Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review
Abstract
1. Introduction
2. Results
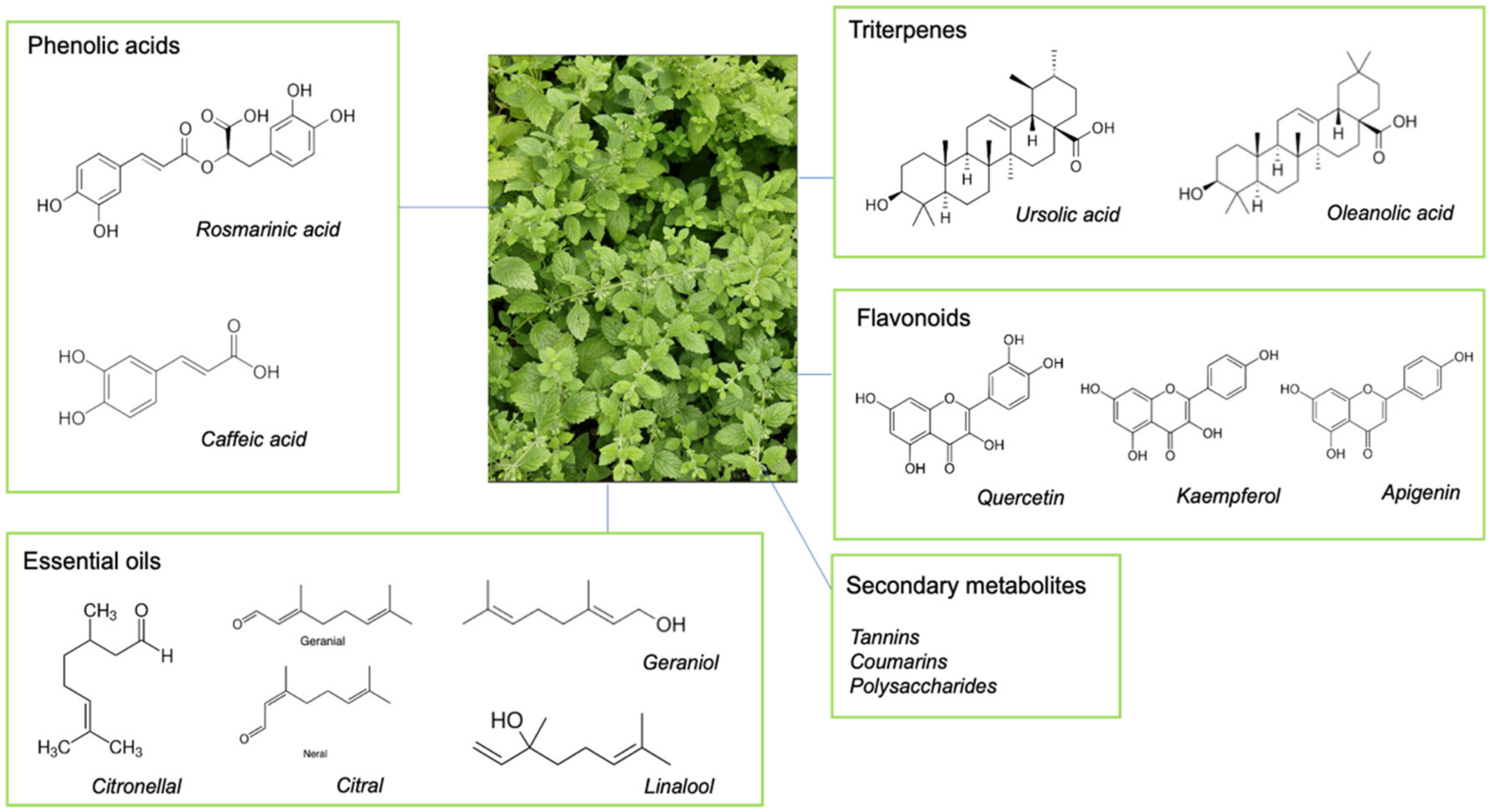
2.1. Key Chemical Components of Lemon Balm
2.2. Biological Mechanisms of Action of Lemon Balm
- GABAergic activity: Lemon balm influences GABAergic activity through various mechanisms, primarily by enhancing the activity of GABA, an inhibitory neurotransmitter in the central nervous system [11,12]. Rosmarinic acid has been shown to inhibit GABA transaminase, the enzyme responsible for the degradation of GABA, leading to the accumulation of GABA levels in the brain [1,20]. Numerous compounds in lemon balm, including RA, can directly bind to GABA receptors such as GABAA [21], enhancing their inhibitory action [22,23]. This agonist activity at GABAA receptors by RA shares similar mechanisms to approved pharmacological therapies for insomnia such as benzodiazepines [12,24].
- Cholinergic modulation: Lemon balm can also influence cholinergic activity; both RA [25] and terpenoids [26] can inhibit acetylcholinesterase (AChE)—a key therapeutic target for Alzheimer’s management [27]. AChE is the enzyme responsible for breaking down acetylcholine—leading to increased levels of acetylcholine in the brain and enhanced cholinergic transmission. Other, as yet unidentified, compounds in lemon balm have been found to interact directly with nicotinic and muscarinic acetylcholine receptors [28], but it is still unclear how lemon balm or its components interact with subtypes of muscarinic (including M1, M2, and M4 receptors) and nicotinic (including α4β2 and α7 subtypes) receptors [12,29].
- Antioxidant activity: Lemon balm exhibits significant antioxidant activity due to its rich content of phenolic compounds, flavonoids, and essential oils. RA, caffeic acid, and flavonoids are potent antioxidants that help neutralize free radicals, thereby reducing oxidative stress and protecting cells from damage [25,30]. Additionally, there is evidence that lemon balm can inhibit lipid peroxidation [31], chelate metal ions [32], and modulate the activity of antioxidant enzymes [33], making it a valuable herb for protecting against oxidative stress and related cellular damage.
- Anti-inflammatory effects: Lemon balm has demonstrated significant anti-inflammatory activity, attributed to its rich content of phenolic acids, flavonoids, and essential oils. These compounds act through various mechanisms to reduce inflammation. Firstly, lemon balm can inhibit the production of pro-inflammatory cytokines, which play a crucial role in the inflammatory response [34]. Lemon balm also contains compounds that inhibit cyclooxygenase (COX) and lipoxygenase (LOX), which are enzymes involved in the production of inflammatory mediators such as prostaglandins and leukotriennes [35]. Lemon balm can inhibit the NF-κB signalling pathway, a key regulator of inflammation [36]. Finally, lemon balm can modulate the activity of various enzymes involved in inflammation [22].
- Gut microbiota: Lemon balm has been shown to influence the gut microbiota, primarily through its antimicrobial, anti-inflammatory, and antioxidant properties. Extracts of lemon balm exhibited antimicrobial activity against several strains of bacteria, suggesting a role in modulating gut microbiota by inhibiting harmful pathogens, thereby promoting a healthier microbial balance [25]. Some studies suggest that lemon balm may have prebiotic effects promoting the growth of beneficial gut bacteria, such as Bifidobacterium animalis [37]. The anti-inflammatory and antioxidant properties of lemon balm (outlined above) can also help reduce inflammation and protect gut cells from oxidative stress, creating a more favourable environment for beneficial bacteria to thrive [35,36]. Finally, lemon balm has been shown to modulate the overall composition of gut microbiota, promoting a balance that supports health [38]. Together, these effects contribute to a healthier gut environment, supporting the growth of beneficial bacteria and inhibiting pathogenic bacteria.
2.3. Psychological Outcomes Following Lemon Balm Supplementation
2.3.1. Infants and Children
| Citation | Design | Participants | Intervention | Design Method | Findings | |||
|---|---|---|---|---|---|---|---|---|
| No. | Age | Population | Duration | Dosage (E: Experimental Group, C: Control Group) | ||||
| Akbarzadeh et al. (2018) [43] | RCT | 200 | 14–18 | Adolescent females experiencing PMS | 3 consecutive months (d per respective menstrual cycle treatment) | E1: 2 × 0.6g lemon balm capsules/d per cycle (n = 50) E2: CEP per cycle (n = 50) C1: 2 × 0.6 g starch-based placebo capsules/d per cycle and CEP (n = 50) C2: education programme without training (n = 50) | Baseline measurements: Well-being affected by PMS entry (PSST > 20 and GHQ < 23) w 0, 4, 8 & 16: Wellbeing assessment: PSST (emotional, social, and physical specific to PMS) Tolerability: Assessed verbal reports of AEs at w16 | Group E1 (at w 16 compared to Groups E2, C1, and C2):
|
| Gromball et al. (2014) [42] | Prospective multi-centre non-interventional study | 169 | 6–11 | Children with hyperactivity, concentration, and impulsiveness problems (not meeting ADHD in DSM-IV criteria) | 7 weeks | E: average 2 × 0.16 g lemon balm and 0.32 g valerian capsules/d (n = 169). No placebo | Baseline assessment: No ADHD criteria, but existing sleep and concentration problems (parent and paediatrician Obs). w 0, 2, and 7: Concentration and sleep-quality assessments: Researcher visits and parents’ continuous home behaviour Obs scored 0–5 (5 = severe). Tolerability: AEs reported by parents | Group E (at w 7 compared to w 0):
|
| Heydari et al. (2019) [44] | Double-blind RCT | 100 | 14–18 | Adolescent females experiencing PMS | 3 consecutive months (d per respective menstrual cycle treatment) | E: 2 × 0.6 g lemon balm capsules/d (n = 50) C: 2 × 0.6 g starch-based placebo capsules/d (n = 50) | Baseline assessment: Well-being affected by PMS entry (PSST > 20) W 0 and 16: Well-being assessment: GHQ-28 Tolerability: AEs verbally reported at w 16 | Group E (compared to Group C at w 16):
|
| Martinelli et al. (2017) [39] | Multi-centre prospective randomised comparative study | 176 | ≥2 weeks to 4 months | Infants with IC | 28 days | E1: 1 × 1 mL of 0.13 g lemon balm, 0.018 g chamomile, 2 × 109 tyndallized Lactobacillus acidophilus/d (n = 60) E2: 1× Lactobacillus reuteri (108 CFU) in 5 drops/d (n = 60) C: 2 × 15 drops in 0.06 mg Simethicone/d (n = 60) | Baseline assessment: Anxiety entry scoring: Frequent and severity of crying episodes using Rome III criteria D 0, 7, 14, 21, and 28: Anxiety and gastrointestinal assessments: parents filled in maternal diary (crying time frequency and eating habits/stool frequency) Tolerability: assessed AEs throughout | Group E1 (compared to Group E2 and C at d 28):
|
| Müller & Klement (2006) [41] | Open multicentre post-marketing surveillance study | 918 | M = 8.3 | Children with restlessness and dyssomnia | M = 4-weeks (range 3–5 weeks) | E: variable dose of 3.5–4 × 0.08 g lemon balm and 0.16g valerian capsules/d (n = 918) No placebo | Baseline assessment: Restlessness and/or dyssomnia problems >10 months (physician Obs) W 0 and 4: Sleep and restlessness assessment: Physician visits and parents’ feedback at visits Symptom severity then weighted on Obs of efficacy and improvement scoring, 0–5 (1 = very good, 5 = poor) Tolerability: AEs reported by physicians over whole study phase (1 = very good to 5 = poor) | Group E (at w 4 compared to w 0):
|
| Savino et al. (2005) [40] | Double-blind RCT | 93 | 21–60 days | Infants with IC | 21 days (7-day intervention with a 14-day follow up) | E: 2 × 1 mL containing 0.01937 g lemon balm, 0.0328 g chamomile, 0.0355 g fennel powdered sachet/d (n = 41) C: 2 × 1 mL matched placebo with only vitamins, no herbals (n = 47) | Baseline assessment: Anxiety entry scoring: Frequent and severity of crying episodes using Wessel’s criteria (>3 h frequency, >3 wks severity) and 3 d Obs D 0, 7, and 21: Anxiety and gastrointestinal assessments: parents filled in structured diary (crying time frequency/severity and medication administration, AE development) | Group E (compared to Group C)):
|
2.3.2. Young Adults
| Citation | Design | Participants | Intervention | Design Method | Findings | |||
|---|---|---|---|---|---|---|---|---|
| No. | Age | Population | Duration | Dosage (E: Experimental Group, C: Control Group) | ||||
| Beihaghi et al. (2019) [51] | Triple-blind RCT (not mentioned how triple-blind) | 60 | 18–35 | Healthy post caesarean section | 14 days (10-day treatment) | E: 3 × 0.5 g lemon balm capsules/d (n = 30) C: 3 × 0.5 g matching unnamed placebo capsules/d (n = 30) | Baseline measurements: No sign of depression at entry (BDI < 10) At d 0, 3–5, 10, and 14: Mood measurements: EPDS (post-partum blues) Tolerability: Researcher checklist where AEs also assessed | Group E (at d 3–5 compared to Group C):
|
| Kennedy et al. (2002) [45] | Double-blind cross-over RCT | 20 | 18–22 | Healthy | Single dose with 7-day washout between groups | E1: 2 × 0.15 g lemon balm capsules/d E2: 4 × 0.15 g lemon balm capsules/d E3: 6 × 0.15 g lemon balm capsules/d C: 0 mg matching placebo capsules/d In vitro human occipital tissue IC50 cholinergic binding activity | Baseline assessment: Exclusion of smokers (may influence at the nicotinic receptor level) Timepoints 0, 1, 2.5, 4 and 6 h: Cognitive assessment: computerised CDR battery and serial subtraction task (memory and attention) Mood assessment: BL-VAS 100 mm (calmness, alertness, contentedness) | Groups E1–E3 (compared to Group C):
|
| Kennedy et al. (2003) [46] | Double-blind cross-over RCT | 20 | 18–23 | Healthy | Single dose with 7-day washout between groups | E1: 3 × 0.2 g lemon balm capsules/d E2: 5 × 0.2 g lemon balm capsules/d E3: 8 × 0.2 g lemon balm capsules/d C: 0 g inert placebo capsules/d Phytochemical screening prior to capsule preparation assessing human acetylcholine inhibition and receptor-binding properties | Baseline assessment: Chosen IC50 concentrations for nicotinic and muscarinic receptor displacement (0.18 and 3.47 mg ml−1, respectively). Exclusion of smokers (may influence at the nicotinic receptor level) Timepoints 0, 1, 3, and 6 h: Cognitive assessment: Computerised CDR battery and RVIP (memory and attention) Mood assessment: BL-VAS 100 mm (calmness, alertness, contentedness) | Groups E1–E3 (compared to Group C):
|
| Kennedy et al. (2004) [47] | Double-blind cross-over RCT | 18 | Age range not provided in paper M = 29 | Healthy | Single dose with 7-day washout between groups | E1: 2 × 0.15 g lemon balm capsules/d E2: 4 × 0.15 g lemon balm capsules/d C: 0 g inert placebo capsules/d | Timepoints 0 and 1 h: Cognitive stress task: DISS (20 min duration) Mood assessment: BL-VAS 100 mm (alertness, contentedness, calmness) | Groups E1–E2 (at 1 h compared to Group C):
|
| Kennedy et al. (2006) [48] | Double-blind cross-over RCT | 24 | Age range not provided in paper M = 23.48 | Healthy | Single dose with 7-day washout between groups | E1: 3 × 0.08 g lemon balm and 0.12 g valerian capsules/d E2: 6 × 0.08 g lemon balm and 0.12 g valerian capsules/d E3: 9 × 0.08 g lemon balm and 0.12 g valerian capsules/d C: 0 g inactive placebo capsules/d | Baseline assessment Exclusion of smokers (may influence at the nicotinic receptor level) Timepoints 0, 1, 3, and 6 h per block: Cognitive stress task: DISS (20 min duration). Mood assessments: BL-VAS 100 mm (alertness, contentedness, calmness) and STAI (state and trait anxiety) | Group E1–E3 (compared to Group C):
|
| Meier et al. (2018) [49] | Double-blind RCT | 70 | 18–45 M = 26.07 | Healthy males | 4 days | 3 tablets taken p/d for 3 d and 2 tablets taken on d 4: E1: 1 tablet contained “Ze 185” 0.06 g lemon balm, 0.09 g valerian, 0.09 g Butterbur and 0.09 g passionflower tablet/d (n = 23) C1: unnamed placebo tablet (n = 23) C2: no treatment (n = 24) Phytochemical screening of lemon balm: HPLC revealed 11.862 RA mAU | Baseline assessment: Tolerability: HR, BP, serum markers, and AEs Anxiety measurement: STAI Physiological stress measurement: cortisol at d 4: Cognitive stress task: TSST computerised battery Physiological stress measurement: 11 × saliva (cortisol measurements at −50, −35, −20, −10 min pre-TSST and 0, 10, 20, 30, 45, 60, 90 min post TSST) Stress assessment: 5 × STAI (pre-TSST, −45 to TSST completion, immediately post TSST and 30, 90 min post TSST) Tolerability: AEs recorded, HR, BP, serum markers | Group E1 (following TSST on d 4 compared to Group C1 and C2):
|
| Scholey et al. (2014) [29] | 2 × Double-blind crossover RCT | RCT 1: 25 RCT 2: 21 | RCT 1: 18–39 M = 25.3 RCT 2: 21–30 M = 23.6 | Healthy | Single dose with 7-day washout between groups | RCT 1: E1: 1 × 0.3 g lemon balm (RA > 6%) and natural fruit sweetener beverage/d of 480 mL E2: 1 × 0.6 g lemon balm (RA > 6%) and natural fruit sweetener beverage/d of 480 mL E3: 1 × 0.6 g lemon balm (RA > 6%) and artificial sweetener blend of 480 mL C: 1 × 480 mL artificial sweetener placebo beverage/d RCT 2: E1: 1 × 250 g yoghurt with 0.3 g lemon balm (RA > 6%) and natural fruit sweetener E2: 1 × 250 g yoghurt with 0.6 g lemon balm(RA > 6%) and natural fruit sweetener E3: 1 × 250 g yoghurt with 0.6 g lemon balm (RA > 6%) and artificial fruit sweetener C: 1 × 250 g yoghurt with artificial sweetener placebo/d | Baseline assessment: Pilot study of 5 stressed young adults (independent of RCT 1 and 2) found >2% RA bioavailability in 1.8 g lemon balm and 200 mL fruit sweetener drink peaked at 1 h in serum biomarkers when trialling cognitive and mood batteries RCT 1: Timepoints 0, 1, and 3 h: Cognitive stress task: MTF computerised battery (psychomotor tracking, memory, attention) Mood assessments: STAI (state and trait anxiety), DASS (stress) Physiological Stress marker: 1 × saliva sample (cortisol) at 12 p.m. or 4 p.m. RCT 2: Timepoints 0, 1, and 3 h Cognitive stress task: MTF adapted from DISS and 20 min duration (psychomotor, memory, attention) Mood assessments: BL-VAS 100 mm (stress and fatigue) | RCT 1: Group E1–E3 (compared to Group C):
RCT 2: Group E1–E3 (compared to Group C):
No AEs reported in either RCT |
2.3.3. Middle-Aged Adults
| Citation | Design | Participants | Intervention | Design Method | Findings | |||
|---|---|---|---|---|---|---|---|---|
| No. | Age | Population | Duration | Dosage (E: Experimental Group, C: Control Group) | ||||
| Alijaniha et al. (2015) [52] | Double-blind RCT | 55 | 18–60 M = 42 | Healthy with benign heart palpitations | 2 weeks | E: 2 × 0.5 g lemon balm capsules/d (n = 28) C: 2 × 0.5 g breadcrumb capsules/d (n = 27) | Baseline measurements: Safety serum markers, ECG, and self-report heart palpitation perception At w 0 and 2:
| Group E (at w 2 compared to Group C):
|
| Araj-Khoadei et al. (2020) [53] | Double-blind RCT, no placebo | 45 | 18–65 M = 37 | Diagnosed depression (DSM-V criteria) | 8 weeks | E1: 4 × 0.5 g lemon balm capsules/d (n = 16) E2: 4 × 0.5 g lavender capsules/d (n = 17) C: 2 × 10 mg/d fluoxetine (n = 17) Phenolic (Folin–Ciocalteu’s reagent/gallic acid) and flavonoid (rutin) characterisation of herbals revealed lemon balm constituents phenolic at 4.88 mg GA/g and flavonoid at 4.28 RU/g | Baseline measurements: HAM-D for mild to moderate depression (score range 8–24) At w 0, 2, 4, and 8:
| Group E1 (at w 8 compared to Group E2 and Group C):
|
| Bano et al. (2023) [58] | Double-blind RCT | 100 | 18–65 (M = 31) | Healthy with moderate mood or sleep problems | 3 weeks | E: 2 × 0.2 g lemon balm capsules/d with phospholipid (sunflower) carrier (n = 52) C: 2 × 0.2 g matched unnamed placebo capsules/d (n = 48) | Baseline measurements: Moderate depression, anxiety, or stress (DASS-42; ≥14, ≥10, and ≥19, respectively) At w 0 and 3: Sleep and mood assessments: PSQI (sleep quality), DASS-42 (depression, anxiety, stress), PANAS (mood), WEMWBS (well-being), QoL (quality of life) Tolerability: AEs reported throughout study | Group E (at w 3 compared to Group C):
|
| Bongartz et al. (2019) [57] | Double-blind RCT | 50 | 23–64 M = 46 | Healthy, with moderate sleep problems | 6 weeks | E: “IQP-AO-101” sachet/d in 100 mL water containing 0.08 g lemon balm, 0.3 g asparagus extract, 0.01 g zinc, 0.03 g saffron extract, 0.06 g vitamin C, 0.03 IU vitamin E (n = 25) C: matched placebo containing excipient sachet/d in 100 mL water (n = 25) | Baseline measurements: Sleep study entry (PSQI 6–15) and safety serum markers, HR, BP At w 0, 1, 4, and 6: Sleep and mood assessments: mAIS (sleep), POMS-65 (mood), FAIR-2 (attention), sleep diary (sleep) AE self-reports At w 0 and 4: Sleep assessment: Fitbit Flex 2 activity tracker (HR variability) | Group E (at w 6 compared to Group C):
|
| Cerny and Schmid (1999) [56] | Double-blind multi-centred parallel RCT | 98 | 20–70 M = 33 | Healthy, with mild sleep problems | 30 days | E: 3 × 0.08 g lemon balm and 0.12 g valerian tablets/d (n = 66) C: matched placebo without herbals 0.6 g tablets/d (n = 32) | Baseline measurements: verbal report of sleep problems entry and safety serum markers, BP, HR d 0 and 30: Sleep and wellbeing assessments: VAS 100 mm and verbal report Tolerability and AEs: self-report (5-point rating) and physical examination | Group E (at d 30 compared to Group C):
|
| Lemoine et al. (2019) [59] | Open-label pilot study | 40 | 20–75 M = 33 | Healthy, with mild-to-moderate sleep complaints | 2 weeks (with 1-week follow-up) | E: 2 × 0.12 g lemon balm, 0.001 g melatonin, 0.00042 g vitamin B6, 0.0084 g Californian poppy extract, 0.075 g passionflower extract capsules/d (n = 40) | Baseline measurements: ISI (sleep entry 8–21), 1-week run-in fixed sleep schedule, restricted caffeine, and completed sleep diary w 2, 3, and follow-up w 4: Sleep assessment: Sleep diary recording sleep quality (0–10, 10 = good/very good sleep), time to fall asleep, nighttime wakings, daytime naps, and wake-up time Tolerability: AEs reported throughout study | Group E (at post-treatment and follow-up compared to baseline):
|
| Ranjbar et al. (2018) [60] | Double-blind RCT | 45 | 18–60 M = 39 | Insomnia (ICSD II criteria), with co-morbid depression and anxiety | 4 weeks | E: 3 × 0.5 g capsules/d to amount 1 g lemon balm and 0.4 g lavender (n = 23) C: 3 × 0.5 g starch-based capsule/d (n = 22) | Baseline measurements: Homogenous for sleep problems (ISI > 7), depression (BDI > 10I), and anxiety (BAI > 7). W 0 and 4: Sleep and mood assessment: ISI (sleep) and BAI (anxiety) Tolerability: AE self-reports w 0, 2, and 4: Mood assessment: BDI (depression). | Group E (at w 4 compared to Group C):
|
| Shirazi et al. (2021) [55] | Double-blind RCT | 60 | 43–60 M = 52 | Post-menopausal women (hormone and period absence confirmation) with sleep disorders | 8 weeks | E: 1 × 0.5 g lemon balm and fennel fruit extract capsule/d (n = 20) C1: 1 × 0.03 g citalopram capsule/d (n = 20) C2: 0.5 g starch-based placebo (n = 20) | Baseline measurements: Sleep disturbance entry (PSQI ≥ 5) w 0 and 8: Wellbeing and sleep assessment: MENQOL (menopause quality of life) Tolerability: Self-reported AEs at w 8 | Group E (at w 8 compared to Groups C1 and C2):
|
| Taavoni et al. (2013) [54] | Triple-blind RCT | 100 | 50–60 M = 54 | Post-menopausal women with sleep disorders | 4 weeks | E: 2 × 0.08 g lemon balm and 0.16 g valerian capsules/d (n = 50) C: 2 × 0.05 g starch-based capsules/d (n = 50) | Baseline measurements: Sleep disturbance entry (PSQI ≥ 5) w 0 and 4: Sleep assessment: PSQI (sleep quality) Tolerability: verbal report of AEs at w 4 | Group E (at w 4 compared to Group C):
|
2.3.4. Older Adults
| Citation | Design | Participants | Intervention | Design Method | Findings | |||
|---|---|---|---|---|---|---|---|---|
| No. | Age | Population | Duration | Dosage (E: Experimental Group, C: Control Group) | ||||
| Akhondzadeh et al. (2003) [64] | Double-blind RCT | 42 | 65–80 M = 73 | Mild-to-moderate AD (NINCDS/ADRDA diagnosis) | 16 weeks | E: 60 lemon balm drops/d (dose unspecified) (n = 20) C: 60 drops/d matching placebo (unspecified) (n = 15) | Baseline measurements: Cognitive decline assessment ADAS-cog ≥ 12 and CDR-SB ≤ 2 Tolerability: ECG, HR, and BP w 0 to 16 (every 2 weeks): Cognitive and mood assessments: ADAS-cog and CDR-SB Tolerability: ECG, HR, and BP and self-report /observed AEs | Group E (at w 16 compared to Group C):
|
| Haybar et al. (2018) [61] | Double-blind RCT | 73 | 40–75 M = 59 | Adults experiencing CSA | 8 weeks | E: 3 × 1 g lemon balm capsules/d (n = 35) C: 3 × 1 g cornstarch placebo capsules/d (n = 38) | Baseline measurements: 3-day habitual diet and physical activity level (IPAQ) w 0 to 8: Sleep and mood assessments: DASS-21 (anxiety, depression, and stress), PSQI (sleep quality) | Group E (at w 8 compared to Group C):
|
| Noguchi-Shinohara et al. (2020) [66] | Double-blind RCT (24 weeks) followed by 24-week treatment, no placebo | 20 | >60 M = 89.3 | Mild dementia due to AD (n = 20) (MMSE screened between 20 and 26) | 48 weeks | Part 1: 24-week double-blind 500 mg lemon balm capsules or unnamed placebo capsules/d Part 2: 24-week 500 mg lemon balm only/d | w 0, 8, 16, 24, 32, 40, and 48: Tolerability: ECG, urinalysis, haematology, blood chemistry, chest X-ray, HR, and BP w 0, 16, and 24: Mood and cognitive assessments: ADAS-Cog (memory), NPI (agitation), MMSE (executive function, memory), DAD (executive function) |
|
| Soltanpour et al. (2019) [62] | Double-blind RCT | 80 | 30–70 M = 58 | Post-surgery cardiac patients experiencing anxiety and sleep disturbance | 7 days | E: 3 × 0.5 g lemon balm capsules/d (n = 40) C: 3 × 0.5 g wheat starch placebo capsules/d (n = 40) Herbal assay: phenolic (Folins–Ciocalteu’s agent and gallic acid) and flavonoid (rutin) components revealed phenolic and flavonoid, 4.88 GA/g and 4.28 RU/g, respectively | Baseline measurements: No considerable sleep disorder prior to surgery (PSQI < 28) d 0 to 7: Sleep and mood assessment: SMHSQ (sleep quality), HADS (anxiety) | Group E (at d 7 compared to Group C):
|
| Veiskaramian et al. (2021) [63] | Double-blind RCT | 70 | 35–65 M = 57 | ACS patients experiencing stress | 1 day | E: 2 phases (with 90 min interval). Each phase = 2 drops lemon balm oil for 10 min (n = 36) C: 2 phases (with 90 min interval). Each phase = 2 drops sunflower oil for 10 min (n = 34) Phytochemical screening of lemon balm: 24.4% beta caryophyllene, 8.6% geranial, 6.9% 1,8-cineole, 6.7% neral, 5.8% dehydroaromedendrene, 4.8% thymol, and 3.8% α-Pinene components), | Baseline measurements: Cardiac markers: MAP > 70 mm Hg and HR > 60 Stress (DASS-21 > 19) and chest pain (10 cm VAS ≥ 3cm) Timepoints, t1 to 6: Stress assessment: DASS-21 (stress), MAP taken before (t1), 5 mins (t2), and 15 mins (t2) after phase 1. Interval of 90 min followed by t4–6 for phase 2. Threat perception scale as secondary stress measure. | Group E (compared to Group C):
|
| Watson et al. (2019) [65] | Counter-balanced repeated measures, double-blinded design | 49 | >65 M = 89.3 | Dementia (n = 39) and non-dementia (n = 10) living in residential care homes | 2 weeks (with 2-week washout periods)) | E1: 2 drops lemon balm oil/d E2: 2 drops lavender oil/d C: 2 drops placebo sunflower oil oil/d All treatments were applied to a cloth and attached to participant clothing for 2 h inhalation blocks Phytochemical screening of lemon balm revealed beta caryophyllene, germacrene D, citral, and geraniol components | Baseline measurements: Mood and cognitive level entry: MMSE (>10 cognition) and NPI (≥6 agitation frequency) w 0–2, 4–6, and 8–10: Mood & cognitive assessments: CMAI (physical non-aggressive behaviour) and NPI (agitation) | Group E1 (compared to E2 and Group C):
|
2.3.5. Across the Lifespan
| Citation | Design | Participants | Intervention | Design Method | Findings | |||
|---|---|---|---|---|---|---|---|---|
| No. | Age | Population | Duration | Intervention (E: Experimental Group, C: Control Group) | ||||
| Cases et al. (2011) [67] | Open-label pilot | 20 | 18–70 | Healthy with mild anxiety and sleep problems (DSM-IV-TR) | 15 days | E: 2 × 0.3 g lemon balm capsules/d (n = 20) Phytochemical screening of lemon balm: 7.95 ± 0.29% RA; 18.5 ± 0.63% total hydroxycinnamates; flavonoids, including 0.23 ± 0.01% hesperidin, 0.46 ± 0.04% luteolin-3-glucuronide, and 0.69 ± 0.04% total flavonoids; triterpenes, including 0.22 ± 0.03 oleanolic acid and 0.64 ± 0.09% ursolic acid | d 0 and 15: Sleep & stress assessments: FRSA (anxiety manifestations and symptoms), HDRS (insomnia) Clinical improvement for anxiety and sleep assessment: CGI-I ≤ 2 Tolerability: Verbal reports of AEs | Group E (at d 20 compared to d 0):
|
| Chehroudi et al. (2017) [68] | Double-blind RCT | 36 | n.d. | Hospitalised 2nd- and 3rd-degree burns | 20 days | E: 2 × 2.5 g lemon balm tea/d (n = 18) C: 2 × 2.5 g black tea/d (n = 18) | d 0 and 20: Sleep & mood assessments: BDI (depression), Kettle’s (anxiety) and PSQI (sleep quality) Serum antioxidants: 5 mL | Group E (at d 20 compared to Group C):
|
| Lotfi et al. (2019) [69] | Single-blind RCT (only participant blinded) | 94 | 20–75 | ACS with co-morbid anxiety symptoms | 3 days | E: 2 × 3 drops lemon balm oil/d (n = 45) C: 2 × 3 drops odourless sesame placebo oil/d (n = 47) All treatments were applied to a cloth and attached to participant clothing for 30 min twice a day for 3 days | Baseline measurements: olfactory test with coffee sniffing d 0, 2, and 3: Anxiety measurements: STAI (state and trait anxiety) | Group E (at d 3 compared to Group C):
|
| Lotfi et al. (2020) [70] | Single-blind RCT (only participant blinded) | 92 | 20–75 | ACS with co-morbid sleep problems | 3 days | E: 2 × 3 drops lemon balm oil/d (n = 45) C: 2 × 3 drops odourless sesame placebo oil/d (n = 47) All treatments were applied to a cloth and attached to participant clothing for 30 min twice a day for 3 days | Baseline measurements: Olfactory test with coffee sniffing D1 and 3: Sleep assessment: VSH (sleep quality) in 3 subscales, sleep disorder (0–700 points), sleep efficacy (0–500 points), and daytime napping (0–400 points) | Group E (at d 3 compared to Group C):
|
3. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Akram, M.; Abbaass, W.; Semwal, P.; Calina, D. Phytochemical Constituents, Biological Activities, and Health-Promoting Effects of the Melissa officinalis. Oxid. Med. Cell. Longev. 2021, 2021, 6584693. [Google Scholar] [CrossRef] [PubMed]
- Świąder, K.; Startek, K.; Wijaya, C.H. The therapeutic properties of Lemon balm (Melissa officinalis L.): Reviewing novel findings and medical indications. J. Appl. Bot. Food Qual. 2019, 92, 327–335. [Google Scholar]
- Ghazizadeh, J.; Mohammadinasab, R.; Travica, N.; Sadigh-Eteghad, S.; Torbati, M.; Hamedeyazdan, S.; Araj-khodaei, M. Historical Course of Neuropsychiatric Effects of Lemon Balm (Melissa officinalis L.) as a Medicinal Herb. Pharma. Sci. 2021, 28, 14–19. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.–A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef]
- British Herbal Pharmacopoeia; British Herbal Pharmacopoeia: London, UK, 1996.
- European Pharmacopoeia, 4th ed.; Councils of Europe: Strasbourg, France, 2002.
- Iranian Herbal Pharmacopoeia; Ministry of Health Publication: Tehran, Iran, 2002.
- Waheed, K.; Nawaz, H.; Hanif, M.A.; Rehman, R.; Ogunwande, I.A. Lemon Balm. In Medicinal Plants of South Asia; Elsevier: Amsterdam, The Netherlands, 2020; Volume 35, pp. 465–478. [Google Scholar]
- Draginic, N.; Jakovljevic, V.; Andjic, M.; Jeremic, J.; Srejovic, I.; Rankovic, M.; Tomovic, M.; Nikolic Turnic, T.; Svistunov, A.; Bolevich, S.; et al. Melissa officinalis L. as a Nutritional Strategy for Cardioprotection. Front. Physiol. 2021, 12, 661778. [Google Scholar] [CrossRef]
- Sanchez-Medina, A.; Etheridge, C.J.; Hawkes, G.E.; Hylands, P.J.; Pendry, B.A.; Hughes, M.J.; Corcoran, O. Comparison of rosmarinic acid content in commercial tinctures produced from fresh and dried lemon balm (Melissa officinalis). J. Pharm. Pharm. Sci. 2007, 10, 455–463. [Google Scholar] [CrossRef][Green Version]
- Awad, R.; Levac, D.; Cybulska, P.; Merali, Z.; Trudeau, V.L.; Arnason, J.T. Effects of traditionally used anxiolytic botanicals on enzymes of the γ-aminobutyric acid (GABA) system. Can. J. Physiol. Pharmacol. 2007, 85, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Awad, R.; Muhammad, A.; Durst, T.; Trudeau, V.L.; Arnason, J.T. Bioassay-guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 1075–1081. [Google Scholar]
- Sarris, J.; Panossian, A.; Schweitzer, I.; Stough, C.; Scholey, A. Herbal medicine for depression, anxiety and insomnia: A review of psychopharmacology and clinical evidence. Eur. Neuropsychopharmacol. 2011, 21, 841–860. [Google Scholar] [CrossRef]
- WHO. World Health Organisation Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2002; Volume 2. [Google Scholar]
- Ibragić, S.; Salihović, M.; Tahirović, I.; Toromanović, J. Quantification of some phenolic acids in the leaves of Melissa officinalis L. from Turkey and Bosnia. Bull. Chem. Tech. Bosnia Herzeg. 2014, 42, 47–50. [Google Scholar]
- Tantry, M.A.; Bhat, G.A.; Idris, A.; Dar, J.A.; Yousef Al Omar, S.; Masoodi, K.Z.; Shawl, A.S. Sulfated triterpenes from Lemon balm. Helv. Chim. Acta 2014, 97, 1497–1506. [Google Scholar] [CrossRef]
- Mencherini, T.; Picerno, P.; Scesa, C.; Aquino, R. Triterpene, antioxidant, and antimicrobial compounds from Melissa officinalis. J. Nat. Prod. 2007, 70, 1889–1894. [Google Scholar] [CrossRef] [PubMed]
- Cosge, B.; Ipek, A.; Gürbüz, B. GC/MS analysis of herbage essential oil from lemon balms (Melissa officinalis L.) grown in Turkey. J. Appl. Biol. Sci. 2009, 3, 149–152. [Google Scholar]
- Arceusz, A.; Wesolowski, M.; Ulewicz-Magulska, B. Flavonoids and phenolic acids in methanolic extracts, infusions and tinctures from commercial samples of lemon balm. Nat. Prod. Commun. 2015, 10, 977–981. [Google Scholar] [CrossRef]
- Pineau, S.; Legros, C.; Mattei, C. The Medical use of Lemon Balm (Melissa officinalis) and Valerian (Valeriana officinalis) as Naturals: Insight into their Interactions with GABA Transmission. Int. J. Clin. Pharmacol. Pharmacother. 2016, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Eulenburg, V.; Kreis, W.; Villmann, C.; Pischetsrieder, M. Three-Step Test System for the Identification of Novel GABAA Receptor Modulating Food Plants. Plant Foods Hum. Nutr. 2016, 71, 355–360. [Google Scholar] [CrossRef]
- Sadraei, H.; Ghannadi, A.; Malekshahi, K. Relaxant effect of essential oil of Melissa officinalis and citral on rat ileum contractions. Fitoterapia 2003, 74, 445–452. [Google Scholar] [CrossRef]
- Wang, C.C.; Hsieh, P.W.; Kuo, J.R.; Wang, S.J. Rosmarinic acid, a bioactive phenolic compound, inhibits glutamate release from rat cerebrocortical synaptosomes through GABAA receptor activation. Biomolecules 2021, 11, 1029. [Google Scholar] [CrossRef]
- Avidan, A.Y.; Neubauer, D.N. Chronic insomnia disorder. CONTINUUM Lifelong Learn. Neurol. 2017, 23, 1064–1092. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Dorman, H.D.; Oinonen, P.P.; Darwis, Y.; Laakso, I.; Hiltunen, R. Chemical composition and in vitro antioxidative activity of a lemon balm (Melissa officinalis L.) extract. LWT-Food Sci. Technol. 2008, 41, 391–400. [Google Scholar] [CrossRef]
- Savelev, S.; Okello, E.; Perry, N.S.L.; Wilkins, R.M.; Perry, E.K. Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 2003, 75, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Dubey, R. Target Enzyme in Alzheimer’s Disease: Acetylcholinesterase Inhibitors. Curr. Top. Med. Chem. 2019, 19, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Wake, G.; Court, J.; Pickering, A.; Lewis, R.; Wilkins, R.; Perry, E. CNS acetylcholine receptor activity in European medicinal plants traditionally used to improve failing memory. J. Ethnopharmacol. 2000, 69, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Scholey, A.; Gibbs, A.; Neale, C.; Perry, N.; Ossoukhova, A.; Bilog, V.; Buchwald-Werner, S. Anti-stress effects of lemon balm-containing foods. Nutrients. 2014, 6, 4805–4821. [Google Scholar] [CrossRef]
- Moradkhani, H.; Sargsyan, E.; Bibak, H.; Naseri, B.; Sadat-Hosseini, M.; Fayazi-Barjin, A.; Meftahizade, H. Melissa officinalis L., a valuable medicine plant. J. Med. Plants Res. 2010, 4, 2753–2759. [Google Scholar]
- Patora, J.; Majda, T.; Góra, J.; Klimek, B. Variability in the content and composition of essential oil from lemon balm (Melissa officinalis L.) cultivated in Poland. Acta Pol. Pharm. 2003, 60, 395–400. [Google Scholar]
- Dragland, S.; Senoo, H.; Wake, K.; Holte, K.; Blomhoff, R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 2003, 133, 1286–1290. [Google Scholar] [CrossRef]
- Matkowski, A.; Jamiołkowska-Kozłowska, W.; Nawrot, I. Chinese Medicinal Herbs as Source of Antioxidant Compounds—Where Tradition Meets the Future. Curr. Med. Chem. 2013, 20, 984–1004. [Google Scholar]
- Draginic, N.; Andjic, M.; Jeremic, J.; Zivkovic, V.; Kocovic, A.; Tomovic, M.; Milosavljevic, I. Anti-inflammatory and Antioxidant Effects of Melissa officinalis Extracts: A Comparative Study. Iran. J. Pharm. Res. IJPR 2022, 21, e126561. [Google Scholar] [CrossRef]
- Carnat, A.P.; Carnat, A.; Fraisse, D.; Lamaison, J.L. The aromatic and polyphenolic composition of lemon balm (Melissa officinalis L. subsp. officinalis) tea. Pharm. Acta Helv. 1998, 72, 301–305. [Google Scholar] [CrossRef]
- Miraj, S.; Rafieian-Kopaei; Kiani, S. Melissa officinalis L.: A Review study with an antioxidant prospective. J. Evid.-Based Compl. Alter. Med. 2017, 22, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Bilan, M.V.; Lieshchova, M.A.; Brygadyrenko, V.V. Impacts on gut microbiota of rats with high-fat diet supplemented by herbs of Melissa officinalis, Lavandula angustifolia and Salvia officinalis. Regul. Mech. Biosyst. 2023, 14, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kiani, H.S.; Ali, A.; Zahra, S.; Hassan, Z.U.; Kubra, K.T.; Azam, M.; Zahid, H.F. Phytochemical composition and pharmacological potential of lemongrass (cymbopogon) and impact on gut microbiota. Appl. Chem. 2022, 2, 229–246. [Google Scholar] [CrossRef]
- Martinelli, M.; Ummarino, D.; Giugliano, F.P.; Sciorio, E.; Tortora, C.; Bruzzese, D.; Staiano, A. Efficacy Standardized Extract of Matricaria chamomilla L., Melissa officinalis L. and tyndallized bacteria of the species Lactobacillus acidophilus (HA122) in infantile colic-open-label, wound-controlled study. Neurogastroenterol. Motil. 2017, 29, e13145. [Google Scholar] [CrossRef]
- Savino, F.; Cresi, F.; Castagno, E.; Silvestro, L.; Oggero, R. A randomized double-blind placebo-controlled trial of a standardized extract of Matricariae recutita, Foeniculum vulgare and Melissa officinalis (ColiMil®) in the treatment of breastfed colicky infants. Phytother. Res. Int. J. Pharmacol. Toxicol. Eval. Natur. Prod Deriv. 2005, 19, 335–340. [Google Scholar]
- Müller, S.F.; Klement, S. A combination of valerian and lemon balm is effective in the treatment of restlessness and dyssomnia in children. Phytomedicine 2006, 13, 383–387. [Google Scholar] [CrossRef]
- Gromball, J.; Beschorner, F.; Wantzen, C.; Paulsen, U.; Burkart, M. Hyperactivity, concentration difficulties and impulsiveness improve during seven weeks’ treatment with valerian root and lemon balm extracts in primary school children. Phytomedicine 2014, 21, 1098–1103. [Google Scholar] [CrossRef]
- Akbarzadeh, M.; Moshfeghy, Z.; Dehghani, M.; Emamghoreishi, M.; Tavakoli, P.; Zare, N. Comparison of the effect of Melissa officinalis capsule and care educational programs on the intensity of physical, mental and social symptoms of premenstrual syndrome in high school female students. Int. J. Women’s Heal. Reprod. Sci. 2018, 16, 18–26. [Google Scholar] [CrossRef]
- Heydari, N.; Dehghani, M.; Emamghoreishi, M.; Akbarzadeh, M. Effect of Melissa officinalis capsule on the mental health of female adolescents with premenstrual syndrome: A clinical trial study. Int. J. Adolesc. Med. Heal. 2019, 31, 20170015. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Scholey, A.B.; Tildesley, N.T.; Perry, E.K.; Wesnes, K.A. Modulation of mood and cognitive performance following acute administration of Melissa officinalis (lemon balm). Pharmacol. Biochem. Behav. 2002, 72, 953–964. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wake, G.; Savelev, S.; Tildesley, N.T.; Perry, E.K.; Wesnes, K.A.; Scholey, A.B. Modulation of mood and cognitive performance following acute administration of single doses of Melissa officinalis (Lemon balm) with human CNS nicotinic and muscarinic receptor-binding properties. Neuropsychopharmacology 2003, 28, 1871–1881. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Little, W.; Scholey, A.B. Attenuation of laboratory-induced stress in humans after acute administration of Melissa officinalis (Lemon Balm). Psychosom. Med. 2004, 66, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Little, W.; Haskell, C.F.; Scholey, A.B. Anxiolytic effects of a combination of Melissa officinalis and Valeriana officinalis during laboratory-induced stress. Phyto. Res. Int. J. Pharma. Toxicol. Eval. Natur. Prod. Deriva. 2006, 20, 96–102. [Google Scholar]
- Meier, S.; Haschke, M.; Zahner, C.; Kruttschnitt, E.; Drewe, J.; Liakoni, E.; Gaab, J. Effects of a fixed herbal drug combination (Ze 185) to an experimental acute stress setting in healthy men–An explorative randomized placebo-controlled double-blind study. Phytomedicine 2018, 39, 85–92. [Google Scholar] [CrossRef]
- Mirghafourvand, M.; Malakouti, J.; Charandabi, S.M.A.; Khalili, A.F.; Homayi, S.G. The efficacy of lemon balm (Melissa officinalis L.) alone and combined with lemon balm—Nepeta menthoides on premenstrual syndrome and quality of life among students: A randomized controlled trial. J. Herb. Med. 2016, 6, 142–148. [Google Scholar] [CrossRef]
- Beihaghi, M.; Yousefzade, S.; Mazloom, S.R.; Modares Gharavi, M.; Hamedi, S.S. The effect of Melissa Officinalis on postpartum blues in women undergoing caesarean section. J. Midwifery Reprod. Health 2019, 7, 1636–1643. [Google Scholar]
- Alijaniha, F.; Naseri, M.; Afsharypuor, S.; Fallahi, F.; Noorbala, A.; Mosaddegh, M.; Sadrai, S. Heart palpitation relief with Melissa officinalis leaf extract: Double blind, randomized, placebo-controlled trial of efficacy and safety. J. Ethnopharmacol. 2015, 164, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Araj-Khodaei, M.; Noorbala, A.A.; Yarani, R.; Emadi, F.; Emaratkar, E.; Faghihzadeh, S.; Naseri, M. A double-blind, randomized pilot study for comparison of Melissa officinalis L. and Lavandula angustifolia Mill. with Fluoxetine for the treatment of depression. BMC Complement. Med. Ther. 2020, 20, 207. [Google Scholar] [CrossRef]
- Taavoni, S.; Haghani, H. Valerian/lemon balm use for sleep disorders during menopause. Complement. Ther. Clin. Pr. 2013, 19, 193–196. [Google Scholar] [CrossRef]
- Shirazi, M.; Jalalian, M.N.; Abed, M.; Ghaemi, M. The effectiveness of Melissa officinalis L. versus Citalopram on Quality of Life of Menopausal Women with Sleep Disorder: A Randomized Double-Blind Clinical Trial. Revist. Brasil. Ginecolog. Obs. 2021, 43, 126–130. [Google Scholar] [CrossRef]
- Cerny, A.; Schmid, K. Tolerability and efficacy of valerian/lemon balm in healthy volunteers (a double-blind, placebo-controlled, multicentre study). Fitoterapia 1999, 70, 221–228. [Google Scholar] [CrossRef]
- Bongartz, U.; Tan, B.K.; Seibt, S.; Bothe, G.; Uebelhack, R.; Chong, P.W.; Wszelaki, N. Sleep Promoting Effects of IQP-AO-101: A Double-Blind, Randomized, Placebo-Controlled Exploratory Trial. Evid.-Based Complement. Altern. Med. 2019, 2019, 9178218. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Hepsomali, P.; Rabbani, F.; Farooq, U.; Kanwal, A.; Saleem, A.; Khan, A. The possible “calming effect” of subchronic supplementation of a standardised phospholipid carrier-based Melissa officinalis L. extract in healthy adults with emotional distress and poor sleep conditions: Results from a prospective, randomised, double-blinded, placebo-controlled clinical trial. Front. Pharmacol. 2023, 14, 1250560. [Google Scholar]
- Lemoine, P.; Bablon, J.C.; Da Silva, C. A combination of melatonin, vitamin B6 and medicinal plants in the treatment of mild-to-moderate insomnia: A prospective pilot study. Complement. Ther. Med. 2019, 45, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, M.; Salehi, A.; Rezaeizadeh, H.; Zarshenas, M.M.; Sadeghniiat-Haghighi, K.; Mirabzadeh, M.; Firoozabadi, A. Efficacy of a combination of Melissa officinalis L. and Nepeta menthoides Boiss. Buhse on insomnia: A triple-blind, randomized placebo-controlled clinical trial. J. Alternat. Complement. Med. 2018, 24, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Haybar, H.; Javid, A.Z.; Haghighizadeh, M.H.; Valizadeh, E.; Mohaghegh, S.M.; Mohammadzadeh, A. The effects of Melissa officinalis supplementation on depression, anxiety, stress, and sleep disorder in patients with chronic stable angina. Clin. Nutr. ESPEN 2018, 26, 47–52. [Google Scholar] [CrossRef]
- Soltanpour, A.; Alijaniha, F.; Naseri, M.; Kazemnejad, A.; Heidari, M.R. Effects of Melissa officinalis on anxiety and sleep quality in patients undergoing coronary artery bypass surgery: A double-blind randomized placebo-controlled trial. Eur. J. Integr. Med. 2019, 28, 27–32. [Google Scholar] [CrossRef]
- Veiskaramian, A.; Gholami, M.; Yarahmadi, S.; Baharvand, P.A.; Birjandi, M. Effect of aromatherapy with Melissa essential oil on stress and hemodynamic parameters in acute coronary syndrome patients: A clinical trial in the emergency department. Complement. Ther. Clin. Pr. 2021, 44, 101436. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double-blind, randomised, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 2003, 74, 863–866. [Google Scholar] [CrossRef]
- Watson, K.; Hatcher, D.; Good, A. A randomised controlled trial of Lavender (Lavandula angustifolia) and Lemon Balm (Melissa officinalis) essential oils for the treatment of agitated behaviour in older people with and without dementia. Complement. Ther. Med. 2019, 42, 366–373. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Ono, K.; Hamaguchi, T.; Nagai, T.; Kobayashi, S.; Komatsu, J.; Yamada, M. Safety and efficacy of Melissa officinalis extract containing rosmarinic acid in the prevention of Alzheimer’s disease progression. Sci. Rep. 2020, 10, 18627. [Google Scholar] [CrossRef] [PubMed]
- Cases, J.; Ibarra, A.; Feuillère, N.; Roller, M.; Sukkar, S.G. Pilot trial of Melissa officinalis L. leaf extract in the treatment of volunteers suffering from mild-to-moderate anxiety disorders and sleep disturbances. Mediterr. J. Nutr. Metab. 2011, 4, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Chehroudi, S.; Fatemi, M.J.; Isfeedvajani, M.S.; Salehi, S.H.; Akbari, H.; Samimi, R. Effects of Melissa officinalis L. on reducing stress, alleviating anxiety disorders, depression, and insomnia, and increasing total antioxidants in burn patients. Trauma Mon. 2017, 22, 7. [Google Scholar] [CrossRef]
- Lotfi, A.; Shiri, H.; Ilkhani, R.; Sefidkar, R.; Esmaeeli, R. The efficacy of aromatherapy with Melissa officinalis in reducing anxiety in cardiac patients: A randomized clinical trial. Cresc. J. Med. Biolog. Sci. 2019, 6, 293–299. [Google Scholar]
- Lotfi, A.; Mohtashami, J.; Khangholi, Z.A.; Shirmohammadi-Khorram, N. The Efficacy of Aromatherapy with Lemon Balm (Melissa officinalis L.) on Sleep Quality in Cardiac Patients: A Randomized Controlled Trial. arXiv 2020. [Google Scholar] [CrossRef]
- Zeevenhooven, J.; Browne, P.D.; l’Hoir, M.P.; de Weerth, C.; Benninga, M.A. Infant colic: Mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 479–496. [Google Scholar] [CrossRef]
- Guu, T.W.; Aarsland, D.; Ffytche, D. Light, sleep-wake rhythm, and behavioural and psychological symptoms of dementia in care home patients: Revisiting the sundowning syndrome. Int. J. Geriatr. Psychiatry 2022, 37, 1–10. [Google Scholar] [CrossRef]
- Yeung, V.; Sharpe, L.; Geers, A.; Colagiuri, B. Choice, expectations, and the placebo effect for sleep difficulty. Ann. Behav. Med. 2020, 54, 94–107. [Google Scholar] [CrossRef]
- Winsky-Sommerer, R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur. J. Neurosci. 2009, 29, 1779–1794. [Google Scholar] [CrossRef]
- Tubbs, A.S.; Kennedy, K.E.; Alfonso-Miller, P.; Wills, C.C.; Grandner, M.A. A randomized, double-blind, placebo-controlled trial of a polyphenol botanical blend on sleep and daytime functioning. Int. J. Environ. Res. Public Heal. 2021, 18, 3044. [Google Scholar] [CrossRef]
- Puts, N.A.; Edden, R.A. In vivo magnetic resonance spectroscopy of GABA: A methodological review. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 60, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, M.; Nitschke, J.P.; LoParco, S.; Bartz, J.A.; Otto, A.R. Acute psychosocial stress increases cognitive-effort avoidance. Psychol. Sci. 2021, 32, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Lobach, A.R.; Schmidt, F.; Fedrizzi, D.; Müller, S. Toxicological safety evaluation of an aqueous lemon balm (Melissa officinalis) extract. Food Chem. Toxicol. 2024, 187, 114565. [Google Scholar] [CrossRef] [PubMed]
- Edinoff, A.N.; Nix, C.A.; Hollier, J.; Sagrera, C.E.; Delacroix, B.M.; Abubakar, T.; Kaye, A.D. Benzodiazepines: Uses, dangers, and clinical considerations. Neurol. Int. 2021, 13, 594–607. [Google Scholar] [CrossRef]
- Ruangritchankul, S.; Chantharit, P.; Srisuma, S.; Gray, L.C. Adverse drug reactions of acetylcholinesterase inhibitors in older people living with dementia: A comprehensive literature review. Ther. Clin. Risk Manag. 2021, 17, 927–949. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathews, I.M.; Eastwood, J.; Lamport, D.J.; Cozannet, R.L.; Fanca-Berthon, P.; Williams, C.M. Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review. Nutrients 2024, 16, 3545. https://doi.org/10.3390/nu16203545
Mathews IM, Eastwood J, Lamport DJ, Cozannet RL, Fanca-Berthon P, Williams CM. Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review. Nutrients. 2024; 16(20):3545. https://doi.org/10.3390/nu16203545
Chicago/Turabian StyleMathews, Imogen Maria, Jessica Eastwood, Daniel Joseph Lamport, Romain Le Cozannet, Pascale Fanca-Berthon, and Claire Michelle Williams. 2024. "Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review" Nutrients 16, no. 20: 3545. https://doi.org/10.3390/nu16203545
APA StyleMathews, I. M., Eastwood, J., Lamport, D. J., Cozannet, R. L., Fanca-Berthon, P., & Williams, C. M. (2024). Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review. Nutrients, 16(20), 3545. https://doi.org/10.3390/nu16203545