Dietary Intake Is Similar Among Adult Men with Different Levels of Cold-Induced Brown Adipose Tissue Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment of Dietary Intake
2.3. Anthropometry and Body Composition
2.4. Experimental Protocol
2.4.1. Cold Exposure Protocol
2.4.2. PET/CT Examination
2.5. Statistical Analysis
3. Results
3.1. General Description of Study Participants
3.2. Cold-Induced Brown Adipose Tissue Activation
3.3. Comparison of Dietary Intake Variables Between BATnegative and BATpositive Participants
3.4. Associations Between Cold-Induced BAT Activation (SUVlean) and Dietary Intake Among BATpositive Participants
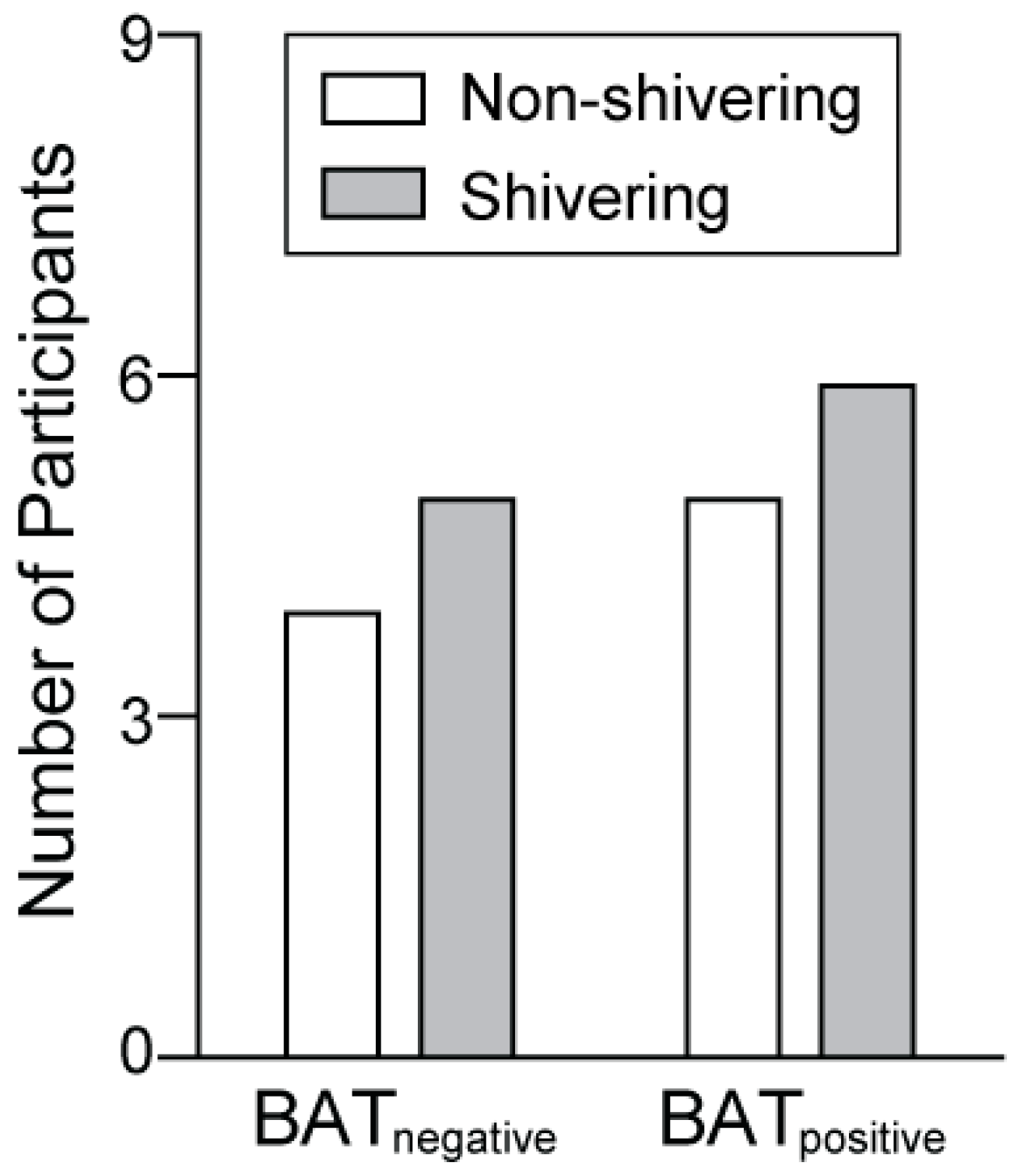
3.5. Comparison of Cold-Induced Shivering Intensity Between BATnegative and BATpositive Participants
3.6. Comparison of Anthropometry and Dietary Intake Variables Between the Non-Shivering and Shivering Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G.; Locke, R.M. Thermogenic Mechanisms in Brown Fat. Physiol. Rev. 1984, 64, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Ricquier, D. Uncoupling Protein 1 of Brown Adipocytes, the Only Uncoupler: A Historical Perspective. Front. Endocrinol. 2011, 2, 85. [Google Scholar] [CrossRef] [PubMed]
- Zingaretti, M.C.; Crosta, F.; Vitali, A.; Guerrieri, M.; Frontini, A.; Cannon, B.; Nedergaard, J.; Cinti, S. The Presence of UCP1 Demonstrates That Metabolically Active Adipose Tissue in the Neck of Adult Humans Truly Represents Brown Adipose Tissue. FASEB J. 2009, 23, 3113–3120. [Google Scholar] [CrossRef]
- Ouellet, V.; Labbé, S.M.; Blondin, D.P.; Phoenix, S.; Guérin, B.; Haman, F.; Turcotte, E.E.; Richard, D.; Carpentier, A.C. Brown Adipose Tissue Oxidative Metabolism Contributes to Energy Expenditure during Acute Cold Exposure in Humans. J. Clin. Investig. 2012, 122, 545–552. [Google Scholar] [CrossRef]
- Nedergaard, J.; Von Essen, G.; Cannon, B. Brown Adipose Tissue: Can It Keep Us Slim? A Discussion of the Evidence for and against the Existence of Diet-Induced Thermogenesis in Mice and Men. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220220. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, W.; Sun, Y.; Dang, Y.; Gong, F.; Zhu, H.; Li, N.; Li, F.; Zhu, Z. Activating Brown Adipose Tissue for Weight Loss and Lowering of Blood Glucose Levels: A MicroPET Study Using Obese and Diabetic Model Mice. PLoS ONE 2014, 9, e113742. [Google Scholar] [CrossRef]
- Cypess, A.M. Does Activating Brown Fat Contribute to Important Metabolic Benefits in Humans? Yes! J. Clin. Investig. 2023, 133, e175282. [Google Scholar] [CrossRef]
- Gupta, R.K. Human Brown Fat and Metabolic Disease: A Heated Debate. J. Clin. Investig. 2023, 133, e176678. [Google Scholar] [CrossRef]
- Nirengi, S.; Stanford, K. Brown Adipose Tissue and Aging: A Potential Role for Exercise. Exp. Gerontol. 2023, 178, 112218. [Google Scholar] [CrossRef]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schöder, H.; et al. Brown Adipose Tissue Is Associated with Cardiometabolic Health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Dinas, P.C.; Nikaki, A.; Jamurtas, A.Z.; Prassopoulos, V.; Efthymiadou, R.; Koutedakis, Y.; Georgoulias, P.; Flouris, A.D. Association between Habitual Physical Activity and Brown Adipose Tissue Activity in Individuals Undergoing PET—CT Scan. Clin. Endocrinol. (Oxf.) 2015, 82, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Heenan, K.A.; Carrillo, A.E.; Fulton, J.L.; Ryan, E.J.; Edsall, J.R.; Rigopoulos, D.; Markofski, M.M.; Flouris, A.D.; Dinas, P.C. Effects of Nutrition/Diet on Brown Adipose Tissue in Humans: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 2752. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, A.E.; Vliora, M. Adipose Tissue Metabolism in Response to Food Intake. Nutrients 2023, 15, 4811. [Google Scholar] [CrossRef] [PubMed]
- Loeliger, R.C.; Maushart, C.I.; Gashi, G.; Senn, J.R.; Felder, M.; Becker, A.S.; Müller, J.; Balaz, M.; Wolfrum, C.; Burger, I.A.; et al. Relation of Diet-Induced Thermogenesis to Brown Adipose Tissue Activity in Healthy Men. Am. J. Physiol.-Endocrinol. Metab. 2021, 320, E93–E101. [Google Scholar] [CrossRef]
- Aita, S.; Matsushita, M.; Yoneshiro, T.; Hatano, T.; Kameya, T.; Ohkubo, I.; Saito, M. Brown Fat-Associated Postprandial Thermogenesis in Humans: Different Effects of Isocaloric Meals Rich in Carbohydrate, Fat, and Protein. Front. Nutr. 2022, 9, 1040444. [Google Scholar] [CrossRef]
- Van Schaik, L.; Kettle, C.; Green, R.; Irving, H.R.; Rathner, J.A. Effects of Caffeine on Brown Adipose Tissue Thermogenesis and Metabolic Homeostasis: A Review. Front. Neurosci. 2021, 15, 621356. [Google Scholar] [CrossRef]
- Fuse, S.; Endo, T.; Tanaka, R.; Kuroiwa, M.; Ando, A.; Kume, A.; Yamamoto, A.; Kuribayashi, K.; Somekawa, S.; Takeshita, M.; et al. Effects of Capsinoid Intake on Brown Adipose Tissue Vascular Density and Resting Energy Expenditure in Healthy, Middle-Aged Adults: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2020, 12, 2676. [Google Scholar] [CrossRef]
- Sun, L.; Camps, S.G.; Goh, H.J.; Govindharajulu, P.; Schaefferkoetter, J.D.; Townsend, D.W.; Verma, S.K.; Velan, S.S.; Sun, L.; Sze, S.K.; et al. Capsinoids Activate Brown Adipose Tissue (BAT) with Increased Energy Expenditure Associated with Subthreshold 18-Fluorine Fluorodeoxyglucose Uptake in BAT-Positive Humans Confirmed by Positron Emission Tomography Scan. Am. J. Clin. Nutr. 2018, 107, 62–70. [Google Scholar] [CrossRef]
- Okla, M.; Kim, J.; Koehler, K.; Chung, S. Dietary Factors Promoting Brown and Beige Fat Development and Thermogenesis. Adv. Nutr. 2017, 8, 473–483. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Matsushita, M.; Hibi, M.; Tone, H.; Takeshita, M.; Yasunaga, K.; Katsuragi, Y.; Kameya, T.; Sugie, H.; Saito, M. Tea Catechin and Caffeine Activate Brown Adipose Tissue and Increase Cold-Induced Thermogenic Capacity in Humans. Am. J. Clin. Nutr. 2017, 105, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska, K.; Adamska-Patruno, E.; Miniewska, K.; Bauer, W.; Mojsak, M.; Kretowski, A. PET/MRI-Evaluated Brown Adipose Tissue Activity May Be Related to Dietary MUFA and Omega-6 Fatty Acids Intake. Sci. Rep. 2022, 12, 4112. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Delgado, G.; Acosta, F.M.; Martinez-Tellez, B.; Finlayson, G.; Gibbons, C.; Labayen, I.; Llamas-Elvira, J.M.; Gil, A.; Blundell, J.E.; Ruiz, J.R. Brown Adipose Tissue Volume and 18F-Fluorodeoxyglucose Uptake Are Not Associated with Energy Intake in Young Human Adults. Am. J. Clin. Nutr. 2020, 111, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Fasoli, L.; Merchan-Ramirez, E.; Martinez-Tellez, B.; Acosta, F.M.; Sanchez-Delgado, G.; Amaro-Gahete, F.J.; Muñoz Hernandez, V.; Martinez-Avila, W.D.; Ortiz-Alvarez, L.; Xu, H.; et al. Association between Dietary Factors and Brown Adipose Tissue Volume/18F-FDG Uptake in Young Adults. Clin. Nutr. 2021, 40, 1997–2008. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Baxa, U.; Niu, G.; Chen, X.; Veech, R.L. A Ketogenic Diet Increases Brown Adipose Tissue Mitochondrial Proteins and UCP1 Levels in Mice. IUBMB Life 2013, 65, 58–66. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Fan, R.; Kim, J.; Shin, S.-H.; Chung, S. Alpha-Linolenic Acid-Enriched Butter Promotes Fatty Acid Remodeling and Thermogenic Activation in the Brown Adipose Tissue. Nutrients 2020, 12, 136. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ide, T. Dietary N-3 Fatty Acids Affect mRNA Level of Brown Adipose Tissue Uncoupling Protein 1, and White Adipose Tissue Leptin and Glucose Transporter 4 in the Rat. Br. J. Nutr. 2000, 84, 175–184. [Google Scholar] [CrossRef]
- Bargut, T.C.L.; Silva-e-Silva, A.C.A.G.; Souza-Mello, V.; Mandarim-de-Lacerda, C.A.; Aguila, M.B. Mice Fed Fish Oil Diet and Upregulation of Brown Adipose Tissue Thermogenic Markers. Eur. J. Nutr. 2016, 55, 159–169. [Google Scholar] [CrossRef]
- Dinas, P.C.; Valente, A.; Granzotto, M.; Rossato, M.; Vettor, R.; Zacharopoulou, A.; Carrillo, A.E.; Davies, N.A.; Gkiata, P.; Jamurtas, A.Z.; et al. Browning Formation Markers of Subcutaneous Adipose Tissue in Relation to Resting Energy Expenditure, Physical Activity and Diet in Humans. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20170008. [Google Scholar] [CrossRef]
- Dinas, P.C.; Krase, A.; Nintou, E.; Georgakopoulos, A.; Granzotto, M.; Metaxas, M.; Karachaliou, E.; Rossato, M.; Vettor, R.; Georgoulias, P.; et al. Human White-Fat Thermogenesis: Experimental and Meta-Analytic Findings. Temperature 2021, 8, 39–52. [Google Scholar] [CrossRef]
- Chen, K.Y.; Cypess, A.M.; Laughlin, M.R.; Haft, C.R.; Hu, H.H.; Bredella, M.A.; Enerbäck, S.; Kinahan, P.E.; van Marken Lichtenbelt, W.; Lin, F.I.; et al. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): Recommendations for Standardized FDG-PET/CT Experiments in Humans. Cell Metab. 2016, 24, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Du Bois, D.; Du Bois, E.F. A Formula to Estimate the Approximate Surface Area If Height and Weight Be Known. 1916. Nutr. 1989, 5, 303–311, discussion 312–313. [Google Scholar]
- Salamone, L.M.; Fuerst, T.; Visser, M.; Kern, M.; Lang, T.; Dockrell, M.; Cauley, J.A.; Nevitt, M.; Tylavsky, F.; Lohman, T.G. Measurement of Fat Mass Using DEXA: A Validation Study in Elderly Adults. J. Appl. Physiol. 2000, 89, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Smith, S.; Linderman, J.; Courville, A.B.; Brychta, R.J.; Dieckmann, W.; Werner, C.D.; Chen, K.Y.; Celi, F.S. Temperature-Acclimated Brown Adipose Tissue Modulates Insulin Sensitivity in Humans. Diabetes 2014, 63, 3686–3698. [Google Scholar] [CrossRef]
- Reed, G.; Hill, J. Measuring the Thermic Effect of Food. Am. J. Clin. Nutr. 1996, 63, 164–169. [Google Scholar] [CrossRef]
- Williams, B.; Poulter, N.R.; Brown, M.J.; Davis, M.; McInnes, G.T.; Potter, J.F.; Sever, P.S.; Thom, S.M. British Hypertension Society Guidelines for Hypertension Management 2004 (BHS-IV): Summary. BMJ 2004, 328, 634–640. [Google Scholar] [CrossRef]
- Vanderstappen, I.; Vandermeersch, E.; Vanacker, B.; Mattheussen, M.; Herijgers, P.; Van Aken, H. The Effect of Prophylactic Clonidine on Postoperative Shivering: A Large Prospective Double-blind Study. Anaesthesia 1996, 51, 351–355. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P.; Virtanen, K.A.; Richard, D.; Haman, F.; Turcotte, É.E. Brown Adipose Tissue Energy Metabolism in Humans. Front. Endocrinol. 2018, 9, 447. [Google Scholar] [CrossRef]
- Laerd Statistics. Chi-Square Test for Association Using SPSS Statistics. Statistical Tutorials and Software Guides. 2016. Available online: https://statistics.laerd.com/ (accessed on 15 September 2024).
- IBM Corp. IBM SPSS Statistics for Windows, version 29; IBM Corp.: Armonk, NY, USA, 2022. [Google Scholar]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Okamatsu-Ogura, Y.; Kameya, T.; Kawai, Y.; Miyagawa, M.; Tsujisaki, M.; Saito, M. Age-Related Decrease in Cold-Activated Brown Adipose Tissue and Accumulation of Body Fat in Healthy Humans. Obesity 2011, 19, 1755–1760. [Google Scholar] [CrossRef]
- Mele, L.; Bidault, G.; Mena, P.; Crozier, A.; Brighenti, F.; Vidal-Puig, A.; Del Rio, D. Dietary (Poly)Phenols, Brown Adipose Tissue Activation, and Energy Expenditure: A Narrative Review. Adv. Nutr. 2017, 8, 694–704. [Google Scholar] [CrossRef]
- Darcy, J.; Tseng, Y.-H. ComBATing Aging—Does Increased Brown Adipose Tissue Activity Confer Longevity? GeroScience 2019, 41, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Sardjoe Mishre, A.S.D.; Martinez-Tellez, B.; Acosta, F.M.; Sanchez-Delgado, G.; Straat, M.E.; Webb, A.G.; Kan, H.E.; Rensen, P.C.N.; Ruiz, J.R. Association of Shivering Threshold Time with Body Composition and Brown Adipose Tissue in Young Adults. J. Therm. Biol. 2022, 108, 103277. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, D.A. Limitations in the Assessment of Dietary Energy Intake by Self-Report. Metabolism 1995, 44, 18–22. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total (n = 23) | BATnegative (n = 11) | BATpositive (n = 12) | p-Value a |
|---|---|---|---|---|
| Age (years) | 36.9 ± 5.2 | 39.1 ± 4.1 | 34.8 ± 5.4 | 0.046 b |
| Height (cm) | 181.1 ± 6.8 | 182.2 ± 7.5 | 180.0 ±6.2 | 0.434 |
| Weight (kg) | 98.0 ± 25.7 | 105.0 ± 30.6 | 91.6 ± 19.4 | 0.234 |
| Body surface area (m2) | 2.2 ± 0.3 | 2.2 ± 0.3 | 2.1 ± 0.2 | 0.258 |
| BMI (kg/m2) | 29.6 ± 6.0 | 31.1 ± 6.7 | 28.2 ± 5.3 | 0.257 |
| Healthy weight, n (%) | 6 (26.1) | 2 (18.2) | 4 (33.3) | 0.640 c |
| Overweight/obese, n (%) | 17 (73.9) | 9 (81.8) | 8 (66.7) | |
| Waist circumference (cm) | 102.9 ± 16.7 | 106.7 ± 19.2 | 99.5 ± 14.0 | 0.309 |
| Waist to hip ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.297 |
| Percent body fat (%) | 29.6 ± 8.9 | 30.4 ± 8.3 | 28.8 ± 9.6 | 0.660 |
| Fat free mass (kg) | 67.7 ± 11.5 | 71.1 ± 15.0 | 64.6 ± 6.0 | 0.177 |
| Systolic blood pressure (mmHg) | 123.2 ± 9.9 | 127.0 ± 7.3 | 119.8 ± 11.0 | 0.079 |
| Diastolic blood pressure (mmHg) | 82.8 ± 7.7 | 84.9 ± 8.2 | 80.9 ± 6.9 | 0.220 |
| Fasting blood glucose (mg/dL) d | 89.5 ± 7.8 | 90.7 ± 7.1 | 88.4 ± 8.5 | 0.504 |
| Variable | Total (n = 23) | BATnegative (n = 11) | BATpositive (n = 12) | p-Value a |
|---|---|---|---|---|
| Kilocalories | 1897.7 ± 565.8 | 2044.4 ± 576.3 | 1763.2 ± 545.0 | 0.242 |
| Carbohydrate (g) | 189.2 ± 75.3 | 210.5 ± 82.5 | 169.7 ± 65.5 | 0.202 |
| Total fiber (g) | 18.0 ± 8.8 | 19.7 ± 9.3 | 16.4 ± 8.4 | 0.375 |
| Total sugar (g) | 70.1 ± 41.6 | 71.4 ± 44.5 | 68.9 ± 40.7 | 0.888 |
| Protein (g) | 94.8 ± 29.4 | 102.0 ± 30.2 | 88.2 ± 28.4 | 0.271 |
| Fat (g) | 84.5 ± 32.0 | 87.7 ± 34.5 | 81.6 ± 30.8 | 0.657 |
| Saturated fat (g) | 30.8 ± 14.7 | 32.6 ± 15.6 | 29.3 ± 14.3 | 0.600 |
| Monounsaturated fat (g) | 31.0 ± 12.6 | 32.4 ± 13.5 | 29.6 ± 12.1 | 0.603 |
| Polyunsaturated fat (g) | 12.9 ± 3.6 | 13.2 ± 4.1 | 12.5 ± 3.2 | 0.634 |
| ω-6 (g) | 11.1 ± 3.3 | 11.5 ± 3.7 | 10.8 ± 3.1 | 0.616 |
| ω-3 (g) | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 | 0.881 |
| ω-6:ω-3 ratio | 9.5 ± 3.2 | 9.3 ± 1.2 | 9.8 ± 4.4 | 0.755 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrillo, A.E.; Dinas, P.C.; Krase, A.; Nintou, E.; Georgakopoulos, A.; Metaxas, M.; Ryan, E.J.; Vliora, M.; Georgoulias, P.; Chatziioannou, S.; et al. Dietary Intake Is Similar Among Adult Men with Different Levels of Cold-Induced Brown Adipose Tissue Activation. Nutrients 2024, 16, 3697. https://doi.org/10.3390/nu16213697
Carrillo AE, Dinas PC, Krase A, Nintou E, Georgakopoulos A, Metaxas M, Ryan EJ, Vliora M, Georgoulias P, Chatziioannou S, et al. Dietary Intake Is Similar Among Adult Men with Different Levels of Cold-Induced Brown Adipose Tissue Activation. Nutrients. 2024; 16(21):3697. https://doi.org/10.3390/nu16213697
Chicago/Turabian StyleCarrillo, Andres E., Petros C. Dinas, Argyro Krase, Eleni Nintou, Alexandros Georgakopoulos, Marinos Metaxas, Edward J. Ryan, Maria Vliora, Panagiotis Georgoulias, Sofia Chatziioannou, and et al. 2024. "Dietary Intake Is Similar Among Adult Men with Different Levels of Cold-Induced Brown Adipose Tissue Activation" Nutrients 16, no. 21: 3697. https://doi.org/10.3390/nu16213697
APA StyleCarrillo, A. E., Dinas, P. C., Krase, A., Nintou, E., Georgakopoulos, A., Metaxas, M., Ryan, E. J., Vliora, M., Georgoulias, P., Chatziioannou, S., & Flouris, A. D. (2024). Dietary Intake Is Similar Among Adult Men with Different Levels of Cold-Induced Brown Adipose Tissue Activation. Nutrients, 16(21), 3697. https://doi.org/10.3390/nu16213697










