Immunomodulatory Effects of a Prebiotic Formula with 2′-Fucosyllactose and Galacto- and Fructo-Oligosaccharides on Cyclophosphamide (CTX)-Induced Immunosuppressed BALB/c Mice via the Gut–Immune Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Animal Experiment Design
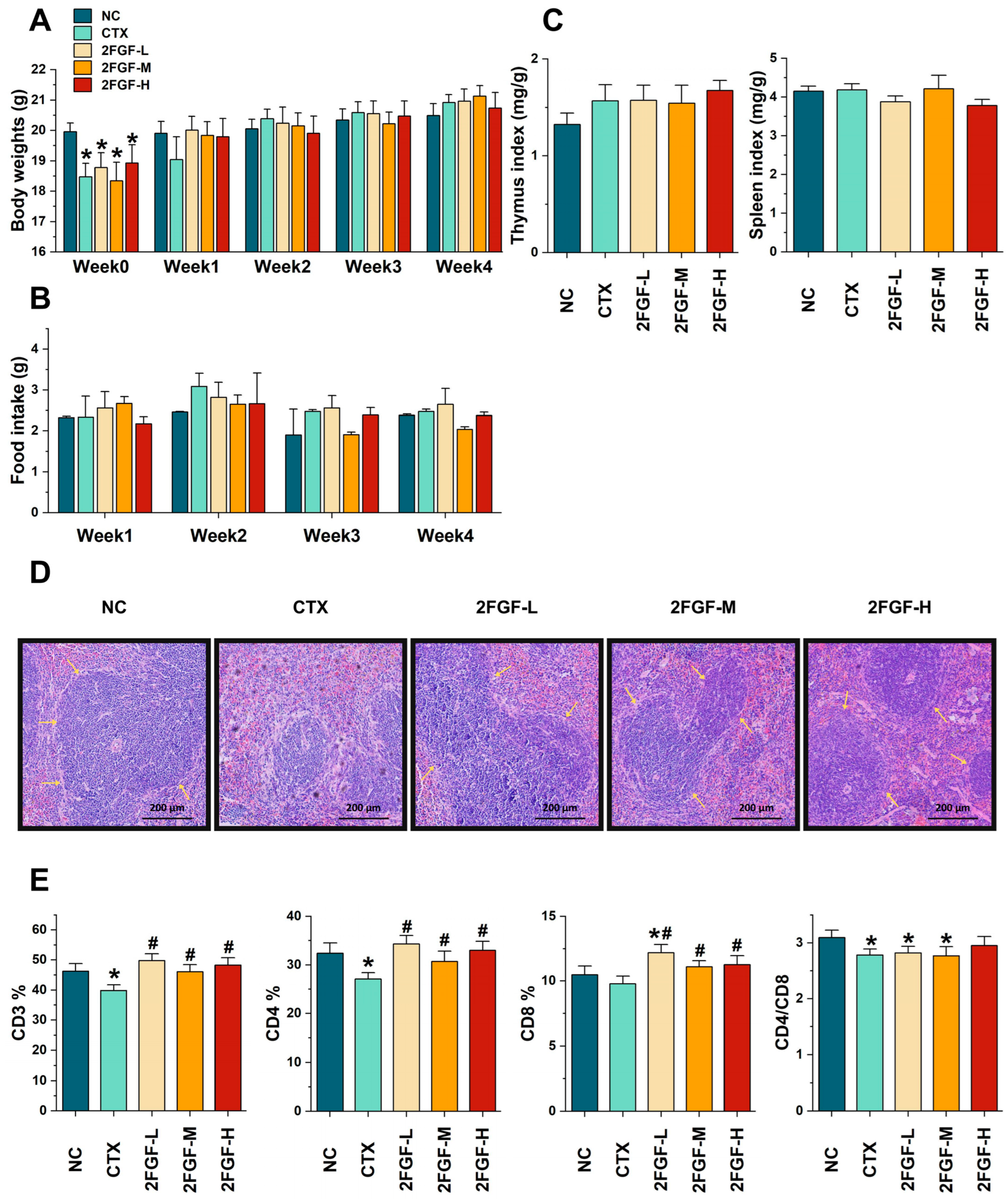
2.3. Determination of the Body Weight, Thymus, and Spleen Indices
2.4. Histological Analysis of the Spleen
2.5. Flow Cytometry Analysis of the Spleen
2.6. Determination of Cytokines
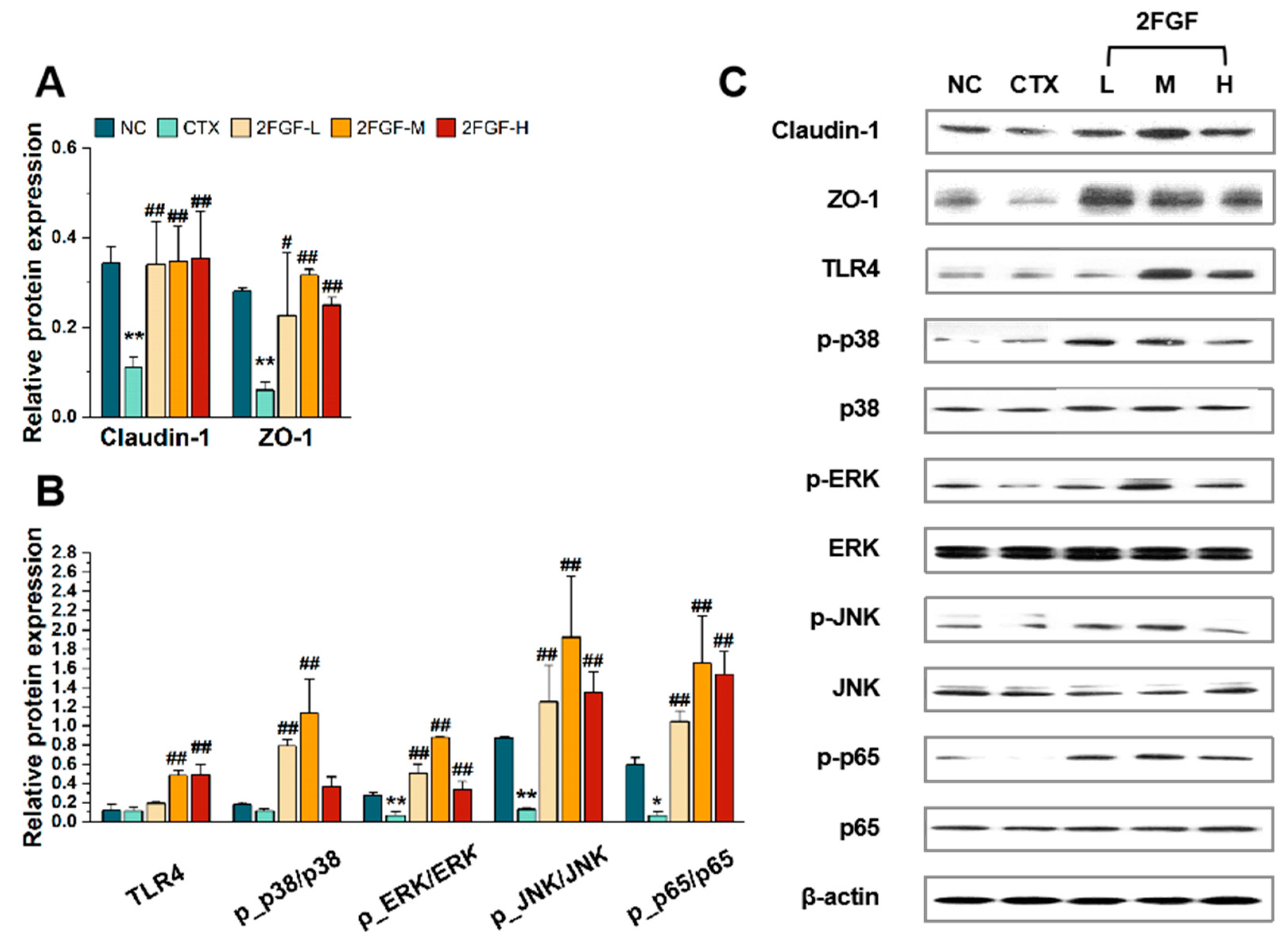
2.7. Western Blot Analysis
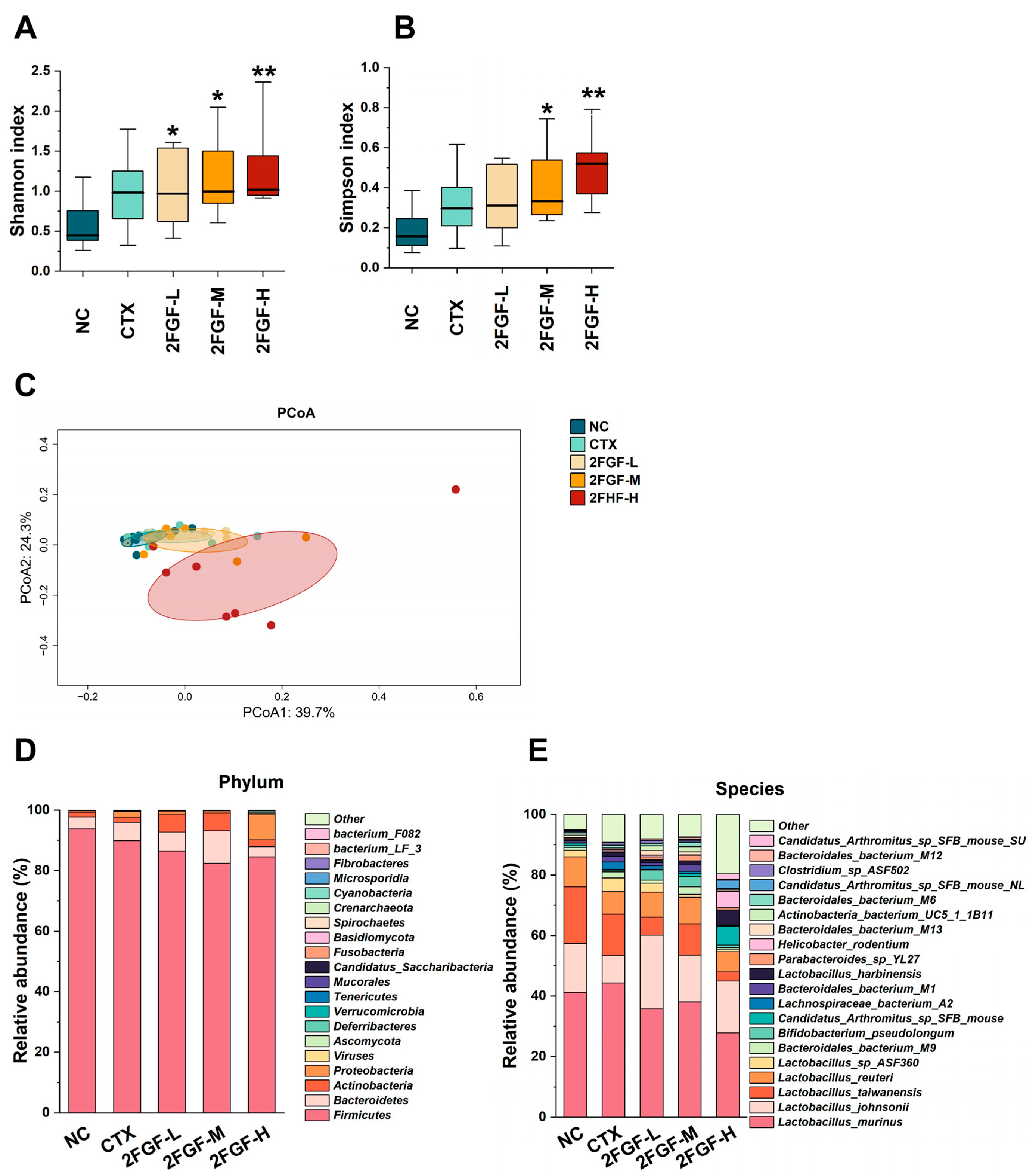
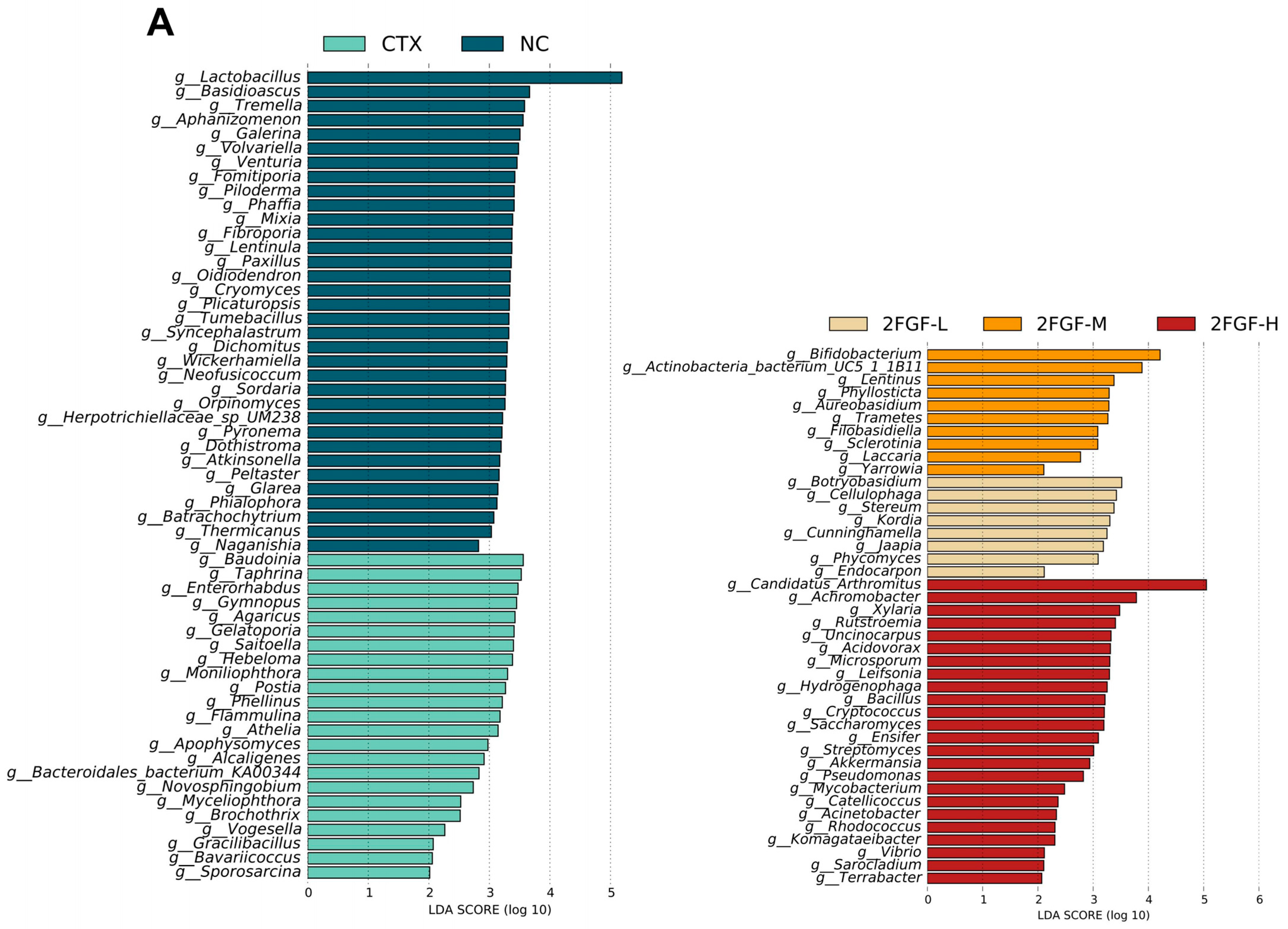
2.8. Metagenomic Analysis of Gut Microbiota
2.9. Statistical Analysis
3. Results
3.1. Effect of 2FGF on CTX-Induced Immunosuppressed Mice
3.1.1. Effect of 2FGF on Body Weight, Food Intake, Immune Organ Indices, Histological Changes, and T Lymphocyte Subgroups
3.1.2. Effect of 2FGF on Serum Cytokine Levels
3.2. Effect of 2FGF on the Intestines of CTX-Induced Immunosuppressed Mice
3.2.1. Effect of 2FGF on Intestinal Epithelial Barrier Functions
3.2.2. Effect of 2FGF on the TLR4/MAPK/NF-κB Pathway
3.2.3. Effect of 2FGF on Gut Microbiota
3.3. Correlation Analysis of Differential Microbiota and Serum Inflammatory Markers
4. Discussion
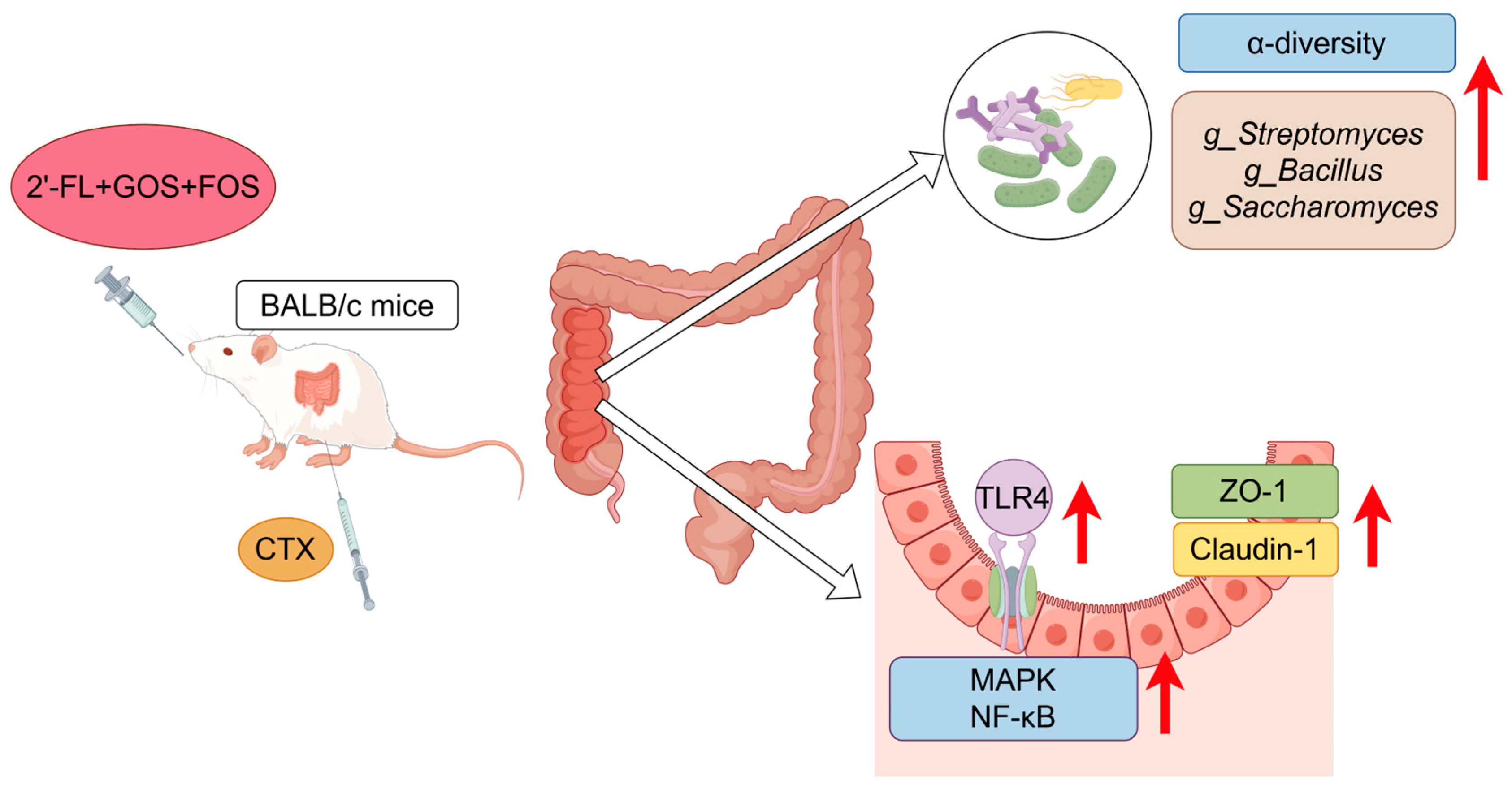
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takeuchi, T.; Nakanishi, Y.; Ohno, H. Microbial Metabolites and Gut Immunology. Annu. Rev. Immunol. 2024, 42, 153–178. [Google Scholar] [CrossRef]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef]
- Vandenplas, Y.; De Greef, E.; Veereman, G. Prebiotics in infant formula. Gut Microbes 2014, 5, 681–687. [Google Scholar] [CrossRef]
- Šuligoj, T.; Vigsnæs, L.K.; Abbeele, P.V.D.; Apostolou, A.; Karalis, K.; Savva, G.M.; McConnell, B.; Juge, N. Effects of Human Milk Oligosaccharides on the Adult Gut Microbiota and Barrier Function. Nutrients 2020, 12, 2808. [Google Scholar] [CrossRef]
- Azagra-Boronat, I.; Massot-Cladera, M.; Knipping, K.; Van’t Land, B.; Stahl, B.; Garssen, J.; Rodríguez-Lagunas, M.J.; Franch, À.; Castell, M.; Pérez-Cano, F.J. Supplementation with 2’-FL and scGOS/lcFOS Ameliorates Rotavirus-Induced Diarrhea in Suckling Rats. Front. Cell. Infect. Microbiol. 2018, 8, 372. [Google Scholar] [CrossRef]
- Xiao, L.; Engen, P.A.; Leusink-Muis, T.; van Ark, I.; Stahl, B.; Overbeek, S.A.; Garssen, J.; Naqib, A.; Green, S.J.; Keshavarzian, A.; et al. The Combination of 2’-Fucosyllactose with Short-Chain Galacto-Oligosaccharides and Long-Chain Fructo-Oligosaccharides that Enhance Influenza Vaccine Responses Is Associated with Mucosal Immune Regulation in Mice. J. Nutr. 2019, 149, 856–869. [Google Scholar] [CrossRef]
- Tian, B.; Jiang, Y.; Liu, R.; Hamed, Y.S.; Rayan, A.M.; Xu, S.; Sun, P.; Yang, K. Positive effects of extracellular polysaccharides from Paecilomyces hepiali on immune-enhancing properties by regulating gut microbiota in cyclophosphamide-induced mice. Int. J. Biol. Macromol. 2024, 274, 133390. [Google Scholar] [CrossRef]
- Skordos, I.; Demeyer, A.; Beyaert, R. Analysis of T cells in mouse lymphoid tissue and blood with flow cytometry. STAR Protoc. 2021, 2, 100351. [Google Scholar] [CrossRef]
- Ma, H.; Mueed, A.; Liu, D.; Ali, A.; Wang, T.; Ibrahim, M.; Su, L.; Wang, Q. Polysaccharides of Floccularia luteovirens regulate intestinal immune response, and oxidative stress activity through MAPK/Nrf2/Keap1 signaling pathway in immunosuppressive mice. Int. J. Biol. Macromol. 2024, 277, 134140. [Google Scholar] [CrossRef]
- Qin, N.; Yang, F.; Li, A.; Prifti, E.; Chen, Y.; Shao, L.; Guo, J.; Le Chatelier, E.; Yao, J.; Wu, L.; et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014, 513, 59–64. [Google Scholar] [CrossRef]
- Jia, D.J.; Wang, Q.W.; Hu, Y.Y.; He, J.M.; Ge, Q.W.; Qi, Y.D.; Chen, L.Y.; Zhang, Y.; Fan, L.N.; Lin, Y.F.; et al. Lactobacillus johnsonii alleviates colitis by TLR1/2-STAT3 mediated CD206+ macrophagesIL-10 activation. Gut Microbes 2022, 14, 2145843. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Mo, S.; Shen, M.; Chen, Y.; Yu, Q.; Li, Z.; Xie, J. Sulfated modification enhances the immunomodulatory effect of Cyclocarya paliurus polysaccharide on cyclophosphamide-induced immunosuppressed mice through MyD88-dependent MAPK/NF-κB and PI3K-Akt signaling pathways. Food Res. Int. 2021, 150, 110756. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.H.; Wang, Y.; Chen, W.M.; Wang, X.M.; Liu, J.; Shao, Y.H.; Tu, Z.C. Galacto-oligosaccharides modified whey protein isolate ameliorates cyclophosphamide-induced immunosuppression. Int. J. Biol. Macromol. 2024, 278, 134642. [Google Scholar] [CrossRef] [PubMed]
- Schoenberger, S.P.; Toes, R.E.; van der Voort, E.I.; Offringa, R.; Melief, C.J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 1998, 393, 480–483. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef]
- Tang, J.; Zhen, H.; Wang, N.; Yan, Q.; Jing, H.; Jiang, Z. Curdlan oligosaccharides having higher immunostimulatory activity than curdlan in mice treated with cyclophosphamide. Carbohydr. Polym. 2019, 207, 131–142. [Google Scholar] [CrossRef]
- Walker, J.A.; McKenzie, A.N.J. T(H)2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Zhao, S.; Peng, X.; Zhou, Q.Y.; Huang, Y.Y.; Rao, X.; Tu, J.L.; Xiao, H.Y.; Liu, D.M. Bacillus coagulans 13002 and fructo-oligosaccharides improve the immunity of mice with immunosuppression induced by cyclophosphamide through modulating intestinal-derived and fecal microbiota. Food Res. Int. 2021, 140, 109793. [Google Scholar] [CrossRef]
- Jo, S.H.; Kim, K.J.; Park, S.Y.; Paik, H.D.; Kim, J.Y. The Human Milk Oligosaccharide 2’-Fucosyllactose Shows an Immune-Enhancing Effect in a Cyclophosphamide-Induced Mouse Model. J. Microbiol. Biotechnol. 2023, 33, 356–362. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H.; Fotopoulou, C.; Cunnea, P.; Zhao, X. Toll-like receptor-targeted anti-tumor therapies: Advances and challenges. Front. Immunol. 2022, 13, 1049340. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Nebreda, A.R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, D.; Fan, H.; Zhang, Y.; LeSage, G.D.; Caudle, Y.; Stuart, C.; Liu, Z.; Yin, D. β-Arrestin 2 negatively regulates Toll-like receptor 4 (TLR4)-triggered inflammatory signaling via targeting p38 MAPK and interleukin 10. J. Biol. Chem. 2014, 289, 23075–23085. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Ryu, J.J.; Jo, Y.K.; Yeo, H.; Kang, S. 2’-Fucosyllactose Attenuates Particulate Matter-Induced Inflammation via Inhibition of Hypoxia-Inducible Factor in Keratinocytes. Biol. Pharm. Bull. 2019, 42, 1620–1627. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut microbiota, intestinal permeability, and systemic inflammation: A narrative review. Intern. Emerg. Med. 2024, 19, 275–293. [Google Scholar] [CrossRef]
- Yin, H.; Li, R.; Liu, J.; Sun, Y.; Zhao, L.; Mou, J.; Yang, J. Fucosylated chondroitin sulfate from sea cucumber Stichopus chloronotus alleviate the intestinal barrier injury and oxidative stress damage in vitro and in vivo. Carbohydr. Polym. 2024, 328, 121722. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, W.; Yin, J.; Zhang, B.; Wang, J.; Wang, S. Differential responses on gut microbiota and microbial metabolome of 2’-fucosyllactose and galactooligosaccharide against DSS-induced colitis. Food Res. Int. 2022, 162, 112072. [Google Scholar] [CrossRef]
- Liao, M.; Zhang, Y.; Qiu, Y.; Wu, Z.; Zhong, Z.; Zeng, X.; Zeng, Y.; Xiong, L.; Wen, Y.; Liu, R. Fructooligosaccharide supplementation alleviated the pathological immune response and prevented the impairment of intestinal barrier in DSS-induced acute colitis mice. Food Funct. 2021, 12, 9844–9854. [Google Scholar] [CrossRef]
- Zhong, L.; Hu, Q.; Zhan, Q.; Zhao, M.; Zhao, L. Oat protein isolate-Pleurotus ostreatus β-glucan conjugate nanoparticles bound to β-carotene effectively alleviate immunosuppression by regulating gut microbiota. Food Funct. 2024, 15, 1867–1883. [Google Scholar] [CrossRef]
- Subedi, U.; Raychaudhuri, S.; Fan, S.; Ogedengbe, O.; Obanda, D.N. Fermenting kale (Brassica oleracea L.) enhances its functional food properties by increasing accessibility of key phytochemicals and reducing antinutritional factors. Food Sci. Nutr. 2024, 12, 5480–5496. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Peng, K.; Xiao, S.; Long, Y.; Yu, Q. The role of Lactobacillus in inflammatory bowel disease: From actualities to prospects. Cell Death Discov. 2023, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, C.; Nie, C.; Yuan, X.; Tu, A.; Li, J. Galactooligosaccharide or 2’-Fucosyllactose Modulates Gut Microbiota and Inhibits LPS/TLR4/NF-κB Signaling Pathway to Prevent DSS-Induced Colitis Aggravated by a High-Fructose Diet in Mice. J. Agric. Food Chem. 2023, 71, 9349–9360. [Google Scholar] [CrossRef] [PubMed]
- Tandon, D.; Haque, M.M.; Gote, M.; Jain, M.; Bhaduri, A.; Dubey, A.K.; Mande, S.S. A prospective randomized, double-blind, placebo-controlled, dose-response relationship study to investigate efficacy of fructo-oligosaccharides (FOS) on human gut microflora. Sci. Rep. 2019, 9, 5473. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, W.; Hu, Z.; Wang, W.; Zhang, H. Marine Streptomyces-Derived Novel Alkaloids Discovered in the Past Decade. Mar. Drugs 2024, 22, 51. [Google Scholar] [CrossRef]
- Kuo, C.L.; Hsin-Hsien Yeh, S.; Chang, T.M.; Wei, A.I.C.; Chen, W.J.; Chu, H.F.; Tseng, A.L.; Lin, P.L.; Lin, Z.C.; Peng, K.T.; et al. Bacillus coagulans BACO-17 ameliorates in vitro and in vivo progression of Rheumatoid arthritis. Int. Immunopharmacol. 2024, 141, 112863. [Google Scholar] [CrossRef]
- Ahmad; Zhang, C.; Wang, Y.; Ullah, H.; Rahman, A.U.; Wei, J.; Qin, Y.H.; Wang, G.; Wang, B.; Li, X. Saccharomyces boulardii (CNCM I-745) alleviates collagen-induced arthritis by partially maintaining intestinal mucosal integrity through TLR2/MYD88/NF-κB pathway inhibition. Int. Immunopharmacol. 2024, 139, 112738. [Google Scholar] [CrossRef]
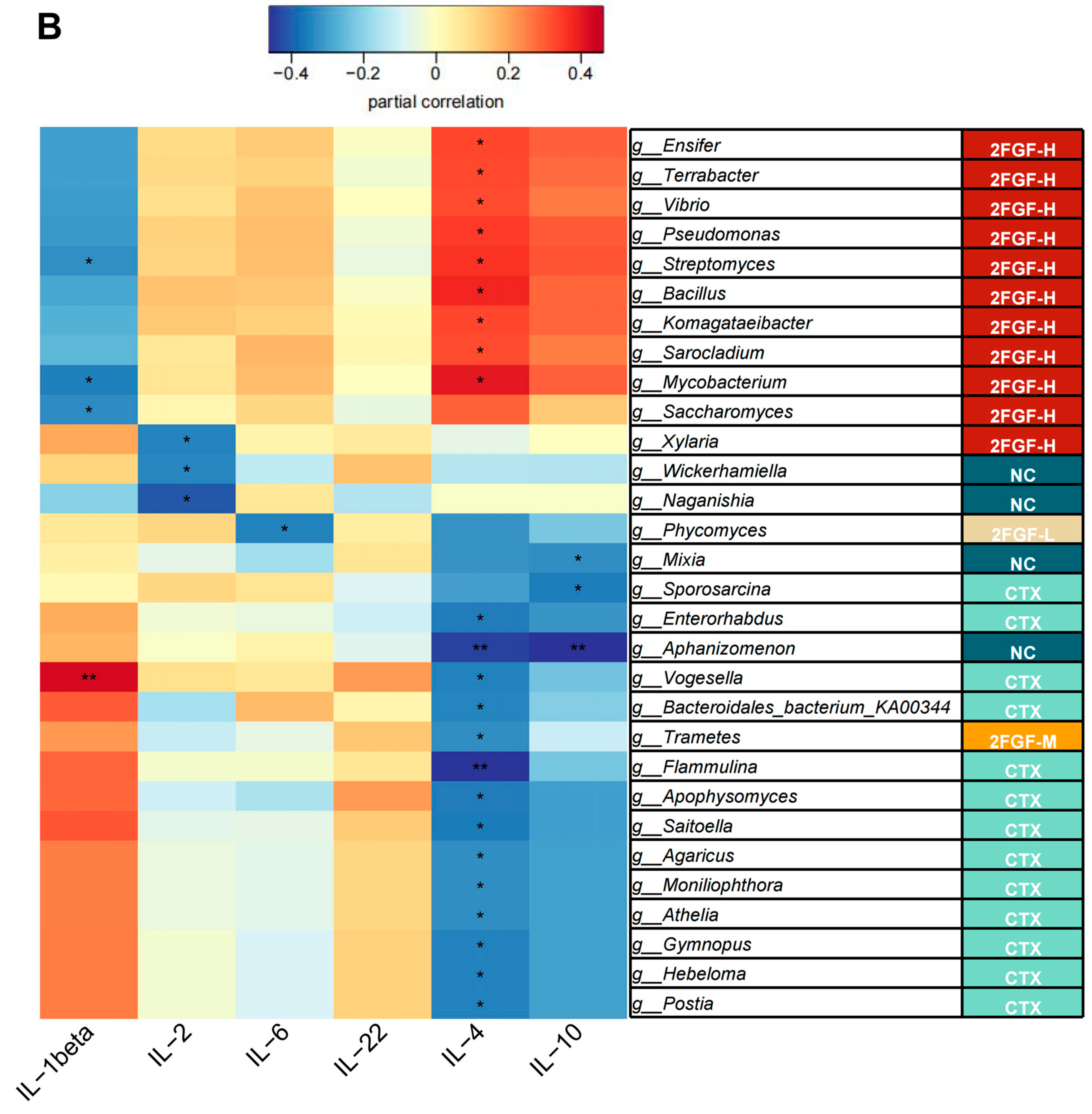
| Group | IL-1β (pg/mL) | IL-2 (pg/mL) | IL-6 (pg/mL) | IL-22 (pg/mL) | IL-4 (pg/mL) | IL-10 (pg/mL) |
|---|---|---|---|---|---|---|
| NC | 0.75 ± 0.41 | 0.93 ± 0.80 | 3.86 ± 2.18 | 6.76 ± 4.37 | 0.49 ± 0.07 | 1.04 ± 0.39 |
| CTX | 0.73 ± 0.39 | 1.33 ± 1.04 | 4.78 ± 2.70 | 5.70 ± 3.82 | 0.46 ± 0.06 | 0.75 ± 0.24 |
| 2FGF-L | 0.76 ± 0.36 | 1.17 ± 0.88 | 3.11 ± 3.25 | 7.83 ± 2.85 | 0.49 ± 0.12 | 1.21 ± 0.97 |
| 2FGF-M | 0.69 ± 0.34 | 1.09 ± 0.75 | 3.65 ± 1.44 | 7.00 ± 5.49 | 0.49 ± 0.04 | 1.48 ± 0.85 # |
| 2FGF-H | 0.82 ± 0.51 | 1.07 ± 1.05 | 4.37 ± 2.84 | 6.42 ± 3.64 | 0.55 ± 0.05 *# | 1.40 ± 0.29 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, W.; Shi, H.; Qian, W.; Meng, L.; Wang, M.; Zhou, Y.; Wen, Z.; Han, M.; Peng, Y.; Li, H.; et al. Immunomodulatory Effects of a Prebiotic Formula with 2′-Fucosyllactose and Galacto- and Fructo-Oligosaccharides on Cyclophosphamide (CTX)-Induced Immunosuppressed BALB/c Mice via the Gut–Immune Axis. Nutrients 2024, 16, 3552. https://doi.org/10.3390/nu16203552
Ye W, Shi H, Qian W, Meng L, Wang M, Zhou Y, Wen Z, Han M, Peng Y, Li H, et al. Immunomodulatory Effects of a Prebiotic Formula with 2′-Fucosyllactose and Galacto- and Fructo-Oligosaccharides on Cyclophosphamide (CTX)-Induced Immunosuppressed BALB/c Mice via the Gut–Immune Axis. Nutrients. 2024; 16(20):3552. https://doi.org/10.3390/nu16203552
Chicago/Turabian StyleYe, Wanyun, Hanxu Shi, Wentao Qian, Liping Meng, Meihua Wang, Yalin Zhou, Zhang Wen, Muke Han, Yile Peng, Hongliang Li, and et al. 2024. "Immunomodulatory Effects of a Prebiotic Formula with 2′-Fucosyllactose and Galacto- and Fructo-Oligosaccharides on Cyclophosphamide (CTX)-Induced Immunosuppressed BALB/c Mice via the Gut–Immune Axis" Nutrients 16, no. 20: 3552. https://doi.org/10.3390/nu16203552
APA StyleYe, W., Shi, H., Qian, W., Meng, L., Wang, M., Zhou, Y., Wen, Z., Han, M., Peng, Y., Li, H., & Xu, Y. (2024). Immunomodulatory Effects of a Prebiotic Formula with 2′-Fucosyllactose and Galacto- and Fructo-Oligosaccharides on Cyclophosphamide (CTX)-Induced Immunosuppressed BALB/c Mice via the Gut–Immune Axis. Nutrients, 16(20), 3552. https://doi.org/10.3390/nu16203552






