Adipocyte FGF21 Signaling Defect Aggravated Adipose Tissue Inflammation in Gestational Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Method
2.1. Animals
2.2. Glucose Tolerance Test
2.3. Assays and Kits
2.4. Cell Culture
2.5. Flow Cytometry
2.6. Quantitative PCR (q-PCR) Analysis
2.7. Western Blot Analysis
2.8. Tissue Histology and Immunohistochemistry
2.9. Statistical Analysis
3. Results
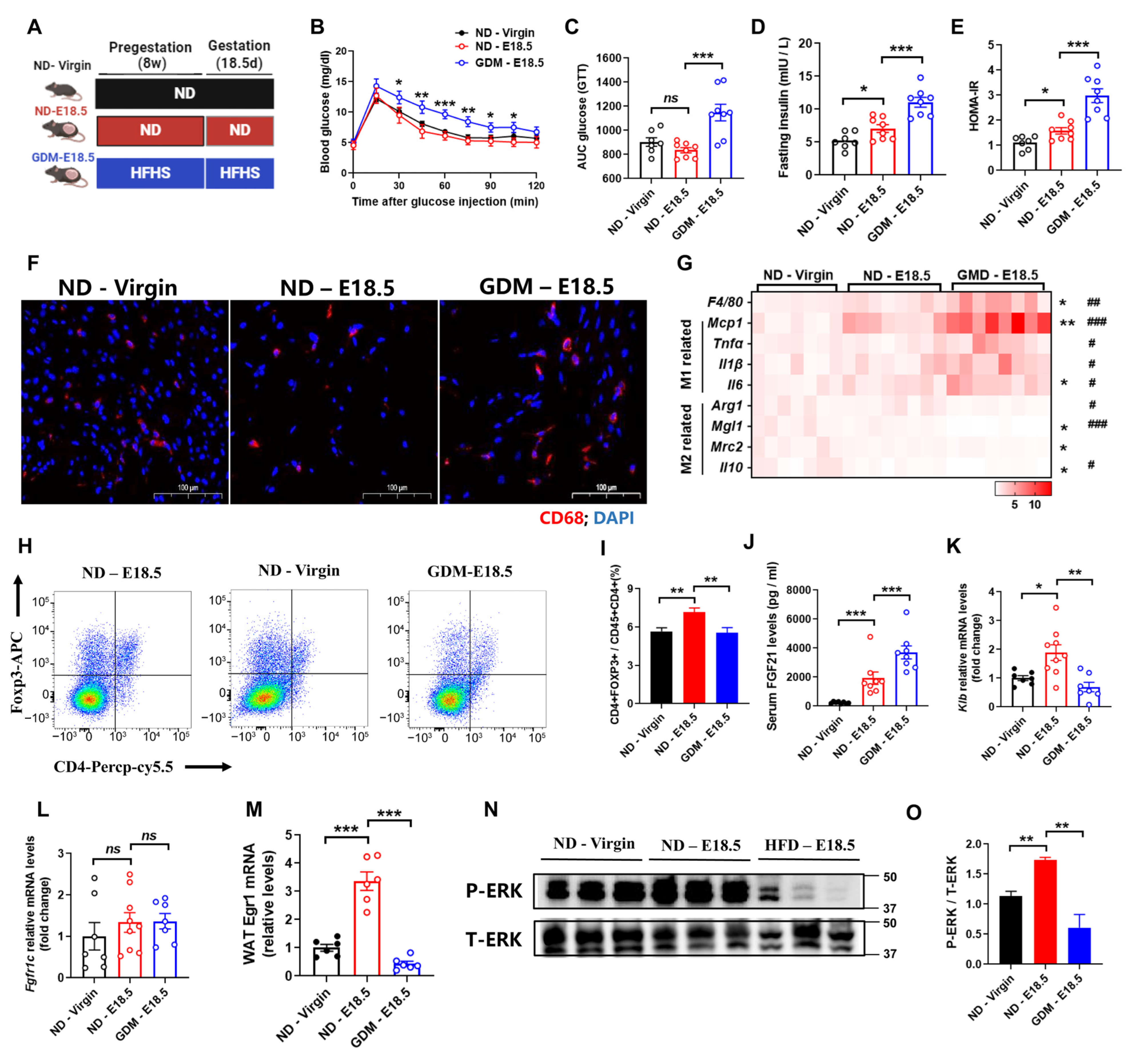
3.1. Gestational Diabetes Mellitus Exhibits Aggravated Adipose Tissues Inflammation and FGF21 Signaling Defects
3.2. FGF21 Is Required for Alleviates Adipose Tissues Inflammation During Pregnancy
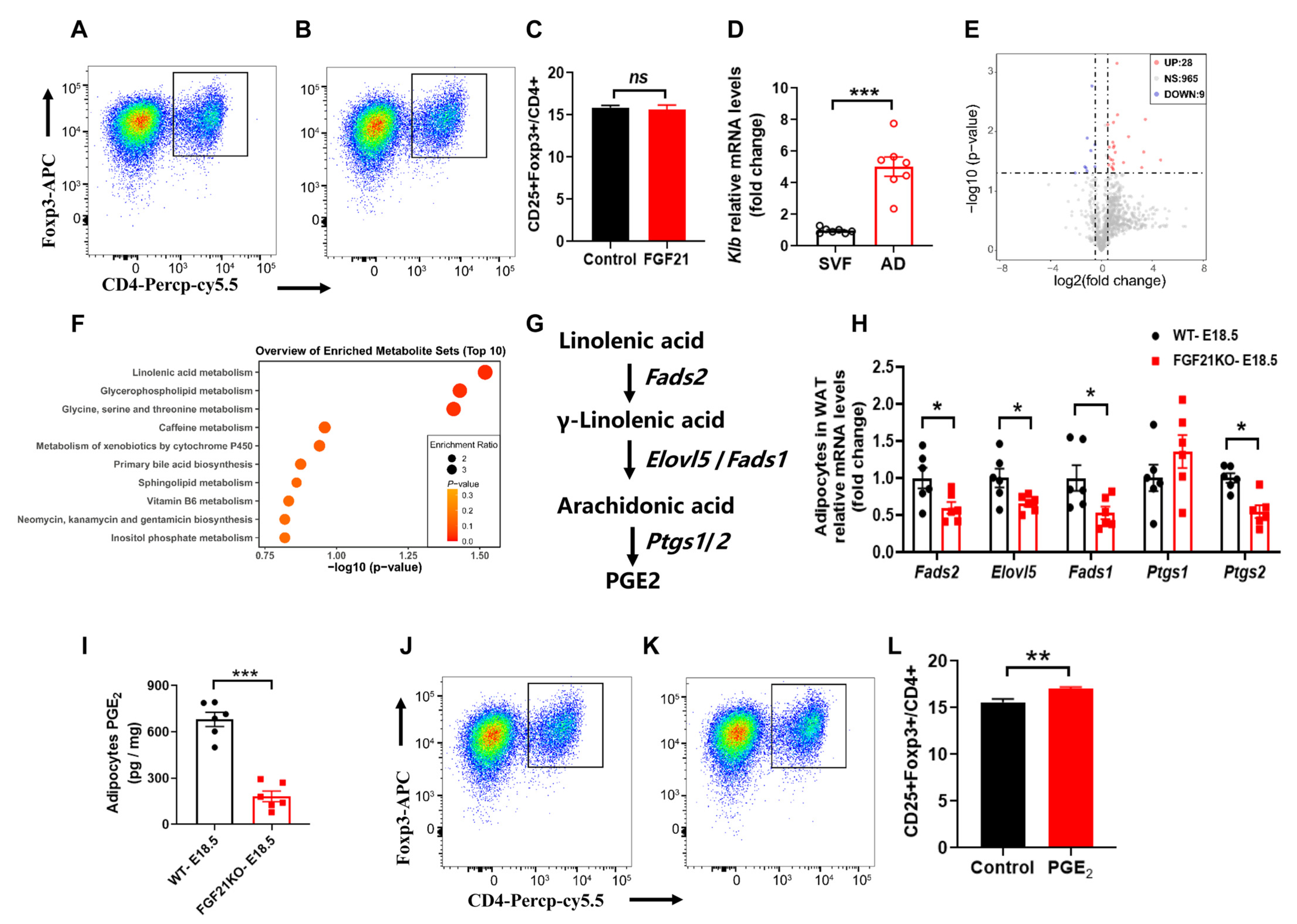
3.3. FGF21 Promotes Tregs Differentiation Through Linolenic Acid-Mediated PGE2 Synthesis in Adipocytes
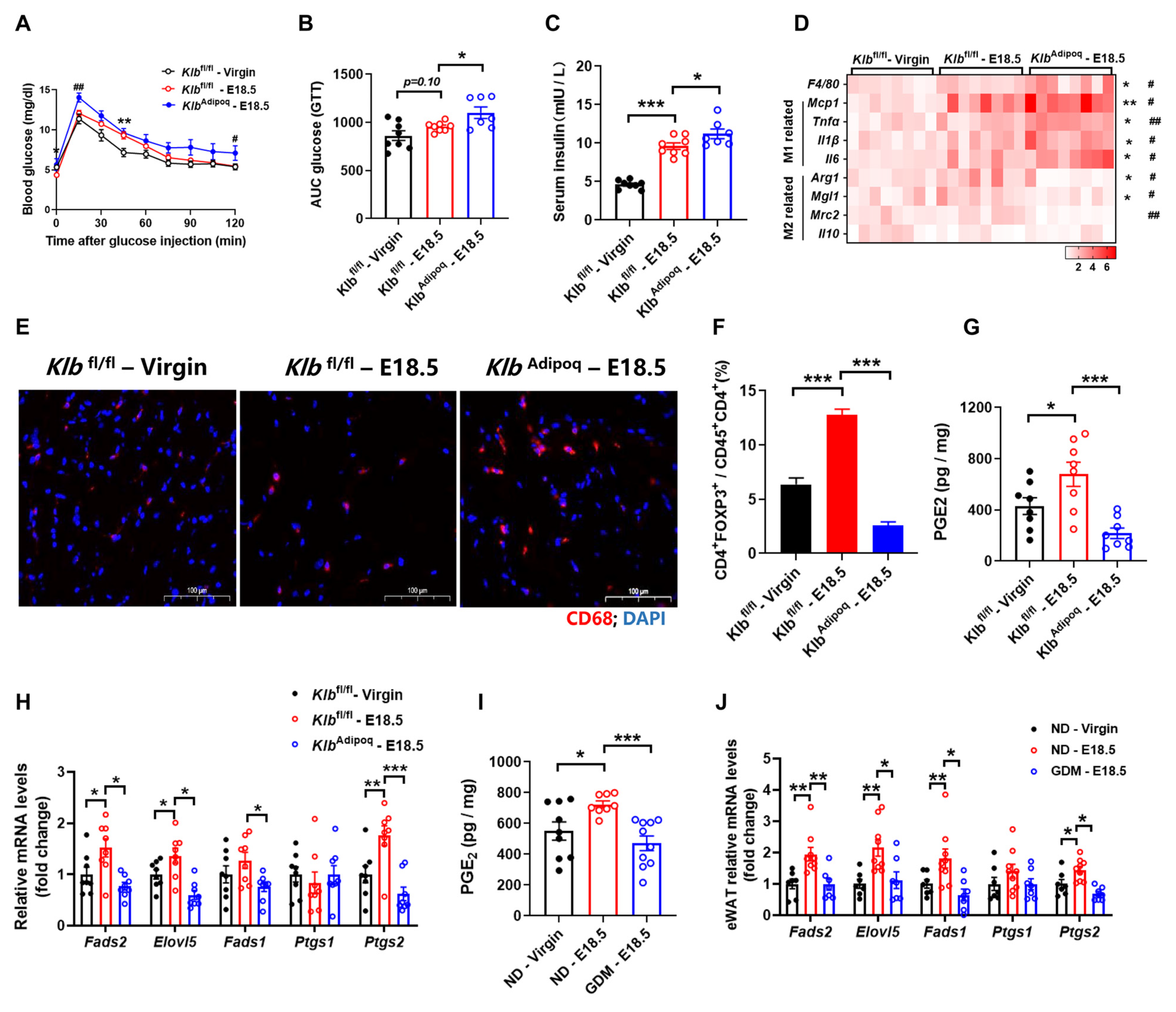
3.4. Deficiency of FGF21 Signaling in Adipocytes Increases Adipose Tissues Inflammation During Pregnancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational diabetes mellitus: Mechanisms, treatment, and complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Piani, F.; Tossetta, G.; Cara-Fuentes, G.; Daniela, M. Diagnostic and prognostic role of CD93 in cardiovascular disease: A systematic review. Biomolecules 2023, 13, 910. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Parrettini, S.; Caroli, A.; Torlone, E. Nutrition and metabolic adaptations in physiological and complicated pregnancy: Focus on obesity and gestational diabetes. Front. Endocrinol. 2020, 11, 611929. [Google Scholar] [CrossRef]
- Corrales, P.; Vidal-Puig, A.; Medina-Gómez, G. Obesity and pregnancy, the perfect metabolic storm. Eur. J. Clin. Nutr. 2021, 75, 1723–1734. [Google Scholar] [CrossRef]
- Winer, S.; Winer, D.A. The adaptive immune system as a fundamental regulator of adipose tissue inflammation and insulin resistance. Immunol. Cell Biol. 2012, 90, 755–762. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Flippo, K.H.; Potthoff, M.J. Metabolic messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Mangelsdorf, D.J. A dozen years of discovery: Insights into the physiology and pharmacology of FGF21. Cell Metab. 2019, 29, 246–253. [Google Scholar] [CrossRef]
- Lee, S.; Choi, J.; Mohanty, J.; Sousa, L.P.; Tome, F.; Pardon, E.; Steyaert, J.; Lemmon, M.A.; Lax, I.; Schlessinger, J. Structures of β-klotho reveal a ‘zip code’-like mechanism for endocrine FGF signalling. Nature 2018, 553, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Kolumam, G.; Stawicki, S.; Chen, Y.; Li, J.; Zavala-Solorio, J.; Phamluong, K.; Feng, B.; Li, L.; Marsters, S. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci. Transl. Med. 2011, 3, 113r–126r. [Google Scholar] [CrossRef] [PubMed]
- Dutchak, P.A.; Katafuchi, T.; Bookout, A.L.; Choi, J.H.; Ruth, T.Y.; Mangelsdorf, D.J.; Kliewer, S.A. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 2012, 148, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef]
- Holland, W.L.; Adams, A.C.; Brozinick, J.T.; Bui, H.H.; Miyauchi, Y.; Kusminski, C.M.; Bauer, S.M.; Wade, M.; Singhal, E.; Cheng, C.C. An FGF21-adiponectin-ceramide axis controls energy expenditure and insulin action in mice. Cell Metab. 2013, 17, 790–797. [Google Scholar] [CrossRef]
- Fisher, F.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 2010, 59, 2781–2789. [Google Scholar] [CrossRef]
- Lu, J.; Gong, Y.; Wei, X.; Yao, Z.; Yang, R.; Xin, J.; Gao, L.; Shao, S. Changes in hepatic triglyceride content with the activation of ER stress and increased FGF21 secretion during pregnancy. Nutr. Metab. 2021, 18, 40. [Google Scholar] [CrossRef]
- Redondo-Angulo, I.; Mas-Stachurska, A.; Sitges, M.; Tinahones, F.J.; Giralt, M.; Villarroya, F.; Planavila, A. Fgf21 is required for cardiac remodeling in pregnancy. Cardiovasc. Res. 2017, 113, 1574–1584. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.; Cui, Z.; Ma, K.; Wu, D.; Luo, J.; Li, F.; Xiong, W.; Rao, S.; Xiang, Q. Lachnospiraceae-derived butyrate mediates protection of high fermentable fiber against placental inflammation in gestational diabetes mellitus. Sci. Adv. 2023, 9, i7337. [Google Scholar] [CrossRef]
- Feuerer, M.; Herrero, L.; Cipolletta, D.; Naaz, A.; Wong, J.; Nayer, A.; Lee, J.; Goldfine, A.B.; Benoist, C.; Shoelson, S. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009, 15, 930–939. [Google Scholar] [CrossRef]
- Kälin, S.; Becker, M.; Ott, V.B.; Serr, I.; Hosp, F.; Mollah, M.M.; Keipert, S.; Lamp, D.; Rohner-Jeanrenaud, F.; Flynn, V.K. A Stat6/Pten axis links regulatory T cells with adipose tissue function. Cell Metab. 2017, 26, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Donato, J., Jr. Programming of metabolism by adipokines during development. Nat. Rev. Endocrinol. 2023, 19, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, G.; Fang, Q.; Zhang, M.; Hui, X.; Sheng, B.; Wu, L.; Bao, Y.; Li, P.; Xu, A. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat. Commun. 2018, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef]
- Hu, C.; Qiao, W.; Li, X.; Ning, Z.; Liu, J.; Dalangood, S.; Li, H.; Yu, X.; Zong, Z.; Wen, Z. Tumor-secreted FGF21 acts as an immune suppressor by rewiring cholesterol metabolism of CD8+ T cells. Cell Metab. 2024, 36, 630–647. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, L.; Lee, J.T.H.; Zhang, J.; Wu, D.; Geng, L.; Wang, Y.; Wong, C.; Xu, A. The FGF21-CCL11 axis mediates beiging of white adipose tissues by coupling sympathetic nervous system to type 2 immunity. Cell Metab. 2017, 26, 493–508. [Google Scholar] [CrossRef]
- Hua, L.; Zhuo, Y.; Jiang, D.; Li, J.; Huang, X.; Zhu, Y.; Li, Z.; Yan, L.; Jin, C.; Jiang, X. Identification of hepatic fibroblast growth factor 21 as a mediator in 17β-estradiol-induced white adipose tissue browning. FASEB J. 2018, 32, 5602–5611. [Google Scholar] [CrossRef]
- Sullivan, A.I.; Jensen-Cody, S.O.; Claflin, K.E.; Vorhies, K.E.; Flippo, K.H.; Potthoff, M.J. Characterization of FGF21 Sites of Production and Signaling in Mice. Endocrinology 2024, 165, e120. [Google Scholar] [CrossRef]
- Ming, A.Y.; Yoo, E.; Vorontsov, E.N.; Altamentova, S.M.; Kilkenny, D.M.; Rocheleau, J.V. Dynamics and distribution of Klothoβ (KLB) and fibroblast growth factor receptor-1 (FGFR1) in living cells reveal the fibroblast growth factor-21 (FGF21)-induced receptor complex. J. Biol. Chem. 2012, 287, 19997–20006. [Google Scholar] [CrossRef]
- Rahman, M.S. Prostacyclin: A major prostaglandin in the regulation of adipose tissue development. J. Cell. Physiol. 2019, 234, 3254–3262. [Google Scholar] [CrossRef]
- Garcia-Alonso, V.; Titos, E.; Alcaraz-Quiles, J.; Rius, B.; Lopategi, A.; Lopez-Vicario, C.; Jakobsson, P.; Delgado, S.; Lozano, J.; Claria, J. Prostaglandin E2 exerts multiple regulatory actions on human obese adipose tissue remodeling, inflammation, adaptive thermogenesis and lipolysis. PLoS ONE 2016, 11, e153751. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Tamura, Y.; Minami, M.; Higuchi, S.; Fujikawa, R.; Ikedo, T.; Nagata, M.; Arai, H.; Murayama, T.; Yokode, M. The prostaglandin E2 receptor EP4 regulates obesity-related inflammation and insulin sensitivity. PLoS ONE 2015, 10, e136304. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.C.; Hsiao, F.C.; Chang, H.M.; Wabitsch, M.; Hsieh, P.S. Importance of adipocyte cyclooxygenase-2 and prostaglandin E2-prostaglandin E receptor 3 signaling in the development of obesity-induced adipose tissue inflammation and insulin resistance. FASEB J. 2016, 30, 2282–2297. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, L.; Yang, Y.; Zhang, H.; Jiang, X.; Jin, C.; Feng, B.; Che, L.; Xu, S.; Lin, Y.; Wu, D.; et al. Adipocyte FGF21 Signaling Defect Aggravated Adipose Tissue Inflammation in Gestational Diabetes Mellitus. Nutrients 2024, 16, 3826. https://doi.org/10.3390/nu16223826
Hua L, Yang Y, Zhang H, Jiang X, Jin C, Feng B, Che L, Xu S, Lin Y, Wu D, et al. Adipocyte FGF21 Signaling Defect Aggravated Adipose Tissue Inflammation in Gestational Diabetes Mellitus. Nutrients. 2024; 16(22):3826. https://doi.org/10.3390/nu16223826
Chicago/Turabian StyleHua, Lun, Yi Yang, Haoqi Zhang, Xuemei Jiang, Chao Jin, Bin Feng, Lianqiang Che, Shengyu Xu, Yan Lin, De Wu, and et al. 2024. "Adipocyte FGF21 Signaling Defect Aggravated Adipose Tissue Inflammation in Gestational Diabetes Mellitus" Nutrients 16, no. 22: 3826. https://doi.org/10.3390/nu16223826
APA StyleHua, L., Yang, Y., Zhang, H., Jiang, X., Jin, C., Feng, B., Che, L., Xu, S., Lin, Y., Wu, D., & Zhuo, Y. (2024). Adipocyte FGF21 Signaling Defect Aggravated Adipose Tissue Inflammation in Gestational Diabetes Mellitus. Nutrients, 16(22), 3826. https://doi.org/10.3390/nu16223826







