Caffeine Intake Alters Recovery Sleep after Sleep Deprivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Inclusion and Exclusion Criteria
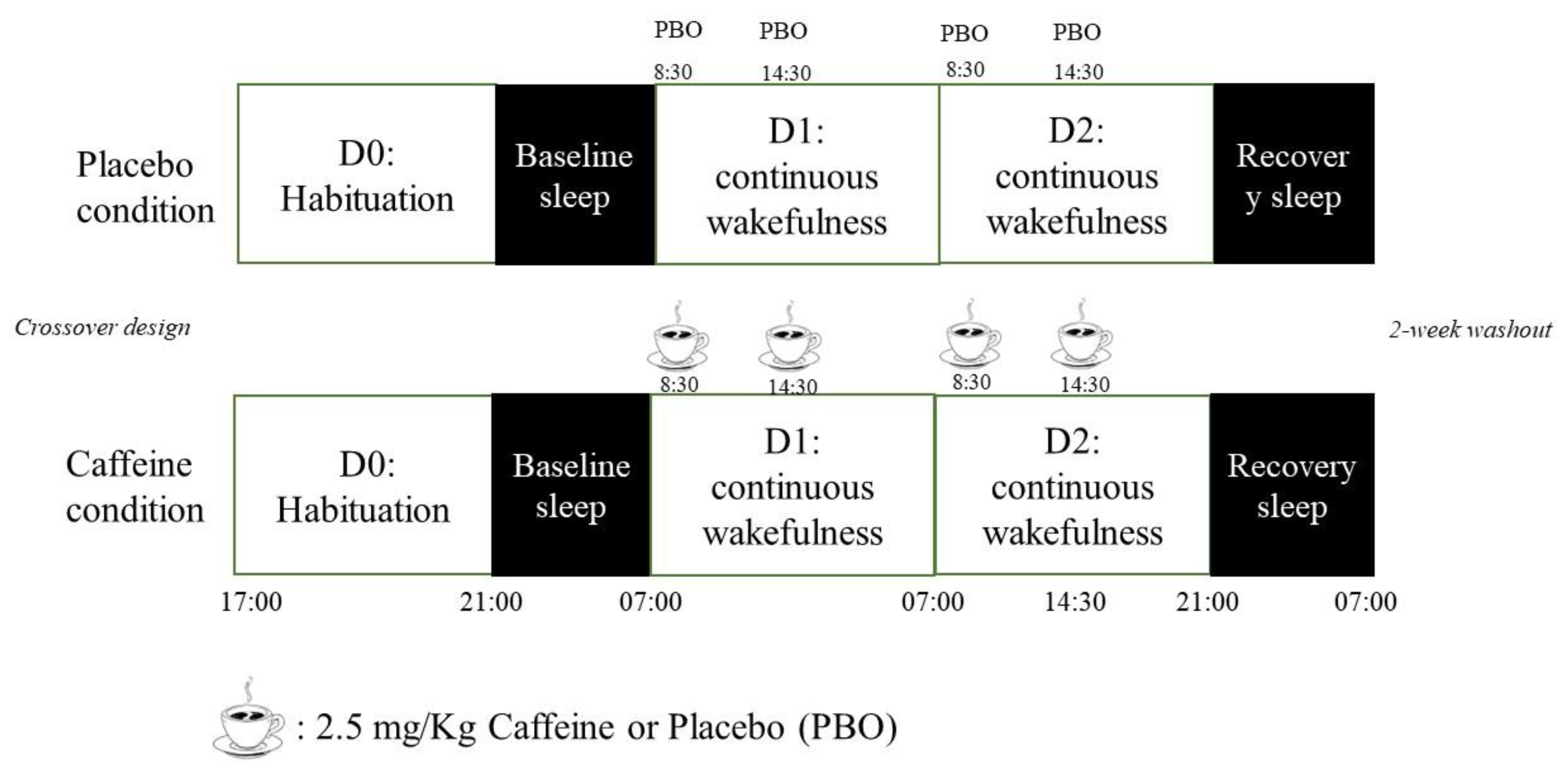
2.3. Sleep Deprivation Session
2.4. Experimental Protocol
2.5. Sleep Recording and Analysis
- Sleep continuity/fragmentation: total awakening frequency per hour of AST; frequency of brief (<2 min) and long (>2 min) awakenings per hour of AST; frequency of awakenings from N1, N2, N3, and REM per minute of that stage.
- Sleep stability: number and average duration of N1, N2, N3, and REM sleep periods not interrupted by a wake period longer than 2 min, frequency of stage transitions (stage transitions were defined as all transitions from one stage to another, including all those to and from wakefulness) calculated per minute of each stage.
- Sleep intensity: EEG power spectral analysis was performed for each EEG frequency band: slow oscillation (0.5–1 Hz), delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), sigma (12–15 Hz), and beta (15–20 Hz). For the present analysis, data derived from the frontal EEG electrode (i.e., Fp1-M1) in NREM stages were used. Artifacts due to electrocardiogram interference were removed using a template subtraction method. Manual visual adjudication was performed by a researcher who was blinded to the subject group, and spectral data with significant artifacts were excluded manually. In accordance with Welch’s method, the spectral power density was calculated using 10 overlapping 4 s sub-epochs for each 30 s epoch, with a 50% tapered cosine window [6].
2.6. Statistical Analysis
3. Results
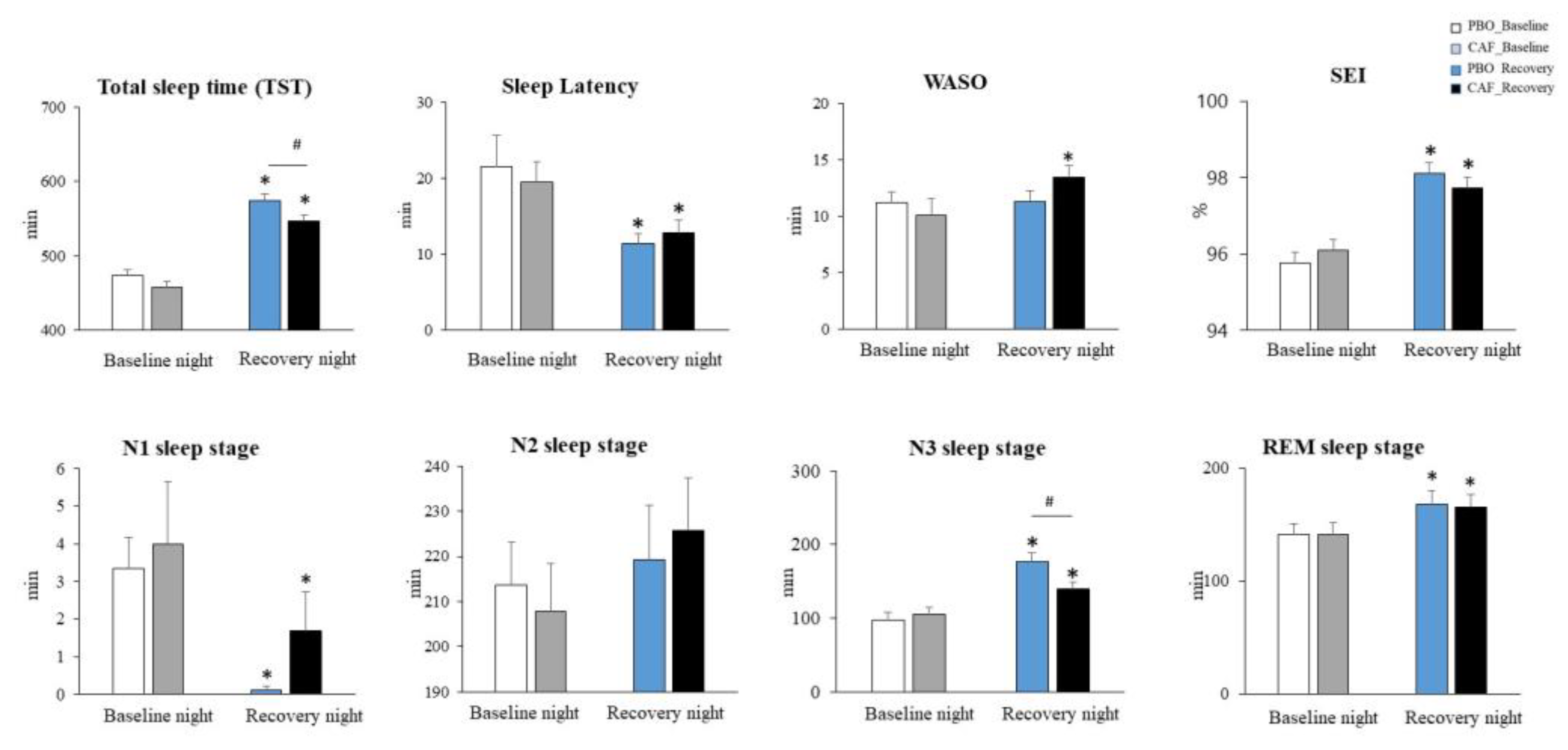
3.1. Sleep Macrostructure Parameters
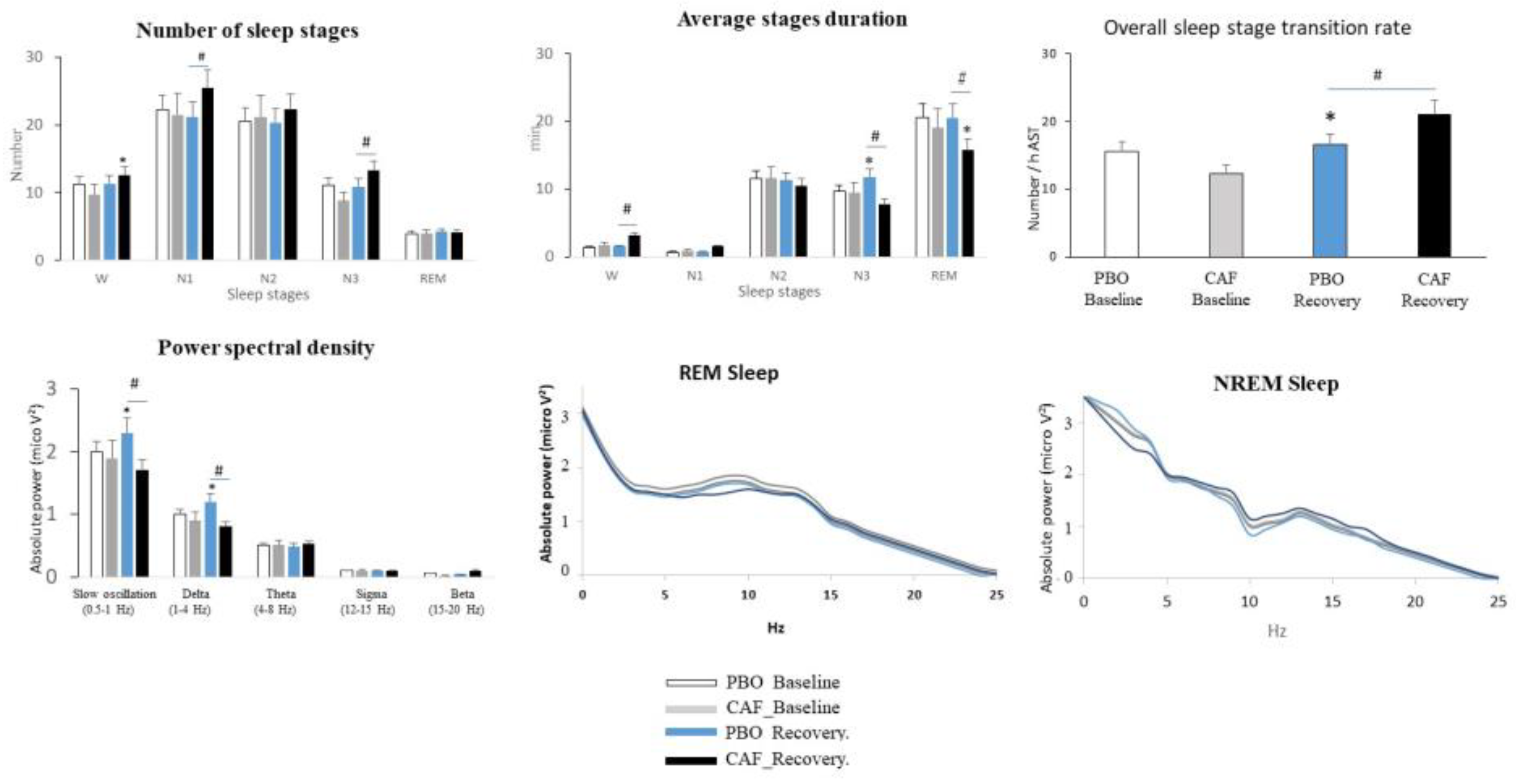
3.2. Sleep Continuity/Fragmentation, Stability, and Organization and EEG Power Density
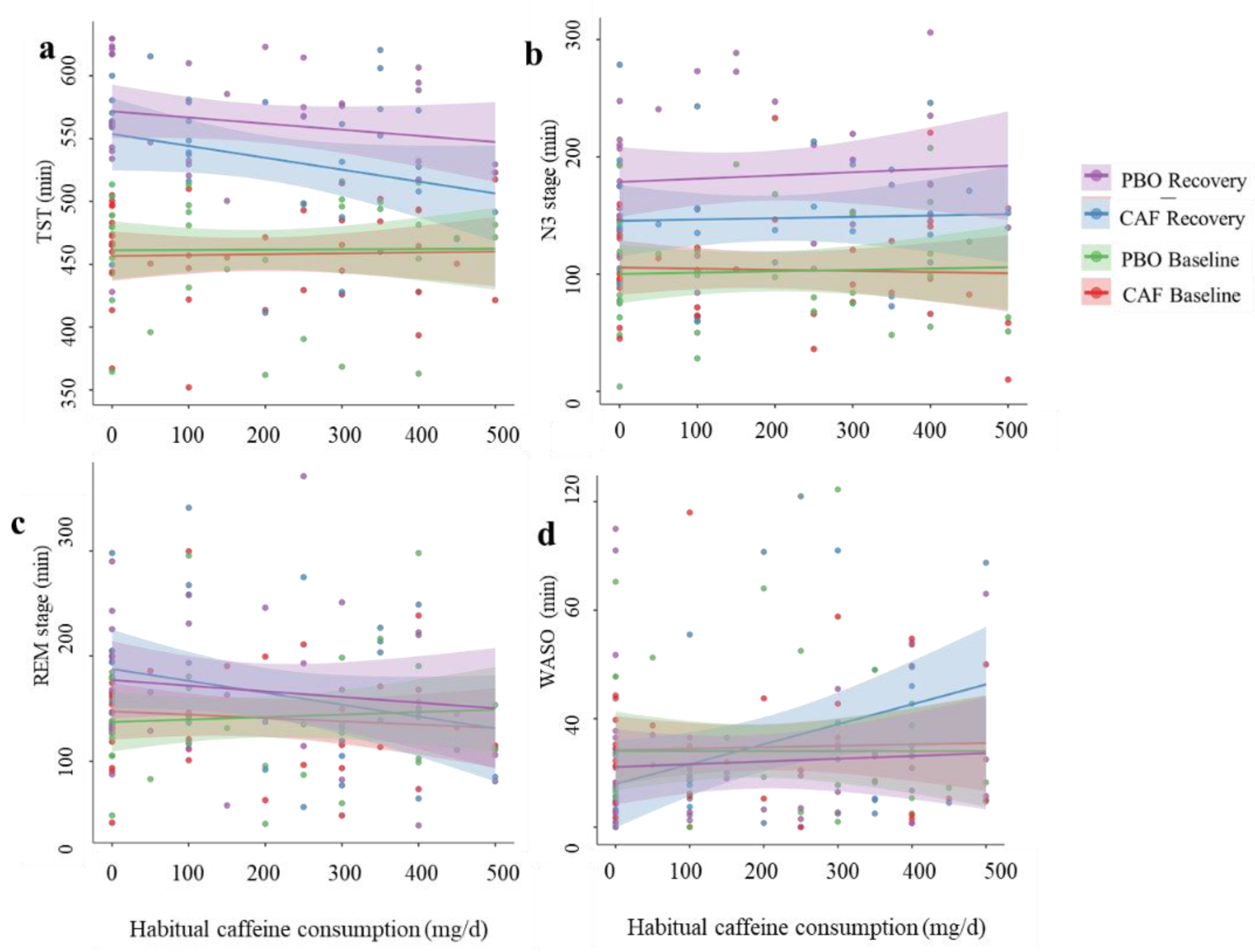
3.3. Correlations between Sleep Parameters and Daily Habitual Caffeine Consumption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alterman, T.; Luckhaupt, S.E.; Dahlhamer, J.M.; Ward, B.W.; Calvert, G.M. Prevalence Rates of Work Organization Characteristics among Workers in the US: Data from the 2010 National Health Interview Survey. Am. J. Ind. Med. 2013, 56, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Martin, D.; Nagaria, Z.; Verceles, A.C.; Jobe, S.L.; Wickwire, E.M. Mental Health Consequences of Shift Work: An Updated Review. Curr. Psychiatry Rep. 2020, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Gander, P.H.; Marshall, N.S.; Harris, R.B.; Reid, P. Sleep, Sleepiness and Motor Vehicle Accidents: A National Survey. Aust. New Zealand J. Public Health 2005, 29, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Li, M.; Ning, Q.; Ma, N. One Night of 10-h Sleep Restores Vigilance after Total Sleep Deprivation: The Role of Delta and Theta Power during Recovery Sleep. Sleep Biol. Rhythm. 2023, 21, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Tharion, W.J.; Shukitt-Hale, B.; Speckman, K.L.; Tulley, R. Effects of Caffeine, Sleep Loss, and Stress on Cognitive Performance and Mood during US Navy SEAL Training. Psychopharmacology 2002, 164, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Quiquempoix, M.; Drogou, C.; Erblang, M.; Van Beers, P.; Guillard, M.; Tardo-Dino, P.-E.; Rabat, A.; Léger, D.; Chennaoui, M.; Gomez-Merino, D.; et al. Relationship between Habitual Caffeine Consumption, Attentional Performance, and Individual Alpha Frequency during Total Sleep Deprivation. Int. J. Environ. Res. Public Health 2023, 20, 4971. [Google Scholar] [CrossRef]
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef]
- Quiquempoix, M.; Sauvet, F.; Erblang, M.; Van Beers, P.; Guillard, M.; Drogou, C.; Trignol, A.; Vergez, A.; Léger, D.; Chennaoui, M.; et al. Effects of Caffeine Intake on Cognitive Performance Related to Total Sleep Deprivation and Time on Task: A Randomized Cross-Over Double-Blind Study. Nat. Sci. Sleep 2022, 14, 457–473. [Google Scholar] [CrossRef]
- Rétey, J.V.; Adam, M.; Gottselig, J.M.; Khatami, R.; Dürr, R.; Achermann, P.; Landolt, H.-P. Adenosinergic Mechanisms Contribute to Individual Differences in Sleep Deprivation-Induced Changes in Neurobehavioral Function and Brain Rhythmic Activity. J. Neurosci. 2006, 26, 10472–10479. [Google Scholar] [CrossRef]
- Killgore, W.; Kahn-Greene, E.T.; Grugle, N.L.; Killgore, D.B.; Balkin, T.J. Sustaining Executive Functions during Sleep Deprivation: A Comparison of Caffeine, Dextroamphetamine, and Modafinil. Sleep 2009, 32, 205–216. [Google Scholar] [CrossRef]
- Hansen, D.A.; Ramakrishnan, S.; Satterfield, B.C.; Wesensten, N.J.; Layton, M.E.; Reifman, J.; Van Dongen, H.P. Randomized, Double-Blind, Placebo-Controlled, Crossover Study of the Effects of Repeated-Dose Caffeine on Neurobehavioral Performance during 48 h of Total Sleep Deprivation. Psychopharmacology 2019, 236, 1313–1322. [Google Scholar] [CrossRef]
- Roehrs, T.; Roth, T. Caffeine: Sleep and Daytime Sleepiness. Sleep Med. Rev. 2008, 12, 153–162. [Google Scholar] [CrossRef]
- Karacan, I.; Thornby, J.I.; Anch, A.M.; Booth, G.H.; Williams, R.L.; Salis, P.J. Dose-Related Sleep Disturbances Induced by Coffee and Caffeine. Clin. Pharmacol. Ther. 1976, 20, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.H.; Arand, D. Caffeine Use as a Model of Acute and Chronic Insomnia. Sleep 1992, 15, 526–536. [Google Scholar] [PubMed]
- Gardiner, C.; Weakley, J.; Burke, L.M.; Roach, G.D.; Sargent, C.; Maniar, N.; Townshend, A.; Halson, S.L. The Effect of Caffeine on Subsequent Sleep: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2023, 69, 101764. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.; Roehrs, T.; Shambroom, J.; Roth, T. Caffeine Effects on Sleep Taken 0, 3, or 6 Hours before Going to Bed. J. Clin. Sleep Med. 2013, 9, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Landolt, H.P.; Dijk, D.-J.; Gaus, S.E.; Borbély, A.A. Caffeine Reduces Low-Frequency Delta Activity in the Human Sleep EEG. Neuropsychopharmacology 1995, 12, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Landolt, H.-P.; Rétey, J.V.; Tönz, K.; Gottselig, J.M.; Khatami, R.; Buckelmüller, I.; Achermann, P. Caffeine Attenuates Waking and Sleep Electroencephalographic Markers of Sleep Homeostasis in Humans. Neuropsychopharmacology 2004, 29, 1933–1939. [Google Scholar] [CrossRef]
- Wesensten, N.J.; Killgore, W.D.; Balkin, T.J. Performance and Alertness Effects of Caffeine, Dextroamphetamine, and Modafinil during Sleep Deprivation. J. Sleep Res. 2005, 14, 255–266. [Google Scholar] [CrossRef]
- Beaumont, M.; Batéjat, D.; Coste, O.; Doireau, P.; Chauffard, F.; Enslen, M.; Lagarde, D.; Pierard, C. Recovery after Prolonged Sleep Deprivation: Residual Effects of Slow-Release Caffeine on Recovery Sleep, Sleepiness and Cognitive Functions. Neuropsychobiology 2005, 51, 16–27. [Google Scholar] [CrossRef]
- Bonnet, M.H.; Arand, D.L. Metabolic Rate and the Restorative Function of Sleep. Physiol. Behav. 1996, 59, 777–782. [Google Scholar] [CrossRef] [PubMed]
- Carrier, J.; Fernandez-Bolanos, M.; Robillard, R.; Dumont, M.; Paquet, J.; Selmaoui, B.; Filipini, D. Effects of Caffeine Are More Marked on Daytime Recovery Sleep than on Nocturnal Sleep. Neuropsychopharmacology 2007, 32, 964–972. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Smith, B.J.; Wright Jr, K.P. Effects of Caffeine on Skin and Core Temperatures, Alertness, and Recovery Sleep during Circadian Misalignment. J. Biol. Rhythm. 2014, 29, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Reissig, C.J.; Strain, E.C.; Griffiths, R.R. Caffeinated Energy Drinks—A Growing Problem. Drug Alcohol Depend. 2009, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Weibel, J.; Lin, Y.-S.; Landolt, H.-P.; Berthomier, C.; Brandewinder, M.; Kistler, J.; Rehm, S.; Rentsch, K.M.; Meyer, M.; Borgwardt, S.; et al. Regular Caffeine Intake Delays REM Sleep Promotion and Attenuates Sleep Quality in Healthy Men. J. Biol. Rhythm. 2021, 36, 384–394. [Google Scholar] [CrossRef]
- Lunsford-Avery, J.R.; Kollins, S.H.; Kansagra, S.; Wang, K.W.; Engelhard, M.M. Impact of Daily Caffeine Intake and Timing on Electroencephalogram-Measured Sleep in Adolescents. J. Clin. Sleep Med. 2022, 18, 877–884. [Google Scholar] [CrossRef]
- Weibel, J.; Lin, Y.-S.; Landolt, H.-P.; Garbazza, C.; Kolodyazhniy, V.; Kistler, J.; Rehm, S.; Rentsch, K.; Borgwardt, S.; Cajochen, C.; et al. Caffeine-Dependent Changes of Sleep-Wake Regulation: Evidence for Adaptation after Repeated Intake. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 99, 109851. [Google Scholar] [CrossRef]
- Corti, R.; Binggeli, C.; Sudano, I.; Spieker, L.; Hänseler, E.; Ruschitzka, F.; Chaplin, W.F.; Lüscher, T.F.; Noll, G. Coffee Acutely Increases Sympathetic Nerve Activity and Blood Pressure Independently of Caffeine Content: Role of Habitual versus Nonhabitual Drinking. Circulation 2002, 106, 2935–2940. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds III, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Horne, J.A.; Ostberg, O. A Self-Assessment Questionnaire to Determine Morningness-Eveningness in Human Circadian Rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar] [PubMed]
- Hughes, J.R.; Higgins, S.T.; Bickel, W.K.; Hunt, W.K.; Fenwick, J.W.; Gulliver, S.B.; Mireault, G.C. Caffeine Self-Administration, Withdrawal, and Adverse Effects among Coffee Drinkers. Arch. Gen. Psychiatry 1991, 48, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Erblang, M.; Drogou, C.; Gomez-Merino, D.; Metlaine, A.; Boland, A.; Deleuze, J.F.; Thomas, C.; Sauvet, F.; Chennaoui, M. The Impact of Genetic Variations in ADORA2A in the Association between Caffeine Consumption and Sleep. Genes 2019, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Ágoston, C.; Urbán, R.; Király, O.; Griffiths, M.D.; Rogers, P.J.; Demetrovics, Z. Why Do You Drink Caffeine? The Development of the Motives for Caffeine Consumption Questionnaire (MCCQ) and Its Relationship with Gender, Age and the Types of Caffeinated Beverages. Int. J. Ment. Health Addict. 2018, 16, 981–999. [Google Scholar] [CrossRef]
- Mayo Clinic Beverage Caffeine Contents. Available online: https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/caffeine/art-20049372 (accessed on 1 June 2022).
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A Review of Caffeine’s Effects on Cognitive, Physical and Occupational Performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef]
- Arnal, P.J.; Thorey, V.; Debellemaniere, E.; Ballard, M.E.; Bou Hernandez, A.; Guillot, A.; Jourde, H.; Harris, M.; Guillard, M.; Van Beers, P.; et al. The Dreem Headband Compared to Polysomnography for Electroencephalographic Signal Acquisition and Sleep Staging. Sleep 2020, 43, zsaa097. [Google Scholar] [CrossRef]
- Conte, F.; Cerasuolo, M.; Fusco, G.; Giganti, F.; Inserra, I.; Malloggi, S.; Di Iorio, I.; Ficca, G. Sleep Continuity, Stability and Organization in Good and Bad Sleepers. J. Health Psychol. 2021, 26, 2131–2142. [Google Scholar] [CrossRef]
- Ficca, G.; Salzarulo, P. What in Sleep Is for Memory. Sleep Med. 2004, 5, 225–230. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- LaJambe, C.M.; Kamimori, G.H.; Belenky, G.; Balkin, T.J. Caffeine Effects on Recovery Sleep Following 27 h Total Sleep Deprivation. Aviat. Space Environ. Med. 2005, 76, 108–113. [Google Scholar] [PubMed]
- Muehlbach, M.J.; Walsh, J.K. The Effects of Caffeine on Simulated Night-Shift Work and Subsequent Daytime Sleep. Sleep 1995, 18, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ortuno, M.; Moore, N.; Taillard, J.; Valtat, C.; Leger, D.; Bioulac, B.; Philip, P. Sleep Duration and Caffeine Consumption in a French Middle-Aged Working Population. Sleep Med. 2005, 6, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Johnson-Greene, D.; Fatis, M.; Sonnek, D.; Shawchuck, C. A Survey of Caffeine Use and Associated Side Effects in a College Population. J. Drug Educ. 1988, 18, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Léger, D.; Debellemaniere, E.; Rabat, A.; Bayon, V.; Benchenane, K.; Chennaoui, M. Slow-Wave Sleep: From the Cell to the Clinic. Sleep Med. Rev. 2018, 41, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Erblang, M.; Sauvet, F.; Drogou, C.; Quiquempoix, M.; Van Beers, P.; Guillard, M.; Rabat, A.; Trignol, A.; Bourrilhon, C.; Erkel, M.-C.; et al. Genetic Determinants of Neurobehavioral Responses to Caffeine Administration during Sleep Deprivation: A Randomized, Cross over Study (NCT03859882). Genes 2021, 12, 555. [Google Scholar] [CrossRef]
- Doty, T.J.; So, C.J.; Bergman, E.M.; Trach, S.K.; Ratcliffe, R.H.; Yarnell, A.M.; Capaldi, V.F.; Moon, J.E.; Balkin, T.J.; Quartana, P.J. Limited Efficacy of Caffeine and Recovery Costs during and Following 5 Days of Chronic Sleep Restriction. Sleep 2017, 40, zsx171. [Google Scholar] [CrossRef]
- Conte, F.; Malloggi, S.; De Rosa, O.; Di Iorio, I.; Romano, F.; Giganti, F.; Ficca, G. Sleep Continuity, Stability and Cyclic Organization Are Impaired in Insomniacs: A Case–Control Study. Int. J. Environ. Res. Public Health 2023, 20, 1240. [Google Scholar] [CrossRef]
- Strauss, M.; Griffon, L.; Van Beers, P.; Elbaz, M.; Bouziotis, J.; Sauvet, F.; Chennaoui, M.; Léger, D.; Peigneux, P. Order Matters: Sleep Spindles Contribute to Memory Consolidation Only When Followed by Rapid-Eye-Movement Sleep. Sleep 2022, 45, zsac022. [Google Scholar] [CrossRef]
- Arnal, P.J.; Sauvet, F.; Leger, D.; Van Beers, P.; Bayon, V.; Bougard, C.; Rabat, A.; Millet, G.Y.; Chennaoui, M. Benefits of Sleep Extension on Sustained Attention and Sleep Pressure before and during Total Sleep Deprivation and Recovery. Sleep 2015, 38, 1935–1943. [Google Scholar] [CrossRef]
- Mander, B.A.; Reid, K.J.; Baron, K.G.; Tjoa, T.; Parrish, T.B.; Paller, K.A.; Gitelman, D.R.; Zee, P.C. EEG Measures Index Neural and Cognitive Recovery from Sleep Deprivation. J. Neurosci. 2010, 30, 2686–2693. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Chai, Y.; Guo, B.; Quan, P.; Rao, H. Sleep Architecture and Sleep EEG Alterations Are Associated with Impaired Cognition Under Sleep Restriction. Nat. Sci. Sleep 2023, 15, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, G.; Gori, S.; Formicola, G.; Gneri, C.; Massetani, R.; Murri, L.; Salzarulo, P. Word Recall Correlates with Sleep Cycles in Elderly Subjects. J. Sleep Res. 1999, 8, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Vyazovskiy, V.V.; Delogu, A. NREM and REM Sleep: Complementary Roles in Recovery after Wakefulness. Neurosci. 2014, 20, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Djonlagic, I.; Saboisky, J.; Carusona, A.; Stickgold, R.; Malhotra, A. Increased Sleep Fragmentation Leads to Impaired Off-Line Consolidation of Motor Memories in Humans. PLoS ONE 2012, 7, e34106. [Google Scholar] [CrossRef]
- Aepli, A.; Kurth, S.; Tesler, N.; Jenni, O.G.; Huber, R. Caffeine Consuming Children and Adolescents Show Altered Sleep Behavior and Deep Sleep. Brain Sci. 2015, 5, 441–455. [Google Scholar] [CrossRef]
- Boulenger, J.-P.; Patel, J.; Post, R.M.; Parma, A.M.; Marangos, P.J. Chronic Caffeine Consumption Increases the Number of Brain Adenosine Receptors. Life Sci. 1983, 32, 1135–1142. [Google Scholar] [CrossRef]
- Jacobson, K.A.; von Lubitz, D.K.; Daly, J.W.; Fredholm, B.B. Adenosine Receptor Ligands: Differences with Acute versus Chronic Treatment. Trends Pharmacol. Sci. 1996, 17, 108–113. [Google Scholar] [CrossRef]
| PBO Baseline | CAF Baseline | PBO Recovery | CAF Recovery | |
|---|---|---|---|---|
| Sleep continuity | ||||
| Awakening (/h·AST) | ||||
| Brief (<2 min) | 3.08 ± 1.23 | 3.12 ± 1.22 | 3.21 ± 1.26 | 4.13 ± 2.22 |
| Long (>2 min) | 0.18 ± 0.11 | 0.20 ± 0.18 | 0.16 ± 0.20 | 0.82 ± 0.20 *# |
| Awakening frequency a | ||||
| From N1 (/min) | 0.12 ± 0.07 | 0.13 ± 0.05 | 0.05 ± 0.06 * | 0.13 ± 0.06 # |
| From N2 (/min) | 0.41 ± 0.03 | 0.41 ± 0.02 | 0.36 ± 0.02 * | 0.48 ± 0.02 # |
| From N3 (/min) | 0.03 ± 0.03 | 0.03 ± 0.04 | 0.02 ± 0.06 | 0.04 ± 0.05 |
| From REM (/min) | 0.04 ± 0.03 | 0.03 ± 0.06 | 0.03 ± 0.02 | 0.03 ± 0.02 |
| Sleep stability, Arousal frequency a | ||||
| N2 to N1 (/min) | 0.13 ± 0.07 | 0.11 ± 0.04 | 0.08 ± 0.08 * | 0.11 ± 0.08 |
| N3 to N2 (/min) | 0.39 ± 0.23 | 0.27 ± 0.22 | 0.25 ± 0.22 | 0.31 ± 0.28 |
| N3 to N1 (/min) | 0.03 ± 0.05 | 0.03 ± 0.04 | 0.01 ± 0.04 * | 0.03 ± 0.03 |
| REM to N1 (/min) | 0.02 ± 0.01 | 0.02 ± 0.03 | 0.04 ± 0.01 | 0.03 ± 0.02 |
| Sleep organization | ||||
| Complete sleep cycles (n) | 0.91 ± 0.81 | 1.12 ± 1.01 | 2.21 ± 1.71 * | 1.22 ± 1.21 |
| Cycle duration (min) | 30.11 ± 22.61 | 32.61 ± 21.10 | 42.41 ± 26.11 | 32.21 ± 20.11 |
| Cycle duration (%AST) | 12.21 ± 14.11 | 12.54 ± 15.11 | 31.12 ± 20.61 * | 13.22 ± 19.11 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauchon, B.; Beauchamps, V.; Gomez-Mérino, D.; Erblang, M.; Drogou, C.; Beers, P.V.; Guillard, M.; Quiquempoix, M.; Léger, D.; Chennaoui, M.; et al. Caffeine Intake Alters Recovery Sleep after Sleep Deprivation. Nutrients 2024, 16, 3442. https://doi.org/10.3390/nu16203442
Pauchon B, Beauchamps V, Gomez-Mérino D, Erblang M, Drogou C, Beers PV, Guillard M, Quiquempoix M, Léger D, Chennaoui M, et al. Caffeine Intake Alters Recovery Sleep after Sleep Deprivation. Nutrients. 2024; 16(20):3442. https://doi.org/10.3390/nu16203442
Chicago/Turabian StylePauchon, Benoit, Vincent Beauchamps, Danielle Gomez-Mérino, Mégane Erblang, Catherine Drogou, Pascal Van Beers, Mathias Guillard, Michaël Quiquempoix, Damien Léger, Mounir Chennaoui, and et al. 2024. "Caffeine Intake Alters Recovery Sleep after Sleep Deprivation" Nutrients 16, no. 20: 3442. https://doi.org/10.3390/nu16203442
APA StylePauchon, B., Beauchamps, V., Gomez-Mérino, D., Erblang, M., Drogou, C., Beers, P. V., Guillard, M., Quiquempoix, M., Léger, D., Chennaoui, M., & Sauvet, F. (2024). Caffeine Intake Alters Recovery Sleep after Sleep Deprivation. Nutrients, 16(20), 3442. https://doi.org/10.3390/nu16203442







