Anti-Inflammatory Effect of Ethanol Extract from Hibiscus cannabinus L. Flower in Diesel Particulate Matter-Stimulated HaCaT Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Preparation of Diesel Particulate Matter (DPM)
2.3. Preparation of Hibiscus cannabinus L. Flower Ethanol Extract (HCFE)
2.4. High-Performance Liquid Chromatography Analysis of Hibiscus cannabinus L. Flower Ethanol Extract (HCFE)
2.5. Analysis of Total Anthocyanin Content
2.6. DPPH Radical Scavenging Assay
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. Measurement of Reactive Oxygen Species (ROS) Production
2.10. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (PCR)
2.11. Western Blotting
2.12. Statistical Analysis
3. Results
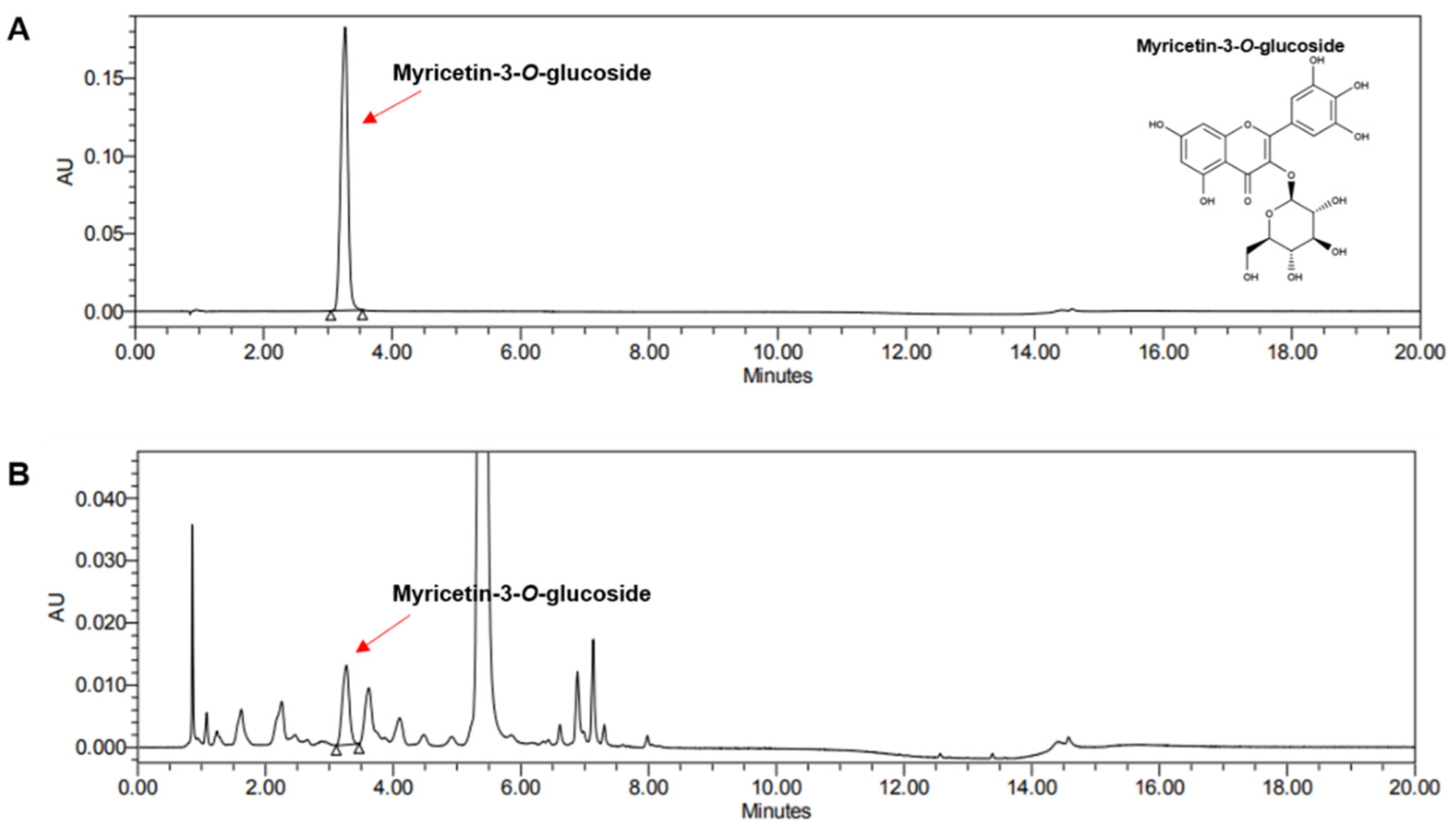
3.1. Ultra Performance Liquid Chromatography Analysis of Hibiscus cannabinus L. Flower Ethanol Extract (HCFE)
3.2. Total Anthocyanin Content of HCFE
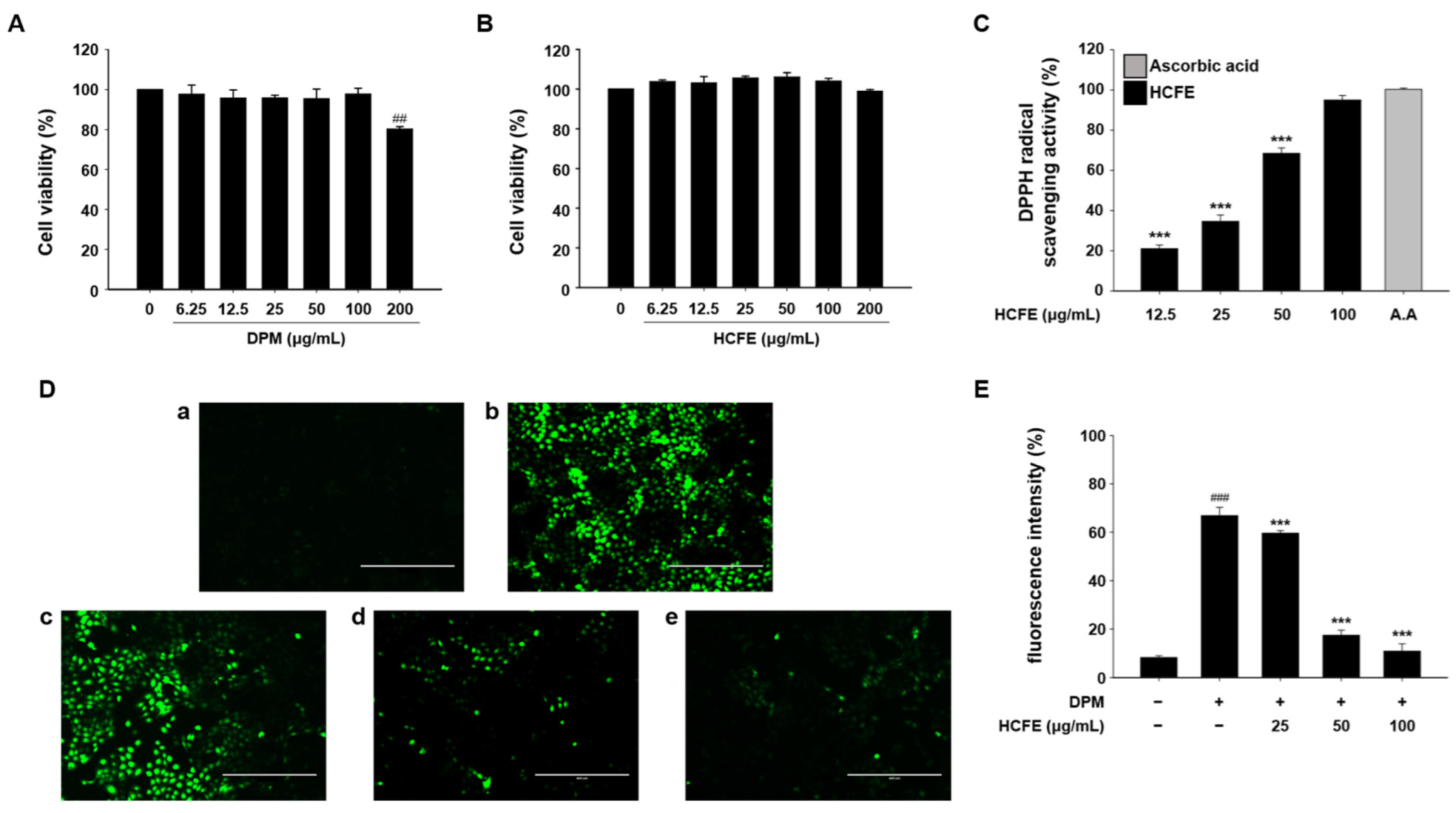
3.3. Antioxidant Effect of HCFE in DPM-Stimulated HaCaT Cells
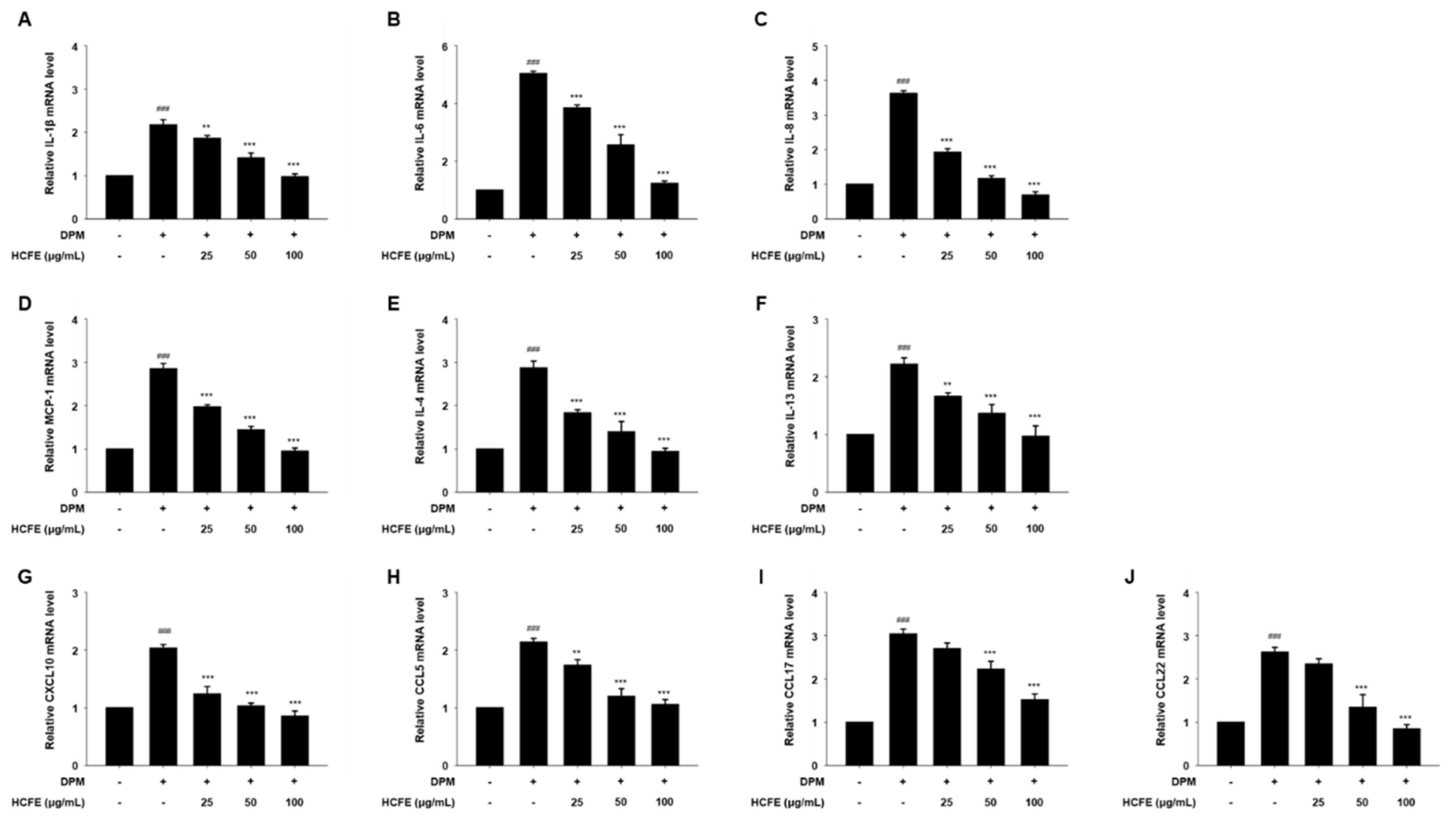
3.4. Anti-Inflammatory and Anti-Atopic Dermatitis Effects on HCFE Through Reduced Gene Expression in DPM-Stimulated HaCaT Cells
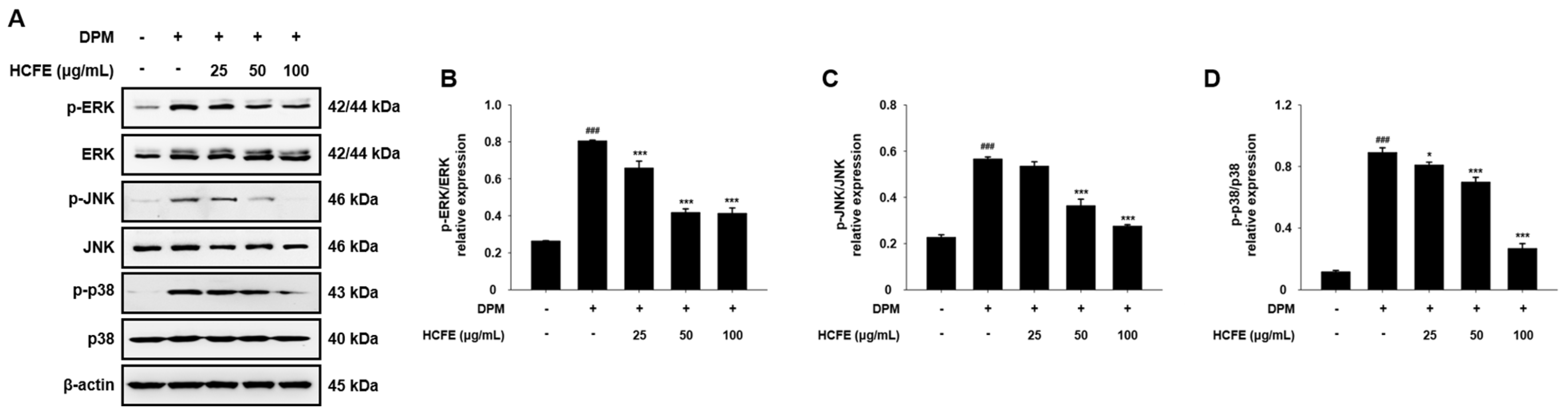
3.5. Inhibitory Effect of HCFE on MAPK Phosphorylation in DPM-Stimulated HaCaT Cells
3.6. Inhibitory Effect of HCFE on NF-κB Translocation to the Nucleus in DPM-Stimulated HaCaT Cells
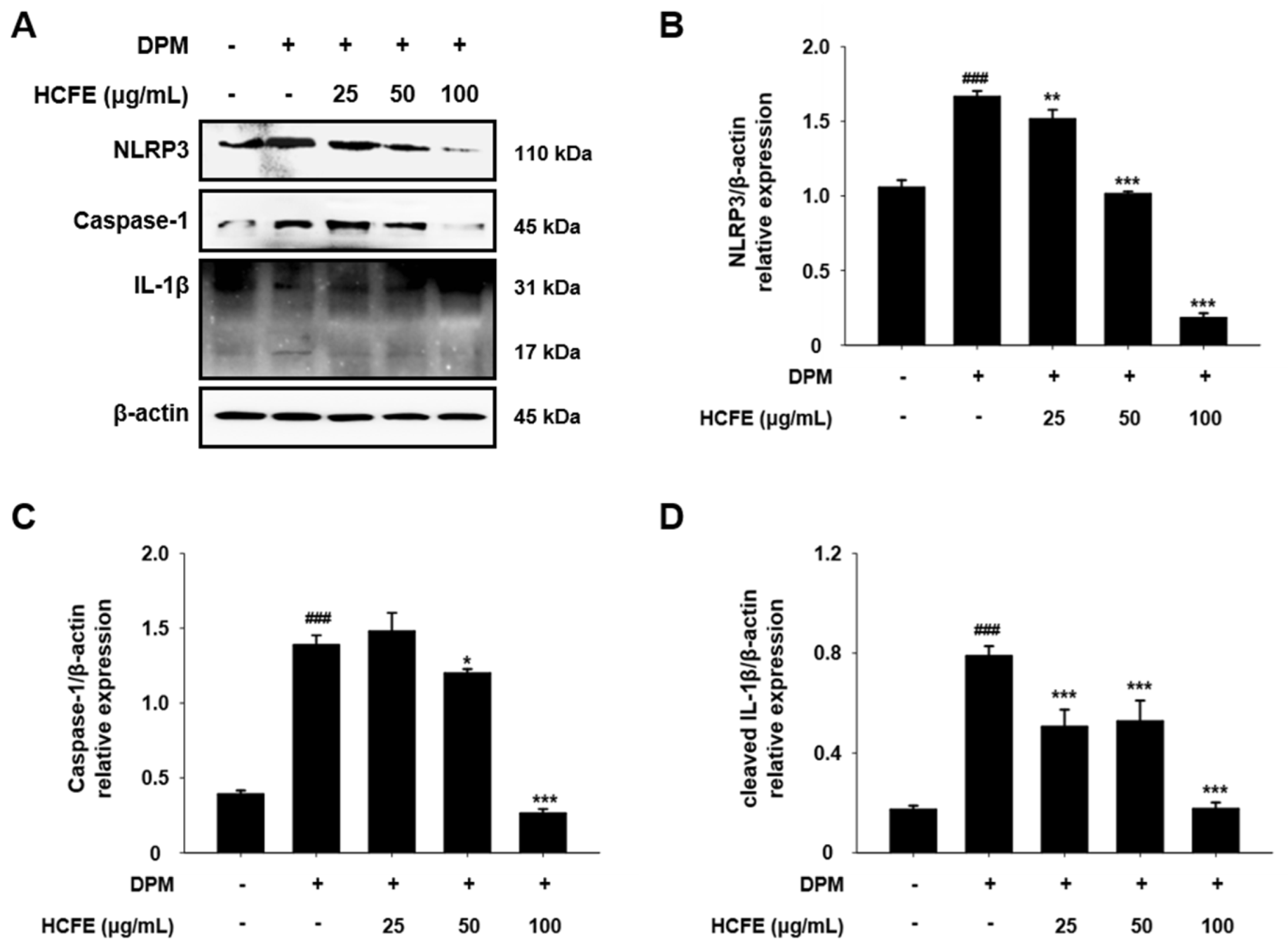
3.7. NLRP3 Inflammasome Down-Regulation by HCFE in DPM-Stimulated HaCaT Cells
3.8. Inhibitory Effect of HCFE on JAK1/STAT6 Pathway in DPM-Stimulated HaCaT Cells
3.9. Up-Regulation of Skin Moisturizing Factors by HCFE in DPM-Stimulated HaCaT Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, J.; Harrison, R.M.; Chen, Q.; Rutter, A.; Schauer, J. Source apportionment of fine particles at urban background and rural sites in the UK atmosphere. Atmos. Environ. 2010, 44, 841–851. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef] [PubMed]
- Alemayehu, Y.A.; Asfaw, S.L.; Terfie, T.A. Exposure to urban particulate matter and its association with human health risks. Environ. Sci. Pollut. Res. Int. 2020, 27, 27491–27506. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chiu, C.-C.; Lee, P.-Y.; Chen, K.-J.; He, C.-X.; Hsu, S.-K.; Cheng, K.-C.J.I.J.o.E.R.; Health, P. The adverse effects of air pollution on the eye: A review. Int. J. Environ. Res. Public Health 2022, 19, 1186. [Google Scholar] [CrossRef] [PubMed]
- Waidyatillake, N.T.; Campbell, P.T.; Vicendese, D.; Dharmage, S.C.; Curto, A.; Stevenson, M. Particulate matter and premature mortality: A Bayesian meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 7655. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM(2.5))-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef] [PubMed]
- Hantrakool, S.; Kumfu, S.; Chattipakorn, S.C.; Chattipakorn, N. Effects of particulate matter on inflammation and thrombosis: Past evidence for future prevention. Int. J. Environ. Res. Public Health 2022, 19, 8771. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fu, J.; Zhou, Y. Research Progress in Atopic March. Front. Immunol. 2020, 11, 1907. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N. Barrier Epithelial Cells and the Control of Type 2 Immunity. Immunity 2015, 43, 29–40. [Google Scholar] [CrossRef]
- Agrawal, R.; Woodfolk, J.A. Skin barrier defects in atopic dermatitis. Curr. Allergy Asthma Rep. 2014, 14, 433. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.M. Significance of Skin Barrier Dysfunction in Atopic Dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Hoisnard, L.; Lebrun-Vignes, B.; Maury, S.; Mahevas, M.; El Karoui, K.; Roy, L.; Zarour, A.; Michel, M.; Cohen, J.L.; Amiot, A.; et al. Adverse events associated with JAK inhibitors in 126,815 reports from the WHO pharmacovigilance database. Sci. Rep. 2022, 12, 7140. [Google Scholar] [CrossRef] [PubMed]
- Stacey, S.K.; McEleney, M. Topical corticosteroids: Choice and application. Am. Fam. Physician 2021, 103, 337–343. [Google Scholar] [PubMed]
- Ahmad Khan, M.S.; Ahmad, I. Herbal Medicine: Current Trends and Future Prospects. In New Look to Phytomedicine; Academic Press: Cambridge, MA, USA, 2019; pp. 3–13. [Google Scholar]
- Ahuja, A.; Gupta, J.; Gupta, R. Miracles of Herbal Phytomedicines in Treatment of Skin Disorders: Natural Healthcare Perspective. Infect. Disord. Drug Targets 2021, 21, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Austin, C.C.; Mondell, C.N.; Clark, D.G.; Wilkie, A.C. Kenaf: Opportunities for an Ancient Fiber Crop. Agronomy 2024, 14, 1542. [Google Scholar] [CrossRef]
- Adnan, M.; Oh, K.K.; Azad, M.O.K.; Shin, M.H.; Wang, M.H.; Cho, D.H. Kenaf (Hibiscus cannabinus L.) Leaves and Seed as a Potential Source of the Bioactive Compounds: Effects of Various Extraction Solvents on Biological Properties. Life 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kwon, S.-J.; Ahn, J.-W.; Jo, Y.D.; Kim, S.H.; Jeong, S.W.; Lee, M.K.; Kim, J.-B.; Kang, S.-Y. Phytochemicals and antioxidant activity in the kenaf plant (Hibiscus cannabinus L.). Plant Biotechnol. 2017, 44, 191–202. [Google Scholar] [CrossRef]
- Abd Ghafar, S.A.; Yazan, L.S.; Tahir, P.M.; Ismail, M. Kenaf seed supercritical fluid extract reduces aberrant crypt foci formation in azoxymethane-induced rats. Exp. Toxicol. Pathol. 2012, 64, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.M.; Kang, M.C.; Jin, M.; Lee, T.H.; Lim, B.O.; Kim, S.Y. Fermented blueberry and black rice containing Lactobacillus plantarum MG4221: A novel functional food for particulate matter (PM 2.5)/dinitrochlorobenzene (DNCB)-induced atopic dermatitis. Food Funct. 2021, 12, 3611–3623. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, F.S.; Li, W.; Beta, T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem. 2008, 109, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Frezzini, M.A.; Castellani, F.; De Francesco, N.; Ristorini, M.; Canepari, S. Application of DPPH assay for assessment of particulate matter reducing properties. Atmosphere 2019, 10, 816. [Google Scholar] [CrossRef]
- Plumb, J.A. Cell sensitivity assays: The MTT assay. Cancer Cell Cult. Methods Protoc. 2004, 88, 165–169. [Google Scholar]
- Eruslanov, E.; Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. Adv. Protoc. Oxidative Stress II 2010, 594, 57–72. [Google Scholar] [CrossRef]
- Nolan, R.; Reeb, K.; Rong, Y.; Matt, S.; Johnson, H.; Runner, K.; Gaskill, P.J.B. Dopamine activates NF-κB and primes the NLRP3 inflammasome in primary human macrophages. Brain Behav. Immun.-Health 2020, 2, 100030. [Google Scholar] [CrossRef]
- Bao, L.; Shi, V.Y.; Chan, L.S. IL-4 regulates chemokine CCL26 in keratinocytes through the Jak1, 2/Stat6 signal transduction pathway: Implication for atopic dermatitis. Mol. Immunol. 2012, 50, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular mechanisms of atopic dermatitis pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef] [PubMed]
- Kaaz, K.; Szepietowski, J.C.; Matusiak, Ł. Influence of itch and pain on sleep quality in atopic dermatitis and psoriasis. Acta Derm.-Venereol. 2019, 99, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Shin, D.B.; Gelfand, J.M. Association between atopic dermatitis and learning disability in children. J. Allergy Clin. Immunol. Pract. 2020, 8, 2808–2810. [Google Scholar] [CrossRef]
- Wahlgren, C.F. Itch and atopic dermatitis: An overview. J. Dermatol. 1999, 26, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, S.F. Atopic Dermatitis: Natural History, Diagnosis, and Treatment. ISRN Allergy 2014, 2014, 354250. [Google Scholar] [CrossRef] [PubMed]
- Montes-Torres, A.; Llamas-Velasco, M.; Pérez-Plaza, A.; Solano-López, G.; Sánchez-Pérez, J. Biological treatments in atopic dermatitis. J. Clin. Med. 2015, 4, 593–613. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-J.; Kim, B.; Lee, K. Air pollution exposure and cardiovascular disease. Toxicol. Res. 2014, 30, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Glencross, D.A.; Ho, T.R.; Camina, N.; Hawrylowicz, C.M.; Pfeffer, P.E. Air pollution and its effects on the immune system. Free Radic. Biol. Med. 2020, 151, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W. Air pollution and skin disorders. Int. J. Womens Dermatol. 2021, 7, 91–97. [Google Scholar] [CrossRef]
- Bonamonte, D.; Filoni, A.; Vestita, M.; Romita, P.; Foti, C.; Angelini, G. The role of the environmental risk factors in the pathogenesis and clinical outcome of atopic dermatitis. Biomed. Res. Int. 2019, 2019, 2450605. [Google Scholar] [CrossRef]
- Sim, Y.Y.; Nyam, K.L. Hibiscus cannabinus L. (kenaf) studies: Nutritional composition, phytochemistry, pharmacology, and potential applications. Food Chem. 2021, 344, 128582. [Google Scholar] [CrossRef]
- Sim, Y.Y.; Tan, C.P.; Cheong, L.Z.; Nyam, K.L. Hibiscus cannabinus L. leaf and seed in cosmetic formulation: An integrated approach as antioxidant and melanogenesis inhibitor. Sustain. Mater. Technol. 2022, 33, e00457. [Google Scholar] [CrossRef]
- Norhisham, D.A.; Saad, N.M.; Ahmad Usuldin, S.R.; Vayabari, D.A.G.; Ilham, Z.; Ibrahim, M.F.; Wan-Mohtar, W.A.A.Q.I. Bioactivities of Kenaf Biomass Extracts: A Review. Processes 2023, 11, 1178. [Google Scholar] [CrossRef]
- Abd Ghafar, S.A.; Ismail, M.; Saiful Yazan, L.; Fakurazi, S.; Ismail, N.; Chan, K.W.; Md Tahir, P. Cytotoxic activity of kenaf seed oils from supercritical carbon dioxide fluid extraction towards human colorectal cancer (HT29) cell lines. Evid. Based Complement. Altern. Med. 2013, 2013, 549705. [Google Scholar] [CrossRef] [PubMed]
- Sim, Y.Y.; Nyam, K.L. Application of Hibiscus cannabinus L.(kenaf) leaves extract as skin whitening and anti-aging agents in natural cosmetic prototype. Ind. Crops Prod. 2021, 167, 113491. [Google Scholar] [CrossRef]
- Sim, Y.Y.; Ong, W.T.J.; Nyam, K.L. Effect of various solvents on the pulsed ultrasonic assisted extraction of phenolic compounds from Hibiscus cannabinus L. leaves. Ind. Crops Prod. 2019, 140, 111708. [Google Scholar] [CrossRef]
- GrKalaiyan, G.; Prabu, K.; Suresh, N.; Suresh, S. Green synthesis of copper oxide spindle like nanostructure using Hibiscus cannabinus flower extract for antibacterial and anticancer activity applications. Results Chem. 2023, 5, 100840. [Google Scholar] [CrossRef]
- Cho, B.O.; Yin, H.H.; Park, S.H.; Byun, E.B.; Ha, H.Y.; Jang, S.I. Anti-inflammatory activity of myricetin from Diospyros lotus through suppression of NF-κB and STAT1 activation and Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated RAW264. 7 macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1520–1530. [Google Scholar] [CrossRef]
- Agraharam, G.; Girigoswami, A.; Girigoswami, K. Myricetin: A multifunctional flavonol in biomedicine. Curr. Pharmacol. Rep. 2022, 8, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.-i.; Mizutani, H. The role of cytokines/chemokines in the pathogenesis of atopic dermatitis. Curr. Probl. Dermatol. 2011, 41, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, X.-K. Oxidative stress in atopic dermatitis. Oxid. Med. Cell Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [PubMed]
- Leuti, A.; Fazio, D.; Fava, M.; Piccoli, A.; Oddi, S.; Maccarrone, M. Bioactive lipids, inflammation and chronic diseases. Adv. Drug Deliv. Rev. 2020, 159, 133–169. [Google Scholar] [CrossRef] [PubMed]
- Fania, L.; Moretta, G.; Antonelli, F.; Scala, E.; Abeni, D.; Albanesi, C.; Madonna, S. Multiple Roles for Cytokines in Atopic Dermatitis: From Pathogenic Mediators to Endotype-Specific Biomarkers to Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 2684. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Hyun, Y.-M.; Kim, S.H.; Ko, M.K.; Park, C.O.; Hyun, J.W. Particulate matter induces inflammatory cytokine production via activation of NFκB by TLR5-NOX4-ROS signaling in human skin keratinocyte and mouse skin. Redox Biol. 2019, 21, 101080. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Moens, U.; Kostenko, S.; Sveinbjørnsson, B. The role of mitogen-activated protein kinase-activated protein kinases (MAPKAPKs) in inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef]
- Suzuki, M.; Tetsuka, T.; Yoshida, S.; Watanabe, N.; Kobayashi, M.; Matsui, N.; Okamoto, T. The role of p38 mitogen-activated protein kinase in IL-6 and IL-8 production from the TNF-α-or IL-1β-stimulated rheumatoid synovial fibroblasts. FEBS Lett. 2000, 465, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Bowers, E.C.; McCullough, S.D.; Morgan, D.S.; Dailey, L.A.; Diaz-Sanchez, D. ERK1/2 and p38 regulate inter-individual variability in ozone-mediated IL-8 gene expression in primary human bronchial epithelial cells. Sci. Rep. 2018, 8, 9398. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Arias-Perez, R.D.; Taborda, N.A.; Gomez, D.M.; Narvaez, J.F.; Porras, J.; Hernandez, J.C. Inflammatory effects of particulate matter air pollution. Environ. Sci. Pollut. Res. Int. 2020, 27, 42390–42404. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.B.; Sivaprasad, U. Th2 Cytokines and Atopic Dermatitis. J. Clin. Cell Immunol. 2011, 2, 110. [Google Scholar] [CrossRef] [PubMed]
- Rerknimitr, P.; Otsuka, A.; Nakashima, C.; Kabashima, K. Skin Barrier Function and Atopic Dermatitis. Curr. Dermatol. Rep. 2018, 7, 209–220. [Google Scholar] [CrossRef]



| Gene | Primer Sequence | |
|---|---|---|
| IL-1β | Forward: | 5′-CTCTCTCACCTCTCCTACTCAC-3′ |
| Reverse: | 5′-ACACTGCCTACTTCTTGCCCC-3′ | |
| IL-4 | Forward: | 5′-ACATTGTCACTGCAAATCGACACC-3′ |
| Reverse: | 5′-TGTCTGTTACGGTCAACTCGGTGC-3′ | |
| IL-6 | Forward: | 5′-CTCCAC AAGCGCCTTCGGTC -3′ |
| Reverse: | 5′-TGTGTGGGGCGGCTACATCT-3′ | |
| IL-8 | Forward: | 5′-ACCGGAGCACTCCATAAGGCA-3′ |
| Reverse: | 5′-AGGCTGCCAAGAGAGCCACG-3′ | |
| IL-13 | Forward: | 5′-ACCACGGTCATTGCTCTCACT-3′ |
| Reverse: | 5′-GTCAGGTTGATGCTCCATAC-3′ | |
| MCP-1 | Forward: | 5′-TCTGTGCCTGCTGCTCATAG-3′ |
| Reverse: | 5′-CAGATCTCCTTGGCCACAAT-3′ | |
| CXCL10 | Forward: | 5′-TTGCTGCCTTATCTTTCTGACTC-3′ |
| Reverse: | 5′-ATGGCCTTCGATTCTGGATT-3′ | |
| CCL5 | Forward: | 5′-CGCTGTCATCCTCATTGCTA-3′ |
| Reverse: | 5′-GCACTTGCCACTGGTGTAGA-3′ | |
| CCL17 | Forward: | 5′-CCATTCCCCTTAGAAAGCTG-3′ |
| Reverse: | 5′-CTCTCAAGGCTTTGCAGGTA-3′ | |
| CCL22 | Forward: | 5′-TGCCGTGATTACGTCCGTTAC-3′ |
| Reverse: | 5′-AAGGCCACGGTCATCAGAGTAG-3′ | |
| GAPDH | Forward: | 5′-GAAGGTGAAGGTCGGAGT-3′ |
| Reverse: | 5′-GAAGATGGTGATGGGATTTC-3′ |
| Sample | Absorbance | A′ | Content (mg/100 mg) | Myricetin-3-O-Glucoside (mg/g) | |||
|---|---|---|---|---|---|---|---|
| pH 1.0 | pH 4.5 | ||||||
| λ 520 nm | λ 700 nm | λ 520 nm | λ 700 nm | ||||
| HCFE | 0.250 | 0.019 | 0.072 | 0.020 | 0.178 | 1.490 | 2.43 ± 0.06 |
| 0.258 | 0.020 | 0.072 | 0.020 | 0.186 | 1.553 | ||
| 0.255 | 0.019 | 0.064 | 0.014 | 0.187 | 1.557 | ||
| Average | 1.53 ± 0.031 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.-Y.; Kim, S.-K.; Lim, D.-W.; Kwon, O.; Choi, Y.-R.; Kang, C.-H.; Lee, Y.J.; Lee, Y.-M. Anti-Inflammatory Effect of Ethanol Extract from Hibiscus cannabinus L. Flower in Diesel Particulate Matter-Stimulated HaCaT Cells. Nutrients 2024, 16, 3805. https://doi.org/10.3390/nu16223805
Han J-Y, Kim S-K, Lim D-W, Kwon O, Choi Y-R, Kang C-H, Lee YJ, Lee Y-M. Anti-Inflammatory Effect of Ethanol Extract from Hibiscus cannabinus L. Flower in Diesel Particulate Matter-Stimulated HaCaT Cells. Nutrients. 2024; 16(22):3805. https://doi.org/10.3390/nu16223805
Chicago/Turabian StyleHan, Ji-Ye, Shin-Kyeom Kim, Do-Won Lim, Osoung Kwon, Yu-Rim Choi, Chan-Ho Kang, Yun Jung Lee, and Young-Mi Lee. 2024. "Anti-Inflammatory Effect of Ethanol Extract from Hibiscus cannabinus L. Flower in Diesel Particulate Matter-Stimulated HaCaT Cells" Nutrients 16, no. 22: 3805. https://doi.org/10.3390/nu16223805
APA StyleHan, J.-Y., Kim, S.-K., Lim, D.-W., Kwon, O., Choi, Y.-R., Kang, C.-H., Lee, Y. J., & Lee, Y.-M. (2024). Anti-Inflammatory Effect of Ethanol Extract from Hibiscus cannabinus L. Flower in Diesel Particulate Matter-Stimulated HaCaT Cells. Nutrients, 16(22), 3805. https://doi.org/10.3390/nu16223805






