, Y.; Yamaguchi, T.; Ban, N.; Kawana, H.; et al. Resveratrol attenuates triglyceride accumulation associated with upregulation of Sirt1 and lipoprotein lipase in 3T3-L1 adipocytes. Mol. Genet. Metab. Rep. 2017, 12, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Auwerx, J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, A.; Corsetti, G. Natural Compounds and Autophagy: Allies Against Neurodegeneration. Front. Cell Dev. Biol. 2020, 8, 555409. [Google Scholar] [CrossRef]
- Ulakcsai, Z.; Bagamery, F.; Vincze, I.; Szoko, E.; Tabi, T. Protective effect of resveratrol against caspase 3 activation in primary mouse fibroblasts. Croat. Med. J. 2015, 56, 78–84. [Google Scholar] [CrossRef]
- Ulakcsai, Z.; Bagaméry, F.; Szökő, É.; Tábi, T. The role of autophagy induction in the mechanism of cytoprotective effect of resveratrol. Eur. J. Pharm. Sci. 2018, 123, 135–142. [Google Scholar] [CrossRef]
- Varga, K.; Paszternák, A.; Kovács, V.; Guczogi, A.; Sikur, N.; Patakfalvi, D.; Bagaméry, F.; Szökő, É.; Tábi, T. Differential Cytoprotective Effect of Resveratrol and Its Derivatives: Focus on Antioxidant and Autophagy-Inducing Effects. Int. J. Mol. Sci. 2024, 25, 11274. [Google Scholar] [CrossRef]
- Morselli, E.; Maiuri, M.C.; Markaki, M.; Megalou, E.; Pasparaki, A.; Palikaras, K.; Criollo, A.; Galluzzi, L.; A Malik, S.; Vitale, I.; et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010, 1, e10. [Google Scholar] [CrossRef]
- Singh, R.; Xiang, Y.; Wang, Y.; Baikati, K.; Cuervo, A.M.; Luu, Y.K.; Tang, Y.; Pessin, J.E.; Schwartz, G.J.; Czaja, M.J. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Investig. 2009, 119, 3329–3339. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W. Lipophagy: Molecular Mechanisms and Implications in Metabolic Disorders. Mol. Cells 2020, 43, 686–693. [Google Scholar] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikur, N.; Böröczky, C.; Paszternák, A.; Gyöngyössy, R.; Szökő, É.; Varga, K.; Tábi, T. Resveratrol and Its Derivatives Diminish Lipid Accumulation in Adipocytes In Vitro—Mechanism of Action and Structure–Activity Relationship. Nutrients 2024, 16, 3869. https://doi.org/10.3390/nu16223869
Sikur N, Böröczky C, Paszternák A, Gyöngyössy R, Szökő É, Varga K, Tábi T. Resveratrol and Its Derivatives Diminish Lipid Accumulation in Adipocytes In Vitro—Mechanism of Action and Structure–Activity Relationship. Nutrients. 2024; 16(22):3869. https://doi.org/10.3390/nu16223869
Chicago/Turabian StyleSikur, Noémi, Csenge Böröczky, Alexandra Paszternák, Ramá Gyöngyössy, Éva Szökő, Kamilla Varga, and Tamás Tábi. 2024. "Resveratrol and Its Derivatives Diminish Lipid Accumulation in Adipocytes In Vitro—Mechanism of Action and Structure–Activity Relationship" Nutrients 16, no. 22: 3869. https://doi.org/10.3390/nu16223869
APA StyleSikur, N., Böröczky, C., Paszternák, A., Gyöngyössy, R., Szökő, É., Varga, K., & Tábi, T. (2024). Resveratrol and Its Derivatives Diminish Lipid Accumulation in Adipocytes In Vitro—Mechanism of Action and Structure–Activity Relationship. Nutrients, 16(22), 3869. https://doi.org/10.3390/nu16223869






