1. Introduction
Prostate cancer (PCa) is the most prevalent cancer in elderly men, characterized by its aggressive nature leading to metastasis and, frequently, fatality [
1]. It is currently the second leading cause of death for men in Western societies [
2]. Prostate cancer’s pathogenesis remains unclear. PCa development and progression involve various factors, including aging, environmental factors, lifestyle choices, physical activity, genetic alterations, and hormonal influences. Oxidative stress (OS) is thought to contribute directly to the development of prostate cancer [
3]. Therefore, OS represents a potential therapeutic target for addressing prostate conditions such as prostatic hypertrophy, cancer, or chronic prostatitis. Oxidative stress in cells results from an imbalance between oxidants and antioxidants, leading to damaged lipids, proteins, and DNA structures. Experimental data suggest that cancer cells, as a result of genetic mutations, have reduced activity in certain antioxidant enzymes compared with normal cells [
4]. This diminished enzymatic activity can induce oxidative stress, leading to damage to cellular components and structures in cancerous cells. Among the diverse strategies employed in cancer treatment, one approach involves deliberately elevating reactive oxygen species (ROS) levels within cancer cells. Chemotherapeutic agents like cisplatin, arsenic trioxide, and anthracycline antibiotics operate through this mechanism [
5].
Selenium (Se), a micronutrient, participates in various physiological processes [
6]. Its importance lies in its role in the catalytic centers of antioxidant enzymes, including selenium-dependent glutathione peroxidase (Se-GPx) and thioredoxin reductase (TrxR) [
7]. Selenite, in erythrocytes, when interacting with glutathione, forms biologically active selenodiglutathione, with potent anticancer properties that induce cancer cell apoptosis. Inorganic selenium compounds (+4), more than organic ones (+2), have been found to inhibit cancer cell growth and proliferation due to their pro-oxidant properties, particularly at doses exceeding therapeutic levels [
8]. This phenomenon presents potential for anticancer therapy; however, the high toxicity of commercially available inorganic Se (+4) compounds limits their applicability. As a result, there is an ongoing search for selenium (+4) compounds that combine high chemoactivity with low toxicity. Consequently, the synthesis of novel chemopreventive compounds is crucial, with a focus on evaluating their efficacy against various cancer types.
Selol is a selenintriglyceride compound derived from sunflower oil. It has lower toxicity compared with sodium selenite (+4) and has no mutagenic effects [
9]. In addition, Selol shows strong cytostatic activity against cancer cell lines, with no side effects on normal cells. Selol is a formulation with potential anticancer activity. Previous in vitro studies demonstrated Selol’s significant antitumor effects on human HL-60 leukemia cells, including drug-resistant variants (HL-60/Dox and HL-60/Vinc) [
10], as well as HeLa [
11] and Caco-2 cancer cells [
12]. Książek et al. observed that prostate cancer cells, LNCaP, were more susceptible to apoptosis induction caused by the presence of ROS compared with normal prostate cells—PNT1A [
13]. The safety of Selol for normal cells was confirmed in PC12 cells. Selol, through the regulation of free radical levels, the enhancement of the antioxidant system, and the inhibition of apoptosis, protects against oxidative damage and death induced by SNP. Selol is believed to induce the production of ROS in cancer cells, leading to excessive oxidative stress. Healthy cells can manage this stress, while in cancer cells, it may result in apoptosis [
14].
Under conditions of chronic oxidative stress, cells activate defensive mechanisms, primarily through the activation of phase 2 enzymes via the Nrf2/ARE signaling pathway. In vivo experiments conducted on a healthy animal model have shown that Selol significantly influences changes in non-enzymatic antioxidants (thiols) and the intracellular and extracellular redox status [
15].
However, these findings have not been validated in a cancer animal model, where the tumor itself affects the organism’s redox status. Considering the physical conditions, our previous study showed that treating mice with Selol resulted in a drop in the rate of body mass reduction and stopped the increase in plasma prostatic specific antigen (PSA) levels. The expression of genes, involved in oxidative stress following treatment with Selol, in LNCaP cells, did not change. However, it is important to remember that gene expression is not directly linked to enzyme activity. Many factors influence the formation of an active protein [
16].
Our goal was to investigate the impact of long-term Selol administration on tumor growth and the oxidative–antioxidative status in both blood and the tumor, which could be crucial for the formulation’s effectiveness. We assessed the levels of the antioxidant enzymes glutathione S-transferase (GST), glutathione peroxidase (GPx), and thioredoxin reductase (TrxR), regulated by the Nrf2/ARE pathway, in both the blood and tumor of mice with xenografted LNCaP prostate cancer. Additionally, the study considered the marker of oxidative damage malondialdehyde (MDA) and tumor oxygen-radical absorbance capacity (ORAC) and determined the selenium levels. Prostate-specific antigen (PSA) concentrations before and after Selol administration were also determined. Therefore, it was decided to perform a macroscopic examination of the results obtained and analyze the alterations in the tumor at the histopathological level. Histopathological examinations like morphology and p53, BCL2, and Ki-67 expression were conducted.
2. Materials and Methods
2.1. Compound Characterization
The synthesis of Selol was carried out in the Department of Drugs Analysis at Warsaw Medical University (Patent Pol. PL 176,530 (Cl. A61K31/095)). Selol is a mixture of selenitriglicerides obtained by the chemical modification of sunflower oil, in which a minimum of 11 distinct selenium-containing triglycerol derivatives were identified by using mass spectrometry [
17]. In the experiments, Selol 5% at a dose of 17 mg Se/kg body mass was used.
2.2. Ethics Statement
All animal experiments were conducted in accordance with the guidelines set forth by European Communities Directive 2010/63/EU. Ethical approval for the study was obtained from the IV Local Ethics Committee for Animal Experimentation in Warsaw, Poland, under protocol number 33/2009 dated 1 April 2009. Every effort was undertaken to minimize animal distress and to limit the number of animals used. The animal experiments adhered to the ARRIVE guidelines.
2.3. Animal Model
Adult, immunodeficient, sexually mature male NSG mice (NOD.Cg-Prkdc/sc dIl2rg), (approximately 27–35 g, 12 weeks old) were purchased from Charles River Laboratories (Germany). Animals were housed in cages (Centre for Postgraduate Medical Training in Warsaw) maintained under controlled environmental conditions at 20 ± 2 °C room temperature, 40 ± 5% relative humidity, and a 12 h light/dark cycle with a dawn/dusk effect. Mice were fed a complete feed mixture for laboratory animals (LSM, Agropol, Lublin, Poland) and had access to water ad libitum. To reduce stress, the animals were handled for 10 minutes each day and acclimated to the oral gavage procedure over a period of one week prior to the start of the experiments.
2.4. Experimental Protocol
The induction of the tumor was performed in accordance with the results of the pilot studies. The procedure involved implanting 5 million LNCaP cells (an epithelial cell line derived from human prostate carcinoma) into the shoulder of mice. Cells were suspended in Matrigel (Corning® Matrigel® Matrix GFR PhenolRF Mouse; Sigma-Aldrich, Sant Louis, MI, USA), a vital protein mixture that promotes optimal cell culture growth, minimizes single-cell dispersion, and contains essential growth factors.
The animal study lasted eight weeks. Tumors were induced in healthy NSG mice (n = 12). At the end of the fifth week after LNCaP cell inoculation, a significant darkening of the skin at the injection site and the development of tumors were observed. Then, the mice were randomly assigned to the following four main groups: (1) Ca—mice with xenografted LNCaP prostate cancer (n = 6); (2) Control—mice without prostatic tumors (n = 5); (3) Control + Se—mice without prostatic tumors treated with Selol (n = 5); (4) Ca + Se—mice with xenografted LNCaP prostate cancer treated with Selol (n = 6). Each of the study groups (Control + Se and Ca + Se) was supplemented daily per os with a single dose of Selol diluted with vegetable oil equivalent to 17 mg Se/kg body weight (which is approx. 20% of LD50—dose based on the in vivo results from the Selol toxicity study; unpublished data). The Control and Ca groups were fed the standard diet with the same rate of pure vegetable oil (placebo) as the study groups. Placebo and Selol were administered to the animals over three weeks. During the treatment period, all mice were weighed and observed for changes in their behavior twice a week. At the end of the experiment, mice were anesthetized with halothane, the blood was collected for the biochemical measurements, and the tumors were isolated from each mouse for further investigation. In the fifth and eighth weeks of the experiment, PSA concentrations were determined to confirm the presence of prostate cancer and/or the effect of Selol supplementation on the marker.
2.5. Biochemical Analysis
The animals underwent a 12-h fast before being sacrificed. Blood samples were collected into heparinized tubes and centrifuged at 1000× g for 15 min at 4 °C to obtain plasma. Red blood cells were washed twice with 0.9% NaCl, and the plasma was refrigerated and stored at −80 °C until analysis. On the analysis day, the samples were thawed at room temperature. Red blood cells intended for enzymatic analysis were hemolyzed by using an equal volume of 3 mM phosphate buffer at pH 7.4 containing 1 mM EDTA. The hemolysates were centrifuged at 1000× g for 20 min at 4 °C, and the supernatants were used for further measurements. The tumors were extracted, weighed, and divided into small fragments. The tumor fragments were briefly exposed to liquid nitrogen for preservation before being stored at −80 °C. All tumor samples were processed within a two months. Before measurement, the tumors were homogenized by using a manual glass homogenizer. For enzyme activity measurements, the samples were homogenized in a chilled medium consisting of 5 mM phosphate buffer, 0.25 mM sucrose, and 0.5 mM EDTA at pH 7.2. Meanwhile, for MDA measurements, a cold medium containing 5 mM pyrophosphate buffer at pH 7.4 was used. The cytosolic fraction of the homogenates was separated by centrifugation at 10,000× g for 20 min at 4 °C.
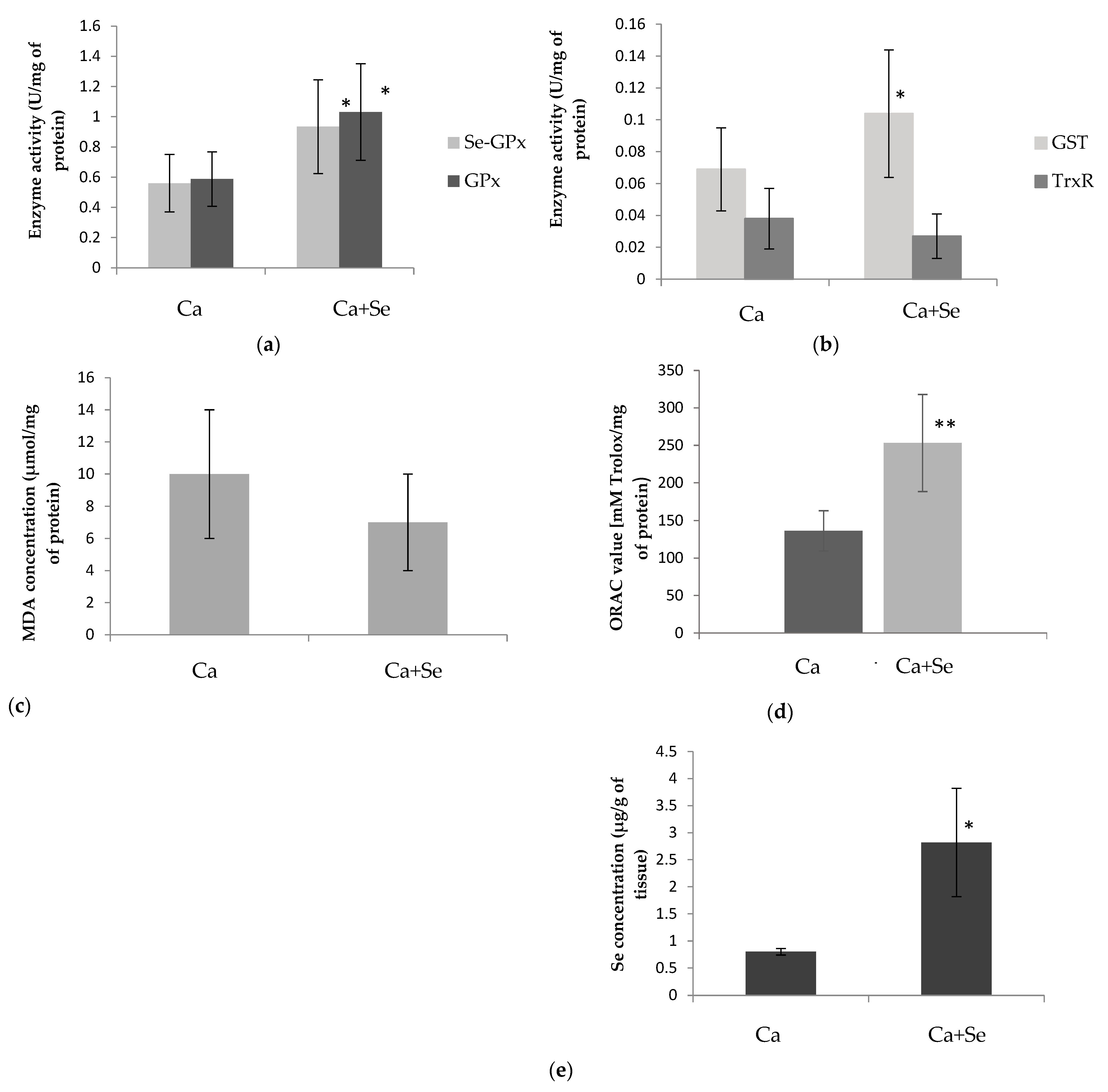
The following parameters were determined in plasma, erythrocytes, and tumor homogenate supernatants: selenium-dependent glutathione peroxidase (Se-GPx) and total glutathione peroxidase (GPx) activities, and thioredoxin reductase (TrxR) and glutathione S-transferase (GST) activities. Furthermore, plasma prostate-specific antigen (PSA) levels, tumor ORAC values (antioxidant capacity), concentrations of malondialdehyde (MDA) in both plasma and tumors (a marker of lipid peroxidation), and selenium concentrations in erythrocytes and tumors were assessed.
2.5.1. Determination of Enzyme Activity
Selenium-dependent glutathione peroxidase (Se-GPx) and glutathione peroxidase (GPx) activities in plasma, red blood cells, and tumor homogenates were determined spectrophotometrically at a wavelength of 340 nm, using a method originally developed by Paglia and Valentine, modified by Wendel [
18,
19]. The reaction was carried out at 25 °C in 50 mM sodium phosphate buffer containing 0.40 mM EDTA at pH 7.0. A supernatant/plasma volume of 10 µL was utilized for enzyme activity analysis. In the final reaction mixture (total volume of 220 µL), the concentrations were as follows: reduced glutathione (GSH) at 1.0 mM, NADPH at 65 µM, and sodium azide at 0.17 mM. For the Se-GPx activity assay, a substrate tert-butyl hydroperoxide was employed at a concentration of 0.02 mM. Cumene hydroperoxide at 1.05 mM concentration was used for the GPx activity determination.
Glutathione S-transferase (GST) activity in plasma, red blood cells, and tumor homogenates was quantified by using spectrophotometric methods at a wavelength of 340 nm, employing the Habig assay [
20]. The enzymatic reaction took place at 25 °C in 50 mM sodium phosphate buffer supplemented with 0.50 mM EDTA at pH 7.5. A 10 µL aliquot of supernatant/plasma was utilized to assess enzyme activity. The final reaction mixture, with a total volume of 200 µL, contained 2 mM reduced glutathione (GSH) and 1 mM 1-chloro-2,4-dinitrobenzene (CDNB) as the substrate.
Thioredoxin reductase (TrxR) activity in plasma, red blood cells, and tumor homogenates was assessed spectrophotometrically at a wavelength of 412 nm, using the modifications outlined by Hill et al. [
21]. The enzymatic reaction was conducted at 37 °C in 50 mM sodium phosphate buffer supplemented with 1 mM EDTA at pH 7.0. A 10 μL of the supernatant was used for the enzyme activity analysis. In the final reaction mixture, with a volume of 200 μL, the concentrations were as follows: 4 mM 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) as the substrate, 2 μM nicotinamide adenine dinucleotide phosphate (NADPH) as the enzymatic reaction cofactor, and 1 mM auranofin (ATM) as a specific inhibitor of the enzyme under study. Thioredoxin reductase activity was determined by measuring the difference in enzymatic activity between samples tested without and with the inhibitor [
22,
23].
2.5.2. Determination of Malondialdehyde Concentration
The concentration of malondialdehyde (MDA) in both plasma and tumor homogenates was quantified by using an ELISA spectrophotometric assay kit (Wuhan EIAAB Science Co., Ltd., Wuhan, China), following the manufacturer’s instructions.
2.5.3. Determination of Oxygen-Radical Absorbance Capacity (ORAC)
The ORAC-FL assay, as described by Ou et al. [
24], involved measuring the antioxidant capacity of samples. Hitachi F-7000 (Hitachi, Tokyo, Japan) spectrofluorometer with excitation at 485 nm and emission at 520 nm was used to determine the ORAC value of supernatants of tumor tissues. Black 96-well plates (Greiner Bio-One, Kremsmünster, Austria) were employed in the assay. All solutions (fluorescein and AAPH) were freshly prepared in PBS buffer at pH 7.4 daily. The reaction mixture consisted of 13 mM AAPH and fluorescein at 40 nM.
2.5.4. Determination of Prostate-Specific Antigen (PSA) Concentration
The PSA levels in the mice’s plasma were assessed twice, labeled as PSA1 and PSA2. At the end of the fifth week, 150 µL of blood was extracted from the tail of each mouse for the initial PSA measurement (PSA1). After three weeks of administering Selol/placebo, the mice were humanely sacrificed, and their blood was gathered for the subsequent PSA level analysis (PSA2). Following collection, the blood was centrifuged to obtain plasma for the evaluation of PSA levels by using immunodetection (Human PSA-total ELISA Kit; Sigma-Aldrich, Sant Louis, MI, USA; as recommended by the manufacturer).
2.5.5. Determination of Protein and Hemoglobin Concentration
The protein concentration in tumor tissue supernatants was determined by using a spectrophotometric assay with Bradford reagent (Sigma-Aldrich, Sant Louis, MI, USA). The absorbance of the protein-bound Coomassie Brilliant Blue G-250 dye was read at 595 nm. Protein concentrations were calculated based on a standard curve prepared by using bovine serum albumin (BSA) as the reference standard. The hemoglobin concentration of red blood cell (RBC) hemolysates was assessed spectrophotometrically at 546 nm by using a standard immunodetection assay (Human hemoglobin ELISA Kit; Sigma-Aldrich, Sant Louis, MI, USA; as recommended by the manufacturer). Measurements of enzymatic activity, hemoglobin concentration, and protein absorbance were conducted by using a spectrophotometer microplate reader (Synergy MX, BioTek® Instruments, Inc., Winooski, VT, USA).
2.5.6. Determination of Selenium Concentration
To quantify the complete selenium (Se) content in red blood cells and tumor tissues, an inductively coupled plasma mass spectrometer (ICP-MS) (Thermo Fisher Scientific, Waltham, MA, USA) was used [
25,
26]. Tissue samples were homogenized in a mixture of 65% HNO
3 and 30% H
2O
2 (in a 3:1 ratio). Subsequently, homogenized samples were transferred to Teflon crucibles and subjected to mineralization by using a microwave mineralizer. For erythrocyte samples, 100 μL aliquots were transferred to Teflon crucibles containing a mineralization mixture as mentioned above, followed by mineralization. The samples were then used for ICPMS analyses, with quality control samples.
2.6. Histopathological Examination
Histopathological evaluation was performed on tissue sections from tumor-bearing animals (n = 5). The lesions assessed were nodules with macroscopic diameters ranging from 3 to 4 mm in control animals and from 3 to 5 mm in animals treated with the active substance. After evaluation, tissues were processed by embedding in 10% buffered formalin, sectioned into 7 µm slices, and stained with hematoxylin and eosin (H&E). Examination of the prepared slides was conducted by using an, Olympus BX41 microscope with an Olympus DP25 camera and cellSens software For immunohistochemical evaluation, slides were cut to a thickness of 4 µm on salinized slides and transferred to a hothouse (50 °C). Staining was performed with DAKO antibodies by using Dako AutostainerLink 48 platform. Slides were transferred to buffer (Target Retrieval Solution EnVision FLEX) at pH 9.0 or 6.0 at PTLink to open antigenic dominance EnVision Detection Kit (Env FLEX, High pH, DAKO) was used. Antibodies against p53, BCL2, and Ki-67 (DAKO Omnis, Agilent Technologies, Santa Clara, CA, USA were used to assess protein expression levels and to evaluate tumor characteristics. Slides were stained with hematoxylin, washed with water, dehydrated with a series of alcohols, overexposed with xylene, and sealed in BDX.
2.7. Statistical Analysis
The data are presented as the means ± standard deviation (SD). Statistical analyses were performed by using one-way ANOVA to compare means among multiple groups, followed by Tukey’s and Dunnett’s post-hoc tests for pairwise comparisons. The Mann–Whitney U test was used for comparing two independent groups, and the Spearman correlation and multiple regression test was used to assess the strength and direction of the association between two and more than two variables, respectively. A p-value of less than 0.05 was considered significant. Statistical tests were performed by using Statistica software (version 10, StatSoft, TIBCO Software, Warsaw, Poland).
4. Discussion
This study explores the biochemical mechanism of action of Selol, a novel organic selenium compound with promising anticancer and pro-oxidant properties. It investigates how Selol induces oxidative stress, focusing on its impact on cells with heightened metabolic activity and oncogenic signaling. The production of reactive oxygen species (ROS) by pro-oxidant compounds can cause damage to cellular structures, ultimately resulting in the death of cancer cells. This discovery presents a promising opportunity for the development of therapeutic approaches in cancer treatment [
27,
28].
The presented study shows that Selol administration to animals with prostate cancer increased antioxidant enzyme activity and selenium concentration in tumor tissues. The ORAC value, indicating the antioxidant capacity of tumor tissues, was observed to be higher after Selol treatment. This elevated ORAC value is likely associated with the presence of selenium. It is probable that other tissues or organs in tumor-bearing mice treated with Selol would similarly exhibit enhanced ORAC values. Consistent with this, our recent studies in healthy mice have demonstrated significant increases in ORAC levels in both blood and organs following Selol supplementation [
29]. However, there were no significant changes in the concentration of MDA (marker of oxidative stress) in the tumor tissues of the Selol-treated group. It is possible that the levels of oxidative stress in cancer cells may not always correlate with the level of lipid peroxidation products. The concentration of 4-hydroxynonenal, an aldehyde produced during lipid peroxidation, varies depending on the type of cancer and the stage of the tumor [
29]. This variation might be due to different levels of activity of aldehyde-metabolizing enzymes that are affected by the progression of cancer, thereby impacting the concentration of aldehyde products from lipid peroxidation [
30]. Selol can induce the expression of these enzymes, in the same way as antioxidant enzymes, as they share a common pathway for activation (e.g., through the Nrf2/ARE pathway, which is activated by oxidative stress) [
31]. Therefore, the lack of difference in MDA levels despite ongoing oxidative stress may be justified. According to Ksiazek et al., malignant prostate cells (LNCaP) have a weaker antioxidant defense compared with normal cells (PNT1A). Therefore, tumors inducing antioxidant enzymes in response to oxidative stress might not sufficiently counteract its effects, leading to apoptotic changes in cancer cells [
13]. In summary, the increase in antioxidant enzyme activity and antioxidant capacity value and the lack of differences in MDA levels in Ca-treated mice may suggest the presence of oxidative stress. This may lead to molecular changes that can result in cell death but also exert anticancer effects. To confirm this hypothesis, further investigation into other markers of oxidation is necessary.
As mentioned previously, after treatment with Selol, we observed an increase in the activity of antioxidant enzymes in tumors, specifically GSH-dependent ones, like Se-GPx, GPx, and GST. The presence of ROS (induced by Selol) causes oxidative stress, leading to the oxidation of sulfhydryl groups and a decrease in the concentration of intracellular GSH. Then, ROS activate the Nrf2-ARE pathway, which leads to the production of antioxidants and an increase in antioxidant enzyme levels in tissues. However, the heightened activity of these antioxidants, including GPx and GST, can deplete further the reduced form of GSH, which serves as a substrate for these enzymes [
31]. The enzyme that reduces GSH is TrxR. Here, we observed higher activity of Se-GPx, GPx, and GST as well as antioxidant capacity in the tumor, leading to the consumption of GSH and its conversion into GSSG. However, TrxR activity remained unchanged, indicating no significant effect on the reduction of oxidized proteins that may accumulate in the cell. These findings are supported by previous research, which observed higher GSSG and lower GSH levels in the tumor after Selol treatment [
15]. Further decline in glutathione concentration may result from aldehyde products formed during lipid peroxidation, reacting with glutathione (GSH) and forming rapidly eliminated conjugates [
32]. All these changes lead to a decrease in the concentration of reduced GSH and an increase in the oxidative form of GSSG. This enhances the oxidoreductive potential in cancer cells, inducing them to undergo apoptosis [
33,
34]. Interestingly, despite administering Selol, our previous research on the same experimental group showed no significant changes in the expression of oxidative stress-related genes, such as GPx and GST, within the tumor [
13]. This finding highlights the importance of phenotypic studies, including the measurement of enzymatic activities, which, although more time-consuming than gene expression screenings, provide crucial insights that might be overlooked in genetic analyses.
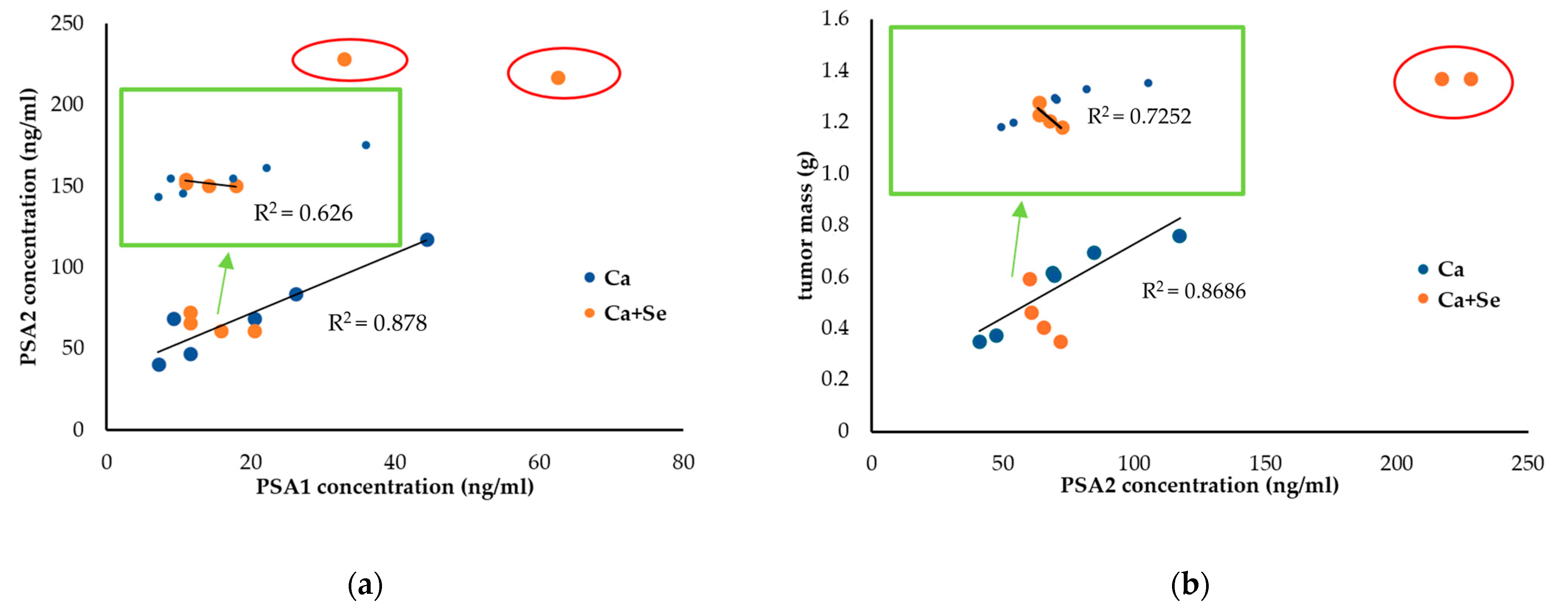
In evaluating the effects of Selol administration on tumor size, mouse weight (as a measure of overall well-being), and PSA levels at the end of the experiment, significant variability was observed in the treated group (CV = 62% vs. 31% for tumor weight and 70% vs. 40% for PSA2 levels). This variation suggests the presence of ongoing processes, potentially necrosis, with dynamics that vary among individuals. Based on the notable fluctuations in PSA levels and tumor sizes observed in the study, particularly after excluding individuals with large tumor masses and elevated PSA values (as shown in
Section 3.6), our findings indicate that Se may be particularly effective against smaller tumors. This suggests its potential role in preventing metastasis or serving as a complementary treatment alongside therapies like chemotherapy or surgery, especially in advanced cancer stages. The obtained results support the in vitro studies on the Selol interaction with standard chemotherapy drugs, which have shown that it can significantly enhance the antiproliferative effects of doxorubicin, especially in cells resistant to the drug [
11]. Additionally, in situations of vincristine-induced hyperalgesia, Selol improved the analgesic effects of fentanyl, buprenorphine, and morphine [
10]. However, this hypothesis must be verified.
The elevated PSA levels observed at the end of the experiment in the small-tumor group treated with Selol could be attributed to a temporary rise in PSA, often referred to as a “PSA flare”. This phenomenon is likely caused by the breakdown of tumor cells, which releases PSA into the bloodstream as the cancer cells are destroyed [
35,
36]. This transient increase in PSA is not necessarily indicative of treatment failure. Instead, it reflects the dynamic process of tumor response and should be interpreted in conjunction with other clinical findings and diagnostic tests. Proper evaluation of this PSA fluctuation is essential to distinguishing between actual disease progression and a temporary flare-up due to effective treatment. Furthermore, considering the variations in oxidative stress markers, including MDA and ORAC, as well as PSA levels and animal weights, we can conclude that smaller tumors may be more sensitive to the pro-oxidant effects of Selol.
The tumor morphology in mice treated with Selol differed from that in the placebo group. These differences, supported by histopathological studies, suggest the onset of tumor cell necrosis in the Selol-treated group. Given that the tumors of animals in the Ca + Se group exhibited a different consistency, it may turn out that they are more sensitive to chemotherapy or other types of anticancer therapy. It is worth conducting research in this direction, which suggests promising new therapeutic options for palliative care, especially for patients in the final stages of cancer.
Furthermore, histological study revealed that Selol treatment influences tumors by inducing tumor cell degeneration, focal necrosis, and constriction of tumor cell fields by connective tissue. However, there was no observed effect on the degradation of the mutated p53 gene. The proliferative activity of tumor tissue remained unaffected, and there was no suppression of apoptosis, as evidenced by the absence of BCL2 oncogene expression. The mechanism of Selol’s action in this specific type of cancer may differ from its effects in other cell lines, such as A545, where both apoptosis and necrosis contribute to its mechanism [
37].
In reassessing the effects of Selol on biochemical parameters, this study found no significant changes in the activities of selenium-dependent glutathione peroxidase (Se-GPx) and glutathione S-transferase (GST) in the plasma and erythrocytes of tumor-bearing mice following Selol supplementation. Additionally, the levels of MDA were comparable in both groups. However, in healthy mice, supplementation with Selol increased the activity of antioxidant enzymes in red blood cells and SeGSHPx activity and MDA concentration in plasma, which supports the previous finding that long-term Selol intake affects antioxidant enzyme activity in the blood of healthy animals [
27,
38]. The lack of increased antioxidant enzyme activity and the elevation in MDA levels in the plasma of tumor-bearing mice after Selol administration may be attributed to their pre-existing high levels due to the cancerous process. Tumors initiate processes like division, metabolism, and inflammatory cytokine release, leading to increased reactive oxygen species (ROS) production. This aligns with previous studies indicating heightened oxidative stress in tumor cells, impacting antioxidant enzyme activity. In various human tumors, reduced levels of superoxide dismutase and catalase activities have been observed, accompanied by an increase in GSH-dependent enzymes and thioredoxin reductase activity [
37].