The Garlic Compound, Diallyl Trisulfide, Attenuates Benzo[a]Pyrene-Induced Precancerous Effect through Its Antioxidant Effect, AhR Inhibition, and Increased DNA Repair in Human Breast Epithelial Cells
Abstract
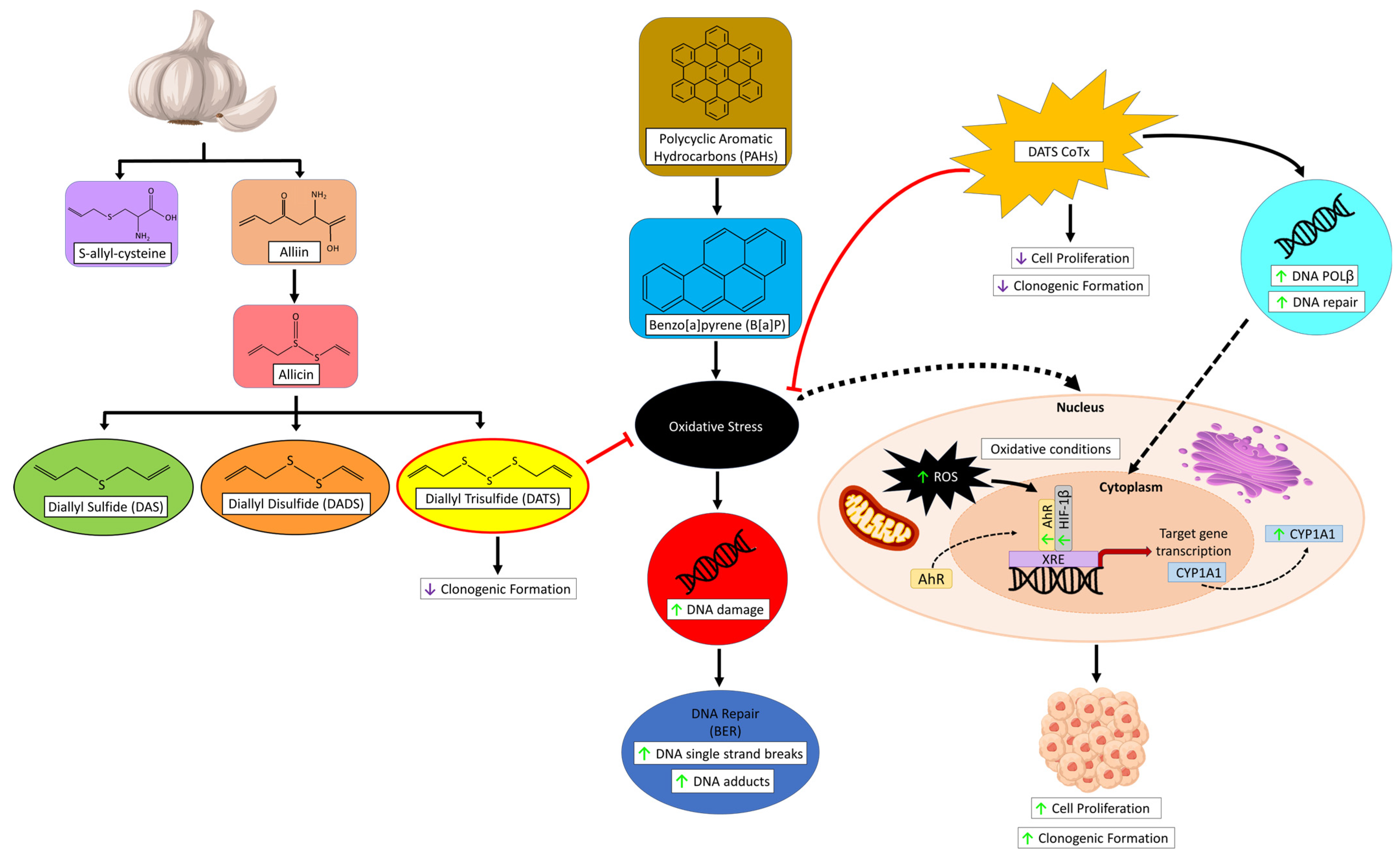
1. Introduction
2. Materials and Methods
2.1. The Cell Line, Chemicals, and Reagents
2.2. Cell Culture
2.3. Cell Treatments
2.4. Cell Harvesting
2.5. Determination of Cell Viability
2.6. Bromodeoxyuridine (BrdU) Cell Proliferation Assay
2.7. Clonogenic Formation Assay
2.8. ROS Detection Assay
2.9. 8-Hydroxy-2-Deoxyguanosine (8-OHdG) Detection
2.10. Western Blotting
2.11. ProteinSimple (Wes) Capillary Electrophoresis Western Analysis
2.12. Statistical Analysis
3. Results
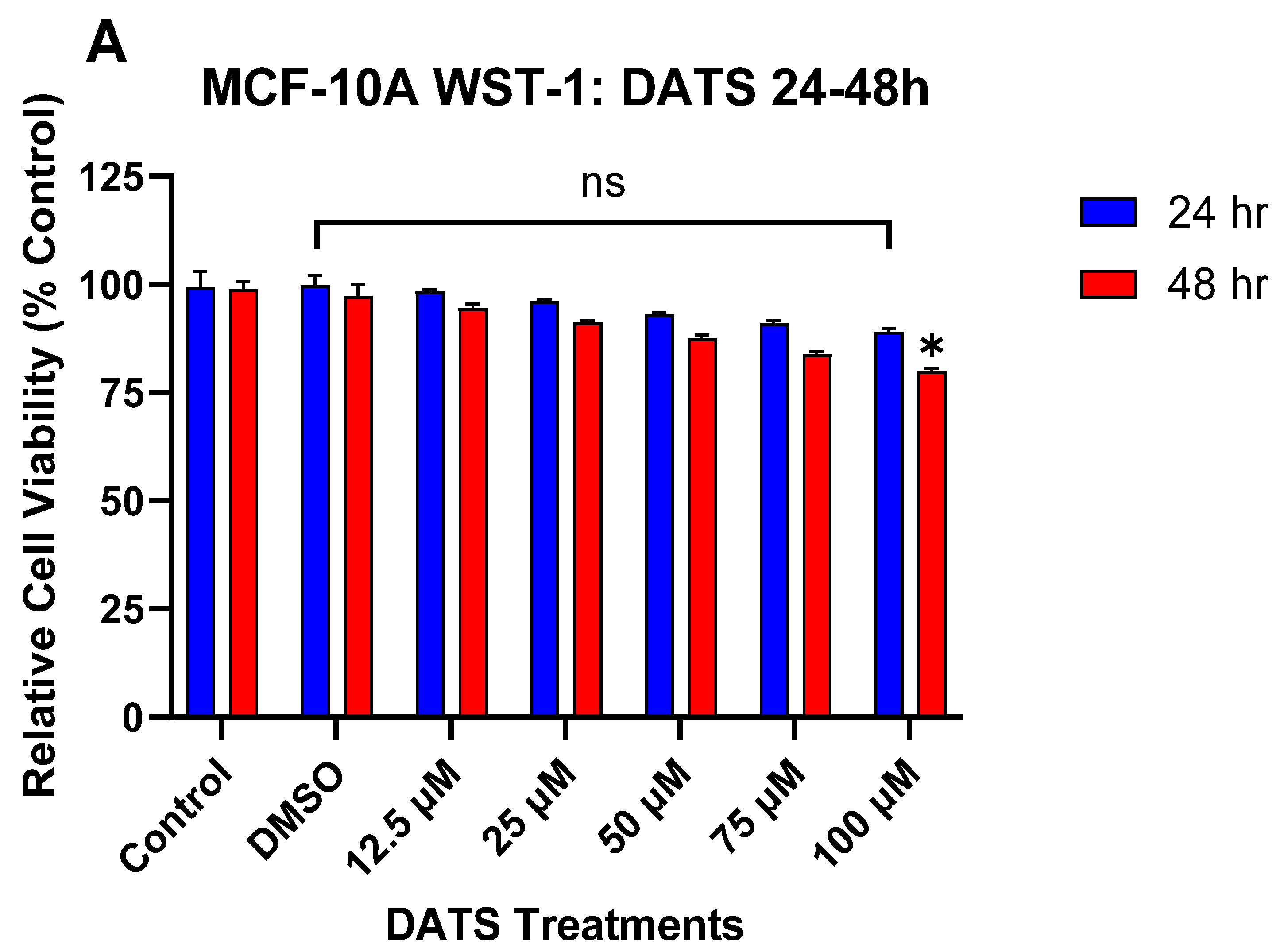
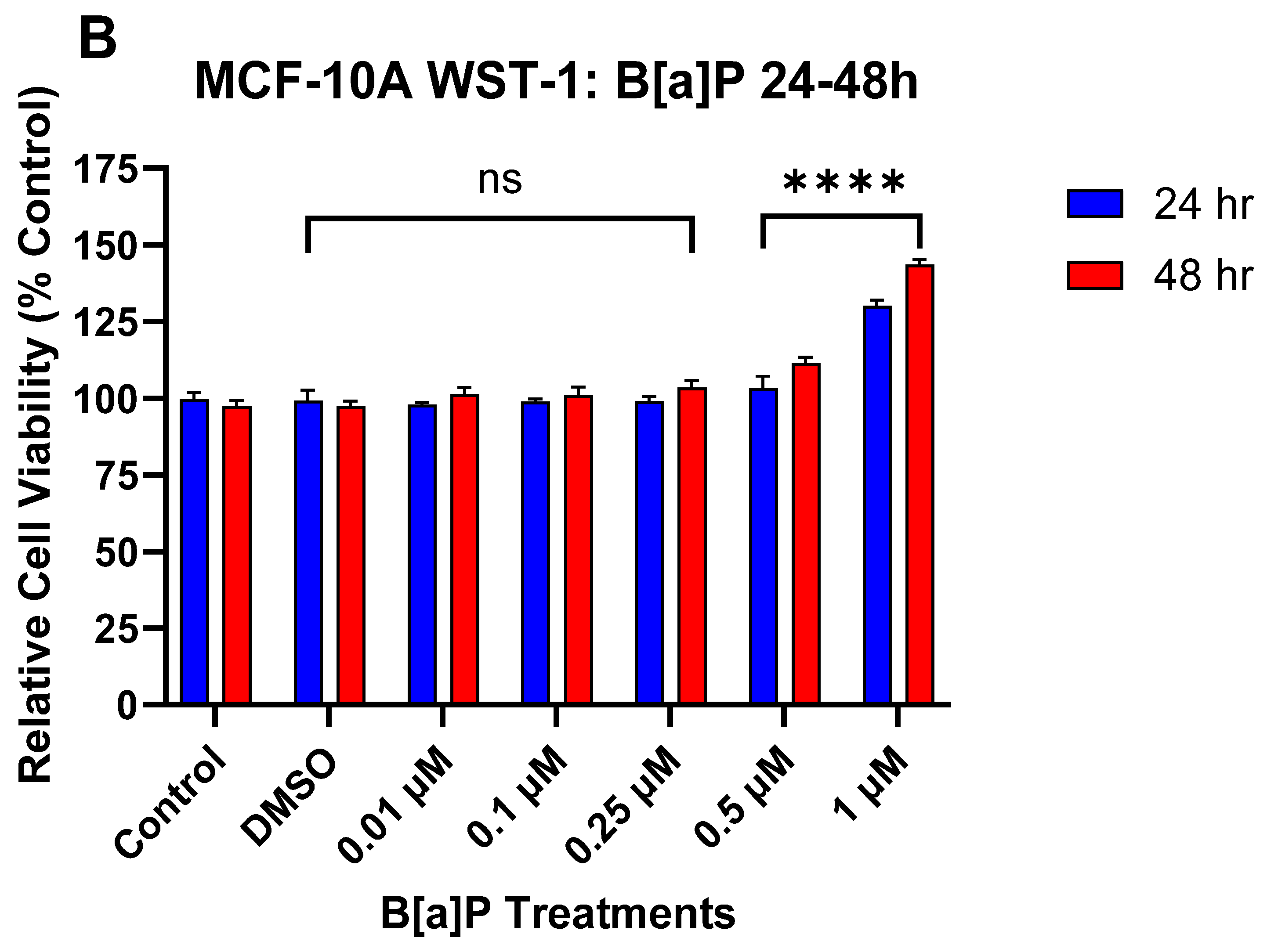
3.1. The Effect of DATS and B[a]P on Cell Viability in MCF-10A Cells
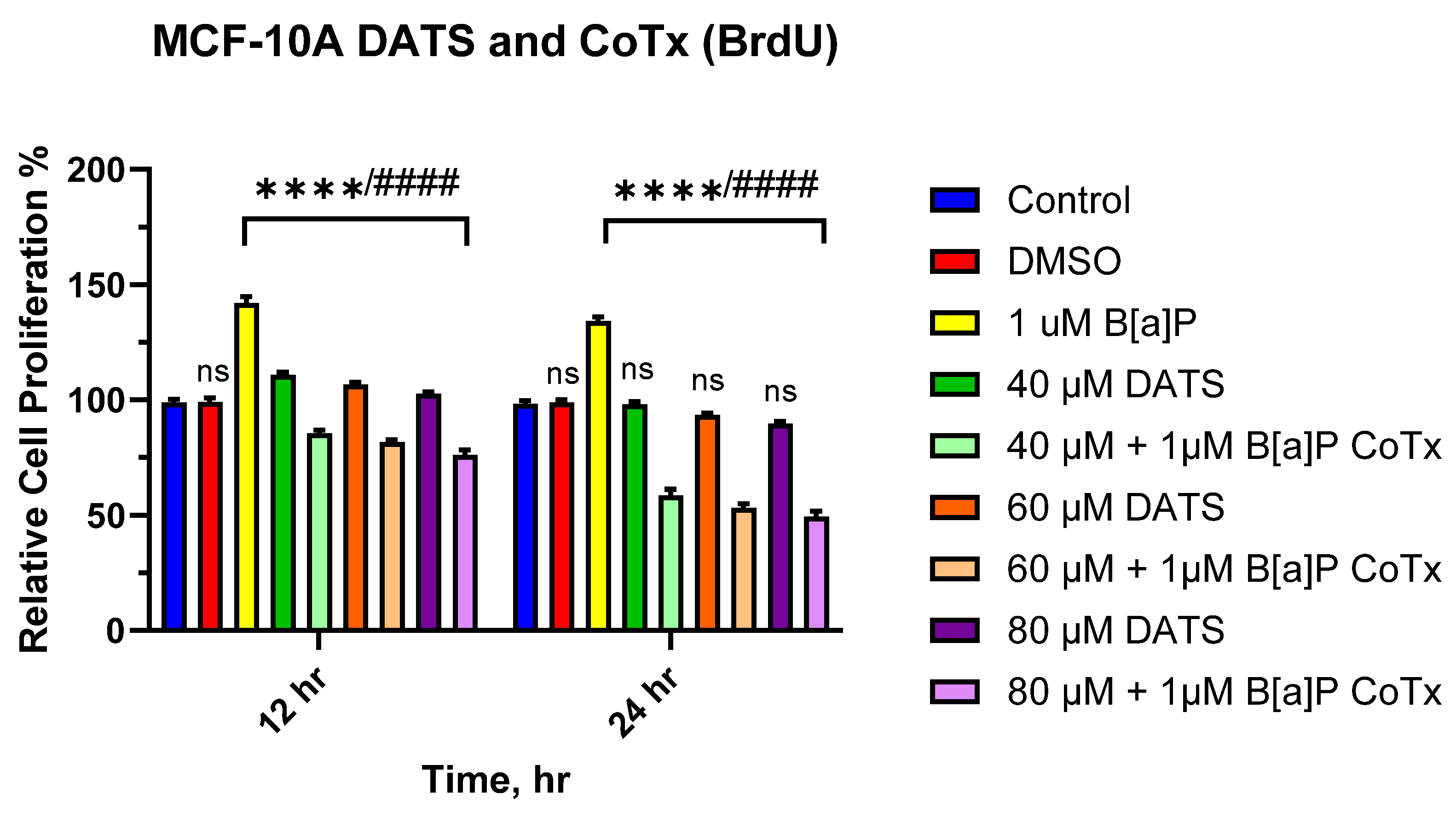
3.2. DATS Inhibits B[a]P-Induced Cell Proliferation Using BrdU Proliferation Assay on MCF-10A Cells
3.3. DATS Inhibits B[a]P-Induced Colony Formation in MCF-10A Cells
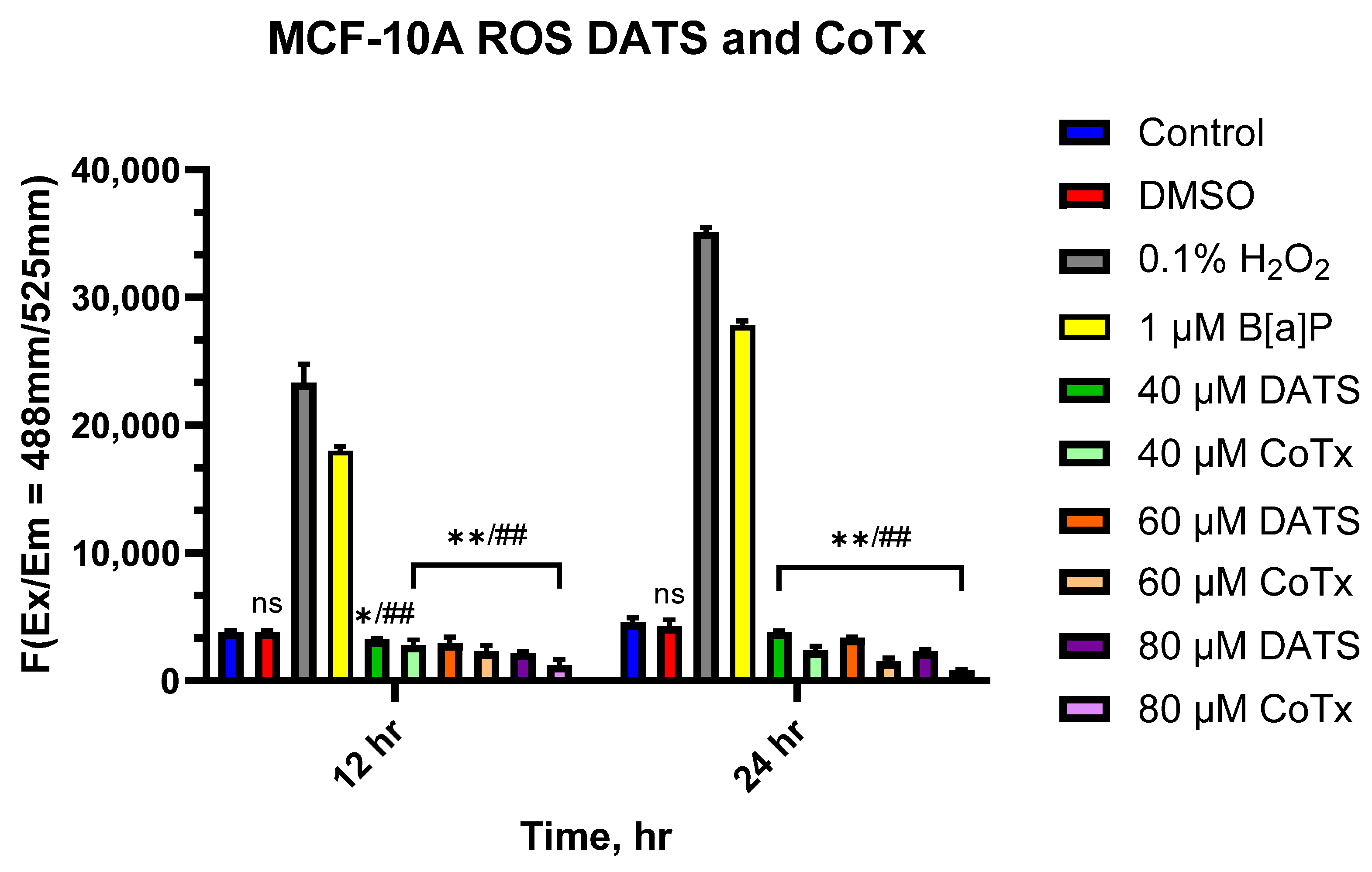
3.4. DATS Suppresses the Accumulation of ROS in B[a]P-Treated MCF-10A Cells
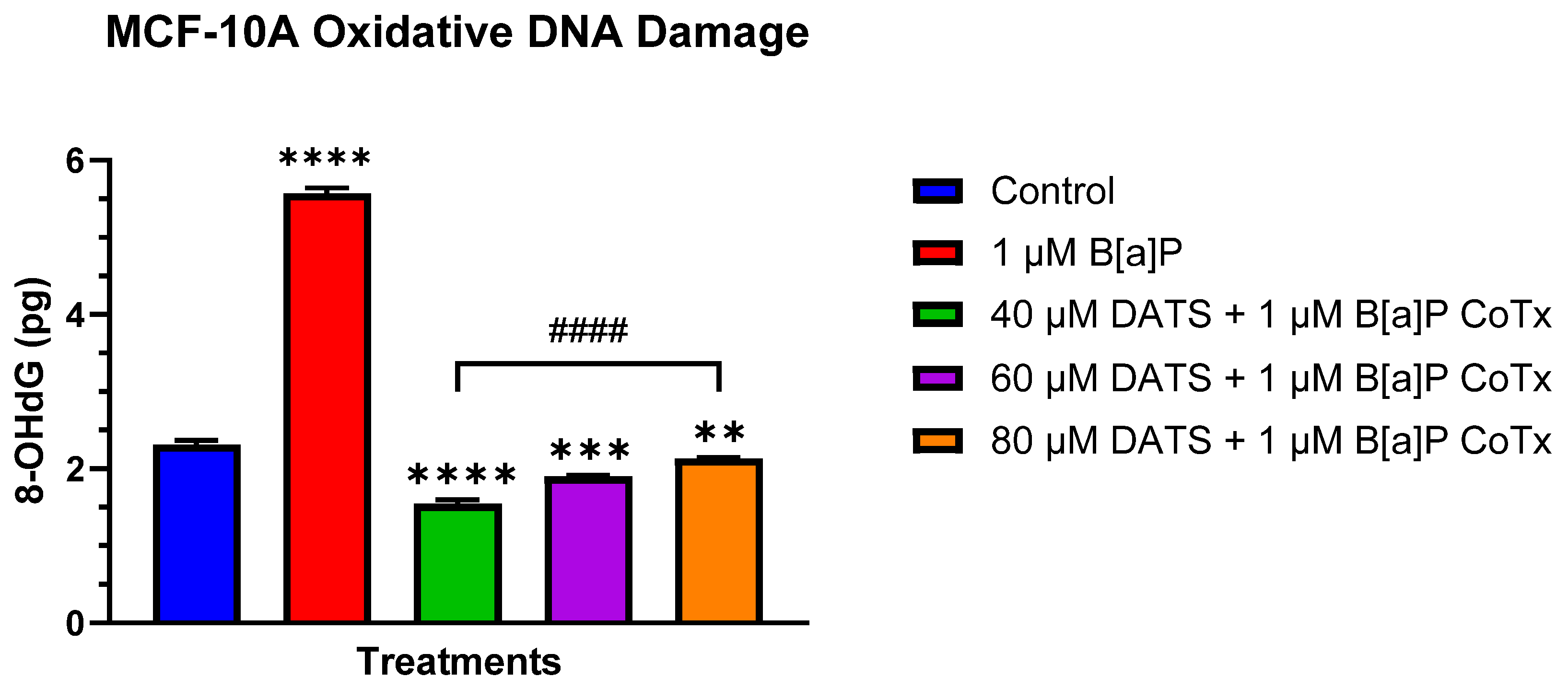
3.5. DATS Inhibits B[a]P-Induced Oxidative (8-OHdG) DNA Damage in MCF-10A Cells
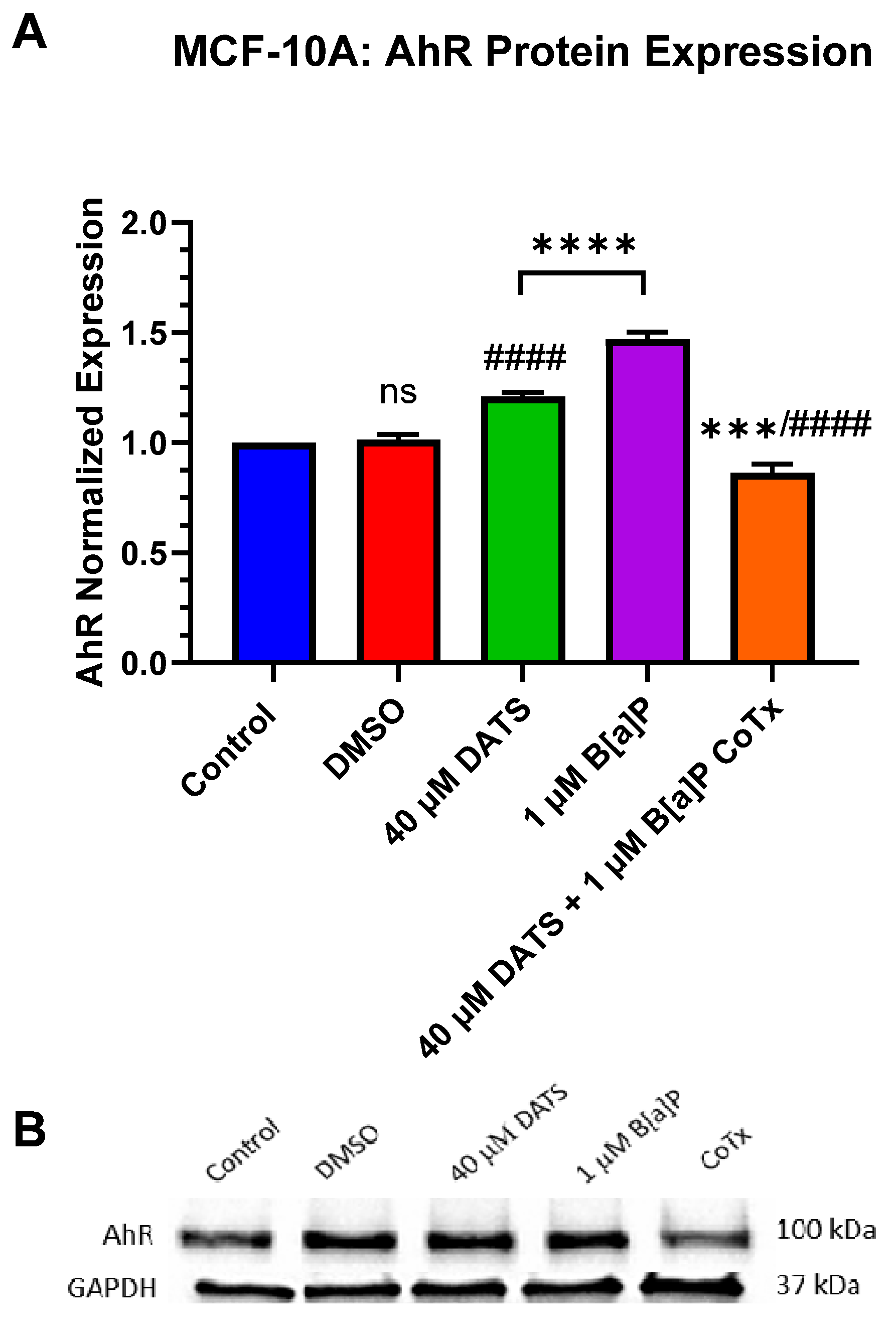
3.6. Decrease in Aryl Hydrocarbon Receptor (AhR) Protein Expression by DATS in B[a]P-Treated MCF-10A Cells
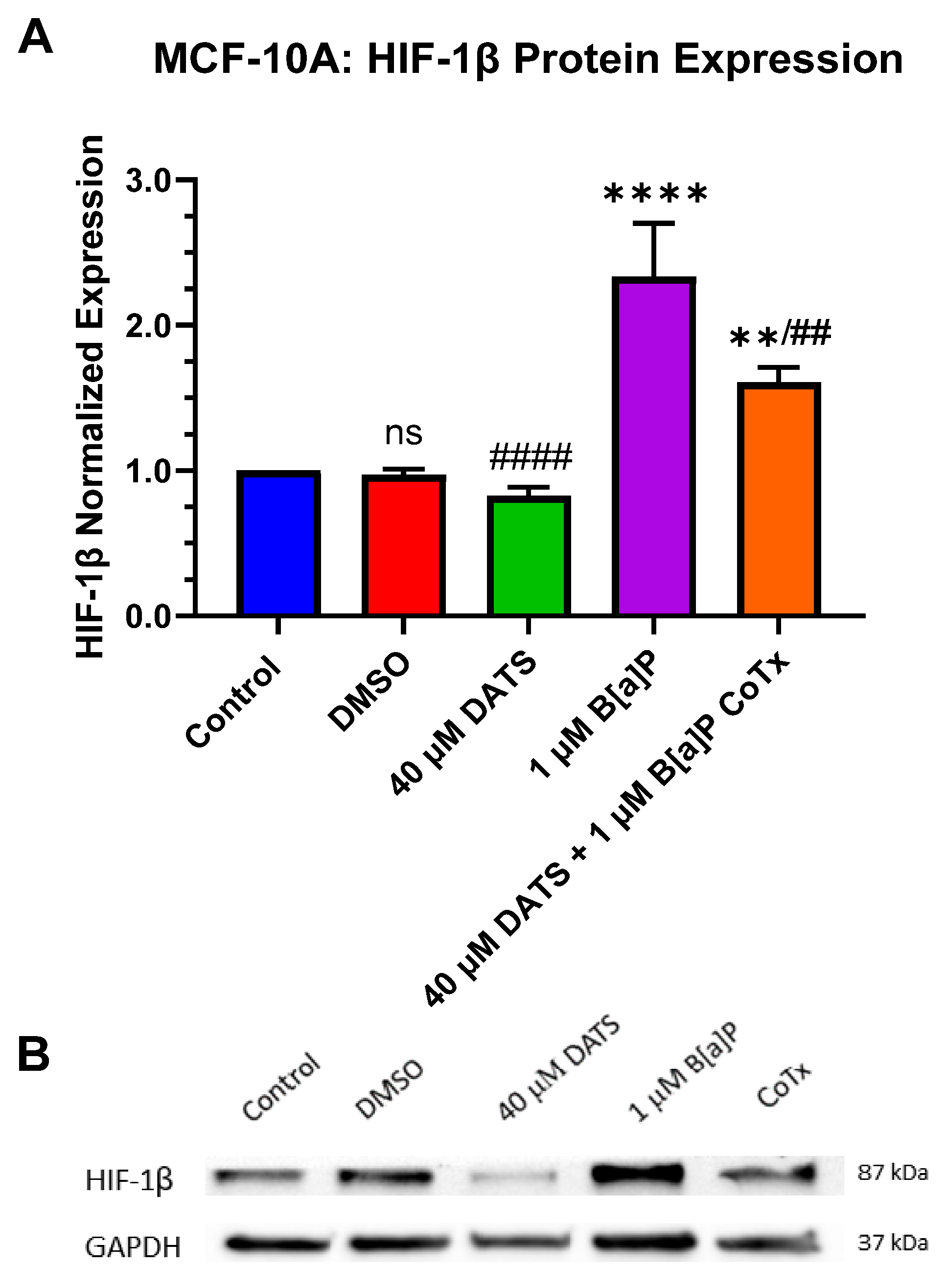
3.7. Decrease in Hypoxia-Inducible Factor-1beta/Aryl Hydrocarbon Receptor Nuclear Translocator (HIF-1β/ARNT) Protein Expression by DATS in B[a]P-Treated MCF-10A Cells
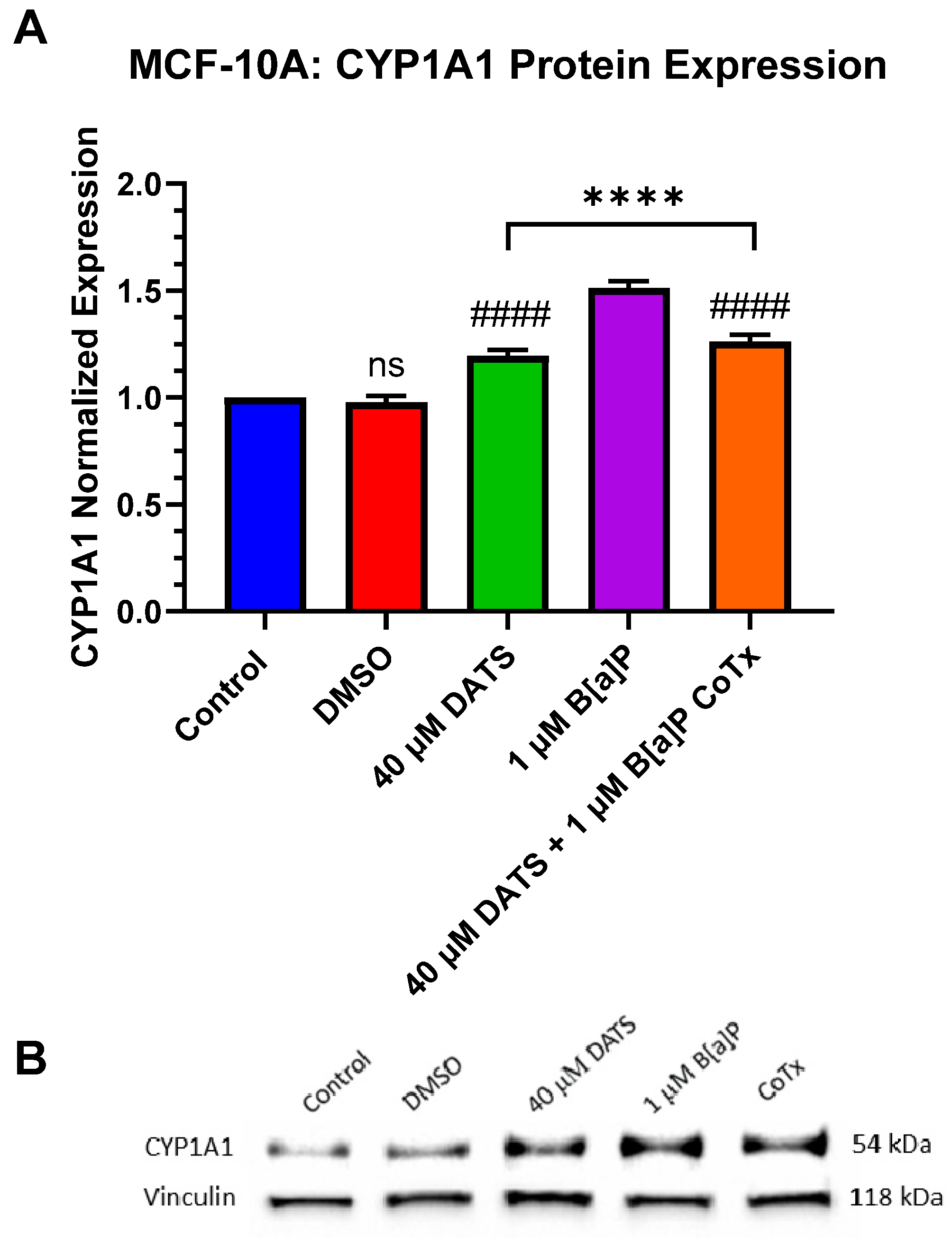
3.8. Decrease in Cytochrome P450 1A1 (CYP1A1) Protein Expression by DATS in B[a]P-Treated MCF-10A Cells
3.9. Induction of DNA Polymerase Beta (POLβ) Protein Expression by DATS in B[a]P-Treated MCF-10A Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.Y.; Zhu, X.S.; Xu, H.Y.; Zhao, Z.X.; Li, S.Y.; Li, S.Z.; Cai, J.H.; Cao, J.M. Diallyl trisulfide suppresses tumor growth through the attenuation of Nrf2/Akt and activation of p38/JNK and potentiates cisplatin efficacy in gastric cancer treatment. Acta Pharmacol. Sin. 2017, 38, 1048–1058. [Google Scholar] [PubMed]
- Espinoza, T.; Valencia, E.; Albarrán, M.; Díaz, D.; Quevedo, R.; Díaz, O.; Bastías, J. Garlic (Allium sativum L.) and Its beneficial properties for health: A review. Agroindustrial Sci. 2020, 10, 103–115. [Google Scholar] [CrossRef]
- Ansary, J.; Forbes-Hernandez, T.Y.; Gil, E.; Cianciosi, D.; Zhang, J.; Elexpuru-Zabaleta, M.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Potential Health Benefit of Garlic Based on Human Intervention Studies: A Brief Overview. Antioxidants 2020, 9, 619. [Google Scholar] [CrossRef]
- Kim, S.H.; Hahm, E.R.; Singh, K.B.; Singh, S.V. Diallyl Trisulfide Inhibits Leptin-induced Oncogenic Signaling in Human Breast Cancer Cells but Fails to Prevent Chemically-induced Luminal-type Cancer in Rats. J. Cancer Prev. 2020, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.R.; Singh, S.V. Diallyl trisulfide inhibits estrogen receptor-alpha activity in human breast cancer cells. Breast Cancer Res. Treat. 2014, 144, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.P.; Horton, M.; Kang, J.; Mak, V.; Luchtenborg, M.; Moller, H. Lung cancer incidence and survival in England: An analysis by socioeconomic deprivation and urbanization. J. Thorac. Oncol. 2011, 6, 2005–2010. [Google Scholar] [CrossRef]
- Hall, S.A.; Kaufman, J.S.; Millikan, R.C.; Ricketts, T.C.; Herman, D.; Savitz, D.A. Urbanization and breast cancer incidence in North Carolina, 1995–1999. Ann. Epidemiol. 2005, 15, 796–803. [Google Scholar] [CrossRef]
- Bukowska, B.; Mokra, K.; Michalowicz, J. Benzo[a]pyrene-Environmental Occurrence, Human Exposure, and Mechanisms of Toxicity. Int. J. Mol. Sci. 2022, 23, 6348. [Google Scholar] [CrossRef]
- Bukowska, B. Hemoglobin adducts as biomarkers of human exposure to selected xenobiotics. Postep. Hig. Med. Dosw. 2015, 69, 668–680. [Google Scholar] [CrossRef]
- Teitelbaum, S.L.; Belpoggi, F.; Reinlib, L. Advancing research on endocrine disrupting chemicals in breast cancer: Expert panel recommendations. Reprod. Toxicol. 2015, 54, 141–147. [Google Scholar] [CrossRef]
- Liamin, M.; Boutet-Robinet, E.; Jamin, E.L.; Fernier, M.; Khoury, L.; Kopp, B.; Le Ferrec, E.; Vignard, J.; Audebert, M.; Sparfel, L. Benzo[a]pyrene-induced DNA damage associated with mutagenesis in primary human activated T lymphocytes. Biochem. Pharmacol. 2017, 137, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Amadou, A.; Praud, D.; Coudon, T.; Deygas, F.; Grassot, L.; Faure, E.; Couvidat, F.; Caudeville, J.; Bessagnet, B.; Salizzoni, P.; et al. Risk of breast cancer associated with long-term exposure to benzo[a]pyrene (BaP) air pollution: Evidence from the French E3N cohort study. Environ. Int. 2021, 149, 106399. [Google Scholar] [CrossRef]
- Nkrumah-Elie, Y.M.; Reuben, J.S.; Hudson, A.; Taka, E.; Badisa, R.; Ardley, T.; Israel, B.; Sadrud-Din, S.Y.; Oriaku, E.; Darling-Reed, S.F. Diallyl trisulfide as an inhibitor of benzo(a)pyrene-induced precancerous carcinogenesis in MCF-10A cells. Food Chem. Toxicol. 2012, 50, 2524–2530. [Google Scholar] [CrossRef]
- Kanga, K.J.W.; Mendonca, P.; Soliman, K.F.A.; Ferguson, D.T.; Darling-Reed, S.F. Effect of Diallyl Trisulfide on TNF-alpha-induced CCL2/MCP-1 Release in Genetically Different Triple-negative Breast Cancer Cells. Anticancer. Res. 2021, 41, 5919–5933. [Google Scholar] [CrossRef]
- Hosono, T.; Fukao, T.; Ogihara, J.; Ito, Y.; Shiba, H.; Seki, T.; Ariga, T. Diallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of beta-tubulin. J. Biol. Chem. 2005, 280, 41487–41493. [Google Scholar] [CrossRef]
- Wang, J.; Si, L.; Wang, G.; Bai, Z.; Li, W. Increased Sulfiredoxin Expression in Gastric Cancer Cells May Be a Molecular Target of the Anticancer Component Diallyl Trisulfide. Biomed. Res. Int. 2019, 2019, 4636804. [Google Scholar] [CrossRef]
- Na, H.K.; Kim, E.H.; Choi, M.A.; Park, J.M.; Kim, D.H.; Surh, Y.J. Diallyl trisulfide induces apoptosis in human breast cancer cells through ROS-mediated activation of JNK and AP-1. Biochem. Pharmacol. 2012, 84, 1241–1250. [Google Scholar] [CrossRef]
- Borkowska, A.; Sielicka-Dudzin, A.; Herman-Antosiewicz, A.; Wozniak, M.; Fedeli, D.; Falcioni, G.; Antosiewicz, J. Diallyl trisulfide-induced prostate cancer cell death is associated with Akt/PKB dephosphorylation mediated by P-p66shc. Eur. J. Nutr. 2012, 51, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, N.; Li, X.; Li, K.; Tian, J.; Li, J. Diallyl trisulfide induces osteosarcoma cell apoptosis through reactive oxygen species-mediated downregulation of the PI3K/Akt pathway. Oncol. Rep. 2016, 35, 3648–3658. [Google Scholar] [CrossRef]
- Chandra-Kuntal, K.; Lee, J.; Singh, S.V. Critical role for reactive oxygen species in apoptosis induction and cell migration inhibition by diallyl trisulfide, a cancer chemopreventive component of garlic. Breast Cancer Res. Treat. 2013, 138, 69–79. [Google Scholar] [CrossRef]
- Darling-Reed, S.F.; Nkrumah-Elie, Y.; Ferguson, D.T.; Flores-Rozas, H.; Mendonca, P.; Messeha, S.; Hudson, A.; Badisa, R.B.; Tilghman, S.L.; Womble, T.; et al. Diallyl Sulfide Attenuation of Carcinogenesis in Mammary Epithelial Cells through the Inhibition of ROS Formation, and DNA Strand Breaks. Biomolecules 2021, 11, 1313. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; GWasef, L.; Elewa, Y.H.; AAl-Sagan, A.; Abd El-Hack, M.E.; Taha, A.E.; MAbd-Elhakim, Y.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Khan, F.; Alshammari, N.; Saeed, A.; Aqil, F.; Saeed, M. Updates on the anticancer potential of garlic organosulfur compounds and their nanoformulations: Plant therapeutics in cancer management. Front. Pharmacol. 2023, 14, 1154034. [Google Scholar] [CrossRef]
- Lawson, L.D.; Wang, Z.J. Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: Use in measuring allicin bioavailability. J. Agric. Food Chem. 2005, 53, 1974–1983. [Google Scholar] [CrossRef]
- Stan, S.D.; Abtahi, M. Diallyl Trisulfide Induces Apoptosis in Breast Ductal Carcinoma In Situ Derived and Minimally Invasive Breast Cancer Cells. Nutrients 2022, 14, 1455. [Google Scholar] [CrossRef]
- Hung, F.M.; Shang, H.S.; Tang, N.Y.; Lin, J.J.; Lu, K.W.; Lin, J.P.; Ko, Y.C.; Yu, C.C.; Wang, H.L.; Liao, J.C.; et al. Effects of diallyl trisulfide on induction of apoptotic death in murine leukemia WEHI-3 cells in vitro and alterations of the immune responses in normal and leukemic mice in vivo. Environ. Toxicol. 2015, 30, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Xiao, D.; Xiao, H.; Powolny, A.A.; Lew, K.L.; Reilly, M.L.; Zeng, Y.; Wang, Z.; Singh, S.V. Mitochondria-mediated apoptosis by diallyl trisulfide in human prostate cancer cells is associated with generation of reactive oxygen species and regulated by Bax/Bak. Mol. Cancer Ther. 2007, 6, 1599–1609. [Google Scholar] [CrossRef]
- Powolny, A.A.; Singh, S.V. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008, 269, 305–314. [Google Scholar] [CrossRef]
- Gianelle, V.; Colombi, C.; Caserini, S.; Ozgen, S.; Galante, S.; Marongiu, A.; Lanzani, G. Benzo (a) pyrene air concentrations and emission inventory in Lombardy region, Italy. Atmos. Pollut. Res. 2013, 4, 257–266. [Google Scholar] [CrossRef]
- Cavalieri, E.; Rogan, E. The molecular etiology and prevention of estrogen-initiated cancers: Ockham’s Razor: Pluralitas non est ponenda sine necessitate. Plurality should not be posited without necessity. Mol. Asp. Med. 2014, 36, 1–55. [Google Scholar]
- Kennedy, D.O.; Agrawal, M.; Shen, J.; Terry, M.B.; Zhang, F.F.; Senie, R.T.; Motykiewicz, G.; Santella, R.M. DNA repair capacity of lymphoblastoid cell lines from sisters discordant for breast cancer. J. Natl. Cancer Inst. 2005, 97, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Caruso, J.A.; Reiners, J.J., Jr.; Emond, J.; Shultz, T.; Tainsky, M.A.; Alaoui-Jamali, M.; Batist, G. Genetic alteration of chromosome 8 is a common feature of human mammary epithelial cell lines transformed in vitro with benzo[a]pyrene. Mutat. Res. 2001, 473, 85–99. [Google Scholar] [CrossRef]
- Iciek, M.; Kwiecien, I.; Wlodek, L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 247–265. [Google Scholar] [CrossRef]
- Martin, F.L.; Cole, K.J.; Williams, J.A.; Millar, B.C.; Harvey, D.; Weaver, G.; Grover, P.L.; Phillips, D.H. Activation of genotoxins to DNA-damaging species in exfoliated breast milk cells. Mutat. Res. 2000, 470, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.L.; Bender, W.; Fang, Q.; Ludeke, A.; Welch, B. Prevention of chemical carcinogen DNA binding and inhibition of nuclear RNA polymerase activity by organosulfur compounds as the possible mechanisms for their anticancer initiation and proliferation effects. Cancer Detect. Prev. 2003, 27, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Volcho, K.P.; Lavrik, O.I. Inhibition of DNA Repair Enzymes as a Valuable Pharmaceutical Approach. Int. J. Mol. Sci. 2023, 24, 7954. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Wang, R.; Hao, W.; Pan, L.; Boldogh, I.; Ba, X. The roles of base excision repair enzyme OGG1 in gene expression. Cell Mol. Life Sci. 2018, 75, 3741–3750. [Google Scholar] [CrossRef]
- Mandl, M.; Depping, R. Hypoxia-inducible aryl hydrocarbon receptor nuclear translocator (ARNT) (HIF-1beta): Is it a rare exception? Mol. Med. 2014, 20, 215–220. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Y.; Yang, F.; Zhang, J.; Zhang, J.; Yu, W.; Wang, M.; Lv, X.; Li, J.; Bai, T.; et al. Interaction between AhR and HIF-1 signaling pathways mediated by ARNT/HIF-1beta. BMC Pharmacol. Toxicol. 2022, 23, 26. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Liao, Z.; Zhang, Y.; Lei, W.; Shui, X. Aryl hydrocarbon receptor: An emerging player in breast cancer pathogenesis and its potential as a drug target (Review). Mol. Med. Rep. 2024, 29, 11. [Google Scholar] [CrossRef]
- Uno, S.; Sakai, M.; Fujinari, Y.; Hosono, T.; Seki, T.; Makishima, M. Diallyl Trisulfide Enhances Benzo[a]pyrene-induced CYP1A1 Expression and Metabolic Activation in Hepatic HepG2 Cells. Anticancer. Res. 2019, 39, 2369–2375. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Cytochrome P450 CYP1A1: Wider roles in cancer progression and prevention. BMC Cancer 2009, 9, 187. [Google Scholar] [CrossRef]
- Larsen, M.C.; Angus, W.G.; Brake, P.B.; Eltom, S.E.; Sukow, K.A.; Jefcoate, C.R. Characterization of CYP1B1 and CYP1A1 expression in human mammary epithelial cells: Role of the aryl hydrocarbon receptor in polycyclic aromatic hydrocarbon metabolism. Cancer Res. 1998, 58, 2366–2374. [Google Scholar]
- Haque, M.W.; Pattanayak, S.P. Taxifolin Inhibits 7,12-Dimethylbenz(a)anthracene-induced Breast Carcinogenesis by Regulating AhR/CYP1A1 Signaling Pathway. Pharmacogn. Mag. 2018, 13, S749–S755. [Google Scholar] [PubMed]
- Arroo, R.R.J.; Androutsopoulos, V.; Beresford, K.; Ruparelia, K.; Surichan, S.; Wilsher, N.; Potter, G.A. Phytoestrogens as natural prodrugs in cancer prevention: Dietary flavonoids. Phytochem. Rev. 2009, 8, 375–386. [Google Scholar] [CrossRef]
- Chun, H.S.; Kim, H.J.; Choi, E.H. Modulation of cytochrome P4501-mediated bioactivation of benzo[a]pyrene by volatile allyl sulfides in human hepatoma cells. Biosci. Biotechnol. Biochem. 2001, 65, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Sondermann, N.C.; Fassbender, S.; Hartung, F.; Hatala, A.M.; Rolfes, K.M.; Vogel, C.F.A.; Haarmann-Stemmann, T. Functions of the aryl hydrocarbon receptor (AHR) beyond the canonical AHR/ARNT signaling pathway. Biochem. Pharmacol. 2023, 208, 115371. [Google Scholar] [CrossRef]
- Tannheimer, S.L.; Lauer, F.T.; Lane, J.; Burchiel, S.W. Factors influencing elevation of intracellular Ca2+ in the MCF-10A human mammary epithelial cell line by carcinogenic polycyclic aromatic hydrocarbons. Mol. Carcinog. 1999, 25, 48–54. [Google Scholar] [CrossRef]
- Takemura, H.; Nagayoshi, H.; Matsuda, T.; Sakakibara, H.; Morita, M.; Matsui, A.; Ohura, T.; Shimoi, K. Inhibitory effects of chrysoeriol on DNA adduct formation with benzo[a]pyrene in MCF-7 breast cancer cells. Toxicology 2010, 274, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Ciolino, H.P.; Daschner, P.J.; Wang, T.T.; Yeh, G.C. Effect of curcumin on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Biochem. Pharmacol. 1998, 56, 197–206. [Google Scholar] [CrossRef]
- Ramadan, K.; Shevelev, I.; Hubscher, U. The DNA-polymerase-X family: Controllers of DNA quality? Nat. Rev. Mol. Cell Biol. 2004, 5, 1038–1043. [Google Scholar] [CrossRef]
- Yamtich, J.; Sweasy, J.B. DNA polymerase family X: Function, structure, and cellular roles. Biochim. Biophys. Acta 2010, 1804, 1136–1150. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferguson, D.T.; Taka, E.; Messeha, S.; Flores-Rozas, H.; Reed, S.L.; Redmond, B.V.; Soliman, K.F.A.; Kanga, K.J.W.; Darling-Reed, S.F. The Garlic Compound, Diallyl Trisulfide, Attenuates Benzo[a]Pyrene-Induced Precancerous Effect through Its Antioxidant Effect, AhR Inhibition, and Increased DNA Repair in Human Breast Epithelial Cells. Nutrients 2024, 16, 300. https://doi.org/10.3390/nu16020300
Ferguson DT, Taka E, Messeha S, Flores-Rozas H, Reed SL, Redmond BV, Soliman KFA, Kanga KJW, Darling-Reed SF. The Garlic Compound, Diallyl Trisulfide, Attenuates Benzo[a]Pyrene-Induced Precancerous Effect through Its Antioxidant Effect, AhR Inhibition, and Increased DNA Repair in Human Breast Epithelial Cells. Nutrients. 2024; 16(2):300. https://doi.org/10.3390/nu16020300
Chicago/Turabian StyleFerguson, Dominique T., Equar Taka, Samia Messeha, Hernan Flores-Rozas, Sarah L. Reed, Bryan V. Redmond, Karam F. A. Soliman, Konan J. W. Kanga, and Selina F. Darling-Reed. 2024. "The Garlic Compound, Diallyl Trisulfide, Attenuates Benzo[a]Pyrene-Induced Precancerous Effect through Its Antioxidant Effect, AhR Inhibition, and Increased DNA Repair in Human Breast Epithelial Cells" Nutrients 16, no. 2: 300. https://doi.org/10.3390/nu16020300
APA StyleFerguson, D. T., Taka, E., Messeha, S., Flores-Rozas, H., Reed, S. L., Redmond, B. V., Soliman, K. F. A., Kanga, K. J. W., & Darling-Reed, S. F. (2024). The Garlic Compound, Diallyl Trisulfide, Attenuates Benzo[a]Pyrene-Induced Precancerous Effect through Its Antioxidant Effect, AhR Inhibition, and Increased DNA Repair in Human Breast Epithelial Cells. Nutrients, 16(2), 300. https://doi.org/10.3390/nu16020300








