Renal Health Through Medicine–Food Homology: A Comprehensive Review of Botanical Micronutrients and Their Mechanisms
Abstract
1. Introduction
2. Botanical Ingredients with Medicine–Food Homology in China, the US, and Europe
3. Nutrients from These Botanical Food Ingredients with Kidney-Protective Effects
3.1. Flavonoids
3.2. Polysaccharides
3.3. Terpenoids
3.4. Alkaloids
3.5. Others
4. Mechanisms Involved in the Kidney-Protective Effects of Botanical Ingredients with Medicine–Food Homology
4.1. Antioxidation, Anti-Inflammation, and Anti-Fibrosis
4.2. Regulating the “Gut–Kidney Axis”
5. Conclusions and Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Huang, J. Drug-Induced Nephrotoxicity: Pathogenic Mechanisms, Biomarkers and Prevention Strategies. Curr. Drug Metab. 2018, 19, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Mody, H.; Ramakrishnan, V.; Chaar, M.; Lezeau, J.; Rump, A.; Taha, K.; Lesko, L.; Ait-Oudhia, S. A Review on Drug-Induced Nephrotoxicity: Pathophysiological Mechanisms, Drug Classes, Clinical Management, and Recent Advances in Mathematical Modeling and Simulation Approaches. Clin. Pharmacol. Drug Dev. 2020, 9, 896–909. [Google Scholar] [CrossRef]
- Schetz, M.; Dasta, J.; Goldstein, S.; Golper, T. Drug-Induced Acute Kidney Injury. Curr. Opin. Crit. Care 2005, 11, 555–565. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The Gut Microbiota and the Brain–Gut–Kidney Axis in Hypertension and Chronic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Gaitonde, D.Y.; Cook, D.L.; Rivera, I.M. Chronic Kidney Disease: Detection and Evaluation. Am. Fam. Physician 2017, 96, 776–783. [Google Scholar]
- Tain, Y.L.; Chang, C.I.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.F.; Hsu, C.N. Resveratrol Propionate Ester Supplement Exerts Antihypertensive Effect in Juvenile Rats Exposed to an Adenine Diet Via Gut Microbiota Modulation. Nutrients 2024, 16, 2131. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, F.; Pugliese, S.; Cairoli, S.; Krohn, P.; De Stefanis, C.; Raso, R.; Rega, L.R.; Taranta, A.; De Leo, E.; Ciolfi, A.; et al. Ketogenic Diet and Progression of Kidney Disease in Animal Models of Nephropathic Cystinosis. J. Am. Soc. Nephrol. 2024, 10-1681. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, T.; Chen, J.; He, J.; Li, Y.; Chen, C.; Lu, G.; Chen, W. Traditional Chinese Medicine Formulas Alleviate Acute Pancreatitis: Pharmacological Activities and Mechanisms. Pancreas 2021, 50, 1348–1356. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Liang, Y.; Yang, Y.; Liu, Z.; Yao, K.; Chen, Z.; Zhai, S. Chinese Herbal Medicine for the Treatment of Depression: Applications, Efficacies and Mechanisms. Curr. Pharm. Des. 2017, 23, 5180–5190. [Google Scholar] [CrossRef]
- Chan, H.H.L.; Ng, T. Traditional Chinese Medicine (Tcm) and Allergic Diseases. Curr. Allergy Asthma Rep. 2020, 20, 67. [Google Scholar] [CrossRef]
- Wojcikowski, K.; Wohlmuth, H.; Johnson, D.W.; Rolfe, M.; Gobe, G. An In Vitro Investigation of Herbs Traditionally Used for Kidney and Urinary System Disorders: Potential Therapeutic and Toxic Effects. Nephrology 2009, 14, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, H.; Wang, R.; Zhang, C.; Huang, S.; Wu, J.; Mo, P.; Yu, H.; Li, S.; Chen, J.; et al. Formula Restores Iron Metabolism from Dysregulation in Anemic Rats with Adenine-Induced Nephropathy. J. Ethnopharmacol. 2023, 312, 116526. [Google Scholar] [CrossRef] [PubMed]
- Ll, J.; Yu, D.R.; Chen, H.Y.; Zhu, C.F.; Cheng, X.X.; Wang, Y.H.; Ni, J.; Wang, X.J.; Jinag, F. Long-Term Effect of the Treatment of Iga Nephropathy by Tonifying Shen, Activating Blood Stasis, Dispelling Wind-Dampness Combined with Western Medicine. Zhongguo Zhong Xi Yi Jie He Za Zhi 2017, 37, 28–33. [Google Scholar] [PubMed]
- Peng, M.; Cai, P.; Ma, H.; Meng, H.; Xu, Y.; Zhang, X.; Si, G. Chinese Herbal Medicine Shenqi Detoxification Granule Inhibits Fibrosis in Adenine Induced Chronic Renal Failure Rats. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 194–204. [Google Scholar] [PubMed]
- Notice of the National Health Commission on Further Regulating the Management of Health Food Raw Materials (No. 51 of 2002); National Health Commission of the People’s Republic of China: Beijing, China, 2002.
- Wan, F.; Ma, F.; Wu, J.; Qiao, X.; Chen, M.; Li, W.; Ma, L. Effect of Lycium barbarum Polysaccharide on Decreasing Serum Amyloid A3 Expression through Inhibiting Nf-Κb Activation in a Mouse Model of Diabetic Nephropathy. Anal. Cell. Pathol. 2022, 2022, 7847135. [Google Scholar] [CrossRef]
- Li, W.; Yu, L.; Fu, B.; Chu, J.; Chen, C.; Li, X.; Ma, J.; Tang, W. Protective Effects of Polygonatum kingianum Polysaccharides and Aqueous Extract on Uranium-Induced Toxicity in Human Kidney (Hk-2) Cells. Int. J. Biol. Macromol. 2022, 202, 68–79. [Google Scholar] [CrossRef]
- Yue, L.; Wang, W.; Wang, Y.; Du, T.; Shen, W.; Tang, H.; Wang, Y.; Yin, H. Bletilla striata Polysaccharide Inhibits Angiotensin Ii-Induced Ros and Inflammation Via Nox4 and Tlr2 Pathways. Int. J. Biol. Macromol. 2016, 89, 376–388. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, Y.; Tang, L.; Yang, X.; Chen, Z.; Wei, Y.; Shao, X.; Shao, X.; Xin, Z.; Cai, B.; et al. Astragalus Polysaccharide Attenuates Cisplatin-Induced Acute Kidney Injury by Suppressing Oxidative Damage and Mitochondrial Dysfunction. BioMed Res. Int. 2020, 2020, 2851349. [Google Scholar] [CrossRef]
- Zhao, K.; Wu, X.; Han, G.; Sun, L.; Zheng, C.; Hou, H.; Xu, B.B.; El-Bahy, Z.M.; Qian, C.; Kallel, M.; et al. Phyllostachys nigra (Lodd. Ex Lindl.) Derived Polysaccharide with Enhanced Glycolipid Metabolism Regulation and Mice Gut Microbiome. Int. J. Biol. Macromol. 2024, 257 Pt 1, 128588. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef]
- Nicastro, H.L.; Vorkoper, S.; Sterling, R.; Korn, A.R.; Brown, A.G.M.; Maruvada, P.; Oh, A.Y. Opportunities to Advance Implementation Science and Nutrition Research: A Commentary on the Strategic Plan for Nih Nutrition Research. Transl. Behav. Med. 2023, 13, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R. Herbal Medicinal Products Versus Botanical-Food Supplements in the European Market: State of Art and Perspectives. Nat. Prod. Commun. 2015, 10, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Luciano, R.L.; Perazella, M.A. Aristolochic Acid Nephropathy: Epidemiology, Clinical Presentation, and Treatment. Drug Saf. 2015, 38, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ban, T.H.; Min, J.W.; Seo, C.; Kim, D.R.; Lee, Y.H.; Chung, B.H.; Jeong, K.H.; Lee, J.W.; Kim, B.S.; Lee, S.H.; et al. Update of Aristolochic Acid Nephropathy in Korea. Korean J. Intern. Med. 2018, 33, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Dugo, M.; Gatto, R.; Zagatti, R.; Gatti, P.; Cascone, C. Herbal Remedies: Nephrotoxicity and Drug Interactions. G. Ital. Nefrol. 2010, 27 (Suppl. S52), S5–S9. [Google Scholar]
- Yang, H.; Zhao, Y.; Ren, B.; Wu, Y.; Qiu, Z.; Cheng, Y.; Qiu, B. Poria Acid Inhibit the Growth and Metastasis of Renal Cell Carcinoma by Inhibiting the Pi3k/Akt/Nf-Κb Signaling Pathway. Heliyon 2024, 10, e31106. [Google Scholar] [CrossRef]
- Lu, M.; Yin, J.; Xu, T.; Dai, X.; Liu, T.; Zhang, Y.; Wang, S.; Liu, Y.; Shi, H.; Zhang, Y.; et al. Fuling-Zexie Formula Attenuates Hyperuricemia-Induced Nephropathy and Inhibits Jak2/Stat3 Signaling and Nlrp3 Inflammasome Activation in Mice. J. Ethnopharmacol. 2024, 319 Pt 2, 117262. [Google Scholar] [CrossRef]
- Notice on Six New Substances Including Angelica Sinensis and Other Substances Which Are Both Food and Chinese Herbal Medicine According to Tradition (No. 8 of 2019); Food Safety Standards and Evaluation Division, Ed.; National Health Commission of the People’s Republic of China: Beijing, China, 2019.
- Notice on Nine New Substances Including Codonopsis Pilosula and Other Substances Which Are Both Food and Chinese Herbal Medicine According to Tradition (No. 9 of 2023); Food Safety Standards and Evaluation Division, Ed.; National Health Commission of the People’s Republic of China: Beijing, China, 2023.
- Regulations on Catalog of Substances That Are Traditionally Used as Both Food and Herbal Medicine; Food Safety Standards and Evaluation Division, Ed.; National Health Commission of the People’s Republic of China: Beijing, China, 2021.
- Vettorazzi, A.; de Cerain, A.L.; Sanz-Serrano, J.; Gil, A.G.; Azqueta, A. European Regulatory Framework and Safety Assessment of Food-Related Bioactive Compounds. Nutrients 2020, 12, 613. [Google Scholar] [CrossRef]
- Bailey, R.L. Current Regulatory Guidelines and Resources to Support Research of Dietary Supplements in the United States. Crit. Rev. Food Sci. Nutr. 2018, 60, 298–309. [Google Scholar] [CrossRef]
- US Department of Health and Human Services, National Institutes of Health, Office of Dietary Supplements. Dietary Supplement Label Database (Dsld); US Department of Health and Human Services, National Institutes of Health, Office of Dietary Supplements: Bethesda, MD, USA, 2013.
- Liu, Y.; Luo, J.; Peng, L.; Zhang, Q.; Rong, X.; Luo, Y.; Li, J. Flavonoids: Potential Therapeutic Agents for Cardiovascular Disease. Heliyon 2024, 10, e32563. [Google Scholar] [CrossRef]
- Frydman, A.; Weisshaus, O.; Bar-Peled, M.; Huhman, D.V.; Sumner, L.W.; Marin, F.R.; Lewinsohn, E.; Fluhr, R.; Gressel, J.; Eyal, Y. Citrus Fruit Bitter Flavors: Isolation and Functional Characterization of the Gene Cm1,2rhat Encoding a 1,2 Rhamnosyltransferase, a Key Enzyme in the Biosynthesis of the Bitter Flavonoids of Citrus. Plant J. 2004, 40, 88–100. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Chang, L.; Ren, Y.; Sui, M.; Fu, Y.; Zhang, L.; Hao, L. Quercetin Improves Diabetic Kidney Disease by Inhibiting Ferroptosis and Regulating the Nrf2 in Streptozotocin-Induced Diabetic Rats. Ren. Fail. 2024, 46, 2327495. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wen, S.; Wang, J.; Zeng, X.; Yu, H.; Chen, Y.; Zhu, X.; Xu, L. Senolytic Combination of Dasatinib and Quercetin Attenuates Renal Damage in Diabetic Kidney Disease. Phytomedicine 2024, 130, 155705. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.F.; Li, J.Y.; Wei, X.Y.; Ma, S.Q.; Wang, Q.G.; Qi, Z.; Duan, Z.C.; Tan, L.; Tang, H. Preclinical Evidence of Reno-Protective Effect of Quercetin on Acute Kidney Injury: A Meta-Analysis of Animal Studies. Front. Pharmacol. 2023, 14, 1310023. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Kuang, W.; Wang, S.; Zhao, Y. Kaempferol Improves Acute Kidney Injury Via Inhibition of Macrophage Infiltration in Septic Mice. Biosci. Rep. 2023, 43, BSR20230873. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Diao, J.; Dong, J.; Yan, Y.; Chen, Y.; Yan, S.; Liu, C.; He, Z.; He, J.; Zhang, C.; et al. Targeting Rock1 in Diabetic Kidney Disease: Unraveling Mesangial Fibrosis Mechanisms and Introducing Myricetin as a Novel Antagonist. Biomed. Pharmacother. 2024, 171, 116208. [Google Scholar] [CrossRef]
- Yang, Z.J.; Wang, H.R.; Wang, Y.I.; Zhai, Z.H.; Wang, L.W.; Li, L.; Zhang, C.; Tang, L. Myricetin Attenuated Diabetes-Associated Kidney Injuries and Dysfunction Via Regulating Nuclear Factor (Erythroid Derived 2)-Like 2 and Nuclear Factor-Κb Signaling. Front. Pharmacol. 2019, 10, 647. [Google Scholar] [CrossRef]
- Liu, P.; Tang, L.; Li, G.; Wu, X.; Hu, F.; Peng, W. Association between Consumption of Flavonol and Its Subclasses and Chronic Kidney Disease in Us Adults: An Analysis Based on National Health and Nutrition Examination Survey Data from 2007–2008, 2009–2010, and 2017–2018. Front. Nutr. 2024, 11, 1399251. [Google Scholar] [CrossRef]
- Jian, J.; Li, Y.-Q.; Han, R.-Y.; Zhong, X.; Xie, K.-H.; Yan, Y.; Wang, L.; Tan, R.-Z. Isorhamnetin Ameliorates Cisplatin-Induced Acute Kidney Injury in Mice by Activating Slpi-Mediated Anti-Inflammatory Effect in Macrophage. Immunopharmacol. Immunotoxicol. 2024, 46, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yang, L.N.; Yang, L.T.; Liang, Y.; Guo, F.; Fu, P.; Ma, L. Fisetin Ameliorates Fibrotic Kidney Disease in Mice Via Inhibiting Acsl4-Mediated Tubular Ferroptosis. Acta Pharmacol. Sin. 2024, 45, 150–165. [Google Scholar] [CrossRef]
- Zou, T.F.; Liu, Z.G.; Cao, P.C.; Zheng, S.H.; Guo, W.T.; Wang, T.X.; Chen, Y.L.; Duan, Y.J.; Li, Q.S.; Liao, C.Z.; et al. Fisetin Treatment Alleviates Kidney Injury in Mice with Diabetes-Exacerbated Atherosclerosis through Inhibiting Cd36/Fibrosis Pathway. Acta Pharmacol. Sin. 2023, 44, 2065–2074. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, S.; Jiang, H.; Liu, S.; Kong, F. Dia Proteomics Analysis Reveals the Mechanism of Folic Acid-Induced Acute Kidney Injury and the Effects of Icariin. Chem. Biol. Interact. 2024, 390, 110878. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, W.; Zhang, X.; Lv, C.; Lu, J. Icariin, the Main Prenylflavonoid of Epimedii Folium, Ameliorated Chronic Kidney Disease by Modulating Energy Metabolism Via Ampk Activation. J. Ethnopharmacol. 2023, 312, 116543. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Kazeem, A.I.; Wu, B.; Ishokare, L.O.; Arunsi, U.O.; Oyelere, A.K. Apigeninidin-Rich Sorghum bicolor (L. Moench) Extracts Suppress A549 Cells Proliferation and Ameliorate Toxicity of Aflatoxin B1-Mediated Liver and Kidney Derangement in Rats. Sci. Rep. 2022, 12, 7438. [Google Scholar] [CrossRef] [PubMed]
- Amorim, J.M.; de Souza, L.C.R.; de Souza, R.A.L.; da Silva Filha, R.; de Oliveira Silva, J.; de Almeida Araújo, S.; Tagliti, C.A.; Simões, E.S.A.C.; Castilho, R.O. Costus Spiralis Extract Restores Kidney Function in Cisplatin-Induced Nephrotoxicity Model: Ethnopharmacological Use, Chemical and Toxicological Investigation. J. Ethnopharmacol. 2022, 299, 115510. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kim, C.S.; Adams, B.C.; Wilkinson, R.; Hill, M.M.; Shah, A.K.; Mohamed, A.; Dutt, M.; Ng, M.S.Y.; Ungerer, J.P.J.; et al. Human Proximal Tubular Epithelial Cell-Derived Small Extracellular Vesicles Mediate Synchronized Tubular Ferroptosis in Hypoxic Kidney Injury. Redox Biol. 2024, 70, 103042. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, L.; Liu, X.; Gao, L.; Li, Y.; Wu, Y. Baicalein Alleviates Cisplatin-Induced Acute Kidney Injury by Inhibiting Alox12-Dependent Ferroptosis. Phytomedicine 2024, 130, 155757. [Google Scholar] [CrossRef]
- Hu, H.; Li, W.; Hao, Y.; Peng, Z.; Zou, Z.; Liang, W. Baicalin Ameliorates Renal Fibrosis by Upregulating Cpt1α-Mediated Fatty Acid Oxidation in Diabetic Kidney Disease. Phytomedicine 2024, 122, 155162. [Google Scholar] [CrossRef]
- Miguel, V.; Rey-Serra, C.; Tituaña, J.; Sirera, B.; Alcalde-Estévez, E.; Herrero, J.I.; Ranz, I.; Fernández, L.; Castillo, C.; Sevilla, L.; et al. Enhanced Fatty Acid Oxidation through Metformin and Baicalin as Therapy for COVID-19 and Associated Inflammatory States in Lung and Kidney. Redox Biol. 2023, 68, 102957. [Google Scholar] [CrossRef]
- Liu, B.; Deng, Q.; Zhang, L.; Zhu, W. Nobiletin Alleviates Ischemia/Reperfusion Injury in the Kidney by Activating Pi3k/Akt Pathway. Mol. Med. Rep. 2020, 22, 4655–4662. [Google Scholar] [CrossRef]
- Song, J.; Wang, H.; Sheng, J.; Zhang, W.; Lei, J.; Gan, W.; Cai, F.; Yang, Y. Vitexin Attenuates Chronic Kidney Disease by Inhibiting Renal Tubular Epithelial Cell Ferroptosis Via Nrf2 Activation. Mol. Med. 2023, 29, 147. [Google Scholar] [CrossRef]
- Ding, T.; Zhao, T.; Li, Y.; Liu, Z.; Ding, J.; Ji, B.; Wang, Y.; Guo, Z. Vitexin Exerts Protective Effects against Calcium Oxalate Crystal-Induced Kidney Pyroptosis In Vivo and In Vitro. Phytomedicine 2021, 86, 153562. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Qian, L.; He, S.; Zhang, C. Hesperidin Alleviates Zinc-Induced Nephrotoxicity Via the Gut-Kidney Axis in Swine. Front. Cell Infect. Microbiol. 2024, 14, 1390104. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, X.; Chen, J.; Luo, X.; Huang, X.; Zhou, Z.; He, Y.; Feng, S.; Jiao, Y.; Wang, R.; et al. Effects of Hesperidin on the Histological Structure, Oxidative Stress, and Apoptosis in the Liver and Kidney Induced by Nicl(2). Front. Vet. Sci. 2024, 11, 1424711. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.Y.; Choi, H.J.; Kim, K.; Leem, J. Antioxidant, Antiapoptotic, and Anti-Inflammatory Effects of Hesperetin in a Mouse Model of Lipopolysaccharide-Induced Acute Kidney Injury. Molecules 2023, 28, 2759. [Google Scholar] [CrossRef]
- Chen, X.; Wei, W.; Li, Y.; Huang, J.; Ci, X. Hesperetin Relieves Cisplatin-Induced Acute Kidney Injury by Mitigating Oxidative Stress, Inflammation and Apoptosis. Chem. Biol. Interact. 2019, 308, 269–278. [Google Scholar] [CrossRef]
- Chen, C.; Feng, F.; Qi, M.; Chen, Q.; Tang, W.; Diao, H.; Hu, Z.; Qiu, Y.; Li, Z.; Chu, Y.; et al. Dietary Citrus Flavonoids Improved Growth Performance and Intestinal Microbiota of Weaned Piglets Via Immune Function Mediated by Tlr2/Nf-Κb Signaling Pathway. J. Agric. Food Chem. 2024, 72, 16761–16776. [Google Scholar] [CrossRef]
- Chang, Y.W.; Chen, Y.L.; Park, S.H.; Yap, E.E.S.; Sung, W.C. Characterization of Functional Ingredients Extracted with Ethanol Solvents from Ponkan (Citrus reticulata) by-Products Using the Microwave Vacuum Drying Method Combined with Ultrasound-Assisted Extraction. Foods 2024, 13, 2129. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, H.; Hao, S.; Pan, D.; Wang, G.; Yu, W. Evaluation of Phenolic Composition and Antioxidant Properties of Different Varieties of Chinese Citrus. Food Chem. 2021, 364, 130413. [Google Scholar] [CrossRef]
- Kamel, K.M.; El-Raouf, O.M.A.; Metwally, S.A.; El-Latif, H.A.A.; El-sayed, M.E. Hesperidin and Rutin, Antioxidant Citrus Flavonoids, Attenuate Cisplatin-Induced Nephrotoxicity in Rats. J. Biochem. Mol. Toxicol. 2014, 28, 312–319. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, M.J.; Kim, J.Y.; Chung, H.Y.; Choi, J.S. Inhibitory Activity of Flavonoids from Prunus Davidiana and Other Flavonoids on Total Ros and Hydroxyl Radical Generation. Arch. Pharm. Res. 2003, 26, 809–815. [Google Scholar] [CrossRef]
- Mbara, K.C.; Fotsing, M.C.D.; Ndinteh, D.T.; Mbeb, C.N.; Nwagwu, C.S.; Khan, R.; Mokhetho, K.C.; Baijnath, H.; Nlooto, M.; Mokhele, S.; et al. Endoplasmic Reticulum Stress in Pancreatic Β-Cell Dysfunction: The Potential Therapeutic Role of Dietary Flavonoids. Curr. Res. Pharmacol. Drug Discov. 2024, 6, 100184. [Google Scholar] [CrossRef] [PubMed]
- Ramos, F.M.M.; Ribeiro, C.B.; Cesar, T.B.; Milenkovic, D.; Cabral, L.; Noronha, M.F.; Sivieri, K. Lemon Flavonoids Nutraceutical (Eriomin®) Attenuates Prediabetes Intestinal Dysbiosis: A Double-Blind Randomized Controlled Trial. Food Sci. Nutr. 2023, 11, 7283–7295. [Google Scholar] [CrossRef] [PubMed]
- Bellavite, P. Neuroprotective Potentials of Flavonoids: Experimental Studies and Mechanisms of Action. Antioxidants 2023, 12, 280. [Google Scholar] [CrossRef] [PubMed]
- Pabich, M.; Materska, M. Biological Effect of Soy Isoflavones in the Prevention of Civilization Diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]
- Misiakiewicz-Has, K.; Maciejewska-Markiewicz, D.; Szypulska-Koziarska, D.; Kolasa, A.; Wiszniewska, B. The Influence of Soy Isoflavones and Soy Isoflavones with Inulin on Kidney Morphology, Fatty Acids, and Associated Parameters in Rats with and without Induced Diabetes Type 2. Int. J. Mol. Sci. 2024, 25, 5418. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, D.; Yan, P.; Zheng, F.; Zhu, H.; Yuan, Z.; Yang, X.; Zuo, Y.; Chen, C.; Lu, H.; et al. Puerarin Suppresses Macrophage M1 Polarization to Alleviate Renal Inflammatory Injury through Antagonizing Tlr4/Myd88-Mediated Nf-Κb P65 and Jnk/Foxo1 Activation. Phytomedicine 2024, 132, 155813. [Google Scholar] [CrossRef]
- Zhang, S.S.; Zhang, N.N.; Guo, S.; Liu, S.J.; Hou, Y.F.; Li, S.; Ho, C.T.; Bai, N.S. Glycosides and Flavonoids from the Extract of Pueraria Thomsonii Benth Leaf Alleviate Type 2 Diabetes in High-Fat Diet Plus Streptozotocin-Induced Mice by Modulating the Gut Microbiota. Food Funct. 2022, 13, 3931–3945. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, W.; Huang, K.; Gan, F. Ferroptosis Is Involved in Quercetin-Mediated Alleviation of Ochratoxin a-Induced Kidney Damage. Food Chem. Toxicol. 2024, 191, 114877. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Feng, Q.; Yang, M.; Yang, Y.; Nie, J.; Wang, S. Quercetin Prevented Diabetic Nephropathy by Inhibiting Renal Tubular Epithelial Cell Apoptosis Via the Pi3k/Akt Pathway. Phytother. Res. 2024, 38, 3594–3606. [Google Scholar] [CrossRef]
- Sheng, H.; Zhang, D.; Zhang, J.; Zhang, Y.; Lu, Z.; Mao, W.; Liu, X.; Zhang, L. Kaempferol Attenuated Diabetic Nephropathy by Reducing Apoptosis and Promoting Autophagy through Ampk/Mtor Pathways. Front. Med. 2022, 9, 986825. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, J.; Zheng, X.; Song, J.; Yin, L.; Guo, H.; Chen, Q.; Liu, Y.; Ma, Q.; Zhang, H.; et al. Kaempferol Attenuates Doxorubicin-Induced Renal Tubular Injury by Inhibiting Ros/Ask1-Mediated Activation of the Mapk Signaling Pathway. Biomed. Pharmacother. 2023, 157, 114087. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Quan, D.; Chen, K.; Kang, L.; Yang, D.; Wu, H.; Yan, M.; Wu, S.; Lv, L.; Zhang, G. Kaempferol Inhibits Renal Fibrosis by Suppression of the Sonic Hedgehog Signaling Pathway. Phytomedicine 2023, 108, 154246. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, P.; Jiang, J.; Almoallim, H.S.; Alharbi, S.A.; Li, Y. Myricetin Attenuates Ethylene Glycol-Induced Nephrolithiasis in Rats Via Mitigating Oxidative Stress and Inflammatory Markers. Appl. Biochem. Biotechnol. 2023, 196, 5419–5434. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, L.; Zhai, H.; Wang, D.; Li, X.; Wang, Y.; Song, M.; Xu, C. Exploration of the Protective Mechanisms of Icariin against Cisplatin-Induced Renal Cell Damage in Canines. Front. Vet. Sci. 2024, 11, 1331409. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.; Sun, S.; Zhou, S.; Lv, Z.; Wang, R. Icariin Alleviates Renal Inflammation and Tubulointerstitial Fibrosis Via Nrf2-Mediated Attenuation of Mitochondrial Damage. Cell Biochem. Funct. 2024, 42, e4005. [Google Scholar] [CrossRef]
- Duan, S.; Ding, Z.; Liu, C.; Wang, X.; Dai, E. Icariin Suppresses Nephrotic Syndrome by Inhibiting Pyroptosis and Epithelial-to-Mesenchymal Transition. PLoS ONE 2024, 19, e0298353. [Google Scholar] [CrossRef]
- Wu, Q.; Li, W.; Zhao, J.; Sun, W.; Yang, Q.; Chen, C.; Xia, P.; Zhu, J.; Zhou, Y.; Huang, G.; et al. Apigenin Ameliorates Doxorubicin-Induced Renal Injury Via Inhibition of Oxidative Stress and Inflammation. Biomed. Pharmacother. 2021, 137, 111308. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Luo, J.; Jiang, Y.; Li, L.; Chen, Y.; Zhang, L.; Huang, Q.; Cao, Y.; Zhou, P.; et al. Apigenin Ameliorates Hyperuricemic Nephropathy by Inhibiting Urat1 and Glut9 and Relieving Renal Fibrosis Via the Wnt/Β-Catenin Pathway. Phytomedicine 2021, 87, 153585. [Google Scholar] [CrossRef]
- Zamani, F.; Samiei, F.; Mousavi, Z.; Azari, M.R.; Seydi, E.; Pourahmad, J. Apigenin Ameliorates Oxidative Stress and Mitochondrial Damage Induced by Multiwall Carbon Nanotubes in Rat Kidney Mitochondria. J. Biochem. Mol. Toxicol. 2021, 35, 1–7. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, Z.; Xie, M.; Li, S.; Zhang, J.; Zhou, J. Apigenin Attenuates Mesoporous Silica Nanoparticles-Induced Nephrotoxicity by Activating Foxo3a. Biol. Trace Elem. Res. 2022, 200, 2793–2806. [Google Scholar] [CrossRef]
- Azimi, A.; Eidi, A.; Mortazavi, P.; Rohani, A.H. Protective Effect of Apigenin on Ethylene Glycol-Induced Urolithiasis Via Attenuating Oxidative Stress and Inflammatory Parameters in Adult Male Wistar Rats. Life Sci. 2021, 279, 119641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, J.; Dong, W.; Huang, Q.; Wang, X.; Deng, K.; Ali, W.; Song, R.; Zou, H.; Ran, D.; et al. Luteolin Alleviates Cadmium-Induced Kidney Injury by Inhibiting Oxidative DNA Damage and Repairing Autophagic Flux Blockade in Chickens. Antioxidants 2024, 13, 525. [Google Scholar] [CrossRef]
- Xu, X.; Yu, Z.; Han, B.; Li, S.; Sun, Y.; Du, Y.; Wang, Z.; Gao, D.; Zhang, Z. Luteolin Alleviates Inorganic Mercury-Induced Kidney Injury Via Activation of the Ampk/Mtor Autophagy Pathway. J. Inorg. Biochem. 2021, 224, 111583. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Yi, T.; Li, Y.; Zhang, W.; Wang, X.; Liu, J.; Fan, Y.; Ji, J.; Xu, L. Luteolin Attenuates Lupus Nephritis by Regulating Macrophage Oxidative Stress Via Hif-1α Pathway. Eur. J. Pharmacol. 2023, 953, 175823. [Google Scholar] [CrossRef]
- Li, F.; Wei, R.; Huang, M.; Chen, J.; Li, P.; Ma, Y.; Chen, X. Luteolin Can Ameliorate Renal Interstitial Fibrosis-Induced Renal Anaemia through the Sirt1/Foxo3 Pathway. Food Funct. 2022, 13, 11896–11914. [Google Scholar] [CrossRef]
- Awoyomi, O.V.; Adeoye, Y.D.; Oyagbemi, A.A.; Ajibade, T.O.; Asenuga, E.R.; Gbadamosi, I.T.; Ogunpolu, B.S.; Falayi, O.O.; Hassan, F.O.; Omobowale, T.O.; et al. Luteolin Mitigates Potassium Dichromate-Induced Nephrotoxicity, Cardiotoxicity and Genotoxicity through Modulation of Kim-1/Nrf2 Signaling Pathways. Environ. Toxicol. 2021, 36, 2146–2160. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yang, S.; Wei, C.; Lu, G.; Lee, K.; He, J.C.; Liu, R.; Zhong, Y. Puerarin Attenuates Diabetic Kidney Injury through Interaction with Guanidine Nucleotide-Binding Protein Gi Subunit Alpha-1 (Gnai1) Subunit. J. Cell Mol. Med. 2022, 26, 3816–3827. [Google Scholar] [CrossRef]
- Song, Q.; Jian, W.; Zhang, Y.; Li, Q.; Zhao, Y.; Liu, R.; Zeng, Y.; Zhang, F.; Duan, J. Puerarin Attenuates Iron Overload-Induced Ferroptosis in Retina through a Nrf2-Mediated Mechanism. Mol. Nutr. Food Res. 2024, 68, e2300123. [Google Scholar] [CrossRef]
- Jing, G.H.; Liu, Y.D.; Liu, J.N.; Jin, Y.S.; Yu, S.L.; An, R.H. Puerarin Prevents Calcium Oxalate Crystal-Induced Renal Epithelial Cell Autophagy by Activating the Sirt1-Mediated Signaling Pathway. Urolithiasis 2022, 50, 545–556. [Google Scholar] [CrossRef]
- Sun, J.; Wei, S.; Zhang, Y.; Li, J. Protective Effects of Astragalus Polysaccharide on Sepsis-Induced Acute Kidney Injury. Anal. Cell. Pathol. 2021, 2021, 7178253. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Chai, Y.; Li, W.; Liu, X.; Chen, Y. Astragalus Polysaccharide Protects Sepsis Model Rats after Cecum Ligation and Puncture. Front. Bioeng. Biotechnol. 2022, 10, 1020300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, D.; Chen, S.; Wang, Y.; Jiang, H.; Yin, H. A New Glucomannan from Bletilla Striata: Structural and Anti-Fibrosis Effects. Fitoterapia 2014, 92, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, Y.; Li, J.; Zhang, Z.; He, Y.; Zhao, Q.; Yang, H.; Zhou, P. A Proteoglycan Isolated from Ganoderma Lucidum Attenuates Diabetic Kidney Disease by Inhibiting Oxidative Stress-Induced Renal Fibrosis Both In Vitro and In Vivo. J. Ethnopharmacol. 2023, 310, 116405. [Google Scholar] [CrossRef]
- Zhong, D.; Wang, H.; Liu, M.; Li, X.; Huang, M.; Zhou, H.; Lin, S.; Lin, Z.; Yang, B. Ganoderma Lucidum Polysaccharide Peptide Prevents Renal Ischemia Reperfusion Injury Via Counteracting Oxidative Stress. Sci. Rep. 2015, 5, 16910. [Google Scholar] [CrossRef]
- Xie, W.; Huang, Y.-Y.; Chen, H.-G.; Zhou, X. Study on the Efficacy and Mechanism of Lycium Barbarum Polysaccharide against Lead-Induced Renal Injury in Mice. Nutrients 2021, 13, 2945. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Zhang, Q.; Li, F.; Lei, T.; Luo, D.; Zhou, H.; Yang, B. Low Molecular Weight Fucoidan against Renal Ischemia–Reperfusion Injury Via Inhibition of the Mapk Signaling Pathway. PLoS ONE 2013, 8, e56224. [Google Scholar] [CrossRef]
- Li, X.-Y.; Chen, H.-R.; Zha, X.-Q.; Chen, S.; Pan, L.-H.; Li, Q.-M.; Luo, J.-P. Prevention and Possible Mechanism of a Purified Laminaria Japonica Polysaccharide on Adriamycin-Induced Acute Kidney Injury in Mice. Int. J. Biol. Macromol. 2020, 148, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Shao, F.; Zhang, B.; Wang, Z.; Li, X. Low Molecular Weight Fucoidan Can Inhibit the Fibrosis of Diabetic Kidneys by Regulating the Kidney Lipid Metabolism. J. Diabetes Res. 2021, 2021, 7618166. [Google Scholar] [CrossRef]
- Chen, J.; Cui, W.; Zhang, Q.; Jia, Y.; Sun, Y.; Weng, L.; Luo, D.; Zhou, H.; Yang, B. Low Molecular Weight Fucoidan Ameliorates Diabetic Nephropathy Via Inhibiting Epithelial-Mesenchymal Transition and Fibrotic Processes. Am. J. Transl. Res. 2015, 7, 1553–1563. [Google Scholar]
- Li, X.-Y.; Chen, H.-R.; Kuang, D.-D.; Pan, L.-H.; Li, Q.-M.; Luo, J.-P.; Zha, X.-Q. Laminaria Japonica Polysaccharide Attenuates Podocyte Epithelial-Mesenchymal Transformation Via Tgf-Β1-Mediated Smad3 and P38mapk Pathways. Int. J. Biol. Macromol. 2023, 241, 124637. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Li, S.; Chen, Z.; Tan, J.; Yao, J.; Duan, D. Low Molecular Weight Fucoidan Alleviates Diabetic Nephropathy by Binding Fibronectin and Inhibiting Ecm-Receptor Interaction in Human Renal Mesangial Cells. Int. J. Biol. Macromol. 2020, 150, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Zhang, Q.; Zhao, T. Low Molecular Weight Fucoidan and Its Fractions Inhibit Renal Epithelial Mesenchymal Transition Induced by Tgf-Β1 or Fgf-2. Int. J. Biol. Macromol. 2017, 105, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-M.; Jiang, S.; Li, S.-S.; Sun, Y.-S.; Wang, S.-H.; Liu, W.-C.; Wang, Z.; Wang, Y.-P.; Zhang, R.; Li, W. Endoplasmic Reticulum Stress-Activated Perk-Eif2α-Atf4 Signaling Pathway Is Involved in the Ameliorative Effects of Ginseng Polysaccharides against Cisplatin-Induced Nephrotoxicity in Mice. ACS Omega 2021, 6, 8958–8966. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Zhu, M.; Chen, J.; Yang, B.; Lv, Q.; Wang, L.; Guo, S.; Tan, X.; Li, C.; Bu, W.; et al. Characterization of a Novel Polysaccharide from Moutan Cortex and Its Ameliorative Effect on Ages-Induced Diabetic Nephropathy. Int. J. Biol. Macromol. 2021, 176, 589–600. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, L.; Zhu, M.; Yang, B.; Yang, Y.; Jia, X.; Feng, L. Moutan Cortex Polysaccharide Ameliorates Diabetic Kidney Disease Via Modulating Gut Microbiota Dynamically in Rats. Int. J. Biol. Macromol. 2022, 206, 849–860. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Wang, R.; Tang, W.; Kong, L.; Wang, W.; Wang, L.; Zhang, Y.; Ma, W. Plantaginis Semen Polysaccharides Ameliorate Renal Damage through Regulating Nlrp3 Inflammasome in Gouty Nephropathy Rats. Food Funct. 2021, 12, 2543–2553. [Google Scholar] [CrossRef]
- Huang, C.; Yu, J.; Da, J.; Dong, R.; Dai, L.; Yang, Y.; Deng, Y.; Yuan, J. Dendrobium Officinale Kimura & Migo Polysaccharide Inhibits Hyperglycaemia-Induced Kidney Fibrosis Via the Mirna-34a-5p/Sirt1 Signalling Pathway. J. Ethnopharmacol. 2023, 313, 116601. [Google Scholar]
- Wang, X.; Liu, W.; Jin, G.; Wu, Z.; Zhang, D.; Bao, Y.; Shi, W. Salvia Miltiorrhiza Polysaccharides Alleviates Florfenicol Induced Kidney Injury in Chicks Via Inhibiting Oxidative Stress and Apoptosis. Ecotoxicol. Environ. Saf. 2022, 233, 113339. [Google Scholar] [CrossRef]
- Zhang, J.; Bi, R.; Meng, Q.; Wang, C.; Huo, X.; Liu, Z.; Wang, C.; Sun, P.; Sun, H.; Ma, X.; et al. Catalpol Alleviates Adriamycin-Induced Nephropathy by Activating the Sirt1 Signalling Pathway In Vivo and In Vitro. Br. J. Pharmacol. 2019, 176, 4558–4573. [Google Scholar] [CrossRef]
- Cong, C.; Yuan, X.; Hu, Y.; Chen, W.; Wang, Y.; Tao, L. Catalpol Alleviates Ang Ii-Induced Renal Injury through Nf-Κb Pathway and Tgf-Β1/Smads Pathway. J. Cardiovasc. Pharmacol. 2022, 79, e116–e121. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Q.; Meng, X.; Zhao, L.; Zheng, X.; Feng, W. Catalpol Ameliorates Fructose-Induced Renal Inflammation by Inhibiting Tlr4/Myd88 Signaling and Uric Acid Reabsorption. Eur. J. Pharmacol. 2024, 967, 176356. [Google Scholar] [CrossRef] [PubMed]
- Zaaba, N.E.; Al-Salam, S.; Beegam, S.; Elzaki, O.; Yasin, J.; Nemmar, A. Catalpol Attenuates Oxidative Stress and Inflammation Via Mechanisms Involving Sirtuin-1 Activation and Nf-Κb Inhibition in Experimentally-Induced Chronic Kidney Disease. Nutrients 2023, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, T.; Wang, C.; Meng, Q.; Huo, X.; Wang, C.; Sun, P.; Sun, H.; Ma, X.; Wu, J.; et al. Catalpol-Induced Ampk Activation Alleviates Cisplatin-Induced Nephrotoxicity through the Mitochondrial-Dependent Pathway without Compromising Its Anticancer Properties. Oxid. Med. Cell Longev. 2021, 2021, 7467156. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Y.; Lv, Z.; Shu, A.; Du, Q.; Wang, W.; Chen, Y.; Xu, H. Study on the Inhibitive Effect of Catalpol on Diabetic Nephropathy. Life Sci. 2020, 257, 118120. [Google Scholar] [CrossRef]
- Shu, A.; Du, Q.; Chen, J.; Gao, Y.; Zhu, Y.; Lv, G.; Lu, J.; Chen, Y.; Xu, H. Catalpol Ameliorates Endothelial Dysfunction and Inflammation in Diabetic Nephropathy Via Suppression of Rage/Rhoa/Rock Signaling Pathway. Chem.-Biol. Interact. 2021, 348, 109625. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, C.; Kang, K.; Liu, H.; Liu, X.; Jia, X.; Yu, K. Loganin Attenuates Septic Acute Renal Injury with the Participation of Akt and Nrf2/Ho-1 Signaling Pathways. Drug Des. Devel Ther. 2021, 15, 501–513. [Google Scholar] [CrossRef]
- Huang, F.; Wang, X.; Xiao, G.; Xiao, J. Loganin Exerts a Protective Effect on Ischemia-Reperfusion-Induced Acute Kidney Injury by Regulating Jak2/Stat3 and Nrf2/Ho-1 Signaling Pathways. Drug Dev. Res. 2022, 83, 150–157. [Google Scholar] [CrossRef]
- Kong, X.; Zhao, Y.; Wang, X.; Yu, Y.; Meng, Y.; Yan, G.; Yu, M.; Jiang, L.; Song, W.; Wang, B.; et al. Loganin Reduces Diabetic Kidney Injury by Inhibiting the Activation of Nlrp3 Inflammasome-Mediated Pyroptosis. Chem.-Biol. Interact. 2023, 382, 110640. [Google Scholar] [CrossRef]
- Liu, K.; Xu, H.; Lv, G.; Liu, B.; Lee, M.K.K.; Lu, C.; Lv, X.; Wu, Y. Loganin Attenuates Diabetic Nephropathy in C57bl/6j Mice with Diabetes Induced by Streptozotocin and Fed with Diets Containing High Level of Advanced Glycation End Products. Life Sci. 2015, 123, 78–85. [Google Scholar] [CrossRef]
- Li, X.; Ma, A.; Liu, K. Geniposide Alleviates Lipopolysaccharide-Caused Apoptosis of Murine Kidney Podocytes by Activating Ras/Raf/Mek/Erk-Mediated Cell Autophagy. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1524–1532. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, N.; Shi, G.; Wang, H. Geniposide Ameliorated Sepsis-Induced Acute Kidney Injury by Activating Pparγ. Aging 2020, 12, 22744–22758. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qian, N.; Zhu, L.; Fan, L.; Fu, G.; Ma, M.; Bao, J.; Cao, C.; Liang, X. Geniposide Ameliorates Acute Kidney Injury Via Enhancing the Phagocytic Ability of Macrophages Towards Neutrophil Extracellular Traps. Eur. J. Pharmacol. 2023, 957, 176018. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Tan, L.; Yu, S.; Song, J.; Li, Z.; Peng, X.; Wei, Q.; He, Z.; Zhang, W.; Yang, X. Geniposide Reduced Oxidative Stress-Induced Apoptosis in Hk-2 Cell through Pi3k/Akt3/Foxo1 by M6a Modification. Int. Immunopharmacol. 2024, 131, 111820. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Jin, G.; Shi, Z.; Sun, W.; Chen, F. Geniposide Reduces Development of Streptozotocin-Induced Diabetic Nephropathy Via Regulating Nuclear Factor-Kappa B Signaling Pathways Geniposide Reduces Development of Streptozotocin-Induced Diabetic Nephropathy Via Regulating Nuclear Factor-Kappa B Signaling Pathways. Fundam. Clin. Pharmacol. 2017, 31, 54–63. [Google Scholar]
- Dusabimana, T.; Park, E.J.; Je, J.; Jeong, K.; Yun, S.P.; Kim, H.J.; Kim, H.; Park, S.W. Geniposide Improves Diabetic Nephropathy by Enhancing Ulk1-Mediated Autophagy and Reducing Oxidative Stress through Ampk Activation. Int. J. Mol. Sci. 2021, 22, 1651. [Google Scholar] [CrossRef]
- Peng, J.H.; Leng, J.; Tian, H.J.; Yang, T.; Fang, Y.; Feng, Q.; Zhao, Y.; Hu, Y.Y. Geniposide and Chlorogenic Acid Combination Ameliorates Non-Alcoholic Steatohepatitis Involving the Protection on the Gut Barrier Function in Mouse Induced by High-Fat Diet. Front. Pharmacol. 2018, 9, 1399. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Y.; Wang, L.; Sai, N.; Liu, Y.; Ni, J. Morroniside Inhibits H2O2-Induced Podocyte Apoptosis by Down-Regulating Nox4 Expression Controlled by Autophagy in Vitro. Front. Pharmacol. 2020, 11, 533809. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, W.; Ye, S. The Olive Constituent Oleuropein Exerts Nephritic Protective Effects on Diabetic Nephropathy in Db/Db Mice. Arch. Physiol. Biochem. 2019, 128, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Tüfekci, K.K.; Tatar, M. Oleuropein Mitigates Acrylamide-Induced Nephrotoxicity by Affecting Placental Growth Factor Immunoactivity in the Rat Kidney. Eurasian J. Med. 2023, 55, 228–233. [Google Scholar]
- Chen, L.; Cao, G.; Wang, M.; Feng, Y.L.; Chen, D.Q.; Vaziri, N.D.; Zhuang, S.; Zhao, Y.Y. The Matrix Metalloproteinase-13 Inhibitor Poricoic Acid Zi Ameliorates Renal Fibrosis by Mitigating Epithelial-Mesenchymal Transition. Mol. Nutr. Food Res. 2019, 63, e1900132. [Google Scholar] [CrossRef]
- Wang, M.; Chen, D.Q.; Chen, L.; Cao, G.; Zhao, H.; Liu, D.; Vaziri, N.D.; Guo, Y.; Zhao, Y.Y. Novel Inhibitors of the Cellular Renin-Angiotensin System Components, Poricoic Acids, Target Smad3 Phosphorylation and Wnt/Β-Catenin Pathway against Renal Fibrosis. Br. J. Pharmacol. 2018, 175, 2689–2708. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Q.; Wang, Y.N.; Vaziri, N.D.; Chen, L.; Hu, H.H.; Zhao, Y.Y. Poricoic Acid a Activates Ampk to Attenuate Fibroblast Activation and Abnormal Extracellular Matrix Remodelling in Renal Fibrosis. Phytomedicine 2020, 72, 153232. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Q.; Wu, X.Q.; Chen, L.; Hu, H.H.; Wang, Y.N.; Zhao, Y.Y. Poricoic Acid a as a Modulator of Tph-1 Expression Inhibits Renal Fibrosis Via Modulating Protein Stability of Β-Catenin and Β-Catenin-Mediated Transcription. Ther. Adv. Chronic Dis. 2020, 11, 2040622320962648. [Google Scholar] [CrossRef]
- Chen, D.Q.; Feng, Y.L.; Chen, L.; Liu, J.R.; Wang, M.; Vaziri, N.D.; Zhao, Y.Y. Poricoic Acid a Enhances Melatonin Inhibition of Aki-to-Ckd Transition by Regulating Gas6/Axlnfκb/Nrf2 Axis. Free Radic. Biol. Med. 2019, 134, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Q.; Chen, L.; Guo, Y.; Wu, X.Q.; Zhao, T.T.; Zhao, H.L.; Zhang, H.J.; Yan, M.H.; Zhang, G.Q.; Li, P. Poricoic Acid a Suppresses Renal Fibroblast Activation and Interstitial Fibrosis in Uuo Rats Via Upregulating Sirt3 and Promoting Β-Catenin K49 Deacetylation. Acta Pharmacol. Sin. 2023, 44, 1038–1050. [Google Scholar] [CrossRef]
- Li, Q.; Ming, Y.; Jia, H.; Wang, G. Poricoic Acid a Suppresses Tgf-Β1-Induced Renal Fibrosis and Proliferation Via the Pdgf-C, Smad3 and Mapk Pathways. Exp. Ther. Med. 2021, 21, 289. [Google Scholar] [CrossRef]
- Chung, S.; Yoon, H.E.; Kim, S.J.; Kim, S.J.; Koh, E.S.; Hong, Y.A.; Park, C.W.; Chang, Y.S.; Shin, S.J. Oleanolic Acid Attenuates Renal Fibrosis in Mice with Unilateral Ureteral Obstruction Via Facilitating Nuclear Translocation of Nrf2. Nutr. Metab. 2014, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Nataraju, A.; Saini, D.; Ramachandran, S.; Benshoff, N.; Liu, W.; Chapman, W.; Mohanakuma, T.r. Oleanolic Acid, a Plant Triterpenoid, Significantly Improves Survival and Function of Islet Allograft. Transplantation 2009, 88, 987–994. [Google Scholar] [CrossRef]
- Alqrad, M.A.I.; El-Agamy, D.S.; Ibrahim, S.R.M.; Sirwi, A.; Abdallah, H.M.; Abdel-Sattar, E.; El-Halawany, A.M.; Elsaed, W.M.; Mohamed, G.A. Sirt1/Nrf2/Nf-Κb Signaling Mediates Anti-Inflammatory and Anti-Apoptotic Activities of Oleanolic Acid in a Mouse Model of Acute Hepatorenal Damage. Medicina 2023, 59, 1351. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, J.Y.; Wei, Z.T.; Liu, S.K.; Sun, J.L.; Mao, Y.H.; Xu, Y.D.; Yang, Y. Therapeutic Effect and Mechanism of Combination Therapy with Ursolic Acid and Insulin on Diabetic Nephropathy in a Type I Diabetic Rat Model. Front. Pharmacol. 2022, 13, 969207. [Google Scholar] [CrossRef]
- Wang, K.; Xu, X.; Shan, Q.; Ding, R.; Lyu, Q.; Huang, L.; Chen, X.; Han, X.; Yang, Q.; Sang, X.; et al. Integrated Gut Microbiota and Serum Metabolomics Reveal the Protective Effect of Oleanolic Acid on Liver and Kidney-Injured Rats Induced by Euphorbia Pekinensis. Phytother. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luan, Z.L.; Huo, X.K.; Zhang, M.; Morisseau, C.; Sun, C.P.; Hammock, B.D.; Ma, X.C. Direct Targeting of Seh with Alisol B Alleviated the Apoptosis, Inflammation, and Oxidative Stress in Cisplatin-Induced Acute Kidney Injury. Int. J. Biol. Sci. 2023, 19, 294–310. [Google Scholar] [CrossRef] [PubMed]
- Luan, Z.L.; Ming, W.H.; Sun, X.W.; Zhang, C.; Zhou, Y.; Zheng, F.; Yang, Y.L.; Guan, Y.F.; Zhang, X.Y. A Naturally Occurring Fxr Agonist, Alisol B 23-Acetate, Protects against Renal Ischemia-Reperfusion Injury. Am. J. Physiol. Renal Physiol. 2021, 321, F617–F628. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, M.C.; Chen, Y.Y.; Chen, L.; Wang, Y.N.; Vaziri, N.D.; Miao, H.; Zhao, Y.Y. Alisol B 23-Acetate Attenuates Ckd Progression by Regulating the Renin-Angiotensin System and Gut-Kidney Axis. Ther. Adv. Chronic Dis. 2020, 11, 2040622320920025. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hao, X.; Li, X.; Li, Q.; Fang, X. Effects of Ginsenoside Rh2 on Cisplatin-Induced Nephrotoxicity in Renal Tubular Epithelial Cells by Inhibiting Endoplasmic Reticulum Stress. J. Biochem. Mol. Toxicol. 2024, 38, e23768. [Google Scholar] [CrossRef]
- Guo, J.; Chen, L.; Ma, M. Ginsenoside Rg1 Suppresses Ferroptosis of Renal Tubular Epithelial Cells in Sepsis-Induced Acute Kidney Injury Via the Fsp1-Coq(10)-Nad(P)H Pathway. Curr. Med. Chem. 2024, 31, 2119–2132. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, R.; Han, J. Ginsenoside Rh4 Facilitates the Sensitivity of Renal Cell Carcinoma to Ferroptosis Via the Nrf2 Pathway. Arch. Esp. Urol. 2024, 77, 119–128. [Google Scholar] [CrossRef]
- Hwang, H.J.; Hong, S.H.; Moon, H.S.; Yoon, Y.E.; Park, S.Y. Ginsenoside Rh2 Sensitizes the Anti-Cancer Effects of Sunitinib by Inducing Cell Cycle Arrest in Renal Cell Carcinoma. Sci. Rep. 2022, 12, 19752. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, Y.; Li, P.; Jiang, Y.; Song, D. Ginsenoside Rg3 Induces Mesangial Cells Proliferation and Attenuates Apoptosis by Mir-216a-5p/Mapk Pathway in Diabetic Kidney Disease. Aging 2024, 16, 9933–9943. [Google Scholar] [CrossRef]
- Sui, Z.; Sui, D.; Li, M.; Yu, Q.; Li, H.; Jiang, Y. Ginsenoside Rg3 Has Effects Comparable to Those of Ginsenoside Re on Diabetic Kidney Disease Prevention in Db/Db Mice by Regulating Inflammation, Fibrosis and Pparγ. Mol. Med. Rep. 2023, 27, 84. [Google Scholar] [CrossRef]
- Liu, Y.; Mou, L.; Yi, Z.; Lin, Q.; Banu, K.; Wei, C.; Yu, X. Integrative Informatics Analysis Identifies That Ginsenoside Re Improves Renal Fibrosis through Regulation of Autophagy. J. Nat. Med. 2024, 78, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Shi, Q.; Liu, Y.; Han, M.; Su, Y.; Sun, R.; Zhou, H.; Li, W.; Li, W. Ginsenoside Rg1 Treatment Alleviates Renal Fibrosis by Inhibiting the Nox4-Mapk Pathway in T2dm Mice. Ren. Fail. 2023, 45, 2197075. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Liu, L.; Jia, Y.; Lei, L.; Liu, J.; Zhu, S.; Zhou, H.; Chen, R.; Lu, H.A.J.; Yang, B. Ganoderma Triterpenes Retard Renal Cyst Development by Downregulating Ras/Mapk Signaling and Promoting Cell Differentiation. Kidney Int. 2017, 92, 1404–1418. [Google Scholar] [CrossRef]
- Geng, X.Q.; Ma, A.; He, J.Z.; Wang, L.; Jia, Y.L.; Shao, G.Y.; Li, M.; Zhou, H.; Lin, S.Q.; Ran, J.H.; et al. Ganoderic Acid Hinders Renal Fibrosis Via Suppressing the Tgf-Β/Smad and Mapk Signaling Pathways. Acta Pharmacol. Sin. 2020, 41, 670–677. [Google Scholar] [CrossRef]
- Shao, G.; He, J.; Meng, J.; Ma, A.; Geng, X.; Zhang, S.; Qiu, Z.; Lin, D.; Li, M.; Zhou, H.; et al. Ganoderic Acids Prevent Renal Ischemia Reperfusion Injury by Inhibiting Inflammation and Apoptosis. Int. J. Mol. Sci. 2021, 22, 10229. [Google Scholar] [CrossRef]
- Cai, L.; Horowitz, M.; Islam, M.S. Potential Therapeutic Targets for the Prevention of Diabetic Nephropathy: Glycyrrhetinic Acid. World J. Diabetes 2023, 14, 1717–1720. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, T.; Li, Y.; Guo, F.; Jin, X. Glycyrrhizic Acid Prevents Diabetic Nephropathy by Activating Ampk/Sirt1/Pgc-1α Signaling in Db/Db Mice. J. Diabetes Res. 2017, 2017, 2865912. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Li, Y.; Hu, X.; Qiu, Y.; Li, S.; Xie, Y.; Xu, C.; Lu, C.; Chen, G.; Yang, J. Glycyrrhizic Acid Improves Tacrolimus-Induced Renal Injury by Regulating Autophagy. Faseb J. 2023, 37, e22749. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Choi, A.; Seo, N.; Lim, J.S.; You, J.S.; Chung, Y.E. Protective Effect of Glycyrrhizin, a Direct Hmgb1 Inhibitor, on Post-Contrast Acute Kidney Injury. Sci. Rep. 2021, 11, 15625. [Google Scholar] [CrossRef]
- Li, Z.; Yan, M.; Cao, L.; Fang, P.; Guo, Z.; Hou, Z.; Zhang, B. Glycyrrhetinic Acid Accelerates the Clearance of Triptolide through P-Gp in Vitro. Phytother. Res. 2017, 31, 1090–1096. [Google Scholar] [CrossRef]
- Jiang, Y.; Cai, C.; Zhang, P.; Luo, Y.; Guo, J.; Li, J.; Rong, R.; Zhang, Y.; Zhu, T. Transcriptional Profile Changes after Treatment of Ischemia Reperfusion Injury-Induced Kidney Fibrosis with 18β-Glycyrrhetinic Acid. Ren. Fail. 2022, 44, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, J.; Song, Z.; Li, T.; Li, Z.; Gong, X. Tetramethylpyrazine Attenuates Renal Tubular Epithelial Cell Ferroptosis in Contrast-Induced Nephropathy by Inhibiting Transferrin Receptor and Intracellular Reactive Oxygen Species. Clin. Sci. 2024, 138, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Duan, Y.; Zheng, J.; Ye, Z.; Hei, T.K. Tetramethylpyrazine Prevents Contrast-Induced Nephropathy Via Modulating Tubular Cell Mitophagy and Suppressing Mitochondrial Fragmentation, Ccl2/Ccr2-Mediated Inflammation, and Intestinal Injury. Oxid. Med. Cell Longev. 2019, 2019, 7096912. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, A.; Wang, Z.; Sun, X.; Dong, M.; Qi, F.; Wang, L.; Zhang, Y.; Du, P. Tetramethylpyrazine Alleviates Acute Kidney Injury by Inhibiting Nlrp3/Hif-1α and Apoptosis. Mol. Med. Rep. 2020, 22, 2655–2664. [Google Scholar] [CrossRef]
- Rai, U.; Kosuru, R.; Prakash, S.; Tiwari, V.; Singh, S. Tetramethylpyrazine Alleviates Diabetic Nephropathy through the Activation of Akt Signalling Pathway in Rats. Eur. J. Pharmacol. 2019, 865, 172763. [Google Scholar] [CrossRef]
- Jing, M.; Cen, Y.; Gao, F.; Wang, T.; Jiang, J.; Jian, Q.; Wu, L.; Guo, B.; Luo, F.; Zhang, G.; et al. Nephroprotective Effects of Tetramethylpyrazine Nitrone Tbn in Diabetic Kidney Disease. Front. Pharmacol. 2021, 12, 680336. [Google Scholar] [CrossRef]
- Hu, J.; Gu, W.; Ma, N.; Fan, X.; Ci, X. Leonurine Alleviates Ferroptosis in Cisplatin-Induced Acute Kidney Injury by Activating the Nrf2 Signalling Pathway. Br. J. Pharmacol. 2022, 179, 3991–4009. [Google Scholar] [CrossRef]
- Yin, X.; Gao, Q.; Li, C.; Yang, Q.; Dong, H.; Li, Z. Leonurine Alleviates Vancomycin Nephrotoxicity Via Activating Pparγ and Inhibiting the Tlr4/Nf-Κb/Tnf-A Pathway. Int. Immunopharmacol. 2024, 131, 111898. [Google Scholar] [CrossRef]
- Cheng, H.; Bo, Y.; Shen, W.; Tan, J.; Jia, Z.; Xu, C.; Li, F. Leonurine Ameliorates Kidney Fibrosis Via Suppressing Tgf-Β and Nf-Κb Signaling Pathway in Uuo Mice. Int. Immunopharmacol. 2015, 25, 406–415. [Google Scholar] [CrossRef]
- Xu, D.; Chen, M.; Ren, X.; Ren, X.; Wu, Y. Leonurine Ameliorates Lps-Induced Acute Kidney Injury Via Suppressing Ros-Mediated Nf-Κb Signaling Pathway. Fitoterapia 2014, 97, 148–155. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, X.; Huang, L.; Lu, Z.; Wu, A.; Guo, S.; Li, C.; Mao, W.; Xie, Y.; Xu, P.; et al. Novel Insights into the Protective Effects of Leonurine against Acute Kidney Injury: Inhibition of Er Stress-Associated Ferroptosis Via Regulating Atf4/Chop/Acsl4 Pathway. Chem. Biol. Interact. 2024, 395, 111016. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, E.H.M.; Shalkami, A.-G.S.; Khalaf, M.M.; Mohamed, W.R.; Hemeida, R.A.M. The Impact of Keap1/Nrf2, P38Mapk/Nf-Κb and Bax/Bcl2/Caspase-3 Signaling Pathways in the Protective Effects of Berberine against Methotrexate-Induced Nephrotoxicity. Biomed. Pharmacother. 2019, 109, 47–56. [Google Scholar] [CrossRef]
- Fouad, G.I.; Ahmed, K.A. The Protective Impact of Berberine against Doxorubicin-Induced Nephrotoxicity in Rats. Tissue Cell 2021, 73, 101612. [Google Scholar] [CrossRef]
- Malaviya, A.N. Landmark Papers on the Discovery of Methotrexate for the Treatment of Rheumatoid Arthritis and Other Systemic Inflammatory Rheumatic Diseases: A Fascinating Story. Int. J. Rheum. Dis. 2016, 19, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Domitrovic, R.; Cvijanovic, O.; Pernjak-Pugel, E.; Skoda, M.; Mikelic, L.; Crncevic-Orlic, Z. Berberine Exerts Nephroprotective Effect against Cisplatin-Induced Kidney Damage through Inhibition of Oxidative/Nitrosative Stress, Inflammation, Autophagy and Apoptosis. Food Chem. Toxicol. 2013, 62, 397–406. [Google Scholar] [CrossRef]
- Adil, M.; Kandhare, A.D.; Dalvi, G.; Ghosh, P.; Venkata, S.; Raygude, K.S.; Bodhankar, S.L. Ameliorative Effect of Berberine against Gentamicin-Induced Nephrotoxicity in Rats Via Attenuation of Oxidative Stress, Inflammation, Apoptosis and Mitochondrial Dysfunction. Ren. Fail. 2016, 38, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Kumas, M.; Esrefoglu, M.; Karatas, E.; Duymac, N.; Kanbay, S.; Ergun, I.S.; Uyuklu, M.; Kocyigit, A. Investigation of Dose-Dependent Effects of Berberine against Renal Ischemia/Reperfusion Injury in Experimental Diabetic Rats. Nefrologia 2019, 39, 411–423. [Google Scholar] [CrossRef]
- Lu, J.; Yi, Y.; Pan, R.; Zhang, C.; Han, H.; Chen, J.; Liu, W. Berberine Protects Hk-2 Cells from Hypoxia/Reoxygenation Induced Apoptosis Via Inhibiting Sphk1 Expression. J. Nat. Med. 2018, 72, 390–398. [Google Scholar] [CrossRef]
- Visnagri, A.; Kandhare, A.D.; Bodhankar, S.L. Renoprotective Effect of Berberine Via Intonation on Apoptosis and Mitochondrial-Dependent Pathway in Renal Ischemia Reperfusion-Induced Mutilation. Ren. Fail. 2015, 37, 482–493. [Google Scholar] [CrossRef]
- Zhang, X.; He, H.; Liang, D.; Jiang, Y.; Liang, W.; Chi, Z.-H.; Ma, J. Protective Effects of Berberine on Renal Injury in Streptozotocin (Stz)-Induced Diabetic Mice. Int. J. Mol. Sci. 2016, 17, 1327. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, Z.; Zhang, X.; Wu, A.; Huang, Y.; Miao, Y.; Yang, M. Effect of Berberine on the Renal Tubular Epithelial-to- Mesenchymal Transition by Inhibition of the Notch/Snail Pathway in Diabetic Nephropathy Model Kkay Mice. Drug Des. Dev. Ther. 2017, 11, 1065–1079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guan, T.; Yang, B.; Chi, Z.; Wan, Q.; Gu, H.F. Protective Effect of Berberine on High Glucose and Hypoxia-Induced Apoptosis Via the Modulation of Hif-1α in Renal Tubular Epithelial Cells. Am. J. Transl. Res. 2019, 11, 669–682. [Google Scholar] [PubMed]
- Wang, F.-M.; Yang, Y.-J.; Ma, L.-L.; Tian, X.-J.; He, Y.-Q. Berberine Ameliorates Renal Interstitial Fibrosis Induced by Unilateral Ureteral Obstruction in Rats. Nephrology 2014, 19, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Yu, H.; Fu, J.; Hu, J.; Xu, H.; Zhang, Z.; Bu, M.; Yang, X.; Zhang, H.; Lu, J.; et al. Berberine Ameliorates Chronic Kidney Disease through Inhibiting the Production of Gut-Derived Uremic Toxins in the Gut Microbiota. Acta Pharm. Sin. B 2023, 13, 1537–1553. [Google Scholar] [CrossRef]
- Peerapen, P.; Thongboonkerd, V. Protective Roles of Trigonelline against Oxalate-Induced Epithelial-to-Mesenchymal Transition in Renal Tubular Epithelial Cells: An in Vitro Study. Food Chem. Toxicol. 2020, 135, 110915. [Google Scholar] [CrossRef]
- Peerapen, P.; Boonmark, W.; Putpeerawit, P.; Sassanarakkit, S.; Thongboonkerd, V. Proteomic and Computational Analyses Followed by Functional Validation of Protective Effects of Trigonelline against Calcium Oxalate-Induced Renal Cell Deteriorations. Comput. Struct. Biotechnol. J. 2023, 21, 5851–5867. [Google Scholar] [CrossRef]
- Sheweita, S.A.; ElHady, S.A.; Hammoda, H.M. Trigonella Stellata Reduced the Deleterious Effects of Diabetes Mellitus through Alleviation of Oxidative Stress, Antioxidant- and Drug-Metabolizing Enzymes Activities. J. Ethnopharmacol. 2020, 256, 112821. [Google Scholar] [CrossRef]
- Chen, C.; Ma, J.; Miao, C.S.; Zhang, H.; Zhang, M.; Cao, X.; Shi, Y. Trigonelline Induces Autophagy to Protect Mesangial Cells in Response to High. Glucose Via Activating the Mir-5189-5p-Ampk Pathway. Phytomedicine 2021, 92, 153614. [Google Scholar] [CrossRef]
- Gong, M.; Guo, Y.; Dong, H.; Wu, W.; Wu, F.; Lu, F. Trigonelline Inhibits Tubular Epithelial-Mesenchymal Transformation in Diabetic Kidney Disease Via Targeting Smad7. Biomed. Pharmacother. 2023, 168, 115747. [Google Scholar] [CrossRef]
- Peerapen, P.; Boonmark, W.; Thongboonkerd, V. Trigonelline Prevents Kidney Stone Formation Processes by Inhibiting Calcium Oxalate Crystallization, Growth and Crystal-Cell Adhesion, and Downregulating Crystal Receptors. Biomed. Pharmacother. 2022, 149, 112876. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, K.; Gao, Y.; Qiao, R.; Tang, N.; Dong, D.; Li, X.Q.; Nong, Q.; Luo, D.Q.; Xiao, Q.; et al. Piperlonguminine Attenuates Renal Fibrosis by Inhibiting Trpc6. J. Ethnopharmacol. 2023, 313, 116561. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yang, J.; Li, Y.; Yuan, L.; Liu, F.; Yuan, Y.; Tang, X. Matrine Alleviates Cisplatin-Induced Acute Kidney Injury by Inhibiting Mitochondrial Dysfunction and Inflammation Via Sirt3/Opa1 Pathway. J. Cell Mol. Med. 2022, 26, 3702–3715. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Chen, T.; Hao, Z.; Yang, X.; Wang, M.; Zhang, Z.; Hao, S.; Lang, F.; Hao, H. Oxymatrine Alleviates Gentamicin-Induced Renal Injury in Rats. Molecules 2022, 27, 6209. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Liu, X.; Wang, M.; Chen, H.; Chen, Z.; Qiu, T. Oxymatrine Ameliorates Renal Ischemia-Reperfusion Injury from Oxidative Stress through Nrf2/Ho-1 Pathway. Acta Cir. Bras. 2015, 30, 422–429. [Google Scholar] [CrossRef]
- Yao, M.; Lian, D.; Wu, M.; Zhou, Y.; Fang, Y.; Zhang, S.; Zhang, W.; Yang, Y.; Li, R.; Chen, H.; et al. Isoliensinine Attenuates Renal Fibrosis and Inhibits Tgf-Β1/Smad2/3 Signaling Pathway in Spontaneously Hypertensive Rats. Drug Des. Devel Ther. 2023, 17, 2749–2762. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Xu, Z.; Zhang, X.; Tan, X.; He, N.; Shen, J.; Dong, J. Liensinine Pretreatment Reduces Inflammation, Oxidative Stress, Apoptosis, and Autophagy to Alleviate Sepsis Acute Kidney Injury. Int. Immunopharmacol. 2023, 122, 110563. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhong, J.; Chen, W.; Li, X.; Liu, H.; Li, Y.; Xiong, W.; Li, H. Neferine Alleviates Acute Kidney Injury by Regulating the Ppar-A/Nf-Κb Pathway. Clin. Exp. Nephrol. 2024, 1–19. [Google Scholar] [CrossRef]
- Li, H.; Chen, W.; Chen, Y.; Zhou, Q.; Xiao, P.; Tang, R.; Xue, J. Neferine Attenuates Acute Kidney Injury by Inhibiting Nf-Κb Signaling and Upregulating Klotho Expression. Front. Pharmacol. 2019, 10, 1197. [Google Scholar] [CrossRef]
- Hongmei, H.; Maojun, Y.; Ting, L.I.; Dandan, W.; Ying, L.I.; Xiaochi, T.; Lu, Y.; Shi, G.U.; Yong, X.U. Neferine Inhibits the Progression of Diabetic Nephropathy by Modulating the Mir-17-5p/Nuclear Factor E2-Related Factor 2 Axis. J. Tradit. Chin. Med. 2024, 44, 44–53. [Google Scholar]
- Li, H.; Ge, H.; Song, X.; Tan, X.; Xiong, Q.; Gong, Y.; Zhang, L.; He, Y.; Zhang, W.; Zhu, P.; et al. Neferine Mitigates Cisplatin-Induced Acute Kidney Injury in Mice by Regulating Autophagy and Apoptosis. Clin. Exp. Nephrol. 2023, 27, 122–131. [Google Scholar] [CrossRef]
- Li, H.; Tang, Y.; Wen, L.; Kong, X.; Chen, X.; Liu, P.; Zhou, Z.; Chen, W.; Xiao, C.; Xiao, P.; et al. Neferine Reduces Cisplatin-Induced Nephrotoxicity by Enhancing Autophagy Via the Ampk/Mtor Signaling Pathway. Biochem. Biophys. Res. Commun. 2017, 484, 694–701. [Google Scholar] [CrossRef]
- Yin, W.; Wang, J.H.; Liang, Y.M.; Liu, K.H.; Chen, Y.; Chen, Y. Neferine Targeted the Nlrc5/Nlrp3 Pathway to Inhibit M1-Type Polarization and Pyroptosis of Macrophages to Improve Hyperuricemic Nephropathy. Curr. Mol. Med. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Wang, M.X.; Zhao, X.J.; Chen, T.Y.; Liu, Y.L.; Jiao, R.Q.; Zhang, J.H.; Ma, C.H.; Liu, J.H.; Pan, Y.; Kong, L.D. Nuciferine Alleviates Renal Injury by Inhibiting Inflammatory Responses in Fructose-Fed Rats. J. Agric. Food Chem. 2016, 64, 7899–7910. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, B.; Fan, Y.; Liu, M.; Han, B.; Meng, Y.; Xu, X.; Song, Z.; Liu, X.; Hao, Q.; et al. Nuciferine Protects against Folic Acid-Induced Acute Kidney Injury by Inhibiting Ferroptosis. Br. J. Pharmacol. 2021, 178, 1182–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Chen, Z.; Zhu, Q.; Guo, Z.; Sun, X.; Jiang, L.; Li, J.; Wang, N.; Yao, X.; Zhang, C.; et al. Curcumin Inhibits Pat-Induced Renal Ferroptosis Via the P62/Keap1/Nrf2 Signalling Pathway. Toxicology 2024, 506, 153863. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Tian, L.; Guo, P.; Liu, S.; Chen, H.; Sun, L. Curcumin Attenuates Lupus Nephritis by Inhibiting Neutrophil Migration Via Pi3k/Akt/Nf-Κb Signalling Pathway. Lupus Sci. Med. 2024, 11, e001220. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. The Ameliorative Effect of Turmeric (Curcuma longa Linn) Extract and Its Major Constituent, Curcumin, and Its Analogs on Ethanol Toxicity. Phytother. Res. 2024, 38, 2165–2181. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, Q.Q.; Shu, H.P.; Tu, Y.C.; Liao, Q.Q.; Yao, L.J. Curcumin Ameliorates Focal Segmental Glomerulosclerosis by Inhibiting Apoptosis and Oxidative Stress in Podocytes. Arch. Biochem. Biophys. 2023, 746, 109728. [Google Scholar] [CrossRef]
- Altamimi, J.Z.; AlFaris, N.A.; Al-Farga, A.M.; Alshammari, G.M.; BinMowyna, M.N.; Yahya, M.A. Curcumin Reverses Diabetic Nephropathy in Streptozotocin-Induced Diabetes in Rats by Inhibition of Pkcβ/P(66)Shc Axis and Activation of Foxo-3a. J. Nutr. Biochem. 2021, 87, 108515. [Google Scholar] [CrossRef]
- Feng, L.; Lin, Z.; Tang, Z.; Zhu, L.; Xu, S.; Tan, X.; Wang, X.; Mai, J.; Tan, Q. Emodin Improves Renal Fibrosis in Chronic Kidney Disease by Regulating Mitochondrial Homeostasis through the Mediation of Peroxisome Proliferator-Activated Receptor-Gamma Coactivator-1 Alpha (Pgc-1α). Eur. J. Histochem. 2024, 68, 3917. [Google Scholar] [CrossRef]
- Dong, X.; Wen, R.; Xiong, Y.; Jia, X.; Zhang, X.; Li, X.; Zhang, L.; Li, Z.; Zhang, S.; Yu, Y.; et al. Emodin Alleviates Crs4-Induced Mitochondrial Damage Via Activation of the Pgc1α Signaling. Phytother. Res. 2024, 38, 1345–1357. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Li, G.; Zhou, S.; Wang, R.; Long, Q.; Wang, M.; Li, L.; Huang, H.; Ba, Y. Emodin Ameliorates Renal Injury and Fibrosis Via Regulating the Mir-490-3p/Hmga2 Axis. Front. Pharmacol. 2023, 14, 1042093. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Deng, L.; Li, J.; Chen, M.; Liu, Y.; Hu, Y.; Zhong, W. Emodin Retarded Renal Fibrosis through Regulating Hgf and Tgfβ-Smad Signaling Pathway. Drug Des. Devel Ther. 2020, 14, 3567–3575. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Gao, Y.; Wang, X.; Wu, X.; Zou, D.; Zhu, Z.; Han, Z.; Wang, T.; Shi, Y. Emodin Mitigates Podocytes Apoptosis Induced by Endoplasmic Reticulum Stress through the Inhibition of the Perk Pathway in Diabetic Nephropathy. Drug Des. Devel Ther. 2018, 12, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- Kuang, B.C.; Wang, Z.H.; Hou, S.H.; Zhang, J.; Wang, M.Q.; Zhang, J.S.; Sun, K.L.; Ni, H.Q.; Gong, N.Q. Methyl Eugenol Protects the Kidney from Oxidative Damage in Mice by Blocking the Nrf2 Nuclear Export Signal through Activation of the Ampk/Gsk3β Axis. Acta Pharmacol. Sin. 2023, 44, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Fathy, M.; Abdel-Latif, R.; Abdelgwad, Y.M.; Othman, O.A.; Abdel-Razik, A.H.; Dandekar, T.; Othman, E.M. Nephroprotective Potential of Eugenol in a Rat Experimental Model of Chronic Kidney Injury; Targeting Nox, Tgf-Β, and Akt Signaling. Life Sci. 2022, 308, 120957. [Google Scholar] [CrossRef]
- Oikawa, D.; Yamashita, S.; Takahashi, S.; Waki, T.; Kikuchi, K.; Abe, T.; Katayama, T.; Nakayama, T. (+)-Sesamin, a Sesame Lignan, Is a Potent Inhibitor of Gut Bacterial Tryptophan Indole-Lyase That Is a Key Enzyme in Chronic Kidney Disease Pathogenesis. Biochem. Biophys. Res. Commun. 2022, 590, 158–162. [Google Scholar] [CrossRef]
- Altyar, A.E.; Albadrani, G.M.; Farouk, S.M.; Alamoudi, M.K.; Sayed, A.A.; Mohammedsaleh, Z.M.; Al-Ghadi, M.Q.; Saleem, R.M.; Sakr, H.I.; Abdel-Daim, M.M. The Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Effects of Sesamin against Cisplatin-Induced Renal and Testicular Toxicity in Rats. Ren. Fail. 2024, 46, 2378212. [Google Scholar] [CrossRef]
- Rousta, A.M.; Mirahmadi, S.M.; Shahmohammadi, A.; Nourabadi, D.; Khajevand-Khazaei, M.R.; Baluchnejadmojarad, T.; Roghani, M. Protective Effect of Sesamin in Lipopolysaccharide-Induced Mouse Model of Acute Kidney Injury Via Attenuation of Oxidative Stress, Inflammation, and Apoptosis. Immunopharmacol. Immunotoxicol. 2018, 40, 423–429. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, Y.; Deng, J.; Zhang, C.; Zhang, J.; Cheng, Y.; Luo, X.; Han, B.; Yang, H. Sesamin Ameliorates High-Fat Diet-Induced Dyslipidemia and Kidney Injury by Reducing Oxidative Stress. Nutrients 2016, 8, 276. [Google Scholar] [CrossRef]
- Zhang, N.; Zhou, J.; Zhao, L.; Zhao, Z.; Wang, S.; Zhang, L.; Zhou, F. Ferulic Acid Supplementation Alleviates Hyperuricemia in High-Fructose/Fat Diet-Fed Rats Via Promoting Uric Acid Excretion and Mediating the Gut Microbiota. Food Funct. 2023, 14, 1710–1725. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, B.; Zhao, X.; Lin, Y.; Zhuang, Y.; Guo, J.; Wang, S. Chlorogenic Acid Prevents Hyperuricemia Nephropathy Via Regulating Tmao-Related Gut Microbes and Inhibiting the Pi3k/Akt/Mtor Pathway. J. Agric. Food Chem. 2022, 70, 10182–10193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, B.; Zhao, X.; Lin, Y.; Wang, J.; Wang, X.; Hu, N.; Wang, S. Chlorogenic Acid Supplementation Ameliorates Hyperuricemia, Relieves Renal Inflammation, and Modulates Intestinal Homeostasis. Food Funct. 2021, 12, 5637–5649. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Dai, C.; Hao, Z.; Tang, Q.; Wang, H.; Wang, J.; Zhao, H. Chlorogenic Acid Prevents Vancomycin-Induced Nephrotoxicity without Compromising Vancomycin Antibacterial Properties. Phytother. Res. 2020, 34, 3189–3199. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Yi, Y.-L.; Guo, S.; Zhang, F.; Yan, H.; Zhan, Z.-L.; Zhu, Y.; Duan, J.-A. Isolation, Structural Characterization and Bioactivities of Polysaccharides from Laminaria Japonica: A Review. Food Chem. 2022, 370, 131010. [Google Scholar] [CrossRef]
- Lu, J.; Yao, J.; Pu, J.; Wang, D.; Liu, J.; Zhang, Y.; Zha, L. Transcriptome Analysis of Three Medicinal Plants of the Genus Polygonatum: Identification of Genes Involved in Polysaccharide and Steroidal Saponins Biosynthesis. Front. Plant Sci. 2023, 14, 1293411. [Google Scholar] [CrossRef]
- Ge, J.; Liu, Z.; Zhong, Z.; Wang, L.; Zhuo, X.; Li, J.; Jiang, X.; Ye, X.-Y.; Xie, T.; Bai, R. Natural Terpenoids with Anti-Inflammatory Activities: Potential Leads for Anti-Inflammatory Drug Discovery. Bioorganic Chem. 2022, 124, 105817. [Google Scholar] [CrossRef] [PubMed]
- Galappaththi, M.C.A.; Patabendige, N.M.; Premarathne, B.M.; Hapuarachchi, K.K.; Tibpromma, S.; Dai, D.-Q.; Suwannarach, N.; Rapior, S.; Karunarathna, S.C. A Review of Ganoderma Triterpenoids and Their Bioactivities. Biomolecules 2022, 13, 24. [Google Scholar] [CrossRef]
- Lu, D.; Yang, Y.; Du, Y.; Zhang, L.; Yang, Y.; Tibenda, J.J.; Nan, Y.; Yuan, L. The Potential of Glycyrrhiza from “Medicine Food Homology” in the Fight against Digestive System Tumors. Molecules 2023, 28, 7719. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Yang, L.; Kang, S.; Yan, G.; Han, Y.; Fang, H.; Sun, H. Potential of Alisols as Cancer Therapeutic Agents: Investigating Molecular Mechanisms, Pharmacokinetics and Metabolism. Biomed. Pharmacother. 2023, 168, 115722. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, J.H. A Review on the Medicinal Potentials of Ginseng and Ginsenosides on Cardiovascular Diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef]
- Yokozawa, T.; Liu, Z.W.; Dong, E. A Study of Ginsenoside-Rd in a Renal Ischemia-Reperfusion Model. Nephron 1998, 78, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Kouda, R.; Yakushiji, F. Recent Advances in Iridoid Chemistry: Biosynthesis and Chemical Synthesis. Chem.—Asian J. 2020, 15, 3771–3783. [Google Scholar] [CrossRef] [PubMed]
- Danielewski, M.; Matuszewska, A.; Nowak, B.; Kucharska, A.Z.; Sozański, T. The Effects of Natural Iridoids and Anthocyanins on Selected Parameters of Liver and Cardiovascular System Functions. Oxid. Med. Cell Longev. 2020, 2020, 2735790. [Google Scholar] [CrossRef]
- Bridi, R.; von Poser, G.L.; de Carvalho Meirelles, G. Iridoids as a Potential Hepatoprotective Class: A Review. Mini Rev. Med. Chem. 2023, 23, 452–479. [Google Scholar] [PubMed]
- Zhou, T.Y.; Tian, N.; Li, L.; Yu, R. Iridoids Modulate Inflammation in Diabetic Kidney Disease: A Review. J. Integr. Med. 2024, 22, 210–222. [Google Scholar] [CrossRef]
- Kou, Y.; Li, Z.; Yang, T.; Shen, X.; Wang, X.; Li, H.; Zhou, K.; Li, L.; Xia, Z.; Zheng, X.; et al. Therapeutic Potential of Plant Iridoids in Depression: A Review. Pharm. Biol. 2022, 60, 2167–2181. [Google Scholar] [CrossRef]
- Zhang, F.; Yan, Y.; Xu, J.K.; Zhang, L.M.; Li, L.; Chen, X.; Li, D.X.; Peng, Y.; Yang, H.; Li, L.Z.; et al. Simultaneous Determination of Thirteen Iridoid Glycosides in Crude and Processed Fructus Corni from Different Areas by Uplc-Ms/Ms Method. J. Chromatogr. Sci. 2024, 62, 562–569. [Google Scholar] [CrossRef]
- Cheng, C.; Li, Z.; Zhao, X.; Liao, C.; Quan, J.; Bode, A.M.; Cao, Y.; Luo, X. Natural Alkaloid and Polyphenol Compounds Targeting Lipid Metabolism: Treatment Implications in Metabolic Diseases. Eur. J. Pharmacol. 2020, 870, 172922. [Google Scholar] [CrossRef]
- Li, J.; Gong, X. Tetramethylpyrazine: An Active Ingredient of Chinese Herbal Medicine W Ith Therapeutic Potential in Acute Kidney Injury and Renal Fibrosis. Front. Pharmacol. 2022, 13, 820071. [Google Scholar] [CrossRef]
- Wu, D.; Wen, W.; Qi, C.L.; Zhao, R.X.; Lü, J.H.; Zhong, C.Y.; Chen, Y.Y. Ameliorative Effect of Berberine on Renal Damage in Rats with Diabetes Induced by High-Fat Diet and Streptozotocin. Phytomedicine 2012, 19, 712–718. [Google Scholar] [CrossRef]
- Tang, L.; Lv, F.; Liu, S.; Zhang, S. Effect of Berberine on Expression of Transforming Growth Factor-Beta1 and Type Iv Collagen Proteins in Mesangial Cells of Diabetic Rats with Nephropathy. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Medica 2011, 36, 3494–3497. [Google Scholar]
- Chen, Q.; Li, D.; Wu, F.; He, X.; Zhou, Y.; Sun, C.; Wang, H.; Liu, Y. Berberine Regulates the Metabolism of Uric Acid and Modulates Intestinal Flora in Hyperuricemia Rats Model. Comb. Chem. High. Throughput Screen. 2023, 26, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Wu, M.; Chen, T.; Tang, W.; Li, P.; Chen, J. Berberine Attenuates Hyperuricemia by Regulating Urate Transporters and Gut Microbiota. Am. J. Chin. Med. 2022, 50, 2199–2221. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Han, P.; Ma, S.; Peng, R.; Wang, C.; Kong, W.; Cong, L.; Fu, J.; Zhang, Z.; Yu, H.; et al. Abnormal Metabolism of Gut Microbiota Reveals the Possible Molecular Mechanism of Nephropathy Induced by Hyperuricemia. Acta Pharm. Sin. B 2020, 10, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, J.; Wei, H.; Wang, X.; Xie, M.; Jiang, Y.; Zhou, J. Matrine-Induced Nephrotoxicity Via Gsk-3β/Nrf2-Mediated Mitochondria-Dependent Apoptosis. Chem. Biol. Interact. 2023, 378, 110492. [Google Scholar] [CrossRef]
- Wang, X.; Lin, Z.; Li, T.; Zhu, W.; Huang, H.; Hu, J.; Zhou, J. Sodium Selenite Prevents Matrine-Induced Nephrotoxicity by Suppressing Ferroptosis Via the Gsh-Gpx4 Antioxidant System. Biol. Trace Element Res. 2024, 202, 4674–4686. [Google Scholar] [CrossRef]
- Wang, H.-W.; Shi, L.; Xu, Y.-P.; Qin, X.-Y.; Wang, Q.-Z. Oxymatrine Inhibits Renal Fibrosis of Obstructive Nephropathy by Downregulating the Tgf-Β1-Smad3 Pathway. Ren. Fail. 2016, 38, 945–951. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, R.; Zhang, L.; Zhu, Z. Simple Phenylpropanoids: Recent Advances in Biological Activities, Biosynthetic Pathways, and Microbial Production. Nat. Prod. Rep. 2024, 41, 6–24. [Google Scholar] [CrossRef]
- Zhu, X.; Quan, Y.-Y.; Yin, Z.-J.; Li, M.; Wang, T.; Zheng, L.-Y.; Feng, S.-Q.; Zhao, J.-N.; Li, L. Sources, Morphology, Phytochemistry, Pharmacology of Curcumae Longae Rhizoma, Curcumae Radix, and Curcumae Rhizoma: A Review of the Literature. Front. Pharmacol. 2023, 14, 1229963. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Cas, M.D.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef]
- Xu, X.; Wang, H.; Guo, D.; Man, X.; Liu, J.; Li, J.; Luo, C.; Zhang, M.; Zhen, L.; Liu, X. Curcumin Modulates Gut Microbiota and Improves Renal Function in Rats with Uric Acid Nephropathy. Ren. Fail. 2021, 43, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Mohtashami, L.; Amiri, M.S.; Ayati, Z.; Ramezani, M.; Jamialahmadi, T.; Emami, S.A.; Sahebkar, A. Ethnobotanical Uses, Phytochemistry and Pharmacology of Different Rheum Species (Polygonaceae): A Review. Adv. Exp. Med. Biol. 2021, 1308, 309–352. [Google Scholar]
- Zeng, Y.Q.; Dai, Z.; Lu, F.; Lu, Z.; Liu, X.; Chen, C.; Qu, P.; Li, D.; Hua, Z.; Qu, Y.; et al. Emodin Via Colonic Irrigation Modulates Gut Microbiota and Reduces Uremic Toxins in Rats with Chronic Kidney Disease. Oncotarget 2016, 7, 17468–17478. [Google Scholar] [CrossRef]
- Tucker, P.S.; Scanlan, A.T.; Dalbo, V.J. Chronic Kidney Disease Influences Multiple Systems: Describing the Relationship between Oxidative Stress, Inflammation, Kidney Damage, and Concomitant Disease. Oxidative Med. Cell. Longev. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Hsu, C.-N. Perinatal Oxidative Stress and Kidney Health: Bridging the Gap between Animal Models and Clinical Reality. Antioxidants 2023, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Mapuskar, K.A.; Pulliam, C.F.; Zepeda-Orozco, D.; Griffin, B.R.; Furqan, M.; Spitz, D.R.; Allen, B.G. Redox Regulation of Nrf2 in Cisplatin-Induced Kidney Injury. Antioxidants 2023, 12, 1728. [Google Scholar] [CrossRef]
- Honda, T.; Hirakawa, Y.; Nangaku, M. The Role of Oxidative Stress and Hypoxia in Renal Disease. Kidney Res. Clin. Pract. 2019, 38, 414–426. [Google Scholar] [CrossRef] [PubMed]
- Allameh, H.; Fatemi, I.; Malayeri, A.R.; Nesari, A.; Mehrzadi, S.; Goudarzi, M. Pretreatment with Berberine Protects against Cisplatin-Induced Renal Injury in Male Wistar Rats. Naunyn-Schmiedebergs Arch. Pharmacol. 2020, 393, 1825–1833. [Google Scholar] [CrossRef]
- Verma, V.K.; Malik, S.; Mutneja, E.; Sahu, A.K.; Rupashi, K.; Dinda, A.K.; Arya, D.S.; Bhatia, J. Mechanism Involved in Fortification by Berberine in Cddp-Induced Nephrotoxicity. Curr. Mol. Pharmacol. 2020, 13, 342–352. [Google Scholar] [CrossRef]
- El-Horany, H.E.-S.; Gaballah, H.H.; Helal, D.S. Berberine Ameliorates Renal Injury in a Rat Model of D-Galactose-Induced Aging through a Pten/Akt-Dependent Mechanism. Arch. Physiol. Biochem. 2020, 126, 157–165. [Google Scholar] [CrossRef]
- Hasanein, P.; Riahi, H. Preventive Use of Berberine in Inhibition of Lead-Induced Renal Injury in Rats. Environ. Sci. Pollut. Res. 2018, 25, 4896–4903. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Anderson, M.D.; Sutton, T.A. Pathophysiology of Acute Kidney Injury. Compr. Physiol. 2012, 2, 1303–1353. [Google Scholar] [PubMed]
- Fu, Y.; Xiang, Y.; Li, H.; Chen, A.; Dong, Z. Inflammation in Kidney Repair: Mechanism and Therapeutic Potential. Pharmacol. Ther. 2022, 237, 108240. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Sulbaran, J.A.; Pedreañez, A.; Vargas, R.; Hernandez-Fonseca, J.P. Apoptosis in Post-Streptococcal Glomerulonephritis and Mechanisms for Failed of Inflammation Resolution. Pediatr. Nephrol. 2024, 39, 1709–1724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Deng, Z.; Wang, Y. Molecular Insight in Intrarenal Inflammation Affecting Four Main Types of Cells in Nephrons in Iga Nephropathy. Front. Med. 2023, 10, 1128393. [Google Scholar] [CrossRef]
- Vallés, P.G.; Lorenzo, A.F.G.; Garcia, R.D.; Cacciamani, V.; Benardon, M.E.; Costantino, V.V. Toll-Like Receptor 4 in Acute Kidney Injury. Int. J. Mol. Sci. 2023, 24, 1415. [Google Scholar] [CrossRef]
- Yeh, T.H.; Tu, K.C.; Wang, H.Y.; Chen, J.Y. From Acute to Chronic: Unraveling the Pathophysiological Mechanisms of the Progression from Acute Kidney Injury to Acute Kidney Disease to Chronic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 1755. [Google Scholar] [CrossRef]
- Lovisa, S.; Zeisberg, M.; Kalluri, R. Partial Epithelial-to-Mesenchymal Transition and Other New Mechanisms of Kidney Fibrosis. Trends Endocrinol. Metab. 2016, 27, 681–695. [Google Scholar] [CrossRef]
- La Russa, A.; Serra, R.; Faga, T.; Crugliano, G.; Bonelli, A.; Coppolino, G.; Bolignano, D.; Battaglia, Y.; Ielapi, N.; Costa, D.; et al. Kidney Fibrosis and Matrix Metalloproteinases (Mmps). Front. Biosci.-Landmark 2024, 29, 192. [Google Scholar] [CrossRef]
- Evenepoel, P.; Poesen, R.; Meijers, B. The Gut-Kidney Axis. Pediatr. Nephrol. 2017, 32, 2005–2014. [Google Scholar] [CrossRef]
- Ramya Ranjan Nayak, S.P.; Boopathi, S.; Haridevamuthu, B.; Arockiaraj, J. Toxic Ties: Unraveling the Complex Relationship between Endocrine Disrupting Chemicals and Chronic Kidney Disease. Environ. Pollut. 2023, 338, 122686. [Google Scholar] [CrossRef] [PubMed]
- Hayeeawaema, F.; Muangnil, P.; Jiangsakul, J.; Tipbunjong, C.; Huipao, N.; Khuituan, P. A Novel Model of Adenine-Induced Chronic Kidney Disease-Associated Gastrointestinal Dysfunction in Mice: The Gut-Kidney Axis. Saudi J. Biol. Sci. 2023, 30, 103660. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ma, J.; Li, R. Alterations of Gut Microbiota in Biopsy-Proven Diabetic Nephropathy and a Long History of Diabetes without Kidney Damage. Sci. Rep. 2023, 13, 12150. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xie, S.; Lv, D.; Zhang, Y.; Deng, J.; Zeng, L.; Chen, Y. A Reduction in the Butyrate Producing Species Roseburia Spp. and Faecalibacterium prausnitzii Is Associated with Chronic Kidney Disease Progression. Antonie Van Leeuwenhoek 2016, 109, 1389–1396. [Google Scholar] [CrossRef]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of Urease- and Uricase-Containing, Indole- and P-Cresol-Forming and Contraction of Short-Chain Fatty Acid-Producing Intestinal Microbiota in Esrd. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef]
- Marques, F.Z.; Nelson, E.; Chu, P.Y.; Horlock, D.; Fiedler, A.; Ziemann, M.; Tan, J.K.; Kuruppu, S.; Rajapakse, N.W.; El-Osta, A.; et al. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation 2017, 135, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Tang, Z.; Zhu, C.; Li, F.; Chen, S.; Han, X.; Zheng, R.; Hu, X.; Lin, R.; Pei, Q.; et al. Intestinal Cxcr6+ Ilc3s Migrate to the Kidney and Exacerbate Renal Fibrosis Via Il-23 Receptor Signaling Enhanced by Pd-1 Expression. Immunity 2024, 57, 1306–1323. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut Microbiota Induce Igf-1 and Promote Bone Formation and Growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef]
- Gleeson, P.J.; Benech, N.; Chemouny, J.; Metallinou, E.; Berthelot, L.; da Silva, J.; Bex-Coudrat, J.; Boedec, E.; Canesi, F.; Bounaix, C.; et al. The Gut Microbiota Posttranslationally Modifies Iga1 in Autoimmune Glomerulonephritis. Sci. Transl. Med. 2024, 16, eadl6149. [Google Scholar] [CrossRef]
- Lan, T.; Tang, T.; Li, Y.; Duan, Y.; Yuan, Q.; Liu, W.; Ren, Y.; Li, N.; Liu, X.; Zhang, Y.; et al. Ftz Polysaccharides Ameliorate Kidney Injury in Diabetic Mice by Regulating Gut-Kidney Axis. Phytomedicine 2023, 118, 154935. [Google Scholar] [CrossRef]
- Kunter, U.; Seikrit, C.; Floege, J. Novel Agents for Treating Iga Nephropathy. Curr. Opin. Nephrol. Hypertens. 2023, 32, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-W.; Han, P.; Fu, J.; Yu, H.; Xu, H.; Hu, J.-C.; Lu, J.-Y.; Yang, X.-Y.; Zhang, H.-J.; Bu, M.-M.; et al. Gut microbiota-based metabolites of Xiaoyao Pills (a typical Traditional Chinese medicine) ameliorate depression by inhibiting fatty acid amide hydrolase levels in brain. J. Ethnopharmacol. 2023, 313, 116555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-W.; Gao, C.-S.; Zhang, H.; Yang, J.; Wang, Y.-P.; Pan, L.-B.; Yu, H.; He, C.-Y.; Luo, H.-B.; Zhao, Z.-X.; et al. Morinda officinalis oligosaccharides increase serotonin in the brain and ameliorate depression via promoting 5-hydroxytryptophan production in the gut microbiota. Acta Pharm. Sin. B 2022, 12, 3298–3312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, Q.; Ma, S.-R.; Zhao, Z.-X.; Pan, L.-B.; Cong, L.; Han, P.; Peng, R.; Yu, H.; Lin, Y.; et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct. Target. Ther. 2021, 6, 77. [Google Scholar] [CrossRef]
- Feng, R.; Shou, J.-W.; Zhao, Z.-X.; He, C.-Y.; Ma, C.; Huang, M.; Fu, J.; Tan, X.-S.; Li, X.-Y.; Wen, B.-Y.; et al. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci. Rep. 2015, 5, 12155. [Google Scholar] [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef]
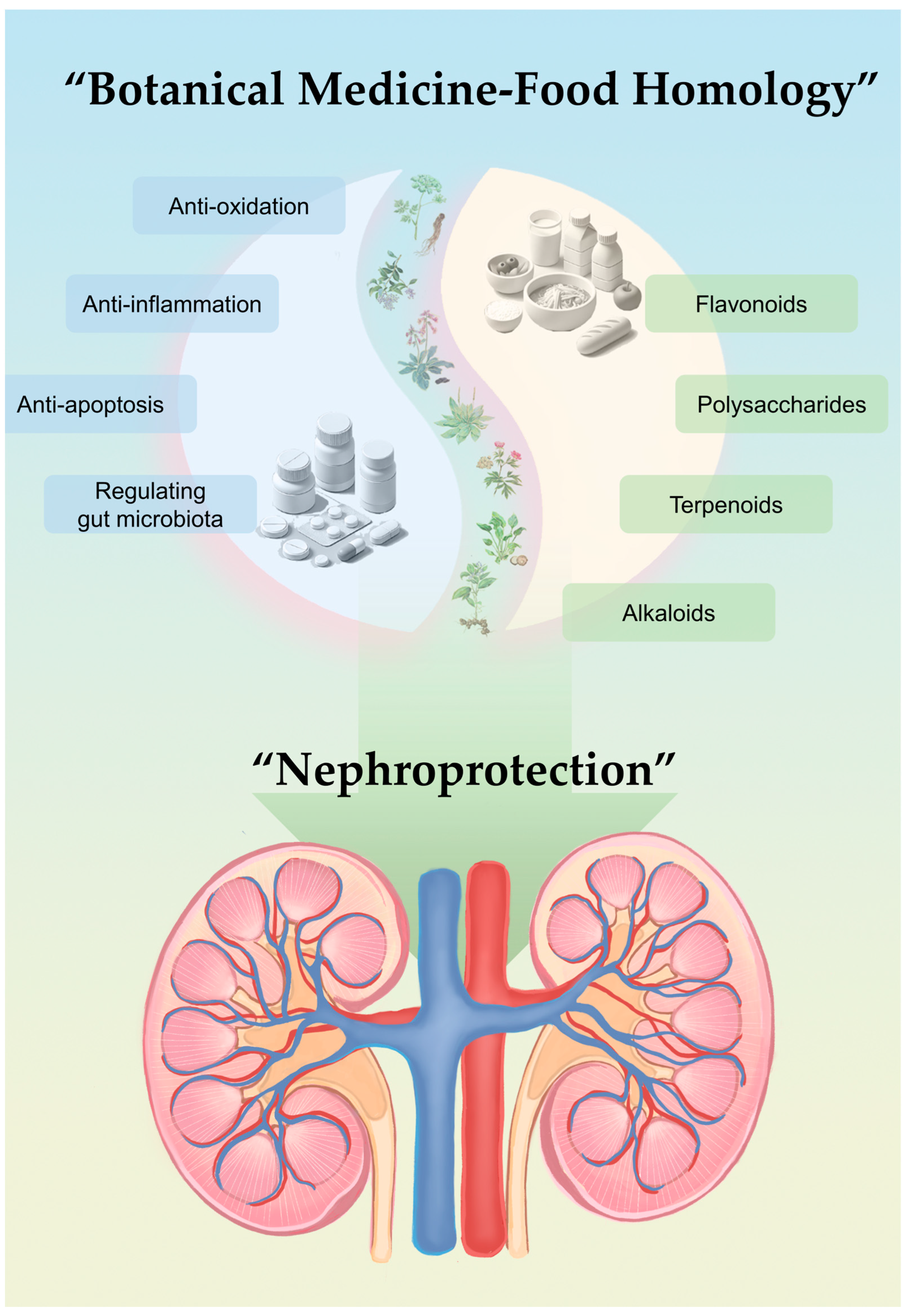
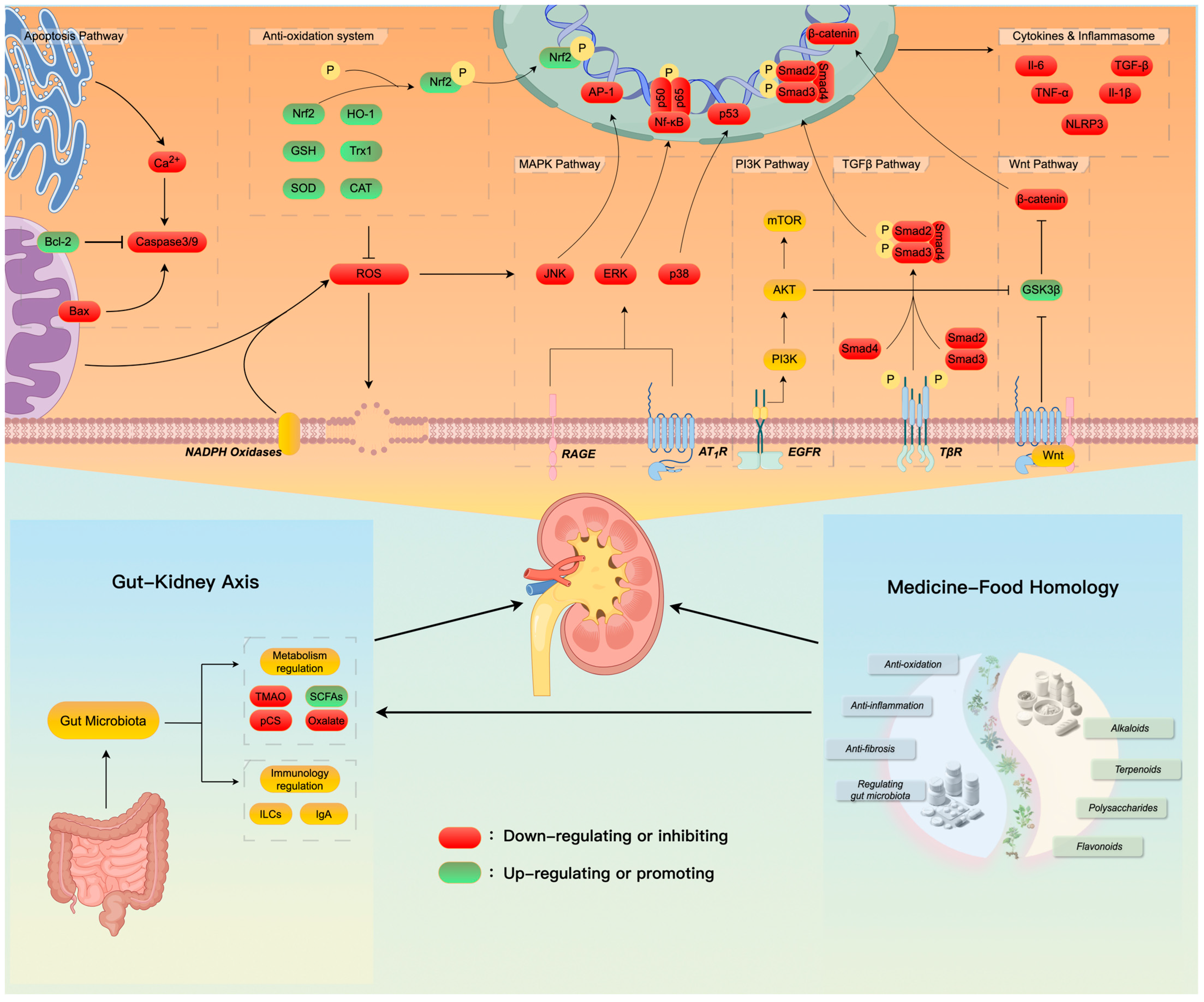
| Latin Name | English Common Name | EMA Herbal Medicine | EFSA Compendium | NIH DSLD |
|---|---|---|---|---|
| Eugenia caryophyllata Thunb. | Clove | √ | √ | √ |
| Illicium verum Hook. f. | Star anise | √ | √ | |
| Canavalia gladiata (Jacq.) DC. | Sword bean | √ | ||
| Foeniculum vulgare Mill. | Fennel | √ | √ | √ |
| Cirsium setosum (Willd.) MB. | Thistle | |||
| Dioscorea opposita Thunb. | Chinese yam | √ | ||
| Crataegus pinnatifida Bge. var. major N.E.Br./Crataegus pinnatifida Bge. | Hawthorn | √ | √ | √ |
| Portulaca oleracea L. | Purslane | √ | √ | |
| Prunus mume (Sieb.) Sieb. et Zucc. | Japanese apricot | √ | √ | |
| Chaenomeles speciosa (Sweet) Nakai | Flowering quince | √ | ||
| Cannabis sativa L. | Hemp | √ | √ | |
| Citrus aurantium L. var. amara Engl. | Bitter orange | |||
| Polygonatum odoratum (Mill.) Druce | Solomon’s seal | √ | ||
| Glycyrrhiza uralensis Fisch./Glycyrrhiza inflata Bat./Glycyrrhiza glabra L. | Licorice | √ | √ | √ |
| Angelica dahurica (Fisch. ex Hoffm.) Benth. et Hook. f./Angelica dahurica (Fisch. ex Hoffm.) Benth. et Hook. f. var. formosana (Boiss.) Shan et Yuan | Chinese angelica | √ | ||
| Ginkgo biloba L. | Ginkgo | √ | √ | √ |
| Dolichos lablab L. | Hyacinth bean | √ | √ | |
| Dimocarpus longan Lour. | Longan | √ | √ | |
| Cassia obtusifolia L./Cassia tora L. | Sicklepod | √ | ||
| Lilium lancifolium Thunb./Lilium pumilum DC. | Tiger lily | √ | √ | |
| Myristica fragrans Houtt. | Nutmeg | √ | √ | |
| Cinnamomum cassia Presl | Cassia | √ | √ | √ |
| Phyllanthus emblica L. | Amla | √ | ||
| Citrus medica L. var. sarcodactylis Swingle | Fingered citron | |||
| Prunus armeniaca L. var. ansu Maxim/Prunus sibirica L./Prunus mandshurica (Maxim) Koehne/Prunus armeniaca L. | Chinese apricot | √ | √ | |
| Hippophae rhamnoides L. | Sea buckthorn | |||
| Euryale ferox Salisb. | Euryale ferox | √ | ||
| Zanthoxylum schinifolium Sieb. et Zucc./Zanthoxylum bungeanum Maxim. | Sichuan pepper | √ | ||
| Vigna umbeuata Ohwi et Ohashi/Vigna angularis Ohwi et Ohashi | Azuki bean | |||
| Hordeum vulgare L. | Barley | √ | √ | |
| Laminaria japonica Aresch./Ecklonia kurome Okam. | Kunbu | |||
| Ziziphus jujuba Mill. | Jujube | √ | √ | |
| Siraitia grosvenorii (Swingle.) C.Jeffrey ex A. M. Lu et Z. Y.Zhang | Luohanguo siraitia fruit | √ | ||
| Prunus japonica Thunb. | Pruni semen | √ | ||
| Lonicera japonica Thunb. | Japanese honeysuckle | √ | ||
| Canarium album Raeusch. | White canarium | √ | ||
| Houttuynia cordata Thunb. | Fish mint | √ | ||
| Zingiber officinale Rosc. | Ginger | √ | √ | √ |
| Hovenia dulcis Thunnb./Hovenia acerba Lindl./Hovenia trichocarpa Chun et Tsiang | Japanese raisin tree | √ | ||
| Lycium barbarum L. | Goji berry | √ | √ | |
| Gardenia jasminoides Ellis | Cape jasmine | √ | √ | |
| Amomum villosum Lour./Amomum villosum Lour. var. xanthioides T. L. Wu et Senjen/Amomum longiligulare T. L. Wu | Villus amomum | √ | √ | |
| Sterculia lychnophora Hance | Malva nut tree | √ | √ | |
| Poria cocos (Schw.) Wolf | Poria, fu ling | |||
| Citrus medica L./Citrus wilsonii Tanaka | Citron | √ | √ | |
| Mosla chinensis Maxim./Mosla chinensis Maxim. cv. Jiangxiangru | Chinese mosla | √ | ||
| Prunus persica (L.) Batsch/Prunus davidiana (Carr.) Franch. | Peach | √ | √ | |
| Morus alba L. | White mulberry | √ | √ | |
| Citrus reticulata Blanco | Mandarin orange | √ | √ | |
| Platycodon grandiflorum (Jacq.) A. DC. | Balloon flower | √ | √ | |
| Alpinia oxyphylla Miq. | Sharp-leaf galangal | √ | √ | |
| Nelumbo nucifera Gaertn. | Lotus leaf | √ | √ | |
| Raphanus sativus L. | Radish seed | √ | √ | |
| Alpinia officinarum Hance | Lesser galangal | √ | √ | |
| Lophatherum gracile Brongn. | Lophatherum | √ | √ | |
| Glycine max (L.) Merr. | Soybean | √ | √ | √ |
| Chrysanthemum morifolium Ramat. | Chrysanthemum | √ | √ | |
| Cichorium glandulosum Boiss. et Huet/Cichorium intybus L. | Chicory | √ | ||
| Polygonatum kingianum Coll.et Hemsl./Polygonatum sibiricum Red./Polygonatum cyrtonema Hua | Polygonati rhizoma | √ | ||
| Sinapis alba L. | White mustard | √ | √ | |
| Perilla frutescens (L.) Britton | Perilla | √ | √ | |
| Pueraria lobata (Willd.) Ohwi/Pueraria thomsonii Benth. | Kudzu | √ | √ | |
| Sesamum indicum L. | Sesame | √ | √ | |
| Piper nigrum L. | Black pepper | √ | √ | |
| Sophora japonica L. | Sophora | √ | √ | |
| Taraxacum mongolicum Hand. Mazz./Taraxacum borealisinense Kitam. | Dandelion | √ | √ | √ |
| Torreya grandis Fort. | Torreya | √ | √ | |
| Ziziphus jujuba Mill. var. spinosa (Bunge) Hu ex H. F. Chou | Chinese date | |||
| Imperata cylindrical Beauv. var. major (nees) C. E. Hubb. | Cogongrass | |||
| Phragmites communis Trin. | Reed | √ | √ | |
| Mentha haplocalyx Briq. | Chinese mint | √ | √ | √ |
| Coix lacryma-jobi L. var. mayuen (Roman.) Stapf | Job’s tears | √ | √ | |
| Allium macrostemon Bge./Allium chinense G. Don | Chinese onion | √ | ||
| Rubus chingii Hu | Chinese raspberry | √ | √ | √ |
| Pogostemon cablin (Blanco) Benth./Agastache rugosus (Fisch. et Mey.) O. Ktze. | Patchouli | √ | √ | |
| Angelica sinensis (Oliv.) Diels | Angelica root | √ | √ | √ |
| Kaempferia galanga L. | Aromatic ginger | √ | √ | |
| Crocus sativus L. | Saffron | √ | √ | |
| Amomum tsao-ko Crevost & Lemarié | Cardamom | √ | √ | |
| Curcuma longa L. | Turmeric | √ | √ | √ |
| Piper longum L. | Long pepper | √ | √ | |
| Codonopsis pilosula (Franch.) Nannf./Codonopsis pilosula Nannf. var. modesta (Nannf.) L. T. Shen/Codonopsis tangshen Oliv. | Dangshen | √ | √ | |
| Cistanche deserticola Y. C. Ma | Desert cistanche | √ | √ | |
| Dendrobium officinale Kimura et Migo | Chinese orchid | √ | √ | |
| Panax quinquefolius L. | American ginseng | √ | √ | |
| Astragalus membranaceus (Fisch.) Bge. | Mongolian milkvetch (huangqi) | √ | √ | |
| Ganoderma lucidum (Leyss. ex Fr.) Karst./Ganoderma sinense Zhao, Xu et Zhang | Ganoderma | |||
| Cornus officinalis Sieb. et Zucc. | Japanese cornel dogwood | √ | ||
| Gastrodia elata Bl. | Gastrodia | √ | √ | |
| Eucommia ulmoides Oliv. | Hardy rubber tree | √ | √ |
| Botanical Resources | Compounds | Models | Effects | Ref. |
|---|---|---|---|---|
| Flavonoids | ||||
| Allium macrostemon Bge., Crocus sativus L., Plantago asiatica L. S | Quercetin | DN rats, ochratoxin A-induced AKI mice | ↓ PI3K/AKT signaling ↑ Nrf2/HO-1, fatty acid oxidation | [37,38,39,74,75] |
| Glycine max (L.) Merr., Lonicera japonica Thunb., Ginkgo biloba L., Lycium barbarum L., Plantago asiatica L. S | Kaempferol | CLP-induced AKI mice, DN mice, DOX-induced AKI mice | ↓ ICAM-1, VCAM-1, MCP-1, caspase-3, Bax, MAPK signaling, TGF-β1, and α-SMA ↑ Bcl-2, SOD, and GSH | [40,76,77,78] |
| Myricaceae, Polygonaceae, Primulaceae, Pinaceae, and Anacardiaceae | Myricetin | DN mice, EG-induced AKI mice | ↓ IL-1β, TNF-α, ROCK1/ERK/P38 signaling, and NF-κB signaling ↑ Nrf2, CAT, and SOD | [41,42,79] |
| Ginkgo biloba L., Hippophae rhamnoides L., Citrus reticulata Blanco, Citrus aurantium L., and Citrus medica L. | Isorhamnetin | LPS-induced AKI mice | ↓ IL-1β, IL-6, and TNF-α and M1 macrophage ↑ M2 macrophage | [43,44] |
| Epimedium brevicornu Maxim. S | Icariin | Adenine/UUO-induced CKD rats, DOX-induced AKI mice | ↓ TGF-β, α-SMA, and E-cadherin ↑ Nrf2/HO-1, SOD, CAT | [47,48,80,81,82] |
| Plantago asiatica L. S | Apigenin | DOX/EG/oxonate-induced AKI mice, UAN mice | ↓ Oxidative and nitrosative stress, IL-6, TNF-α, NLRP3, caspase-1, IL-1β, TGF-β, Wnt/β-catenin signaling | [49,50,83,84,85,86,87] |
| Citrus reticulata Blanco, Citrus aurantium L., Citrus medica L. | Nobiletin | IRI-induced AKI mice | ↑ PI3K/AKT signaling | [55] |
| Crataegus pinnatifida Bge. | Vitexin | UUO-induced CKD mice, oxalate-induced AKI mice | ↓ NLRP3, caspase-1, and IL-1β ↑ Nrf2/HO-1, SOD, GSH | [56,57] |
| Citrus reticulata Blanco, Citrus aurantium L., Citrus medica L. | Hesperidin, hesperetin | LPS/Zn-induced AKI mice, DDP-induced HK-2 cells | ↓ p53, caspase-3 ↑ Nrf2/HO-1, SOD, GSH, and CAT Regulating gut microbiota | [58,59,60,61] |
| Plantago asiatica L. S | Luteolin | Cd/HgCl2/K2Cr2O7-induced AKI mice, LN mice | ↓ HIF-1α, α-SMA, collagen I, and fibronectin ↑ Nrf2/HO-1, GSH, SOD, CAT, and AMPK/mTOR autophagy | [88,89,90,91,92] |
| Pueraria lobata (Willd.) Ohwi | Puerarin | DN mice, UUO-induced CKD mice | ↑ Nrf2, cAMP/PKA/CREB ↓ TLR4/MyD8, and M1 macrophage | [72,93,94,95] |
| Polysaccharides | ||||
| Astragalus membranaceus (Fisch.) Bge. | / | DDP-induced AKI mice | ↓ ROS generation and mitochondrial vacuolation | [19] |
| / | LPS-induced AKI mice | ↓ Caspase-3/9 and Bax ↑ Bcl-2 | [96] | |
| / | LPS-induced AKI mice | Regulating gut microbiota ↑ SCFAs | [97] | |
| Bletilla striataS | Mw: 260 kDa | Ang II-induced HMCs | ↓ ROS generation and NOX4 | [18] |
| / | TGF-β-induced HMCs | ↓ TGF-β, and α-SMA | [98] | |
| Ganoderma lucidum (Leyss. ex Fr.) Karst. | Mw: 72.9 kDa; Ara:Gal:Rha:Glc = 0.08:0.21:0.24:0.47 | DN mice | ↓ Collagen-1, fibronectin, α-SMA, TGF- β, and MAPK/NF-κB signaling | [99] |
| / | IRI-induced AKI mice | ↓ p53, caspase-3, Bax, cytochrome c, and ER stress ↑ Bcl-2 | [100] | |
| Lycium barbarum L. | Ara:Gal:Glc:GalA:Man:Rha = 12.25: 8.66: 7.66: 2.86: 1.70: 1.00 | DN mice | ↓ TNF-α, IL-1β, IL-6, and NF-κB signaling | [16] |
| / | Lead-induced AKI mice | ↓ Bax and caspase-3 ↑ Bcl-2 | [101] | |
| Laminaria japonica Aresch./Ecklonia kurome Okam. | Mw: 7 kDa | IRI-induced AKI mice | ↓ p53, Bax, MMP, and cytochrome c ↑ Bcl-2 | [102] |
| Mw: 1960 kDa | DOX-induced AKI mice | ↓ TNF-α, IL-1β, MCP-1, and podocyte injury | [103] | |
| Mw: 8.84 kDa; Fuc:Gal:Man:Glc:Rha:Xyl = 1:0.057:0.041:0.008:0.029:0.019 | DN mice | ↓ collagen-1, fibronectin, and α-SMA | [104] | |
| Mw: 7 kDa | DN mice | ↓ α-SMA, fibronectin, and TGF-β/Smad ↑ E-cadherin | [105] | |
| / | DOX-induced CKD mice | ↓ α-SMA, fibronectin, and TGF-β/Smad | [106] | |
| Mw: 7.774 kDa | AGE-induced HRMCs | ↓ Fibronectin | [107] | |
| Mw: 8.84 kDa | TGF-β1- or FGF-2-induced HK-2 cells | ↓ α-SMA, MMP9, and EMT | [108] | |
| Polygonatum kingianum Coll.et Hemsl./Polygonatum sibiricum Red./Polygonatum cyrtonema Hua | Mw: 141 kDa; Gal:GalA:Ara:Glc = 57.67:26.82:4.59:4.54 | Uranium-induced HK-2 cells | ↓ ROS ↑ GSK-3β/Fyn/Nrf2 | [17] |
| Panax ginseng C. A. Mey. S | Glc:Gal:Ara:GalA:Rha:Man = 76.7:6.5:5.1:9.2:1.4:1.1 | DDP-induced AKI mice | ↓ p53, caspase-3, caspase-6, and ER stress by PERK/eIF2α/ATF4 signaling | [109] |
| Paeonia × suffruticosaS | Mw: 164 kDa; D-Glc:L-Ara = 3.31:2.25; | DN rats | ↓ TGF-β, ICAM-1, and VCAM-1 | [110] |
| Mw: 164 kDa; Ara = 3.31:2.25 | DN rats | Regulating gut microbiota ↑ SCFAs | [111] | |
| Plantago asiaticaS | / | Adenine-induced rats, UAN | ↓ IL-6, TNF-α, NLRP3, and caspase-1 | [112] |
| Dendrobium officinale Kimura et Migo | / | HG-induced HK-2 cells, db/db mice | ↓ TGF-β, and α-SMA ↑ SIRT1 | [113] |
| Salvia miltiorrhiza Bunge S | / | Florfenicol-induced AKI broilers | ↓ p53, caspase-3 ↑ Nrf2/HO-1 | [114] |
| Phyllostachys nigraS | Mw: 34 kDa | DN mice | Regulating gut microbiota ↑ Lactobacillales | [20] |
| Terpenoids | ||||
| Rehmannia glutinosaS, Plantago asiaticaS, Scrophularia ningpoensisS, and Crocus sativus L. | Catalpol | DN mice, DOX/Ang II/Fru/DDP-induced AKI mice, adenine-induced CKD mice | ↓ TRPC6, NF-κB, TGF-β1/Smad, TLR4/MyD88 signaling, RAGE/RhoA/ROCK signaling ↑ AMPK signaling | [115,116,117,118,119,120,121] |
| Cornus officinalis Sieb. et Zucc. | Loganin | DN mice, IRI/DOX/CLP-induced AKI mice | ↓ NLRP3, AGE/RAGE signaling ↑ Nrf2/HO-1 | [122,123,124,125] |
| Gardenia jasminoides Ellis, Eucommia ulmoides Oliv., Rehmannia glutinosa S, and Scrophularia ningpoensis S | Geniposide | DN mice, CLP-induced AKI mice, H2O2-induced HK-2 cells | ↓ ICAM-1, TNF-α, IL-1, IL-6, NF-κB, and NETs ↑ GSK3β, AMPK-PI3K/AKT, Bcl-2 Regulating gut microbiota | [126,127,128,129,130,131,132] |
| Cornus officinalis Sieb. et Zucc. | Morroniside | H2O2-induced podocytes | ↓ NOX4 | [133] |
| Canarium album Raeusch. | Oleuropein | DN mice, acrylamide-induced AKI mice | ↓ TNF-α, IFN-γ, IL-2, IL-6, and IL-17α ↑ SOD, GSH-Px, and CAT | [134,135] |
| Poria cocos (Schw.) Wolf. | Poricoic acid (A, B, C, D, E, F, G, H, AM, AE, BM, DM, and derivatives) | UUO-induced CKD mice | ↓ MMP-13, Wnt/β-catenin, TGF-β/Smad3/MAPK ↑ AMPK, Nrf2 | [136,137,138,139,140,141,142] |
| Ligustrum lucidum Ait. S | Oleanic acid | UUO-induced CKD mice, DN mice | ↓ NF-κB/TNF-α, TGF-β ↑ Nrf2/SIRT1/HO-1 Regulating gut microbiota | [143,144,145,146,147] |
| Alisma orientatle (Sam.) Juzep. S | Alisol (A, B, and derivatives) | IRI/DDP-induced AKI mice, UUO-induced CKD mice | ↓ ICAM-1, MCP-1, COX-2, iNOS, IL-6, TNF-α, FXR activation, TGF-β/Smad3 ↑ SOD and GSH and HO-1 | [148,149,150] |
| Panax ginseng C. A. Mey. | Ginsenosides | DDP-induced AKI mice, renal carcinoma, DN mice, UUO-induced CKD mice | ↓ ER stress, lipid peroxidation, PPARγ, and NOX4-MAPK pathways and TGF-β | [151,152,153,154,155,156,157,158] |
| Ganoderma lucidum (Leyss. ex Fr.) Karst. | Ganoderic acid, ganodermanontriol, lucidenic acid | ADPKD mice, IRI-induced AKI mice, UUO-induced CKD mice | ↓ Ras/MAPK, TGF-β/Smad, IL-6, COX-2, and iNOS ↓ TLR4/MyD88/NF-κB, and caspase-3 | [159,160,161] |
| Glycyrrhiza uralensis Fisch. | Glycyrrhizinic acid, glycyrrhizic acid | DN mice, TAC/PC-induced AKI mice | ↓ ROS, IL-1β, IL-6, TNF-α, CCR2 ↑ AMPK/SIRT1/PGC-1α Regulates autophagy | [162,163,164,165,166,167] |
| Alkaloids | ||||
| Ligusticum sinense Chuanxiong S | Tetramethylpyrazine | PC/IRI-induced AKI rats, DN rats | ↓ CCL2/CCR2, ROS, NLRP3, TNF-α ↑ AKT, Bcl-2 | [168,169,170,171,172] |
| Leonurus japonicus Houtt. S | Leonurine | DDP/LPS/vancomycin-induced AKI mice, UUO-induced CKD mice | ↑ Nrf2 ↓ TLR4/MyD88/NF-κB, TNF-α, IL-1β, TGF-β/Smad3 | [173,174,175,176,177] |
| Coptis chinensis | Berberine | IRI/DOX/DDP/MTX/gentamicin-induced AKI, DN mice, and UUO-induced CKD mice, UAN mice | ↓ IL-6, IL-10, TGF-β/Smad3, mitochondrial stress, and ER stress ↑ Nrf2, MDA, SOD, CAT, GSH, and Bcl-2 Regulating gut microbiota | [178,179,180,181,182,183,184,185,186,187,188,189,190] |
| Trigonella foenum-graecum L. S | Trigonelline | ON mice, DN mice | ↓ EMT, ROS, α-SMA ↑ AMPK pathway | [191,192,193,194,195,196] |
| Piper longum L. S | Piperlonguminine | UUO-induced CKD mice | ↓ TRPC6 | [197] |
| Sophora japonica L. | Matrine, oxymatrine | IRI/DDP/gentamicin-induced AKI mice, UUO-induced CKD mice | ↓ IL-6, IL-10, TGF-β/Smad3 | [198,199,200] |
| Nelumbo nucifera Gaertn. | Liensinine, Isoliensinine | IRI-induced AKI rats, LPS-induced AKI mice | ↓ TGF-β1/Smad3 | [201,202] |
| Nelumbo nucifera Gaertn. | Neferine, nuciferine | UAN rats, LPS/IRI-induced AKI mice, DN mice | ↓ TLR4/MyD88/NF-κB, IL-1β ↑ Bcl-2 | [203,204,205,206,207,208,209,210] |
| Others | ||||
| Curcuma longa L. | Curcumin | LN mice, patulin-induced AKI mice, CKD patients, UAN rats | ↑ Nrf2/FOXO-3a, GSH ↓ PI3K/AKT/NF-κB, TGF-β, ROS Regulating gut microbiota | [211,212,213,214,215] |
| Rheum palmatum L. | Emodin, aloe emodin, rhein | UUO-induced CKD mice/rats, DN mice | ↓ IL-1β, TGF-β, PERK-eIF2α Regulating gut microbiota | [216,217,218,219,220] |
| Eugenia caryophyllata Thunb. | Eugenol | IRI/patulin-induced AKI mice | ↓ TGF-β ↑ Nrf2 | [221,222] |
| Sesamum indicum L. | Sesamin | CKD mice, DDP/LPS-induced AKI mice | ↑ GSH, CAT, and SOD Regulating gut microbiota | [223,224,225,226] |
| Ligusticum sinense Chuanxiong S | Ferulic acid | UAN mice | ↓ TLR4/NF-κB Regulating gut microbiota | [227] |
| Lonicera japonica Thunb. | Chlorogenic acid | UAN mice | ↓ IL-1β, TNF-α, IL-6 Regulating gut microbiota | [228,229,230] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Song, J.-Y.; Feng, R.; Hu, J.-C.; Xu, H.; Ye, M.-L.; Jiang, J.-D.; Chen, L.-M.; Wang, Y. Renal Health Through Medicine–Food Homology: A Comprehensive Review of Botanical Micronutrients and Their Mechanisms. Nutrients 2024, 16, 3530. https://doi.org/10.3390/nu16203530
Zhao Y, Song J-Y, Feng R, Hu J-C, Xu H, Ye M-L, Jiang J-D, Chen L-M, Wang Y. Renal Health Through Medicine–Food Homology: A Comprehensive Review of Botanical Micronutrients and Their Mechanisms. Nutrients. 2024; 16(20):3530. https://doi.org/10.3390/nu16203530
Chicago/Turabian StyleZhao, Yi, Jian-Ye Song, Ru Feng, Jia-Chun Hu, Hui Xu, Meng-Liang Ye, Jian-Dong Jiang, Li-Meng Chen, and Yan Wang. 2024. "Renal Health Through Medicine–Food Homology: A Comprehensive Review of Botanical Micronutrients and Their Mechanisms" Nutrients 16, no. 20: 3530. https://doi.org/10.3390/nu16203530
APA StyleZhao, Y., Song, J.-Y., Feng, R., Hu, J.-C., Xu, H., Ye, M.-L., Jiang, J.-D., Chen, L.-M., & Wang, Y. (2024). Renal Health Through Medicine–Food Homology: A Comprehensive Review of Botanical Micronutrients and Their Mechanisms. Nutrients, 16(20), 3530. https://doi.org/10.3390/nu16203530






