The Relationships among the Urinary Iodine Concentration, Selenium Intake, and Thyroid Antibodies in Adults, Including the Interaction between Iodine and Selenium: National Health and Nutrition Examination Survey 2007–2012
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
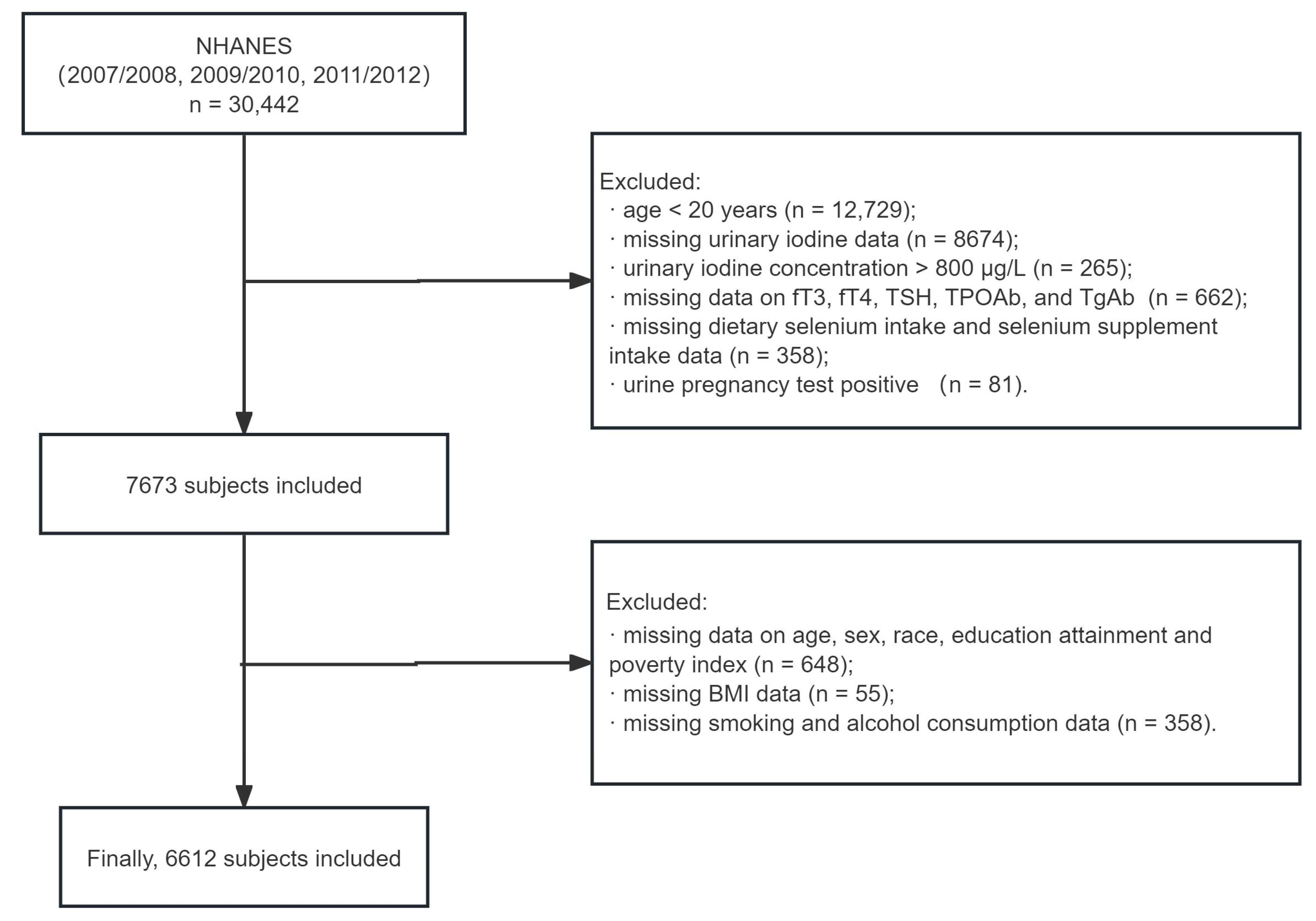
2.2. Study Population
2.3. Study Variables
- Thyroid Function and Antibodies
- The UIC
- Selenium Intake
- Other Variables
2.4. Data Analysis
3. Results
3.1. Associations between the UIC and Thyroid Antibody Positivity
3.2. Selenium Intake Stratification
3.3. Associations between Selenium Intake and Thyroid Antibody Levels
3.4. Interaction and Subgroup Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Teng, D.; Ba, J.; Chen, B.; Du, J.; He, L.; Lai, X.; Teng, X.; Shi, X.; Li, Y.; et al. Efficacy and Safety of Long-Term Universal Salt Iodization on Thyroid Disorders: Epidemiological Evidence from 31 Provinces of Mainland China. Thyroid 2020, 30, 568–579. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, W.; Li, Q.; Jia, X.; Yao, Q.; Song, R.; Qin, Q.; Zhang, J.A. U-shaped relationship between iodine status and thyroid autoimmunity risk in adults. Eur. J. Endocrinol. 2019, 181, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Teng, D.; Yang, W.; Shi, X.; Li, Y.; Ba, J.; Chen, B.; Du, J.; He, L.; Lai, X.; Li, Y.; et al. An Inverse Relationship between Iodine Intake and Thyroid Antibodies: A National Cross-Sectional Survey in Mainland China. Thyroid 2020, 30, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Teng, D.; Shan, Z.; Teng, X.; Guan, H.; Yu, X.; Fan, C.; Chong, W.; Yang, F.; Dai, H.; et al. Antithyroperoxidase and Antithyroglobulin Antibodies in a Five-Year Follow-Up Survey of Populations with Different Iodine Intakes. J. Clin. Endocrinol. Metab. 2008, 93, 1751–1757. [Google Scholar] [CrossRef]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef]
- Köhrle, J.; Jakob, F.; Contempré, B.; Dumont, J.E. Selenium, the thyroid, and the endocrine system. Endocr. Rev. 2005, 26, 944–984. [Google Scholar] [CrossRef]
- Contempré, B.; Duale, N.L.; Dumont, J.E.; Ngo, B.; Diplock, A.T.; Vanderpas, J. Effect of selenium supplementation on thyroid hormone metabolism in an iodine and selenium deficient population. Clin. Endocrinol. 1992, 36, 579–583. [Google Scholar] [CrossRef]
- Contempre, B.; Le Moine, O.; Dumont, J.E.; Denef, J.F.; Many, M.C. Selenium deficiency and thyroid fibrosis. A key role for macrophages and transforming growth factor beta (TGF-beta). Mol. Cell. Endocrinol. 1996, 124, 7–15. [Google Scholar] [CrossRef]
- Contempre, B.; Dumont, J.E.; Denef, J.F.; Many, M.C. Effects of selenium deficiency on thyroid necrosis, fibrosis and proliferation: A possible role in myxoedematous cretinism. Eur. J. Endocrinol. 1995, 133, 99–109. [Google Scholar] [CrossRef]
- Schmutzler, C.; Mentrup, B.; Schomburg, L.; Hoang-Vu, C.; Herzog, V.; Köhrle, J. Selenoproteins of the thyroid gland: Expression, localization and possible function of glutathione peroxidase 3. Biol. Chem. 2007, 388, 1053–1059. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Köhrle, J. The impact of iron and selenium deficiencies on iodine and thyroid metabolism: Biochemistry and relevance to public health. Thyroid 2002, 12, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, A.A.; Karaźniewicz-Łada, M.; Filipowicz, D.; Ruchała, M.; Główka, F.K. Metabolic Characteristics of Hashimoto’s Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management-An Overview. Int. J. Mol. Sci. 2022, 23, 6580. [Google Scholar] [CrossRef]
- Huwiler, V.V.; Maissen-Abgottspon, S.; Stanga, Z.; Mühlebach, S.; Trepp, R.; Bally, L.; Bano, A. Selenium Supplementation in Patients with Hashimoto Thyroiditis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Thyroid 2024, 34, 295–313. [Google Scholar] [CrossRef]
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat. 1 2013, 1–37. [Google Scholar] [PubMed]
- World Health Organizations. Urinary Iodine Concentrations for Determining Iodine Status in Populations; World Health Organizations: Geneva, Switzerland, 2013. [Google Scholar]
- Jin, M.; Zhang, Z.; Li, Y.; Teng, D.; Shi, X.; Ba, J.; Chen, B.; Du, J.; He, L.; Lai, X.; et al. U-Shaped Associations Between Urinary Iodine Concentration and the Prevalence of Metabolic Disorders: A Cross-Sectional Study. Thyroid 2020, 30, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Teng, X.; Shan, Z.; Teng, W.; Fan, C.; Wang, H.; Guo, R. Experimental study on the effects of chronic iodine excess on thyroid function, structure, and autoimmunity in autoimmune-prone NOD.H-2h4 mice. Clin. Exp. Med. 2009, 9, 51–59. [Google Scholar] [CrossRef]
- Latrofa, F.; Fiore, E.; Rago, T.; Antonangeli, L.; Montanelli, L.; Ricci, D.; Provenzale, M.A.; Scutari, M.; Frigeri, M.; Tonacchera, M.; et al. Iodine Contributes to Thyroid Autoimmunity in Humans by Unmasking a Cryptic Epitope on Thyroglobulin. J. Clin. Endocrinol. Metab. 2013, 98, E1768–E1774. [Google Scholar] [CrossRef]
- Pedersen, I.B.; Knudsen, N.; Carlé, A.; Vejbjerg, P.; Jørgensen, T.; Perrild, H.; Ovesen, L.; Rasmussen, L.B.; Laurberg, P. A cautious iodization programme bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin. Endocrinol. 2011, 75, 120–126. [Google Scholar] [CrossRef]
- Aghini Lombardi, F.; Fiore, E.; Tonacchera, M.; Antonangeli, L.; Rago, T.; Frigeri, M.; Provenzale, A.M.; Montanelli, L.; Grasso, L.; Pinchera, A.; et al. The effect of voluntary iodine prophylaxis in a small rural community: The Pescopagano survey 15 years later. J. Clin. Endocrinol. Metab. 2013, 98, 1031–1039. [Google Scholar] [CrossRef]
- Zaletel, K.; Gaberscek, S.; Pirnat, E. Ten-year follow-up of thyroid epidemiology in Slovenia after increase in salt iodization. Croat. Med. J. 2011, 52, 615–621. [Google Scholar] [CrossRef]
- Duntas, L.H. The catalytic role of iodine excess in loss of homeostasis in autoimmune thyroiditis. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Khattak, R.M.; Ittermann, T.; Nauck, M.; Below, H.; Völzke, H. Monitoring the prevalence of thyroid disorders in the adult population of Northeast Germany. Popul. Health Metr. 2016, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Mao, J.; Zhao, J.; Lu, J.; Yan, L.; Du, J.; Lu, Z.; Wang, H.; Xu, M.; Bai, X.; et al. Decreased Thyroid Peroxidase Antibody Titer in Response to Selenium Supplementation in Autoimmune Thyroiditis and the Influence of a Selenoprotein P Gene Polymorphism: A Prospective, Multicenter Study in China. Thyroid 2018, 28, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Chada, S.; Whitney, C.; Newburger, P.E. Post-transcriptional regulation of glutathione peroxidase gene expression by selenium in the HL-60 human myeloid cell line. Blood 1989, 74, 2535–2541. [Google Scholar] [CrossRef]
- Flohe, L.; Günzler, W.A.; Schock, H.H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973, 32, 132–134. [Google Scholar] [CrossRef]
- Chanoine, J.P. Selenium and thyroid function in infants, children and adolescents. BioFactors 2003, 19, 137–143. [Google Scholar] [CrossRef]
- Gelen, V.; Şengül, E.; Yıldırım, S.; Senturk, E.; Tekin, S.; Kükürt, A. The protective effects of hesperidin and curcumin on 5-fluorouracil-induced nephrotoxicity in mice. Environ. Sci. Pollut. Res. Int. 2021, 28, 47046–47055. [Google Scholar] [CrossRef]
- Nettore, I.C.; De Nisco, E.; Desiderio, S.; Passaro, C.; Maione, L.; Negri, M.; Albano, L.; Pivonello, R.; Pivonello, C.; Portella, G.; et al. Selenium supplementation modulates apoptotic processes in thyroid follicular cells. BioFactors 2017, 43, 415–423. [Google Scholar] [CrossRef]
- Yu, J.; Shen, S.; Yan, Y.; Liu, L.; Luo, R.; Liu, S.; Wu, Y.; Li, Y.; Jiang, J.; Ying, H. Iodide Excess Inhibits Thyroid Hormone Synthesis Pathway Involving XBP1-Mediated Regulation. Nutrients 2023, 15, 887. [Google Scholar] [CrossRef]
- Chen, X.; Huang, H.; Liang, B.; Zhou, J. Abnormal Iodine Nutrition-Induced ER Stress Upregulates MCP-1 Expression Through P38/MAPK Signaling Pathway in Thyroid Cells. Biol. Trace Elem. Res. 2019, 191, 98–103. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Cai, Y.; Guo, Y.; Song, F.; Hu, Y.; Li, L.; Zhu, L. The association between dietary selenium intake and Hashimoto’s thyroiditis among US adults: National Health and Nutrition Examination Survey (NHANES), 2007–2012. J. Endocrinol. Investig. 2023, 46, 1385–1395. [Google Scholar] [CrossRef] [PubMed]



| UIC (μg/L) | p-Value 2 | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Total n = 6612 (100%) 1 | <100 n = 2140 (34%) 1 | 100 to <200 n = 2227 (32%) 1 | 200 to <300 n = 1131 (17%) 1 | 300 to <500 n = 826 (12%) 1 | 500 to ≤800 n = 288 (4.0%) 1 | |
| Age (years) | 46 (33, 59) | 45 (32, 56) | 46 (33, 59) | 48 (35, 61) | 48 (34, 61) | 50 (35, 62) | 0.015 |
| Sex | <0.001 | ||||||
| Men | 3365 (48.11%) | 976 (41.83%) | 1127 (48.38%) | 631 (55.97%) | 469 (53.06%) | 162 (51.06%) | |
| Women | 3247 (51.89%) | 1164 (58.17%) | 1100 (51.62%) | 500 (44.03%) | 357 (46.94%) | 126 (48.94%) | |
| Race | 0.195 | ||||||
| Non-Hispanic White | 3193 (71.68%) | 1020 (70.88%) | 1044 (71.43%) | 560 (72.72%) | 421 (73.92%) | 148 (69.26%) | |
| Non-Hispanic Black | 1307 (10.01%) | 473 (10.96%) | 440 (10.24%) | 215 (9.54%) | 129 (7.62%) | 50 (9.38%) | |
| Mexican American | 1046 (7.86%) | 297 (6.97%) | 378 (8.27%) | 191 (8.68%) | 132 (7.67%) | 48 (9.34%) | |
| Other Hispanic | 671 (5.08%) | 200 (4.99%) | 229 (4.76%) | 121 (5.74%) | 92 (5.14%) | 29 (5.44%) | |
| Other/multiracial | 395 (5.37%) | 150 (6.19%) | 136 (5.31%) | 44 (3.32%) | 52 (5.65%) | 13 (6.58%) | |
| Education attainment | 0.404 | ||||||
| Less than 9th grade | 727 (5.56%) | 186 (4.29%) | 242 (5.48%) | 156 (7.57%) | 105 (6.10%) | 38 (6.88%) | |
| 9–11th grade | 1090 (12.15%) | 350 (11.77%) | 362 (11.83%) | 198 (13.46%) | 129 (12.23%) | 51 (12.10%) | |
| High school grad/GED | 1546 (23.92%) | 531 (24.72%) | 503 (23.28%) | 251 (25.18%) | 194 (21.56%) | 67 (24.24%) | |
| Some college or AA degree | 1837 (30.51%) | 608 (30.52%) | 647 (31.84%) | 292 (27.74%) | 211 (30.07%) | 79 (32.72%) | |
| College graduate or above | 1412 (27.87%) | 465 (28.70%) | 473 (27.57%) | 234 (26.05%) | 187 (30.04%) | 53 (24.07%) | |
| Ratio of family income to poverty | 0.009 | ||||||
| ≤1.3 | 2023 (21.45%) | 653 (20.70%) | 660 (20.97%) | 334 (21.24%) | 268 (22.91%) | 108 (28.14%) | |
| 1.3~3.5 | 2497 (33.97%) | 791 (33.35%) | 830 (33.58%) | 468 (40.21%) | 309 (30.91%) | 99 (25.46%) | |
| ≥3.5 | 2092 (44.58%) | 696 (45.95%) | 737 (45.44%) | 329 (38.54%) | 249 (46.18%) | 81 (46.40%) | |
| Smoking status | 0.002 | ||||||
| Current smoker | 1431 (22.40%) | 526 (25.99%) | 466 (22.47%) | 231 (19.80%) | 156 (17.83%) | 52 (16.29%) | |
| Former smoker | 1715 (24.70%) | 514 (24.60%) | 583 (23.81%) | 314 (26.43%) | 219 (22.71%) | 85 (31.67%) | |
| Non-smoker | 3466 (52.90%) | 1100 (49.41%) | 1178 (53.72%) | 586 (53.77%) | 451 (59.47%) | 151 (52.03%) | |
| Alcohol consumption | 0.262 | ||||||
| 1–5 drinks/month | 3266 (49.35%) | 1014 (46.60%) | 1095 (49.36%) | 584 (49.96%) | 427 (55.27%) | 146 (51.77%) | |
| 5–10 drinks/month | 531 (9.66%) | 192 (10.19%) | 175 (10.21%) | 83 (8.72%) | 62 (8.49%) | 19 (8.30%) | |
| 10+ drinks/month | 1005 (18.72%) | 366 (21.26%) | 342 (18.96%) | 148 (17.20%) | 107 (14.64%) | 42 (14.15%) | |
| Non-drinker | 1809 (22.26%) | 567 (21.92%) | 615 (21.47%) | 316 (24.12%) | 230 (21.60%) | 81 (25.78%) | |
| BMI (kg/m2) | 0.001 | ||||||
| Underweight (<18.5) | 101 (1.53%) | 46 (1.71%) | 27 (1.32%) | 16 (1.67%) | 7 (0.92%) | 5 (2.89%) | |
| Normal (18.5 to <25) | 1812 (30.20%) | 727 (37.21%) | 556 (27.23%) | 250 (24.32%) | 203 (27.28%) | 76 (28.34%) | |
| Overweight (25 to <30) | 2270 (33.70%) | 695 (31.57%) | 807 (35.29%) | 390 (35.35%) | 277 (33.26%) | 101 (33.39%) | |
| Obese (30 or greater) | 2429 (34.57%) | 672 (29.51%) | 837 (36.16%) | 475 (38.65%) | 339 (38.54%) | 106 (35.38%) | |
| TSH (mIU/L) | 2.11 (3.40) | 2.04 (3.02) | 2.10 (3.98) | 2.93 (1.59) | 3.06 (1.71) | 2.96 (1.79) | 0.211 |
| fT4 (pmol/L) | 10.26 (2.00) | 10.33 (2.01) | 10.33 (1.91) | 2.35 (4.06) | 1.99 (1.77) | 2.04 (1.45) | 0.250 |
| fT3 (pg/mL) | 3.18 (0.43) | 3.19 (0.36) | 3.20 (0.52) | 10.10 (1.96) | 10.10 (2.16) | 10.19 (2.12) | 0.302 |
| TPOAb-positive | 736 (11.98%) | 237 (12.19%) | 219 (10.59%) | 148 (13.58%) | 96 (11.74%) | 36 (15.56%) | 0.201 |
| TgAb-positive | 461 (7.49%) | 157 (7.51%) | 139 (7.59%) | 84 (6.55%) | 55 (6.23%) | 26 (14.32%) | 0.029 |
| Selenium (μg) | 126.55 (66.48) | 119.93 (64.46) | 126.43 (61.80) | 133.73 (69.56) | 135.23 (78.71) | 126.99 (59.29) | <0.001 |
| UIC (μg/L) | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | p-Value | OR | (95% CI) | p-Value | OR | (95% CI) | p-Value | |
| TPOAb Positive | |||||||||
| UIC < 100 | 1.17 | (0.87, 1.57) | 0.280 | 1.16 | (0.86, 1.57) | 0.314 | 1.17 | (0.85, 1.60) | 0.322 |
| 100 ≤ UIC < 200 | Ref. | Ref. | - | Ref. | Ref. | - | Ref. | Ref. | - |
| 200 ≤ UIC < 300 | 1.33 | (0.93, 1.90) | 0.119 | 1.37 | (0.95, 1.98) | 0.086 | 1.42 | (0.96, 2.08) | 0.074 |
| 300 ≤ UIC < 500 | 1.12 | (0.83, 1.51) | 0.441 | 1.14 | (0.84, 1.55) | 0.381 | 1.13 | (0.83, 1.54) | 0.431 |
| 500 ≤ UIC ≤ 800 | 1.56 | (1.05, 2.31) | 0.029 | 1.55 | (1.06, 2.28) | 0.027 | 1.57 | (1.07, 2.30) | 0.022 |
| TgAb Positive | |||||||||
| UIC < 100 | 0.99 | 0.71, 1.37 | 0.945 | 1.00 | 0.72, 1.39 | 0.988 | 1.00 | 0.70, 1.42 | 0.991 |
| 100 ≤ UIC < 200 | Ref. | Ref. | - | Ref. | Ref. | - | Ref. | Ref. | - |
| 200 ≤ UIC < 300 | 0.85 | 0.54, 1.34 | 0.486 | 0.86 | 0.55, 1.34 | 0.493 | 0.87 | 0.54, 1.38 | 0.531 |
| 300 ≤ UIC < 500 | 0.81 | 0.55, 1.18 | 0.264 | 0.81 | 0.56, 1.17 | 0.249 | 0.78 | 0.54, 1.14 | 0.189 |
| 500 ≤ UIC ≤ 800 | 2.03 | (1.14, 3.62) | 0.017 | 2.01 | (1.13, 3.57) | 0.019 | 2.00 | (1.10, 3.65) | 0.025 |
| TAI | |||||||||
| UIC < 100 | 1.11 | 0.87, 1.42 | 0.393 | 1.11 | 0.86, 1.42 | 0.421 | 1.09 | 0.84, 1.43 | 0.495 |
| 100 ≤ UIC < 200 | Ref. | Ref. | - | Ref. | Ref. | - | Ref. | Ref. | - |
| 200 ≤ UIC < 300 | 1.16 | 0.83, 1.61 | 0.370 | 1.20 | 0.86, 1.66 | 0.277 | 1.22 | 0.86, 1.73 | 0.246 |
| 300 ≤ UIC < 500 | 1.09 | 0.84, 1.42 | 0.513 | 1.11 | 0.85, 1.44 | 0.430 | 1.10 | 0.84, 1.43 | 0.484 |
| 500 ≤ UIC ≤ 800 | 1.61 | (1.07, 2.44) | 0.024 | 1.61 | (1.08, 2.42) | 0.022 | 1.62 | (1.07, 2.45) | 0.024 |
| Characteristic | Overall, N = 6612 (100%) 1 | Selenium (μg) | p-Value 2 | ||
|---|---|---|---|---|---|
| T1, N = 2204 (31%) 1 | T2, N = 2205 (33%) 1 | T3, N = 2203 (37%) 1 | |||
| Age (year) | 46 (33, 59) | 46 (33, 60) | 47 (34, 59) | 45 (32, 57) | 0.030 |
| Sex | <0.001 | ||||
| Men | 3365 (48.11%) | 695 (26.31%) | 1097 (45.85%) | 1573 (68.37%) | |
| Women | 3247 (51.89%) | 1509 (73.69%) | 1108 (54.15%) | 630 (31.63%) | |
| Race | 0.004 | ||||
| Non-Hispanic White | 3193 (71.68%) | 997 (70.55%) | 1035 (69.29%) | 1161 (74.78%) | |
| Non-Hispanic Black | 1307 (10.01%) | 491 (11.78%) | 437 (10.60%) | 379 (7.99%) | |
| Mexican American | 1046 (7.86%) | 373 (7.72%) | 360 (8.61%) | 313 (7.31%) | |
| Other Hispanic | 671 (5.08%) | 247 (5.52%) | 226 (5.40%) | 198 (4.41%) | |
| Other/multiracial | 395 (5.37%) | 96 (4.43%) | 147 (6.09%) | 152 (5.51%) | |
| Education attainment | <0.001 | ||||
| Less than 9th grade | 727 (5.56%) | 329 (7.77%) | 229 (5.35%) | 169 (3.89%) | |
| 9–11th grade | 1090 (12.15%) | 420 (14.60%) | 339 (11.38%) | 331 (10.79%) | |
| High school grad/GED | 1546 (23.92%) | 547 (25.34%) | 505 (24.35%) | 494 (22.34%) | |
| Some college or AA degree | 1837 (30.51%) | 564 (29.82%) | 644 (30.88%) | 629 (30.75%) | |
| College graduate or above | 1412 (27.87%) | 344 (22.47%) | 488 (28.04%) | 580 (32.22%) | |
| Household income | <0.001 | ||||
| ≤1.3 | 2023 (21.45%) | 802 (26.57%) | 653 (21.07%) | 568 (17.51%) | |
| 1.3~3.5 | 2497 (33.97%) | 860 (36.98%) | 839 (34.02%) | 798 (31.40%) | |
| ≥3.5 | 2092 (44.58%) | 542 (36.44%) | 713 (44.91%) | 837 (51.09%) | |
| Smoking status | <0.001 | ||||
| Current smoker | 1431 (22.40%) | 514 (26.33%) | 455 (20.91%) | 462 (20.45%) | |
| Former smoker | 1715 (24.70%) | 519 (20.69%) | 547 (25.23%) | 649 (27.58%) | |
| Non-smoker | 3466 (52.90%) | 1171 (52.98%) | 1203 (53.86%) | 1092 (51.97%) | |
| Alcohol consumption | <0.001 | ||||
| 1–5 drinks/month | 3266 (49.35%) | 1070 (49.88%) | 1088 (47.99%) | 1108 (50.12%) | |
| 5–10 drinks/month | 531 (9.66%) | 138 (7.65%) | 176 (9.20%) | 217 (11.76%) | |
| 10+ drinks/month | 1005 (18.72%) | 215 (13.10%) | 349 (19.89%) | 441 (22.37%) | |
| Non-drinker | 1809 (22.26%) | 781 (29.37%) | 592 (22.93%) | 436 (15.72%) | |
| BMI (kg/m2) | 0.327 | ||||
| Underweight (<18.5) | 101 (1.53%) | 45 (2.13%) | 23 (0.75%) | 33 (1.72%) | |
| Normal (18.5 to <25) | 1812 (30.20%) | 564 (30.17%) | 613 (30.44%) | 635 (30.01%) | |
| Overweight (25 to <30) | 2270 (33.70%) | 766 (34.14%) | 762 (33.57%) | 742 (33.45%) | |
| Obesity (30 or greater) | 2429 (34.57%) | 829 (33.56%) | 807 (35.23%) | 793 (34.82%) | |
| TSH (mIU/L) | 2.11 (3.40) | 2.12 (3.45) | 2.03 (2.31) | 2.16 (4.10) | 0.423 |
| fT4 (pmol/L) | 10.26 (2.00) | 10.33 (2.07) | 10.24 (2.02) | 10.22 (1.91) | 0.502 |
| fT3 (pg/mL) | 3.18 (0.43) | 3.16 (0.41) | 3.17 (0.49) | 3.21 (0.38) | 0.006 |
| TPOAb-positive | 73 (11.98%) | 269 (13.02%) | 249 (12.51%) | 218 (10.64%) | 0.129 |
| TgAb-positive | 46 (7.49%) | 177 (8.95%) | 157 (8.36%) | 127 (5.48%) | 0.011 |
| UIC (μg/L) | 179.93 (139.26) | 166.28 (135.83) | 182.15 (142.31) | 189.34 (138.48) | <0.001 |
| Model 1 | Model 2 | Model 3 | ||||
| β (95% CI) | p | β (95% CI) | p | β (95% CI) | p | |
| TPOAb | −0.049 (−0.092, −0.005) | 0.028 | −0.007 (−0.055, 0.040) | 0.765 | −0.025 (−0.068, 0.019) | 0.257 |
| TgAb | −0.025 (−0.050, 0.000) | 0.054 | −0.015 (−0.048, 0.017) | 0.353 | −0.018 (−0.049, 0.014) | 0.257 |
| Model 1 | Model 2 | Model 3 | ||||
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| TPOAb positive | ||||||
| T1 | Ref. | - | Ref. | - | Ref. | - |
| T2 | 0.995 (0.969, 1.021) | 0.694 | 1.009 (0.983, 1.035) | 0.489 | 0.999 (0.968, 1.032) | 0.967 |
| T3 | 0.976 (0.952, 1.002) | 0.068 | 1.009 (0.980, 1.039) | 0.543 | 0.988 (0.943, 1.035) | 0.598 |
| TgAb positive | ||||||
| T1 | Ref. | - | Ref. | - | Ref. | - |
| T2 | 0.994 (0.971, 1.018) | 0.621 | 1.000 (0.975, 1.026) | 0.975 | 1.000 (0.972, 1.030) | 0.978 |
| T3 | 0.966 (0.941, 0.992) | 0.012 | 0.981 (0.952, 1.011) | 0.206 | 0.985 (0.945, 1.027) | 0.473 |
| Model 1 | p for Interaction | Model 2 | p for Interaction | Model 3 | p for Interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |||||||
| TPOAb Positive | 0.067 * | 0.087 * | 0.097 * | |||||||||
| Selenium T1 | ||||||||||||
| UIC < 100 | 1.39 (0.85, 2.29) | 0.185 | 1.43 (0.88, 2.32) | 0.148 | 1.37 (0.85, 2.22) | 0.185 | ||||||
| 100 ≤ UIC < 200 | Ref. | - | Ref. | - | Ref. | - | ||||||
| 200 ≤ UIC < 300 | 1.83 (1.01, 3.32) | 0.046 | 1.88 (1.02, 3.47) | 0.043 | 1.91 (1.01, 3.61) | 0.046 | ||||||
| 300 ≤ UIC < 500 | 0.60 (0.36, 1.00) | 0.049 | 0.62 (0.36, 1.07) | 0.086 | 0.60 (0.34, 1.04) | 0.065 | ||||||
| 500 ≤ UIC < 800 | 1.12 (0.42, 3.02) | 0.817 | 1.13 (0.42, 3.00) | 0.808 | 1.17 (0.44, 3.14) | 0.745 | ||||||
| Selenium T2 | ||||||||||||
| UIC < 100 | 1.14 (0.72, 1.80) | 0.558 | 1.10 (0.70, 1.74) | 0.665 | 1.09 (0.68, 1.74) | 0.706 | ||||||
| 100 ≤ UIC < 200 | Ref. | - | Ref. | - | Ref. | - | ||||||
| 200 ≤ UIC < 300 | 1.09 (0.70, 1.70) | 0.693 | 1.13 (0.74, 1.72) | 0.566 | 1.16 (0.75, 1.79) | 0.479 | ||||||
| 300 ≤ UIC < 500 | 1.90 (1.17, 3.06) | 0.010 | 1.93 (1.18, 3.15) | 0.010 | 1.82 (1.11, 2.97) | 0.019 | ||||||
| 500 ≤ UIC < 800 | 2.45 (1.29, 4.64) | 0.007 | 2.45 (1.30, 4.60) | 0.006 | 2.60 (1.46, 4.63) | 0.002 | ||||||
| Selenium T3 | ||||||||||||
| UIC < 100 | 0.96 (0.61, 1.51) | 0.848 | 1.03 (0.64, 1.66) | 0.910 | 1.12 (0.70, 1.79) | 0.635 | ||||||
| 100 ≤ UIC < 200 | Ref. | - | Ref. | - | Ref. | - | ||||||
| 200 ≤ UIC < 300 | 1.20 (0.70, 2.06 | 0.507 | 1.25 (0.72, 2.19) | 0.418 | 1.33 (0.74, 2.41) | 0.329 | ||||||
| 300 ≤ UIC < 500 | 0.96 (0.62, 1.48) | 0.842 | 0.96 (0.59, 1.55) | 0.855 | 0.96 (0.59, 1.58) | 0.877 | ||||||
| 500 ≤ UIC < 800 | 1.12 (0.48, 2.60) | 0.782 | 1.11 (0.46, 2.67) | 0.817 | 1.13 (0.47, 2.68) | 0.780 | ||||||
| TgAb Positive | 0.335 | 0.352 | 0.340 | |||||||||
| TAI | 0.235 | 0.264 | 0.283 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Wang, H.; Teng, W.; Shan, Z. The Relationships among the Urinary Iodine Concentration, Selenium Intake, and Thyroid Antibodies in Adults, Including the Interaction between Iodine and Selenium: National Health and Nutrition Examination Survey 2007–2012. Nutrients 2024, 16, 3443. https://doi.org/10.3390/nu16203443
Zhang C, Wang H, Teng W, Shan Z. The Relationships among the Urinary Iodine Concentration, Selenium Intake, and Thyroid Antibodies in Adults, Including the Interaction between Iodine and Selenium: National Health and Nutrition Examination Survey 2007–2012. Nutrients. 2024; 16(20):3443. https://doi.org/10.3390/nu16203443
Chicago/Turabian StyleZhang, Chenyu, Haoyu Wang, Weiping Teng, and Zhongyan Shan. 2024. "The Relationships among the Urinary Iodine Concentration, Selenium Intake, and Thyroid Antibodies in Adults, Including the Interaction between Iodine and Selenium: National Health and Nutrition Examination Survey 2007–2012" Nutrients 16, no. 20: 3443. https://doi.org/10.3390/nu16203443
APA StyleZhang, C., Wang, H., Teng, W., & Shan, Z. (2024). The Relationships among the Urinary Iodine Concentration, Selenium Intake, and Thyroid Antibodies in Adults, Including the Interaction between Iodine and Selenium: National Health and Nutrition Examination Survey 2007–2012. Nutrients, 16(20), 3443. https://doi.org/10.3390/nu16203443






