Self-Initiated Dietary Adjustments Alter Microbiota Abundances: Implications for Perceived Health
Abstract
1. Introduction
2. Materials and Methods
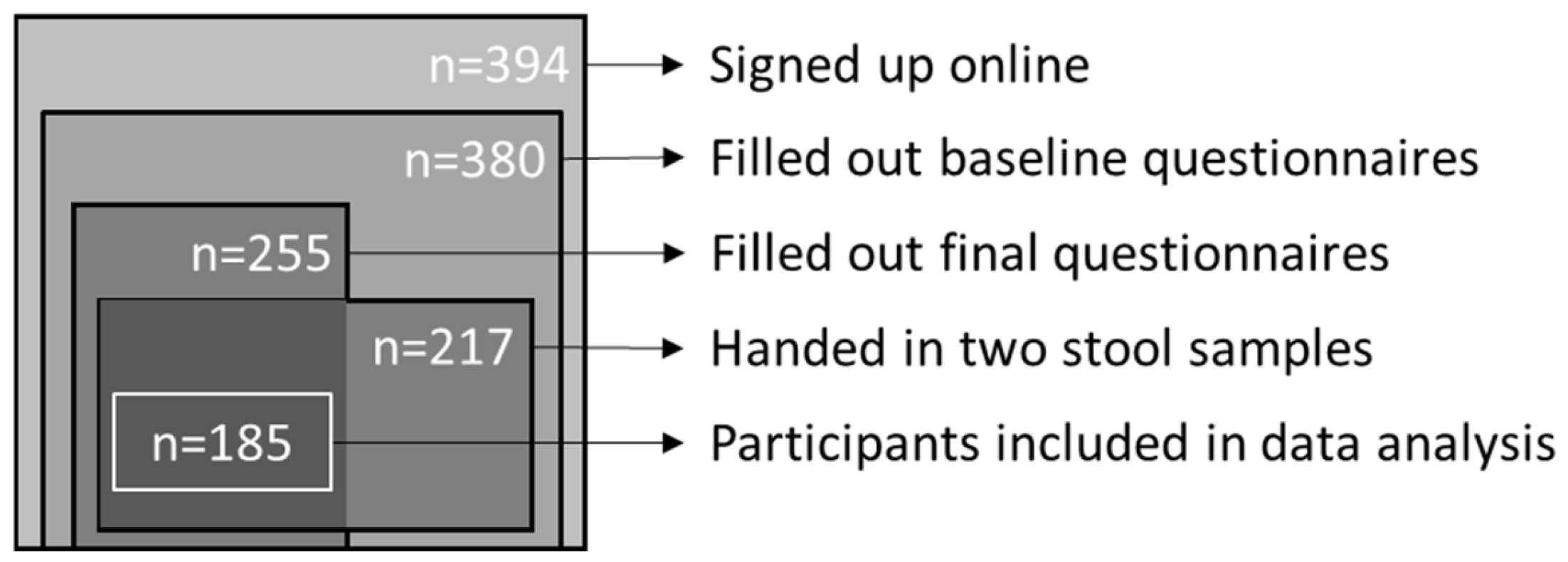
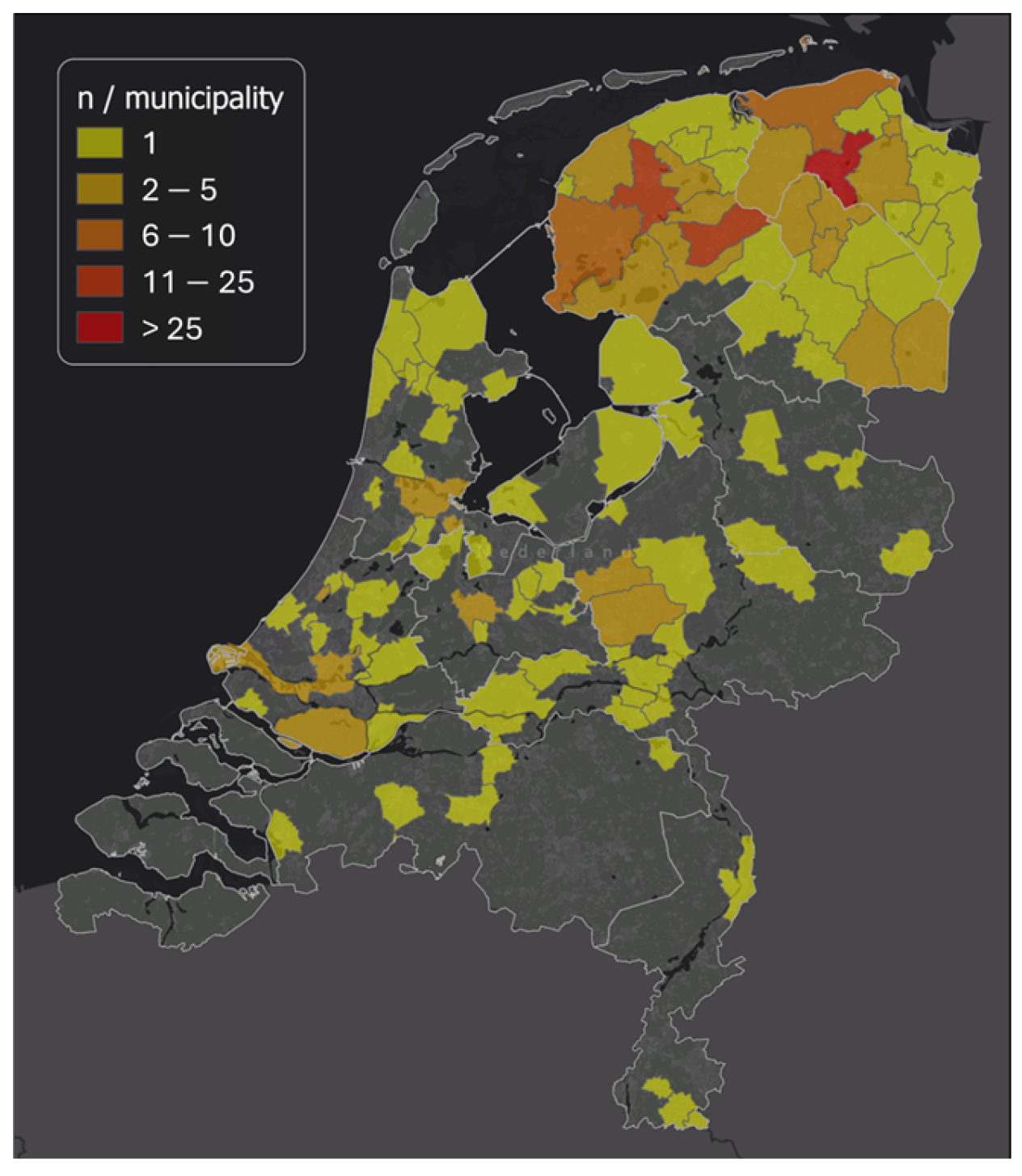
2.1. Study Design
2.2. Questionnaires
2.3. Dietary Intake
2.4. Analysis of Faecal Samples
2.5. Statistical Analysis
3. Results
3.1. Participants and Baseline Values
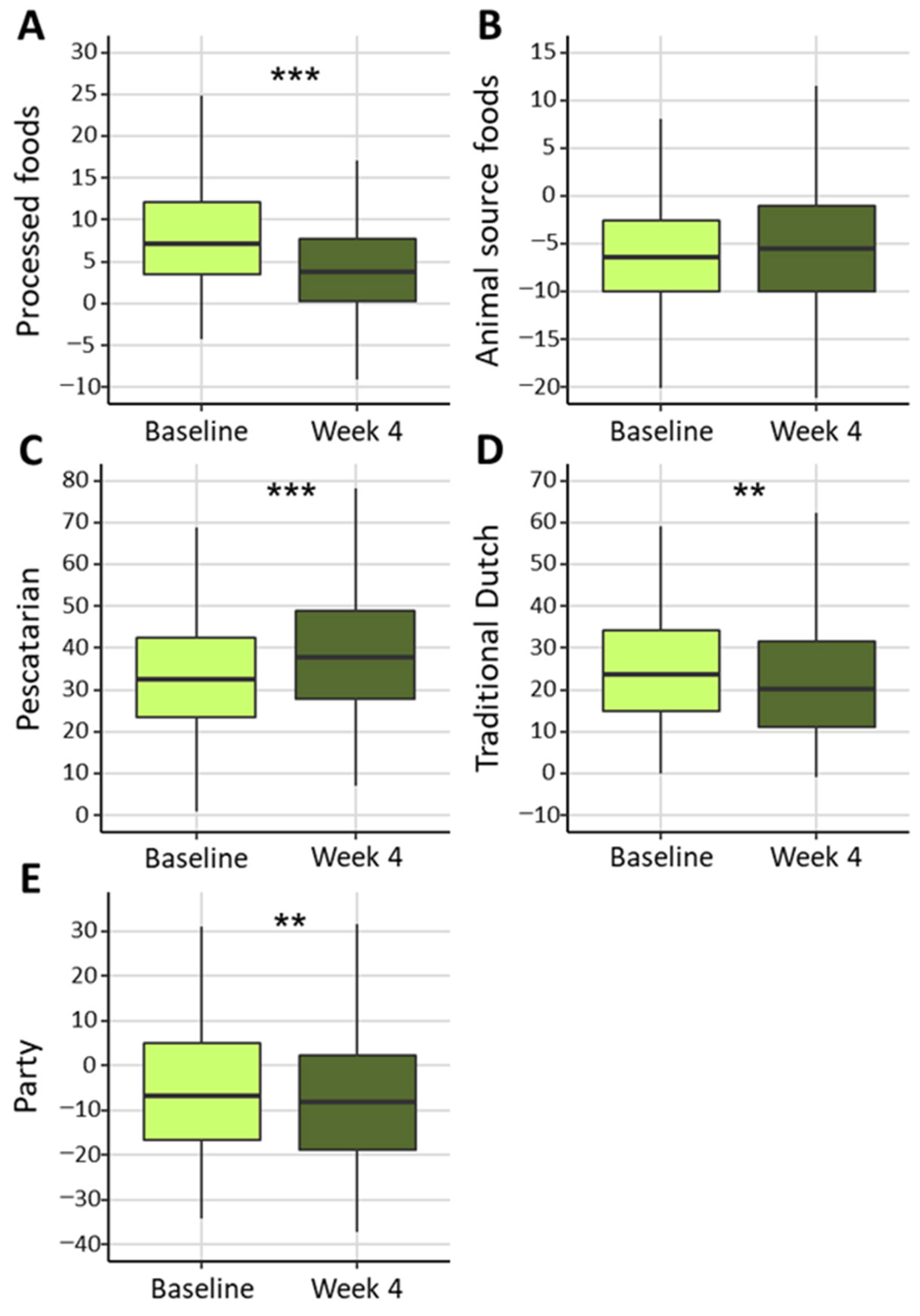
3.2. Dietary Intake and Health Outcomes
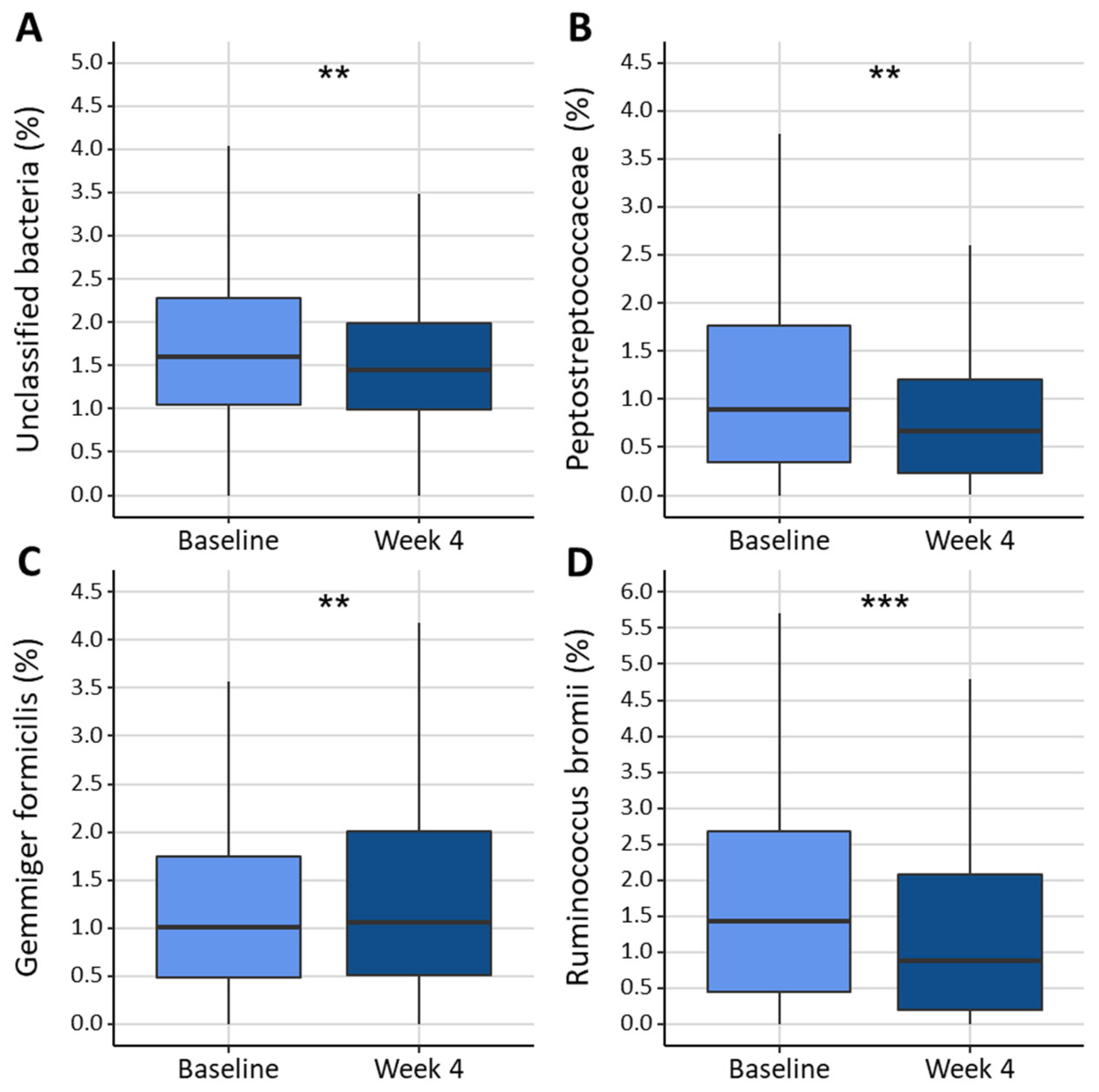
3.3. Gut Microbiota Composition
3.4. Relation Between Dietary Intake and Gut Microbiota
3.5. Relation of Health Outcomes and Gut Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spichak, S.; Bastiaanssen, T.F.S.; Berding, K.; Vlckova, K.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Mining Microbes for Mental Health: Determining the Role of Microbial Metabolic Pathways in Human Brain Health and Disease. Neurosci. Biobehav. Rev. 2021, 125, 698–761. [Google Scholar] [CrossRef] [PubMed]
- Farup, P.G.; Valeur, J. Faecal Microbial Markers and Psychobiological Disorders in Subjects with Morbid Obesity. A Cross-Sectional Study. Behav. Sci. 2018, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Jovel, J.; Patterson, J.; Wang, W.; Hotte, N.; O’Keefe, S.; Mitchel, T.; Perry, T.; Kao, D.; Mason, A.L.; Madsen, K.L.; et al. Characterization of the Gut Microbiome Using 16S or Shotgun Metagenomics. Front. Microbiol. 2016, 7, 459. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Walters, W.A.; Xu, Z.; Knight, R. Meta-Analyses of Human Gut Microbes Associated with Obesity and IBD. FEBS Lett. 2014, 588, 4223–4233. [Google Scholar] [CrossRef]
- Debelius, J.; Song, S.J.; Vazquez-Baeza, Y.; Xu, Z.Z.; Gonzalez, A.; Knight, R. Tiny Microbes, Enormous Impacts: What Matters in Gut Microbiome Studies? Genome Biol. 2016, 17, 217. [Google Scholar] [CrossRef]
- Power, S.E.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F. Intestinal Microbiota, Diet and Health. Br. J. Nutr. 2014, 111, 387–402. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Estimates 2020: Disease Burden by Cause, Age, Sex, by Country and by Region, 2000–2019; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Hernández-Calderón, P.; Wiedemann, L.; Benítez-Páez, A. The Microbiota Composition Drives Personalized Nutrition: Gut Microbes as Predictive Biomarkers for the Success of Weight Loss Diets. Front. Nutr. 2022, 9, 1006747. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Leeming, E.R.; Johnson, A.J.; Spector, T.D.; Le Roy, C.I. Effect of Diet on the Gut Microbiota: Rethinking Intervention Duration. Nutrients 2019, 11, 2862. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e3. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Useros, N.; Nova, E.; González-Zancada, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A. Microbiota and Lifestyle: A Special Focus on Diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Chen, L.; Zhernakova, D.V.; Kurilshikov, A.; Andreu-Sánchez, S.; Wang, D.; Augustijn, H.E.; Vich Vila, A.; Weersma, R.K.; Medema, M.H.; Netea, M.G.; et al. Influence of the Microbiome, Diet and Genetics on Inter-Individual Variation in the Human Plasma Metabolome. Nat. Med. 2022, 28, 2333–2343. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef]
- Califf, K.; Gonzalez, A.; Knight, R.; Caporaso, J.G. The Human Microbiome: Getting Personal. Microbe 2014, 9, 410–415. [Google Scholar]
- Baumert, J.; Meisinger, C.; Lukaschek, K.; Emeny, R.T.; Rückert, I.M.; Kruse, J.; Ladwig, K.H. A Pattern of Unspecific Somatic Symptoms as Long-Term Premonitory Signs of Type 2 Diabetes: Findings from the Population-Based MONICA/KORA Cohort Study, 1984–2009. BMC Endocr. Disord. 2014, 14, 87. [Google Scholar] [CrossRef]
- Appels, A.; Mulder, P. Excess Fatigue as a Precursor of Myocardial Infarction. Eur. Heart J. 1988, 9, 758–764. [Google Scholar] [CrossRef]
- Willems, A.E.M.; Sura-de Jong, M.; van Beek, A.P.; van Dijk, G. Self-Initiated Dietary Changes Reduce General Somatic and Mental Symptoms in a Relatively Healthy Dutch Population. Prev. Med. Rep. 2022, 30, 102004. [Google Scholar] [CrossRef] [PubMed]
- National Collaborating Centre for Mental Health (UK). Depression: The Treatment and Management of Depression in Adults (Updated Edition); British Psychological Society: Leicester, UK, 2010. [Google Scholar]
- Bekhuis, E.; Boschloo, L.; Rosmalen, J.G.M.; de Boer, M.K.; Schoevers, R.A. The Impact of Somatic Symptoms on the Course of Major Depressive Disorder. J. Affect. Disord. 2016, 205, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Angermann, C.E.; Ertl, G. Depression, Anxiety, and Cognitive Impairment. Curr. Heart Fail. Rep. 2018, 15, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Kaul, K.; Tarr, J.M.; Ahmad, S.I.; Kohner, E.M.; Chibber, R. Introduction to Diabetes Mellitus. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2013; pp. 1–11. [Google Scholar]
- Creed, F.H.; Davies, I.; Jackson, J.; Littlewood, A.; Chew-Graham, C.; Tomenson, B.; Macfarlane, G.; Barsky, A.; Katon, W.; McBeth, J. The Epidemiology of Multiple Somatic Symptoms. J. Psychosom. Res. 2012, 72, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Lovibond, P.F.; Lovibond, S.H. The Structure of Negative Emotional States: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav. Res. Ther. 1995, 33, 335–343. [Google Scholar] [CrossRef]
- de Beurs, E.; VanDyck, R.; Marquenie, L.A.; Lange, A.; Blonk, R.W.B.; de Beurs, E.; van Dyck, R.; Marquenie, L.A.; Lange, A.; Blonk, R.W.B. De DASS: Een Vragenlijst Voor Het Meten van Depressie, Angst En Stress. Gedragstherapie 2001, 34, 35–53. [Google Scholar]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B.W. The PHQ-15: Validity of a New Measure for Evaluating the Severity of Somatic Symptoms. Psychosom. Med. 2002, 64, 258–266. [Google Scholar] [CrossRef]
- Andersen, L.G.; Aadahl, M.; Groenvold, M.; Jørgensen, T. Construct Validity of a Revised Physical Activity Scale and Testing by Cognitive Interviewing. Scand. J. Public Health 2010, 38, 707–714. [Google Scholar] [CrossRef]
- Dekker, L.H.; Rijnks, R.H.; Strijker, D.; Navis, G.J. A Spatial Analysis of Dietary Patterns in a Large Representative Population in the North of The Netherlands—The Lifelines Cohort Study. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 166. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Willems, A.E.M.; Sura–de Jong, M.; van Beek, A.P.; Nederhof, E.; van Dijk, G. Effects of Macronutrient Intake in Obesity: A Meta-Analysis of Low-Carbohydrate and Low-Fat Diets on Markers of the Metabolic Syndrome. Nutr. Rev. 2020, 79, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Ze, X.; Duncan, S.H.; Louis, P.; Flint, H.J. Ruminococcus Bromii Is a Keystone Species for the Degradation of Resistant Starch in the Human Colon. ISME J. 2012, 6, 1535–1543. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Horn, W.H.; Finnegan, P.; Newman, J.W.; Marco, M.L.; Keim, N.L.; Kable, M.E. Resistant Starch Type 2 from Wheat Reduces Postprandial Glycemic Response with Concurrent Alterations in Gut Microbiota Composition. Nutrients 2021, 13, 645. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, P.; Yang, X.; Chen, M.; Li, J. Potential of Gut Microbiota for Lipopolysaccharide Biosynthesis in European Women with Type 2 Diabetes Based on Metagenome. Front. Cell Dev. Biol. 2022, 10, 1027413. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, R.; McCormick, K.L.; Zhang, Y.; Lin, X.; Yang, X. The Role of the Gut Microbiota on the Metabolic Status of Obese Children. Microb. Cell Fact. 2021, 20, 53. [Google Scholar] [CrossRef]
- Ning, L.; Zhou, Y.-L.; Sun, H.; Zhang, Y.; Shen, C.; Wang, Z.; Xuan, B.; Zhao, Y.; Ma, Y.; Yan, Y.; et al. Microbiome and Metabolome Features in Inflammatory Bowel Disease via Multi-Omics Integration Analyses across Cohorts. Nat. Commun. 2023, 14, 7135. [Google Scholar] [CrossRef]
- Backer Mortensen, T. Ultra-Processed Foods and the Intestinal Bacterial Flora? Kompass Nutr. Diet. 2022, 2, 29–30. [Google Scholar] [CrossRef]
- de Cuevillas, B.; Riezu-Boj, J.I.; Abete, I.; Zulet, M.A.; Galarregui, C.; Gonzalez-Navarro, C.J.; Milagro, F.I.; Martínez, J.A.; Navas-Carretero, S. Possible Metabolic Interplay between Quality of Life and Fecal Microbiota in a Presenior Population: Preliminary Results. Nutrition 2022, 103–104, 111841. [Google Scholar] [CrossRef]
- Golloso-Gubat, M.J.; Ducarmon, Q.R.; Tan, R.C.A.; Zwittink, R.D.; Kuijper, E.J.; Nacis, J.S.; Santos, N.L.C. Gut Microbiota and Dietary Intake of Normal-Weight and Overweight Filipino Children. Microorganisms 2020, 8, 1015. [Google Scholar] [CrossRef]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fan, D.; Zhang, Y.; Wang, J.; Dong, L.; Hu, Y.; Wang, S. Long-Term Exposure to Advanced Lipid Peroxidation End Products Impairs Cognitive Function through Microbiota-Gut-Brain Axis. Food Chem. 2024, 461, 140864. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Luukkonen, P.; Sädevirta, S.; Yki-Järvinen, H.; Salonen, A. Impact of Short-Term Overfeeding of Saturated or Unsaturated Fat or Sugars on the Gut Microbiota in Relation to Liver Fat in Obese and Overweight Adults. Clin. Nutr. 2021, 40, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat. Med. 2019, 25, 1096–1103. [Google Scholar] [CrossRef]
- Cani, P.D.; de Vos, W.M. Next-Generation Beneficial Microbes: The Case of Akkermansia Muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef]
- Liu, R.; Hong, J.; Xu, X.; Feng, Q.; Zhang, D.; Gu, Y.; Shi, J.; Zhao, S.; Liu, W.; Wang, X.; et al. Gut Microbiome and Serum Metabolome Alterations in Obesity and after Weight-Loss Intervention. Nat. Med. 2017, 23, 859–868. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia Muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Brahe, L.K.; Le Chatelier, E.; Prifti, E.; Pons, N.; Kennedy, S.; Hansen, T.; Pedersen, O.; Astrup, A.; Ehrlich, S.D.; Larsen, L.H. Specific Gut Microbiota Features and Metabolic Markers in Postmenopausal Women with Obesity. Nutr. Diabetes 2015, 5, e159. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, D.; Fang, Z.; Jie, Z.; Qiu, X.; Zhang, C.; Chen, Y.; Ji, L. Human Gut Microbiota Changes Reveal the Progression of Glucose Intolerance. PLoS ONE 2013, 8, e71108. [Google Scholar] [CrossRef]
- Yassour, M.; Lim, M.Y.; Yun, H.S.; Tickle, T.L.; Sung, J.; Song, Y.-M.; Lee, K.; Franzosa, E.A.; Morgan, X.C.; Gevers, D.; et al. Sub-Clinical Detection of Gut Microbial Biomarkers of Obesity and Type 2 Diabetes. Genome Med. 2016, 8, 17. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut Microbiota Dysbiosis Contributes to the Development of Hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A New Functional Genus with Potential Probiotic Properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef] [PubMed]
- Holzhausen, E.A.; Malecki, K.C.; Sethi, A.K.; Gangnon, R.; Cadmus-Bertram, L.; Deblois, C.L.; Suen, G.; Safdar, N.; Peppard, P.E. Assessing the Relationship between Physical Activity and the Gut Microbiome in a Large, Population-Based Sample of Wisconsin Adults. PLoS ONE 2022, 17, e0276684. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, M.; Szulińska, M.; Łoniewski, I.; Kręgielska-Narożna, M.; Skonieczna-Żydecka, K.; Kosciolek, T.; Bezshapkin, V.; Bogdański, P. Treatment With Multi-Species Probiotics Changes the Functions, Not the Composition of Gut Microbiota in Postmenopausal Women With Obesity: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Cell. Infect. Microbiol. 2022, 12. [Google Scholar] [CrossRef]
- Wegmann, U.; Louis, P.; Goesmann, A.; Henrissat, B.; Duncan, S.H.; Flint, H.J. Complete Genome of a New Firmicutes Species Belonging to the Dominant Human Colonic Microbiota (‘Ruminococcus bicirculans’) Reveals Two Chromosomes and a Selective Capacity to Utilize Plant Glucans. Environ. Microbiol. 2014, 16, 2879–2890. [Google Scholar] [CrossRef]
- Averina, O.V.; Zorkina, Y.A.; Yunes, R.A.; Kovtun, A.S.; Ushakova, V.M.; Morozova, A.Y.; Kostyuk, G.P.; Danilenko, V.N.; Chekhonin, V.P. Bacterial Metabolites of Human Gut Microbiota Correlating with Depression. Int. J. Mol. Sci. 2020, 21, 9234. [Google Scholar] [CrossRef]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced Dietary Intake of Carbohydrates by Obese Subjects Results in Decreased Concentrations of Butyrate and Butyrate-Producing Bacteria in Feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The Influence of Diet on the Gut Microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial Degradation of Complex Carbohydrates in the Gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Park, S.-J.; Lee, H.-J. Healthy and Unhealthy Dietary Patterns of Depressive Symptoms in Middle-Aged Women. Nutrients 2024, 16, 776. [Google Scholar] [CrossRef]
- Daïen, C.I.; Pinget, G.V.; Tan, J.K.; Macia, L. Detrimental Impact of Microbiota-Accessible Carbohydrate-Deprived Diet on Gut and Immune Homeostasis: An Overview. Front. Immunol. 2017, 8, 548. [Google Scholar] [CrossRef]
| Mean (SD) | |
|---|---|
| Age (y) | 49.1 (14.9) |
| Gender, female (%) | 80.5 |
| High education (%) | 70.3 |
| Smokers, current (%) | 4.3 |
| Diagnosed with depression (%) | 7.0 |
| High/moderate scores depression (%) | 8.1 |
| High/moderate scores anxiety (%) | 10.8 |
| High/moderate scores stress (%) | 2.2 |
| Probiotic use in the last 6 months (%) | 9.7 |
| Antibiotic use in the last 6 months (%) | 21.1 |
| High blood pressure diagnosis (%) | 8.1 |
| Diabetes Type 2 diagnosis (%) | 2.7 |
| High cholesterol diagnosis (%) | 7.6 |
| Factor Loadings of Food Categories in Identified Dietary Patterns | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Processed Foods | Animal Source Foods | Pescatarian | Traditional Dutch | Party | |||||
| Snacks | 0.754 | Fresh meat | 0.672 | Vegetables | 0.698 | Wholegrain products | 0.694 | Coffee | 0.732 |
| Candy | 0.697 | Eggs | 0.565 | Nuts | 0.696 | Dairy | 0.545 | Alcohol | 0.551 |
| Cookies | 0.648 | Fresh fish | 0.543 | Water | 0.490 | Tea | 0.523 | Processed fish products | 0.341 |
| Sugar sweetened beverages | 0.474 | Processed meat products | 0.412 | Fruit | 0.432 | Processed meat products | 0.407 | Wine | 0.336 |
| Refined grain products | 0.426 | Processed fish products | 0.363 | Processed fish products | 0.373 | Pasta, rice, and potatoes | 0.320 | Vegetables | −0.219 |
| Wine | 0.239 | Fruit | −0.303 | Vegetarian meat replacements | 0.294 | Gluten-free bread replacements | −0.471 | Tea | −0.229 |
| Pasta, rice, and potatoes | 0.210 | Wholegrain products | −0.303 | Pasta, rice and potatoes | 0.240 | Sugar sweetened beverages | −0.242 | ||
| Fruit | −0.201 | Pasta, rice, and potatoes | −0.326 | Fresh fish | 0.237 | Water | −0.407 | ||
| Vegetarian meat replacements | −0.549 | Candy | 0.210 | ||||||
| Refined grain products | −0.273 | ||||||||
| Sugar sweetened beverages | −0.288 | ||||||||
| Baseline | Week 4 | p a | |
|---|---|---|---|
| Caloric intake (kcal) | 1798.3 (259.4) | 1733.3 (266.8) | <0.001 |
| Carbohydrate intake (en%) | 41.4 (3.6) | 40.4 (3.5) | <0.001 |
| Fat intake (en%) | 35.7 (1.6) | 36.0 (1.9) | 0.015 |
| Protein intake (en%) | 17.0 (1.6) | 17.1 (1.5) | 0.096 |
| Saturated fat intake (en%) | 12.5 (0.8) | 12.5 (0.9) | 0.375 |
| Fiber intake (g) | 21.8 (2.9) | 21.6 (3.3) | 0.378 |
| Carbohydrate intake (g) | 185.9 (29.9) | 174.9 (30.2) | <0.001 |
| Fat intake (g) | 76.8 (16.7) | 74.7 (16.3) | <0.001 |
| Protein intake (g) | 71.3 (9.8) | 69.2 (9.8) | <0.001 |
| n | Baseline | n | Week 4 | p a | |
|---|---|---|---|---|---|
| Weight (kg) | 175 | 74.2 (14.0) | 174 | 73.7 (14.1) | <0.001 |
| BMI (kg/m2) | 175 | 24.5 (4.3) | 172 | 24.3 (4.3) | <0.001 |
| Waist circumference (cm) | 174 | 89.3 (12.9) | 178 | 88.7 (13.3) | <0.001 |
| DASS score | 185 | 14.7 (13.9) | 185 | 6.6 (8.7) | <0.001 |
| Depression score | 185 | 5.2 (5.2) | 185 | 2.3 (3.0) | <0.001 |
| Anxiety score | 185 | 4.1 (4.5) | 185 | 2.1 (3.1) | <0.001 |
| Stress score | 185 | 5.2 (5.0) | 185 | 2.2 (3.2) | <0.001 |
| PHQ-15 score | 185 | 7.5 (4.8) | 185 | 4.8 (3.9) | <0.001 |
| Activity pas-2 (METs/24 h) | 180 | 41.0 (5.2) | 177 | 40.9 (4.8) | 0.791 |
| Shannon index | 185 | 5.2 (0.7) | 185 | 5.2 (0.7) | 0.422 |
| Firmicutes/Bacteroidetes ratio | 185 | 3.3 (1.7) | 185 | 4.6 (15.8) | 0.251 |
| B | SE | Adj. r2 | p | |
|---|---|---|---|---|
| Species | ||||
| Blautia obeum | ||||
| Animal source foods | 0.035 | 0.012 | 0.04 | 0.004 |
| Carbohydrate intake (en%) | −0.092 | 0.028 | 0.05 | 0.001 |
| Fat intake (en%) | 0.145 | 0.052 | 0.04 | 0.005 |
| Saturated fat intake (en%) | 0.030 | 0.107 | 0.04 | 0.006 |
| Ruminococcus bromii | ||||
| Animal source foods | −0.067 | 0.018 | 0.06 | <0.001 |
| Carbohydrate intake (en%) | 0.159 | 0.044 | 0.06 | <0.001 |
| Fat intake (en%) | −0.280 | 0.079 | 0.06 | 0.001 |
| Saturated fat intake (en%) | −0.476 | 0.166 | 0.03 | 0.005 |
| Fiber intake | 0.181 | 0.044 | 0.08 | <0.001 |
| Carbohydrate intake (g) | 0.021 | 0.006 | 0.06 | <0.001 |
| Bifidobacterium adolescentis | ||||
| Pescatarian | −0.058 | 0.017 | 0.05 | 0.001 |
| Gracilibacter thermotolerans | ||||
| Pescatarian | 0.039 | 0.013 | 0.07 | 0.003 |
| Flintibacter butyricus | ||||
| Pescatarian | 0.021 | 0.006 | 0.05 | 0.001 |
| Akkermansia muciniphila | ||||
| Saturated fat intake (en%) | 0.591 | 0.196 | 0.06 | 0.003 |
| Genus | ||||
| Prevotella | ||||
| Saturated fat intake (en%) | −1.278 | 0.408 | 0.07 | 0.002 |
| Roseburia | ||||
| Saturated fat intake (en%) | −0.478 | 0.147 | 0.06 | 0.001 |
| Family | ||||
| Bacteroidaceae | ||||
| Fat intake (en%) | 0.709 | 0.268 | 0.12 | 0.009 |
| Saturated fat intake (en%) | 1.514 | 0.554 | 0.13 | 0.007 |
| Erysipelotrichaceae | ||||
| Fat intake (en%) | 0.288 | 0.096 | 0.10 | 0.003 |
| Saturated fat intake (en%) | 0.674 | 0.198 | 0.11 | <0.001 |
| Clostridiaceae | ||||
| Saturated fat intake (en%) | 0.583 | 0.234 | 0.15 | 0.014 |
| B | SE | Adj. r2 | p | |
|---|---|---|---|---|
| Species | ||||
| BMI | ||||
| Eubacterium hallii | 1.470 | 0.336 | 0.14 | <0.001 |
| Waist circumference | ||||
| Eubacterium hallii | 3.117 | 1.038 | 0.19 | 0.003 |
| Eubacterium rectale | 1.603 | 0.480 | 0.20 | 0.001 |
| Genus | ||||
| Stress symptoms | ||||
| Prevotella | 0.176 | 0.051 | 0.12 | <0.001 |
| BMI | ||||
| Clostridium | −0.532 | 0.114 | 0.15 | <0.001 |
| Coprococcus | 0.625 | 0.168 | 0.11 | <0.001 |
| Waist circumference | ||||
| Clostridium | −1.182 | 0.353 | 0.20 | 0.001 |
| Coprococcus | 1.743 | 0.506 | 0.20 | <0.001 |
| Unclassified Lachnospiraceae | 1.630 | 0.480 | 0.20 | <0.001 |
| Family | ||||
| BMI | ||||
| Clostridiaceae | −0.542 | 0.111 | 0.16 | <0.001 |
| Erysipelotrichaceae | −0.465 | 0.138 | 0.10 | <0.001 |
| Lachnospiraceae | 0.103 | 0.036 | 0.08 | 0.005 |
| Peptostreptococcaceae | −0.522 | 0.143 | 0.11 | <0.001 |
| Waist circumference | ||||
| Clostridiaceae | −1.266 | 0.343 | 0.21 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willems, A.; Sura-de Jong, M.; Klaassens, E.; van den Bogert, B.; van Beek, A.; van Dijk, G. Self-Initiated Dietary Adjustments Alter Microbiota Abundances: Implications for Perceived Health. Nutrients 2024, 16, 3544. https://doi.org/10.3390/nu16203544
Willems A, Sura-de Jong M, Klaassens E, van den Bogert B, van Beek A, van Dijk G. Self-Initiated Dietary Adjustments Alter Microbiota Abundances: Implications for Perceived Health. Nutrients. 2024; 16(20):3544. https://doi.org/10.3390/nu16203544
Chicago/Turabian StyleWillems, Anouk, Martina Sura-de Jong, Eline Klaassens, Bartholomeus van den Bogert, André van Beek, and Gertjan van Dijk. 2024. "Self-Initiated Dietary Adjustments Alter Microbiota Abundances: Implications for Perceived Health" Nutrients 16, no. 20: 3544. https://doi.org/10.3390/nu16203544
APA StyleWillems, A., Sura-de Jong, M., Klaassens, E., van den Bogert, B., van Beek, A., & van Dijk, G. (2024). Self-Initiated Dietary Adjustments Alter Microbiota Abundances: Implications for Perceived Health. Nutrients, 16(20), 3544. https://doi.org/10.3390/nu16203544







