Abstract
Background: Metabolic syndrome (MetS) is a set of conditions associated with an increased cardiovascular risk. Several serum fatty acids (FAs) seem to play an essential role in the development of cardiometabolic diseases and mortality. Thus, it is imperative to explore the impact of FAs on MetS parameters, using an early MetS screening tool such as MetScore, which is readily available in clinical practice. Aim: The aim of this study was to assess the potential correlation between serum FAs and cardiovascular risk using a MetScore. Methods: This cross-sectional study involved 41 women with severe obesity. The MetScore was calculated, and participants were categorized into high- and low-cardiovascular-risk groups based on the median MetScore value. Gas chromatography was used to quantify serum FAs. Generalized Linear Models were used to compare group means. The association was assessed through simple logistic regression, and an adjusted logistic regression was conducted to validate the association between Metscore and serum FAs. Results: The high-cardiovascular-risk group exhibited elevated values of HOMA-IR, palmitic, oleic, cis-vaccenic, and monounsaturated fatty acids, as well as the SCD-18C, indicating a heightened cardiovascular risk. Conversely, HDL-c, QUICK, gamma-linolenic, and eicosatetraenoic fatty acids showed lower values compared to the low-risk group. Conclusions: Women with severe obesity and high cardiovascular risk have lower values of some omega-3 and omega-6 FAs, considered cardioprotective and anti-inflammatory, and have higher lipogenic activity and FAs, correlated with high cardiovascular risk. These findings emphasize the need to address lipid metabolism in this population as a therapeutic target to reduce cardiovascular risk. Future research should explore clinical interventions that modulate fatty acid metabolism to mitigate cardiometabolic complications.
1. Introduction
Currently, obesity is one of the most frequent non-communicable diseases (NCDs) worldwide [1]. Hypertrophic adipocytes appear insulin-resistant (IR), leading to greater lipolysis and reduced lipogenesis, resulting in a heightened release of fatty acids (FAs) into the bloodstream. The high efflux of FAs in the bloodstream is one of the important triggering factors of metabolic complications such as glucose disorders, dyslipidemias, and inflammation [2]. The health implications of obesity are profound, contributing to an increased risk of several conditions such as the increased risk for cancer [3], cardiovascular disease (CVD), type 2 diabetes mellitus (DM2), and metabolic syndrome (MetS) [4].
Recent studies have shown that bariatric surgery can significantly alter the FA profile of individuals with obesity. Evaluations conducted before and after surgery indicate that, within one year, there is a notable shift towards an increase in polyunsaturated FAs (PUFAs) and a decrease in saturated FAs (SFAs) and monounsaturated FAs (MUFAs) [2,5]. These changes in FA composition are associated with a reduction in obesity-related comorbidities, including systemic arterial hypertension (SAH), DM2, and non-alcoholic fatty liver disease (NAFLD). Such findings highlight the critical relationship between FA profiles, adiposity, and metabolic disorders, suggesting that weight loss plays a pivotal role in these metabolic improvements [2,6].
MetS is a set of inter-related risk factors associated with CVD and DM2. These factors could include dyslipidemia, IR, SAH, and increased abdominal adiposity [7]. While criteria for the diagnosis of MetS already exist, the most precise strategy to establish this classification is still being discussed. The cardiovascular risk score (MetScore) was developed, aiming at a more accurate and earlier diagnosis of MetS compared to the previously recommended dichotomous classification [8].
As far as we know, no study has evaluated the relationship between serum FAs and cardiovascular risk using a MetScore, which is easily accessible for clinical practice. Since we know that the number of individuals living with severe obesity is growing worldwide [9,10] and that FA disbalance is a factor that contributes to obesity-related disorders [11], it is essential to better understand how serum FAs behave in this population and clarify their associations with cardiovascular risk. In this way, it will be possible to support public policies and guidelines that aim to improve the quality of life of these patients. Thus, the present study aims to assess the potential correlation between serum FAs and cardiovascular risk using a MetScore.
2. Materials and Methods
2.1. Participants
This analytical cross-sectional study was approved by the Research Ethics Committee of the Federal University of Goiás (protocol no 3.251.178) and by the Hospital Estadual Geral de Goiânia Dr. Alberto Rassi (protocol no 961/19).
Data collection was carried out in 2019. Patient recruitment was performed during the first consultation at the HGG outpatient clinic. Patients at this hospital outpatient clinic come from several cities in the state of Goiás and are referred by the State Health Department. During this first consultation, patients were screened according to the non-inclusion criteria. All patients who met the inclusion criteria were invited to participate in the study. Women with body mass index (BMI) above or equal to 40 (BMI ≥ 40 kg/m2), aged between 20 and 59 years [12] were included in the study. The non-inclusion criteria were the presence of acute inflammatory diseases, infectious diseases, neoplastic diseases, genetic syndromes, chronic consumption of alcohol (>30 g/day) and illicit drugs or psychotropic drugs, and pregnancy. All volunteers signed a written informed consent form before participating in the study.
2.2. Study Design
Participant recruitment took place during the first consultation with the surgeon. The patients underwent anthropometric evaluation on the day of the consultation with the dietitian, and the blood sample was collected. The study design is presented in Figure 1.
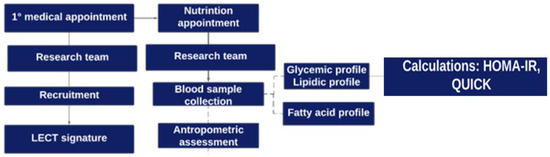
Figure 1.
Graphical illustration of the study design: stages of participant recruitment and data collection.
2.3. Measurements
2.3.1. Anthropometric Assessment
The anthropometric assessment was performed by a trained nutritionist, the mean values of two measurements of weight, height, neck circumference (NC), hip (HC), waist (WC), and waist–hip ratio (WHR) were calculated. The weight was measured using a Líder scale with a capacity of up to 200 kg. The volunteer was dressed in light clothes, without shoes. The circumferences were measured using an inextensible tape with a precision of 0.01 cm.
Height was measured with the patient standing and looking forward, arms extended by the sides, barefoot, leaning the back of the neck, buttocks, and heels against the wall, where the measuring tape was [13]. BMI was obtained by calculating the weight (kg) to height (m2) squared ratio. Abdominal circumference was measured at the largest perimeter identified between the last rib and the iliac crest at the end of expiration [14,15].
2.3.2. Biochemical Exams
Qualified nurses performed blood collection through peripheral puncture. Participants fasted for at least 8 h to a maximum of 12 h before collection. By lipidogram, blood glucose and insulin were evaluated, and the glycated hemoglobin was measured by High-Performance Liquid Chromatography (HPLC). An aliquot of blood was collected and centrifuged, and the serum was stored at −80 °C until the FA dosage. The following markers of insulin resistance and sensitivity were calculated: Homeostasis Model Assessment Insulin Resistance (HOMA-IR) = Fasting insulin (µU/mL) × fasting glucose (mmol/L)/22.5 [16]; Quantitative insulin sensitivity Check Index (QUICKI) = 1/(log fasting insulin (µU/mL) + log fasting glucose (mg/dL) [17].
Based on biochemical tests, the presence of dyslipidemia was classified as follows: Isolated hypercholesterolemia (increase in total cholesterol (TC) and/or LDL-cholesterol (LDL-C)), isolated hypertriglyceridemia (increase in triglycerides (TG)), mixed hyperlipidemia (increase in of TC and TG), isolated decrease in HDL-cholesterol (HDL-C) or associated with an increase in TG or LDL-C [18].
2.3.3. Blood Pressure
Blood pressure data were collected from the medical record, as measured by the nursing team before medical care.
2.3.4. MetScore Calculation
The cardiovascular risk outcome variable was calculated from a proposed score [19]. The formula used the z scores of continuous metabolic risk factors (abdominal circumference, systolic blood pressure, HDL-C cholesterol, triglycerides (TG), and fasting glucose). HDL-C cholesterol and TG were log-transformed before calculating the z scores, as follows:
MetScore = (zAC + zSBP-zlogHDL + zlogTG + zfasting glucose)/5
AC = abdominal circumference; SBP = systolic blood pressure; TG = triglycerides
The patients were grouped into high- and low-cardiovascular-risk groups using the median MetScore as a cutoff point (−0.05), as there is no reference of cutoff point for this population.
2.4. Extraction and Determination of Serum Fatty Acids
2.4.1. Extraction and Methylation of Fatty Acids
The measurement of FAs present in serum was obtained from various lipid fractions, including triglycerides, phospholipids, cholesterol esters, and free FAs. Transesterification was applied directly to the serum samples using acetyl chloride (5% HCl in methanol), which triggered the breakdown of complex lipid molecules and the release of esterified FAs. Additionally, the free FAs already present in the serum were directly methylated. All FAs resulting from this process, both free and previously esterified, were converted into FA methyl esters (FAMEs) [20,21].
2.4.2. Identification and Quantification of Fatty Acid Methyl Esters (FAMEs)
The resulting FAMEs were analyzed using a Varian Model 3900 gas chromatograph (Walnut Creek, CA, USA), coupled with a flame ionization detector (FID) and an automatic sampler CP-8410. Separation was performed on a CP Wax 52 CB capillary column (Varian, Lake Forest, CA, USA) with a thickness of 0.25 μm, an internal diameter of 0.25 mm, and a length of 30 m. Hydrogen was used as the carrier gas at a linear velocity of 22 cm/s. The temperature program was set to 170 °C for 1 min, followed by increases of 2.5 °C/min until 240 °C, with a final time of 5 min. The injector and FID temperatures were maintained at 250 °C and 260 °C, respectively. The identification of FAMEs was performed by comparing the retention times of the samples with those of known standards of FA methyl esters (Supelco, 37 components; Sigma-Aldrich Darmstadt, Alemanha; Mix Me93, Larodan; and Qualmix, PUFA Fish M, Menhaden Oil, Larodan). The concentrations of fatty acids were expressed as percentages of the total fatty acids present [21].
2.5. Desaturases Indexes
The desaturase indexes can demonstrate the indirect action of stearoyl-CoA desaturase 1 (SCD1) activity, indicating de novo lipogenesis of MUFAs (C16:1 and C18:1) from the SFAs (C16:0 and C18:0). The index was obtained by the ratio of fatty acids 16:1n[7]/16:0 (palmitic FA desaturase index (SCD-16)) and 18:1n[9]/18:0 (oleic FA desaturase index (SCD-18)) [22].
2.6. Statistical Analysis
Statistical analyses were performed using the Statistical Software Package for the Social Sciences (SPSS) (version 25; SPSS Inc., Chicago, IL, USA). Initially, a total of 45 volunteers were recruited for the study; however, we experienced a sample loss of 4 participants (approximately 9%), primarily due to incomplete data collection (2 participants) and failure to comply with fasting requirements for the exams (2 participants). In the end, the study included 41 participants.
The sample size calculation was carried out, based on Pearson’s correlation test, between the variables, assuming an α (Rho) of 0.50 and with Power (1 − β) = 0.80 of 0.05; the sample size necessary to validate these analyses is 41 individuals. The Shapiro–Wilk test was used to verify data distribution. The variables studied are described as mean and standard deviation (SD). Individuals were grouped based on the median of the outcome variable (MetScore) into high- and low-cardiovascular-risk groups, and the cutoff value was −0.05. The difference between group means was assessed using the Generalized Linear Model (GLzM). Spearman and Spearman’s coefficients were utilized to verify the MetScore correlations with the other variables. The chi-square test evaluated the relationship between medication intake and cardiovascular risk. Simple logistic regression was used to verify the association between the outcome and the other variables. In addition, a logistic regression model adjusted for age, height, HOMA-IR, HDL-c, and use of hypoglycemic agents was also developed to verify the association between cardiovascular risk and FAs. The association measure for the regressions was the odds ratio with its respective 95% Confidence Interval. The significance level was set at <0.05.
3. Results
3.1. Sample Description and Lipidic, Glycemic Profile
A total of 41 individuals with a mean age of 40.2 ± 8.31 years and a mean BMI of 48.38 ± 6.66 kg/m2 were included in the study following the inclusion criteria. All individuals were classified with increased risk for the development of cardiovascular diseases according to AC, WHR, and NC. About the use of medications, there was no statistical difference between the low- and high-cardiovascular-risk groups. The reference values for all mentioned variables can be found in the footnote of Table 1.

Table 1.
Association between high cardiovascular risk and clinical, anthropometric, and laboratory variables in women with severe obesity.
Regarding the lipid profile, 78.05% of the volunteers had some dyslipidemia, in which 39.02% had isolated hypercholesterolemia, 31.70% had mixed hyperlipidemia, and 7.31% had just hypertriglyceridemia; 60.97% had low HDL-c, and 21.95% had no dyslipidemia. Twenty women (48.78%) presented associated dyslipidemia, consisting of low HDL-c with another type of dyslipidemia. Despite this, only 1 patient reported using statins.
The group with the highest risk was older (p = 0.042), had higher HOMA-IR (p = 0.011), HbA1c (p = 0.002) and VLDL-C (p ≤ 0.001) and QUICKI (p = 0.006). The clinical data of the patients can be observed in Table 1.
3.2. Serum Fatty Acid Profile and Their Association with Cardiovascular Risk
Considering the serum fatty acid profile, 23 FAs were identified, and all were included in the study. Among the 23 FAs identified, six were SFAs (56.18%), twelve were PUFAs (26.64%), and five were MUFAs (17.15%). When evaluating the abundance of omega-3 and 6 fatty acids, these presented averages of 4.96% and 21.68%, respectively. The mean anti-inflammatory omega 3/6 ratio was 0.24, whereas the pro-inflammatory omega 6/3 ratio was 5.23. Table 2 shows the percentage of each FA evaluated in participants.

Table 2.
Analysis of difference between means and logistic regression of fatty acids in women with severe obesity.
Considering the FA profile, the high-risk group had the greatest % of oleic (C18:1n[9]) (p = 0.005), cis-vaccenic (c18:1n[7]) (p = 0.042), and total MUFAs (p = 0.002). There was also a lower % of gamma-linolenic (C18:3n[6]) (p = 0.003) and eicosatetraenoic acid (C20:4n[3]) (p = 0.038). All the differences found between groups were adjusted by age. As for the desaturase indexes, the group with high cardiovascular risk showed greater activity of SCD-18 (p = 0.011). There was no statistically significant difference in the SCD-16C; these data are presented in Table 2.
Logistic regression analysis showed a significant association between cardiovascular risk calculated by the MestScore and glycemic and lipid parameters in women with severe obesity. Furthermore, every increase of one unit of palmitic FA increases the chance of belonging to the high-cardiovascular-risk group by 20%. As for total MUFAs, the chance of belonging to the high-cardiovascular-risk group increases by 42% with an increase of one unit of the total MUFAs and by 27% with an increase in FA c18:1n[9]. The analysis of PUFAs showed that every decrease of one FA C18:3n[6] unit increases the chance of belonging to the high-cardiovascular-risk group by 63%. We also observed that an increase of one unit in SCD-18 increases cardiovascular risk by 673%; these data are presented in Table 2.
The logistic regression model adjusted for each statistically significant FA (p ≤ 0.05) included age, height, HOMA-IR, HDL-c, and use of hypoglycemic agents as adjustment covariates. The adjusted OR comparing cardiovascular risk estimates for women with severe obesity remained statistically significant for all variables except SCD-18C, Table 3.

Table 3.
Adjusted logistic regression analyses of the association between high cardiovascular risk and predictor variables.
The analysis of c16:0 (p = 0.030 OR = 1.36), total MUFAs (p = 0.032 OR= 1.52), and C18:1n[9] (p = 0.045 OR = 1.36) showed that, with each increase of 1 unit of these serum FAs, there is an increase in the individual’s belonging to the high-cardiovascular-risk group of 36%, 52%, and 36%, respectively. As for FA C18:3n[6], it can be detected that every increase of 1 unit reduces the chance of belonging to the high-cardiovascular-risk group by 73%.
Correlations were observed between the MetScore and metabolic parameters, presented in Table 4.

Table 4.
Correlation between Metscore, lipid, glucose, and some fatty acid profile variables.
4. Discussion
Identifying the associations between plasma FAs and cardiovascular risk can support the development of scientific knowledge about the influence of FAs on the cardiometabolic health of individuals with obesity. This association is crucial since plasma FAs play an important role in the metabolic pathways that influence insulin resistance, lipid metabolism, and inflammation. Together, these changes are fundamental in the development of cardiometabolic dysfunction associated with obesity. Addressing these factors may provide information on therapeutic targets to reduce cardiovascular risk in this vulnerable population. In the present study, for the first time, we have identified that women with severe obesity and high cardiovascular risk presented higher proportions of the SFA C16:0, a greater amount of SCD-18 and total MUFAs, and lower proportions of C20:4n3 and C18:3n6, suggesting the worst lipid profile.
In addition, the data also indicate that every increase of one unit of FA C16 increases the individual’s chance of belonging to the high-risk group by 20%. Long-chain fatty acids may contribute to the progression of obesity once they are less oxidized than other types of FAs [11]. Previous studies have demonstrated the relationship between palmitic FAs and cardiovascular health in different populations [11,23,24]. Among the mechanisms by which palmitic acid correlates with worse cardiometabolic outcomes, we highlight its effect on promoting oxidative stress, increasing reactive oxygen species, and the activation of inflammatory signaling [25,26], which favors impairment in hepatic glucose and lipid metabolism, fomenting IR, atherosclerosis, and hypertriglyceridemia [27,28].
In the present study, MUFAs corresponded to an average of 17.15% serum FAs with a higher percentage of total MUFAs in individuals with high cardiovascular risk compared to those with low risk. In an analysis adjusted for age, height, HOMA_IR, and HDL-c, MUFAs remained an independent predictive factor for cardiovascular risk, being able to increase the risk by 39% (Table 3). Wrzosek et al. found similar results in a sample of both women and men with obesity, where higher amounts of serum oleic FAs were found in the group with lower amounts of total PUFAs (considered a worse profile for cardiovascular risk) [2]. These data may be seen as controversial since the dietary intake of MUFAs is associated with reduced cardiometabolic risk. However, it is known that plasma MUFAs do not represent only dietary intake, as they are affected by genetic factors, smoking, physical activity, and especially by de novo lipogenesis that transforms surplus SFAs into MUFAs through the enzymes SCD-1 [29,30].
SCD-1 is a desaturase found mainly in the liver and adipose tissue, responsible for converting a portion of 16:0 into palmitoleic (16:1) and 18:0 into oleic acid (18:1), which might contribute to the higher abundance of MUFAs. Considering that SCD-1 is regulated by factors such as saturated fat intake, carbohydrate consumption [31], and metabolic conditions, its elevated activity is associated with metabolic diseases such as insulin resistance, obesity, type 2 diabetes, and cardiovascular dysfunction, since the excessive production of MUFAs can lead to the accumulation of fat in the liver and adipose tissue, favoring the development of cardiometabolic complications, which have been associated with obesity, IR, DM2, and MetS [32,33].
A recent study found that SCD-1-deficient mice are protected against obesity, MetS, CVD, and NAFLD [34,35,36]. In addition, those animals showed greater thermogenesis and insulin sensitivity [37], although the mechanisms are not completely elucidated. In the studied population, the ability of SCD-18 to increase cardiovascular risk was estimated at 673% for each unit increased in the index (p = 0.023). In agreement, Svendsen et al. obtained significant values of SCD-18, but not of SCD-16, in individuals categorized to be at high cardiometabolic risk [38]. These findings can be attributed to excessive dietary intake of SFAs, simple carbohydrates, and other unhealthy eating habits [32,38] highlighting the importance of guidelines and nutritional guides that address the consumption of fatty acids, simple carbohydrates, and their consequences for health in a practical and easy-to-understand way for the general population.
The activity of desaturases explains the higher proportion of oleic FA (p = 0.005) and vaccenic FA (p = 0.042) in women with high cardiovascular risk. Each unit of oleic FA was associated with a 27% increase in cardiovascular risk (Table 2). Both FAs were also negatively correlated with insulin sensitivity (p = 0.002, r = −0.477; p = 0.013, r = −0.389, respectively), showing their effect on glucose metabolism. Previous studies point out oleic and vaccenic fatty acids as pro-obesogenic and diabetogenic due to their ability to promote IR [38]. Therefore, the hypothesis of endogenous synthesis of MUFAs as one of the etiological factors of glycemic disorders is strengthened [39,40]. However, further studies are needed to investigate the pathophysiological mechanisms [41,42].
De novo lipogenesis-produced fatty acids, including palmitic acid (16:0), cis-palmitoleic acid (16:1n-7), oleic acid (18:1n-9), and cis-vaccenic acid (18:1n-7), are associated with risk factors for cardiovascular diseases, such as adiposity, hypertension, diabetes, inflammation, and cardiovascular mortality [27,40,41,43]. The cis-vaccenic fatty acid can reduce insulin sensitivity through various mechanisms. This fatty acid can activate serine/threonine kinases, which, in turn, decrease the phosphorylation of tyrosine residues on insulin receptor substrates (IRS1/2). This impairs the IRS/phosphatidylinositol 3-kinase (PI3K) signaling pathway, hindering insulin signal transduction and contributing to insulin resistance. Furthermore, free fatty acids, such as cis-vaccenic acid, may lead to the generation of reactive oxygen species and act as modulators of the NLRP3 inflammasome, which may play an important role in insulin resistance [27,31,43].
A recent study showed that women with polycystic ovary syndrome (PCOS) had higher concentrations of cis-vaccenic acid [44]. However, further interventional studies are needed to investigate whether reducing the lipogenesis of vaccenic acid, through dietary or pharmacological interventions, could positively impact insulin sensitivity and cardiovascular outcomes, as this pathway is not entirely clear. Understanding these pathophysiological mechanisms could pave the way for new therapeutic strategies [44].
It is known that IR is closely related to cardiometabolic risk [45], being a parameter that increases the MetScore. The mean values of fasting blood glucose, HbA1c, HOMA-IR, and QUICK, indicate that the patients have disorders in glucose metabolism [16,17,46]. The group with high risk presented worse glucose parameters. In addition, we found that MetScore correlated with HOMA-IR (p = 0.001, r = 0.481), HbA1c (p = 0.00, r = 0.526), and QUICK (p = 0.005, r = −0.430). Furthermore, MetScore also correlated negatively with HDL-C (p = 0.001, r = −0.514) and omega 6 (p = 0.004, r = −0.313), suggesting an intrinsic pathway between glucose lipid metabolism. Finally, the high-risk group presented lower HDL-c (p = 0.008) and higher VLDL-c (p < 0.001).
Regarding the PUFA profile, data revealed an average of 26.64%, of which 21.68% belonged to the omega-6 family and 4.96% from the omega-3 family. Wrzosek et al. found a similar fatty acid profile, with 31.23% of the total PUFAs being 25.92% omega-6 and 1.78% omega-3 [2]. The total PUFAs showed no statistical difference between the groups. However, FA C18:3n[6] (gamma-linolenic) had lower proportions in the high-risk group (p = 0.003). It was also observed that a reduction of 1 unit of GLA increases the chance of belonging to the high-risk group by 63% (Table 2). Moreover, we observed a negative correlation between MetScore and GLA (Table 4). Moderate intake of linoleic acid, in partial replacement of SFAs, was also associated with reduced cardiometabolic risk due to reduced LDL-c through upregulation of the hepatic LDL-c receptor gene, thus leading to clearance of circulating LDL-c [47].
Lower cardiovascular risk may also be associated with higher GLA values due to its beneficial health effects arising mainly from the conversion of GLA into Dihomo-Gamma-Linolenic (DHGLA) which gives rise to metabolites such as cyclooxygenase 1/2 (COX1/2). The COX1/2 increases prostaglandin series 1 (PGE1) and 15-lipoxygenase (15-Lox) forming 15-(S)-hydroxy-8,11,13-eicosatrienoic acid (15-HETrE). DHGLA-derived metabolites suppress inflammation, promote vasodilation, lower blood pressure, and exert antineoplastic effects [38]. Furthermore, both PGE1 and 15-HETrE antagonize the synthesis of pro-inflammatory metabolites derived from Arachidonic Acid (AA), such as series 2 Prostaglandins, series 4 Leukotrienes, HETEs, and series 2 thromboxanes (Figure 2) [48]. GLA is commonly available as a dietary supplement; however, its efficacy in attenuating inflammation through dietary intake remains controversial [49,50,51]. Further research is required to clarify its therapeutic potential in this context [52].
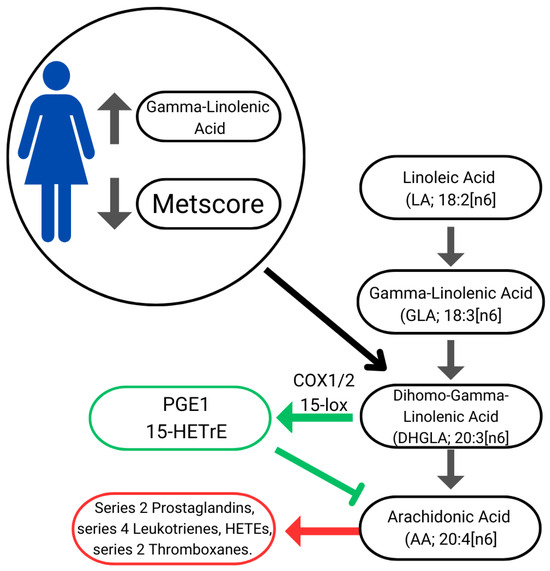
Figure 2.
Illustration of linoleic acid bioconversion pathway, eicosanoid synthesis, and cardiovascular risk. Green arrows: anti-inflammatory pathways; Red arrow: inflammatory pathways.
Women that were grouped in the high-risk group also presented lower proportions of eicosatetraenoic acid (C20:4n[3]) (p = 0.038), corroborating previous findings [53,54]. A recent meta-analysis with a cross-sectional and case–control study identified that higher levels of omega-3 PUFA were associated with a lower prevalence of MetS [53]. Another meta-analysis with prospective cohort studies found higher dietary and plasma omega 3 PUFA levels associated with a significantly low risk of developing MetS [54].
The possible mechanisms for the protective effects of omega 3 PUFA on MetS are related to reduced synthesis of triacylglycerols and LDL-c in the liver, preventing dyslipidemias [55]. Omega-3 can reduce the synthesis of fatty acids in the liver by suppressing the nuclear abundance of the transcription factor SREBP-1c that promotes the activation of essential genes in the regulation of lipogenic enzymes, acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS), which are responsible for catalyzing lipogenesis, increasing the efflux of serum FFAs for the formation of triacylglycerols (Figure 3) [55,56].
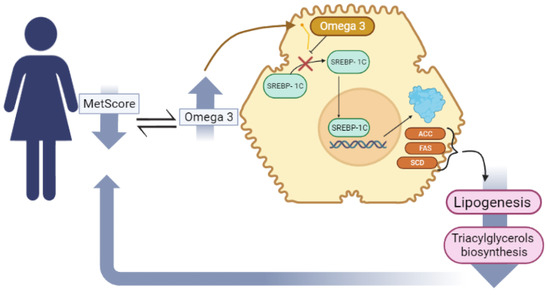
Figure 3.
The mechanism by which PUFA [n3] decreases triacylglycerol biosynthesis.
The omega-3 PUFAs also play a role in improving insulin sensitivity36 and in controlling arterial hypertension as they can reduce the levels of the angiotensin-converting enzyme [57]. However, in the present study, we did not find a correlation between omega 3 PUFAs and glycemic parameters.
Although serum fatty acids are not analyzed in clinical practice, it is essential to understand how they favor cardiovascular health or disease. For the first time in the literature, through the present study, it was possible to demonstrate in women with severe obesity the association of critical fatty acids favoring cardiovascular risk, suggesting that greater attention should be given to nutritional guidelines.
Among the limitations of the study, we mention the fact that the patient’s food context was not analyzed. However, other studies that addressed the same topic did not observe significant differences between dietary intake and the profile of serum fatty acids in relation to cardiovascular risk [2]. Furthermore, it is necessary to carry out longitudinal studies with a larger sample to strengthen the findings of this study.
As strengths, we highlight the use of MetScore for early screening of cardiovascular risk and its use in association with FAs. In addition, the scientific literature lacks data on patients with severe obesity, which requires special attention and a better understanding of the cardiovascular risk to which they may be exposed.
5. Conclusions
Women with severe obesity who have a high cardiovascular risk calculated by MetScore tend to have a worse FA profile, with lower amounts of GLA and FA C20:4n[3], which are considered cardioprotective and anti-inflammatory, and higher percentages of palmitic FA and serum MUFA, which are associated with inflammation and CVD. They also have higher de novo lipogenic activity due to the conversion of SFAs to MUFAs, which is associated with the increased cardiovascular risk demonstrated by the indices of desaturases. These findings emphasize the need to address lipid metabolism in this population as a therapeutic target to reduce cardiovascular risk. Future research should explore clinical interventions that modulate fatty acid metabolism to mitigate cardiometabolic complications.
6. Future Recommendations
We recommend that future research focus on the following points:
- (1)
- There is a need for longitudinal studies to determine the influence of fatty acids on cardiovascular risk over the years.
- (2)
- Clinical trials for dietary interventions should be conducted using different sources of carbohydrates and lipids to verify the influence of serum fatty acids and desaturating enzymes on cardiovascular risk. Furthermore, randomized clinical trials with fatty acid supplementation, which in the present study was shown to be cardioprotective, would help determine their influence on reducing cardiovascular risk.
- (3)
- Evaluation of different populations, mainly longitudinal studies in eutrophic individuals, should be conducted to show the MetS score as a good tool for early screening of cardiovascular risk, through changes in the GA profile over the years.
- (4)
- It is important to conduct controlled and randomized clinical trials involving the supplementation of key fatty acids discussed in this study in order to investigate their potential as a therapeutic option for attenuating inflammation and reducing cardiometabolic alterations.
Author Contributions
Conceptualization, E.S.O., F.M.K., M.A.H. and F.C.C.; Data curation, E.S.O., F.M.K., G.C.L., N.F., G.B.L., R.G.M.W. and G.I.d.M.H.d.S.; Formal analysis, E.S.O., L.M.O. and E.A.S.; Funding acquisition, F.C.C.; Investigation, N.F., G.B.L. and F.C.C.; Methodology, G.C.L., M.A.H., G.B.L., R.G.M.W., G.I.d.M.H.d.S., L.M.O., E.A.S. and F.C.C.; Project administration, G.C.L. and F.C.C.; Resources, M.A.H.; Software, G.I.d.M.H.d.S., L.M.O. and E.A.S.; Supervision, F.C.C.; Validation, G.C.L. and F.C.C.; Visualization, M.A.H.; Writing—original draft, E.S.O., N.F., G.B.L., E.A.S. and F.C.C.; Writing—review and editing, E.S.O., F.M.K., G.C.L., M.A.H., R.G.M.W., G.I.d.M.H.d.S., L.M.O., E.A.S. and F.C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CnPq) grant number [no 434159/2018-2] and “Fundação de Apoio à Pesquisa” (FUNAPE-UFG) grant number [no 01/2022], And The APC was funded by Federal University of Goiás—Postgraduate Program in Health Sciences.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research Ethics Committee of the Federal University of Goiás (protocol no 3.251.178, approval date: 8 April 2019) and by the Hospital Estadual Geral de Goiânia Dr. Alberto Rassi (protocol no 961/19, approval date: 17 June 2019).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to ethical reasons.
Acknowledgments
The authors would like to thank the “Grupo de Estudos da Obesidade (GEO-Goiânia)” and its members, the researchers from the Nutrition Physiology Laboratory of the Federal University of São Paulo and the University of São Paulo, and all the patients who participated in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsdenh, H.; Calvilloi, A.; De Schutter, O.; Devarajan, R.; et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef] [PubMed]
- Wrzosek, M.; Zawadzka, Z.; Sawicka, A.; Bobrowska-Korczak, B.; Białek, A. Impact of Fatty Acids on Obesity-Associated Diseases and Radical Weight Reduction. Obes. Surg. 2022, 32, 428. [Google Scholar] [CrossRef] [PubMed]
- Silveira, E.A.; Kliemann, N.; Noll, M.; Sarrafzadegan, N.; Oliveira, C. Visceral obesity and incident cancer and cardiovascular disease: An integrative review of the epidemiological evidence. Obes. Rev. 2021, 22, e13088. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.J.; Geisler, C. Defining obesity as a disease. Eur. J. Clin. Nutr. 2017, 71, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Wijayatunga, N.N.; Sams, V.G.; Dawson, J.A.; Mancini, M.L.; Mancini, G.J.; Moustaid-Moussa, N. Roux-en-Y gastric bypass surgery alters serum metabolites and fatty acids in patients with morbid obesity. Diabetes Metab. Res. Rev. 2018, 34, e3045. [Google Scholar] [CrossRef]
- Walle, P.; Takkunen, M.; Männistö, V.; Vaittinen, M.; Lankinen, M.; Kärjä, V.; Käkelä, P.; Ågren, J.; Tiainen, M.; Schwab, U.; et al. Fatty acid metabolism is altered in non-alcoholic steatohepatitis independent of obesity. Metabolism 2016, 65, 655–666. [Google Scholar] [CrossRef]
- IDF Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Thivel, D.; Malina, R.M.; Isacco, L.; Aucouturier, J.; Meyer, M.; Duché, P. Metabolic syndrome in obese children and adolescents: Dichotomous or continuous? Metab. Syndr. Relat. Disord. 2009, 7, 549–555. [Google Scholar] [CrossRef]
- Malta, D.C.; da Silva, A.G.; Tonaco, L.A.B.; de Fátima Freitas, M.I.; Velasquez-Melendez, G. Tendência temporal da prevalência de obesidade mórbida na população adulta brasileira entre os anos de 2006 e 2017. Cad. Saúde Pública 2019, 35, e00223518. [Google Scholar] [CrossRef]
- Williamson, K.; Nimegeer, A.; Lean, M. Rising prevalence of BMI ≥40 kg/m2: A high-demand epidemic needing better documentation. Obes. Rev. 2020, 21, e12986. [Google Scholar] [CrossRef]
- Kaikkonen, J.E.; Jula, A.; Viikari, J.S.A.; Juonala, M.; Hutri-Kähönen, N.; Kähönen, M.; Lehtimäki, T.; Raitakari, O.T. Associations of Serum Fatty Acid Proportions with Obesity, Insulin Resistance, Blood Pressure, and Fatty Liver: The Cardiovascular Risk in Young Finns Study. J. Nutr. 2021, 151, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Anjos, C.A.; Clemente, M.; de Fátima Gaspari Dias, J.; Burci, L.M.; de Oliveira Leite, R.; Miguel, M.D. Quality of Life of Elderly People Living in Different Types of Long-term Care Facilities. Braz. J. Pharm. Sci. 2022, 58, e20117. [Google Scholar] [CrossRef]
- Fett, C.A.; Fett, W.C.R.; Marchini, J.S. Comparação entre bioimpendância e antropometria e a relação de índices corporais ao gasto energético de repouso e marcadores bioquímicos sanguíneos em mulheres da normalidade à obesidade. Rev. Bras. Cineantropom Desempenho Hum. 2006, 8, 29–36. [Google Scholar]
- ABESO. Diretrizes Brasileiras de Obesidade—2016; ABESO: São Paulo, Brazil, 2016. [Google Scholar]
- Hingorjo, M.R.; Zehra, S.; Imran, E.; Qureshi, M. Neck circumference: A supplemental tool for the diagnosis of metabolic syndrome. JPMA 2016, 66, 1221–1226. [Google Scholar]
- Stern, S.E.; Williams, K.; Ferrannini, E.; DeFronzo, R.A.; Bogardus, C.; Stern, M.P. Identification of Individuals With Insulin Resistance Using Routine Clinical Measurements. Diabetes 2005, 54, 333–339. [Google Scholar] [CrossRef]
- Placzkowska, S.; Pawlik-Sobecka, L.; Kokot, I.; Piwowar, A. Indirect insulin resistance detection: Current clinical trends and laboratory limitations. Biomed. Pap. 2019, 163, 187–199. [Google Scholar] [CrossRef]
- Faludi, A.A.; Izar, M.C.O.; Saraiva, J.F.K.; Bianco, H.T.; Chacra, A.P.M. Atualização da Diretriz Brasileira de Dislipidemias e Prevenção da Aterosclerose. Arq. Bras. Cardiol. 2017, 109, 1–76. [Google Scholar] [CrossRef]
- Drehmer, M.; Pereira, M.A.; Schmidt, M.I.; Alvim, S.; Lotufo, P.A.; Luft, V.C.; Bruce, D. Total and full-fat, but not low-fat, dairy product intakes are inversely associated with metabolic syndrome in adults. J. Nutr. 2016, 146, 81–89. [Google Scholar] [CrossRef]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef]
- Christie, W. Lipid Analysis; Pergamon Press: Oxford, UK, 1982. [Google Scholar]
- García-Serrano, S.; Moreno-Santos, I.; Garrido-Sánchez, L.; Gutierrez-Repiso, C.; García-Almeida, J.M.; García-Arnés, J.; Rivas-Marín, J.; Gallego-Perales, J.L.; García-Escobar, E.; Rojo-Martinez, G.; et al. Stearoyl-CoA Desaturase-1 Is Associated with Insulin Resistance in Morbidly Obese Subjects. Mol. Med. 2011, 17, 273. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Langley, K.G.; Berglund, N.A.; Kammoun, H.L.; Reibe, S.; Estevez, E.; Weir, J.; Mellett, N.A.; Pernes, G.; Conway, J.R.W.; et al. Evidence that TLR4 Is Not a Receptor for Saturated Fatty Acids but Mediates Lipid-Induced Inflammation by Reprogramming Macrophage Metabolism. Cell Metab. 2018, 27, 1096–1110.e5. [Google Scholar] [CrossRef] [PubMed]
- Perna, M.; Hewlings, S. Saturated Fatty Acid Chain Length and Risk of Cardiovascular Disease: A Systematic Review. Nutrients 2023, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Rogero, M.; Calder, P. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Wenjie, M.; Wu, J.H.Y.; Wang, Q.; Lemaitre, R.N.; Mukamal, K.J.; Djoussé, L.; Irena, K.; Xiaoling, S.; Mary, B.; Joseph, D.; et al. Prospective association of fatty acids in the de novo lipogenesis pathway with risk of type 2 diabetes: The Cardiovascular Health Study. Am. J. Clin. Nutr. 2015, 101, 153. [Google Scholar] [CrossRef]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.H.; Brickey, W.J.; Ting, J.P. Fatty acid-induced NLRP3-PYCARD inflammasome activation interferes with insulin signaling. Nat. Immunol. 2011, 12, 408. [Google Scholar] [CrossRef]
- Hodson, L.; Skeaff, C.M.; Fielding, B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008, 47, 348–380. [Google Scholar] [CrossRef]
- Ravaut, G.; Légiot, A.; Bergeron, K.F.; Mounier, C. Monounsaturated Fatty Acids in Obesity-Related Inflammation. Int. J. Mol. Sci. 2021, 22, 330. [Google Scholar] [CrossRef]
- Alsharari, Z.D.; Leander, K.; Sjögren, P.; Carlsson, A.; Cederholm, T.; de Faire, U.; Hellenius, M.-L.; Marklund, M.; Risérus, U. Association between carbohydrate intake and fatty acids in the de novo lipogenic pathway in serum phospholipids and adipose tissue in a population of Swedish men. Eur. J. Nutr. 2020, 59, 2089–2097. [Google Scholar] [CrossRef]
- Warensjö, E.; Rosell, M.; Hellenius, M.L.; Vessby, B.; De Faire, U.; Risérus, U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: Links to obesity and insulin resistance. Lipids Health Dis. 2009, 8, 37. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Guerendiain, M.; Castellote, A.I.; Estruch, R.; Covas, M.I.; Fitó, M.; Salas-Salvadó, J.; Martínez-González, M.A.; Aros, F.; Lamuela-Raventós, R.M.; et al. Plasma fatty acid composition, estimated desaturase activities, and their relation with the metabolic syndrome in a population at high risk of cardiovascular disease. Clin. Nutr. 2014, 33, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Balatskyi, V.V.; Dobrzyn, P. Role of Stearoyl-CoA Desaturase 1 in Cardiovascular Physiology. Int. J. Mol. Sci. 2023, 24, 5531. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Wang, S.; Rahman, S.M.; Moustaid-Moussa, N. Function and Regulation of Macrophage Stearoyl-CoA Desaturase in Metabolic Disorders. In Stearoyl-CoA Desaturase Genes in Lipid Metabolism; Springer: New York, NY, USA, 2013; pp. 61–71. [Google Scholar] [CrossRef]
- Popeijus, H.E.; Saris, W.H.M.; Mensink, R.P. Role of stearoyl-CoA desaturases in obesity and the metabolic syndrome. Int. J. Obes. 2008, 32, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Lounis, M.A.; Escoula, Q.; Veillette, C.; Bergeron, K.F.; Ntambi, J.M.; Mounier, C. SCD1 deficiency protects mice against ethanol-induced liver injury. Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2016, 1861, 1662–1670. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, K.; Olsen, T.; Nordstrand Rusvik, T.C.; Ulven, S.M.; Holven, K.B.; Retterstøl, K.; Telle-Hansen, V.H. Fatty acid profile and estimated desaturase activities in whole blood are associated with metabolic health. Lipids Health Dis. 2020, 19, 102. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Arancibia-Riveros, C.; Tresserra-Rimbau, A.; Castro-Barquero, S.; Casas, R.; Vázquez-Ruiz, Z.; Ros, E.; Fitó, M.; Estruch, R.; López-Sabate, M.C.; et al. Relationship between estimated desaturase enzyme activity and metabolic syndrome in a longitudinal study. Front. Nutr. 2022, 9, 991277. [Google Scholar] [CrossRef]
- Yu, E.A.; Hu, P.J.; Mehta, S. Plasma fatty acids in de novo lipogenesis pathway are associated with diabetogenic indicators among adults: NHANES 2003–2004. Am. J. Clin. Nutr. 2018, 108, 622. [Google Scholar] [CrossRef]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef]
- Patel, P.S.; Sharp, S.J.; Jansen, E.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Nita, G.F. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: A pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am. J. Clin. Nutr. 2010, 92, 1214–1222. [Google Scholar] [CrossRef]
- Wang, L.; Folsom, A.R.; Zheng, Z.-J.; Pankow, J.S.; Eckfeldt, J.H. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Clin. Nutr. 2003, 78, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, J.; Li, M.; Shang, J.; Bai, X.; Zhang, H.; Wang, Y.; Chen, H.; Song, X. Serum fatty acid profiles associated with metabolic risk in women with polycystic ovary syndrome. Front. Endocrinol. 2023, 14, 1077590. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Leung, J.C.K.; Chan, L.Y.Y.; Yiu, W.H.; Tang, S.C.W. A global perspective on the crosstalk between saturated fatty acids and Toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog. Lipid Res. 2020, 77, 101020. [Google Scholar] [CrossRef] [PubMed]
- Cobas, R.; Rodacki, M.; Giacaglia, L.; Calliari, L.E.P.; Noronha, R.M.; Valerio, C.; Custodio, J.S., Jr.; Scharf, M.; Barcellos, C.R.G.; Tomarchio, M.P.; et al. Diagnóstico do diabetes e rastreamento do diabetes tipo 2. In Diretriz Oficial da Sociedade Brasileira de Diabetes; Conectando Pessoas: Brasília, Brazil, 2022. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Surette, M.E.; Koumenis, I.L.; Edens, M.B.; Tramposch, K.M.; Chilton, F.H. Inhibition of leukotriene synthesis, pharmacokinetics, and tolerability of a novel dietary fatty acid formulation in healthy adult subjects. Clin. Ther. 2003, 25, 948–971. [Google Scholar] [CrossRef]
- Johnson, M.M.; Swan, D.D.; Surette, M.E.; Stegner, J.; Chilton, T.; Fonteh, A.N.; Chilton, F.H. Dietary Supplementation with γ-Linolenic Acid Alters Fatty Acid Content and Eicosanoid Production in Healthy Humans. J. Nutr. 1997, 127, 1435–1444. [Google Scholar] [CrossRef]
- Barham, J.B.; Edens, M.B.; Fonteh, A.N.; Johnson, M.M.; Easter, L.; Chilton, F.H. Addition of Eicosapentaenoic Acid to γ-Linolenic Acid–Supplemented Diets Prevents Serum Arachidonic Acid Accumulation in Humans. J. Nutr. 2000, 130, 1925–1931. [Google Scholar] [CrossRef]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef]
- Guo, X.F.; Li, X.; Shi, M.; Li, D. n-3 Polyunsaturated Fatty Acids and Metabolic Syndrome Risk: A Meta-Analysis. Nutrients 2017, 9, 703. [Google Scholar] [CrossRef]
- Jang, H.; Park, K. Omega-3 and omega-6 polyunsaturated fatty acids and metabolic syndrome: A systematic review and meta-analysis. Clin. Nutr. 2020, 39, 765–773. [Google Scholar] [CrossRef]
- Scorletti, E.; Byrne, C.D. Omega-3 fatty acids and non-alcoholic fatty liver disease: Evidence of efficacy and mechanism of action. Mol. Asp. Med. 2018, 64, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L. Princípios de bioquímica de Lehninger, 6th ed; Artmed Editora: Porto Alegre, Brazil, 2014. [Google Scholar]
- Borghi, C.; Cicero, A.F.G. Omega-3 polyunsaturated fatty acids: Their potential role in blood pressure prevention and management. Heart Int. 2006, 2, 330–337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).