Association of Glutamine and Glutamate Metabolism with Mortality among Patients at Nutritional Risk—A Secondary Analysis of the Randomized Clinical Trial EFFORT
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Randomization and Procedures
2.3. Analysis of Blood Biomarkers
2.4. Outcomes
2.5. Statistical Analysis
3. Results
3.1. Patient Population
3.2. Association of Glutamine and Glutamate with Nutritional Parameters and Inflammation
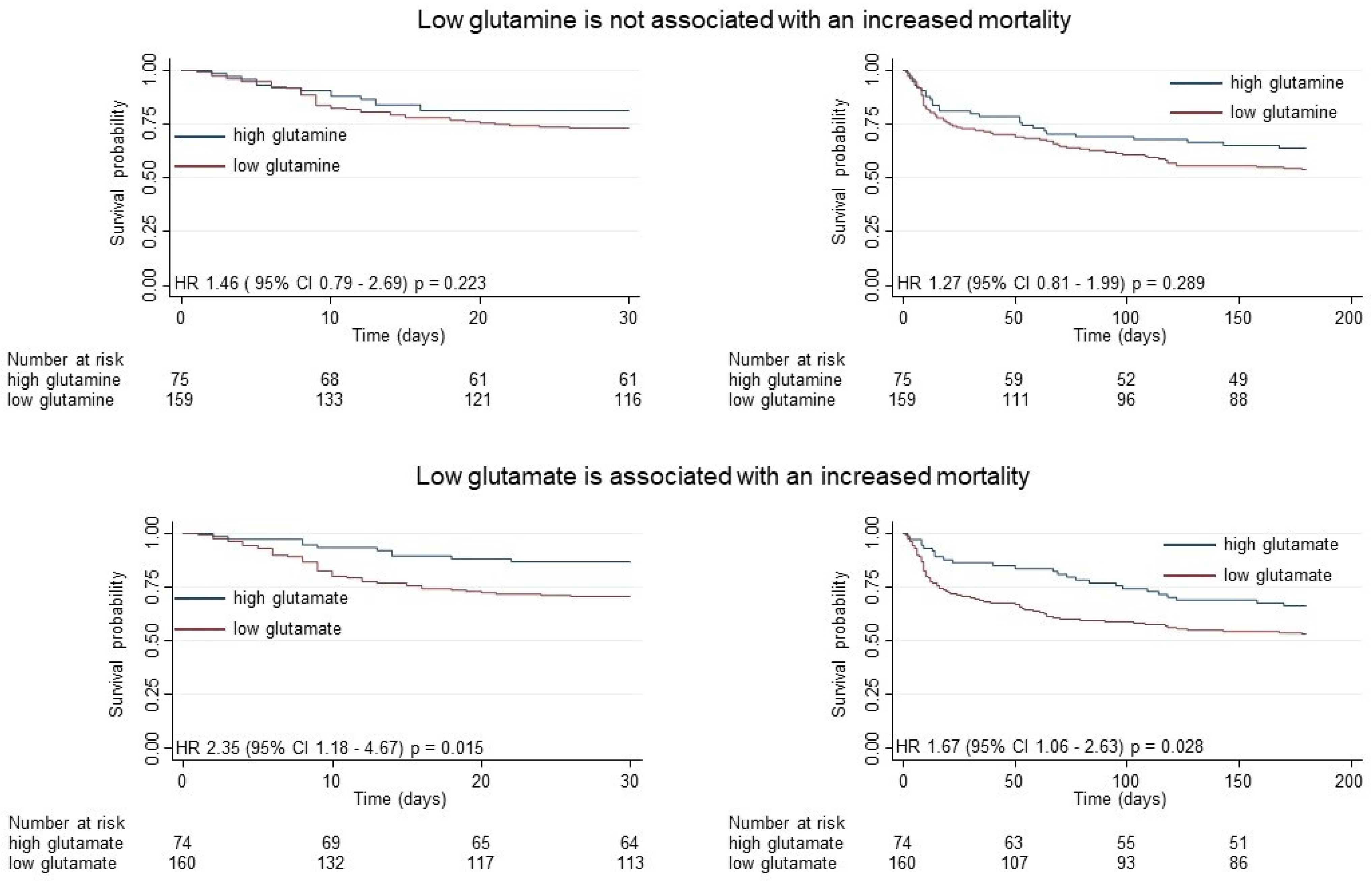
3.3. Prognostic Value of Low Glutamine or Low Glutamate to Predict Clinical Outcomes
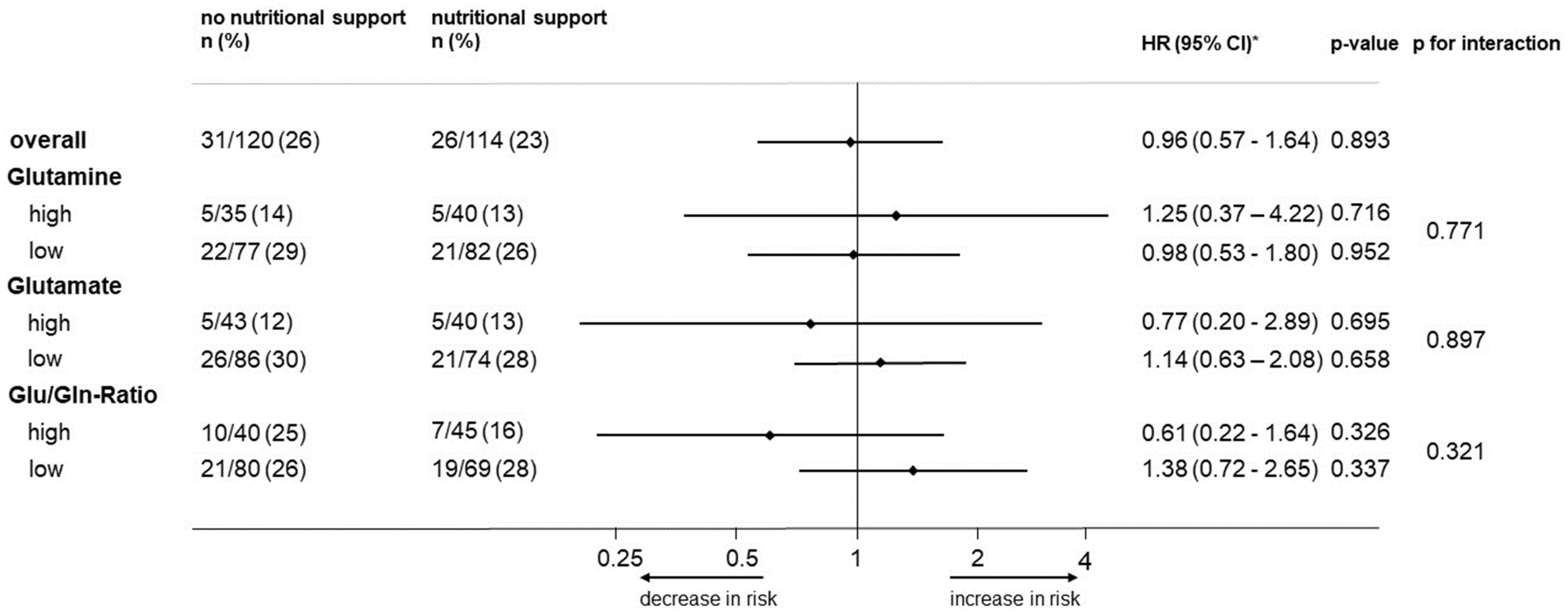
3.4. Association of Glutamine and Glutamate Levels or the Ratio with the Effectiveness of Nutritional Support
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| BMI | body mass index |
| CCI | Charlson comorbidity index |
| CI | confidence interval |
| CRP | C-reactive protein |
| DRM | disease-related malnutrition |
| EFFORT | Effect of Early Nutritional Support on Frailty, Functional Outcomes, and Recovery of Malnourished Medical Inpatients Trial |
| EKNZ | Ethics Committee of Northwest and Central Switzerland |
| GA | glutaminase |
| HR | hazard ratio |
| LC-MS/MS | liquid chromatography coupled to tandem mass spectrometry |
| NH3 | ammonia |
| NRS | nutritional risk screening 2002 |
| OR | odds ratio |
| SD | standard deviation |
| UHPLC | ultra-high-pressure liquid chromatography |
References
- Wischmeyer, P.E. Glutamine: Mode of action in critical illness. Crit. Care Med. 2007, 35 (Suppl. S9), S541–S544. [Google Scholar] [CrossRef] [PubMed]
- Oehler, R.; Pusch, E.; Dungel, P.; Zellner, M.; Eliasen, M.M.; Brabec, M.; Roth, E. Glutamine depletion impairs cellular stress response in human leucocytes. Br. J. Nutr. 2002, 87 (Suppl. S1), S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, V.; Macedo Rogero, M.; Noel Keane, K.; Curi, R.; Newsholme, P. Glutamine: Metabolism and Immune Function, Supplementation and Clinical Translation. Nutrients 2018, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P. Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 2001, 131 (Suppl. S9), 2515S–2522S, discussion 2523S–2534S. [Google Scholar] [CrossRef] [PubMed]
- Curi, R.; Newsholme, P.; Marzuca-Nassr, G.N.; Takahashi, H.K.; Hirabara, S.M.; Cruzat, V.; Krause, M.; de Bittencourt, P.I., Jr. Regulatory principles in metabolism-then and now. Biochem. J. 2016, 473, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.; Funovics, J.; Mühlbacher, F.; Schemper, M.; Mauritz, W.; Sporn, P.; Fritsch, A. Metabolic disorders in severe abdominal sepsis: Glutamine deficiency in skeletal muscle. Clin. Nutr. 1982, 1, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Oudemans-van Straaten, H.M.; Bosman, R.J.; Treskes, M.; van der Spoel, H.J.; Zandstra, D.F. Plasma glutamine depletion and patient outcome in acute ICU admissions. Intensive Care Med. 2001, 27, 84–90. [Google Scholar] [CrossRef]
- Matsuyama, T.; Yoshinaga, S.K.; Shibue, K.; Mak, T.W. Comorbidity-associated glutamine deficiency is a predisposition to severe COVID-19. Cell Death Differ. 2021, 28, 3199–3213. [Google Scholar] [CrossRef]
- Schlemmer, M.; Suchner, U.; Schäpers, B.; Duerr, E.M.; Alteheld, B.; Zwingers, T.; Stehle, P.; Zimmer, H.G. Is glutamine deficiency the link between inflammation, malnutrition, and fatigue in cancer patients? Clin. Nutr. 2015, 34, 1258–1265. [Google Scholar] [CrossRef]
- van Zanten, A.R.; Dhaliwal, R.; Garrel, D.; Heyland, D.K. Enteral glutamine supplementation in critically ill patients: A systematic review and meta-analysis. Crit. Care 2015, 19, 294. [Google Scholar] [CrossRef]
- Heyland, D.; Muscedere, J.; Wischmeyer, P.E.; Cook, D.; Jones, G.; Albert, M.; Elke, G.; Berger, M.M.; Day, A.G. A randomized trial of glutamine and antioxidants in critically ill patients. N. Engl. J. Med. 2013, 368, 1489–1497. [Google Scholar] [CrossRef]
- Heyland, D.K.; Wibbenmeyer, L.; Pollack, J.A.; Friedman, B.; Turgeon, A.F.; Eshraghi, N.; Jeschke, M.G.; Bélisle, S.; Grau, D.; Mandell, S.; et al. A Randomized Trial of Enteral Glutamine for Treatment of Burn Injuries. N. Engl. J. Med. 2022, 387, 1001–1010. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Barker, L.A.; Gout, B.S.; Crowe, T.C. Hospital malnutrition: Prevalence, identification and impact on patients and the healthcare system. Int. J. Env. Res. Public. Health 2011, 8, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Seres, D.; Lobo, D.N.; Gomes, F.; Kaegi-Braun, N.; Stanga, Z. Management of disease-related malnutrition for patients being treated in hospital. Lancet 2021, 398, 1927–1938. [Google Scholar] [CrossRef] [PubMed]
- McWhirter, J.P.; Pennington, C.R. Incidence and recognition of malnutrition in hospital. BMJ 1994, 308, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Tribolet, P.; Bregenzer, T.; Braun, N.; et al. Individualised nutritional support in medical inpatients at nutritional risk: A randomised clinical trial. Lancet 2019, 393, 2312–2321. [Google Scholar] [CrossRef] [PubMed]
- Deutz, N.E.; Matheson, E.M.; Matarese, L.E.; Luo, M.; Baggs, G.E.; Nelson, J.L.; Hegazi, R.A.; Tappenden, K.A.; Ziegler, T.R. Readmission and mortality in malnourished, older, hospitalized adults treated with a specialized oral nutritional supplement: A randomized clinical trial. Clin. Nutr. 2016, 35, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Baumgartner, A.; Bounoure, L.; Bally, M.; Deutz, N.E.; Greenwald, J.L.; Stanga, Z.; Mueller, B.; Schuetz, P. Association of Nutritional Support With Clinical Outcomes Among Medical Inpatients Who Are Malnourished or at Nutritional Risk: An Updated Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1915138. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Sulo, S.; Walzer, S.; Vollmer, L.; Brunton, C.; Kaegi-Braun, N.; Stanga, Z.; Mueller, B.; Gomes, F. Cost savings associated with nutritional support in medical inpatients: An economic model based on data from a systematic review of randomised trials. BMJ Open 2021, 11, e046402. [Google Scholar] [CrossRef]
- Norman, K.; Pirlich, M.; Smoliner, C.; Kilbert, A.; Schulzke, J.D.; Ockenga, J.; Lochs, H.; Reinhold, T. Cost-effectiveness of a 3-month intervention with oral nutritional supplements in disease-related malnutrition: A randomised controlled pilot study. Eur. J. Clin. Nutr. 2011, 65, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Bonilla-Palomas, J.L.; Gamez-Lopez, A.L.; Castillo-Dominguez, J.C.; Moreno-Conde, M.; Lopez Ibanez, M.C.; Alhambra Exposito, R.; Ramiro Ortega, E.; Anguita-Sanchez, M.P.; Villar-Raez, A. Nutritional Intervention in Malnourished Hospitalized Patients with Heart Failure. Arch. Med. Res. 2016, 47, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Merker, M.; Felder, M.; Gueissaz, L.; Bolliger, R.; Tribolet, P.; Kagi-Braun, N.; Gomes, F.; Hoess, C.; Pavlicek, V.; Bilz, S.; et al. Association of Baseline Inflammation With Effectiveness of Nutritional Support Among Patients With Disease-Related Malnutrition: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e200663. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Van den Berghe, G. Nutrition in the acute phase of critical illness. N. Engl. J. Med. 2014, 370, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, J.; Nielsen, E.E.; Korang, S.K.; Halberg Engell, K.; Nielsen, M.S.; Zhang, K.; Didriksen, M.; Lund, L.; Lindahl, N.; Hallum, S.; et al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst. Rev. 2017, 5, CD011598. [Google Scholar] [CrossRef] [PubMed]
- Gariballa, S.; Forster, S. Effects of acute-phase response on nutritional status and clinical outcome of hospitalized patients. Nutrition 2006, 22, 750–757. [Google Scholar] [CrossRef]
- Gariballa, S.; Forster, S.; Walters, S.; Powers, H. A randomized, double-blind, placebo-controlled trial of nutritional supplementation during acute illness. Am. J. Med. 2006, 119, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Kaegi-Braun, N.; Tribolet, P.; Baumgartner, A.; Fehr, R.; Baechli, V.; Geiser, M.; Deiss, M.; Gomes, F.; Kutz, A.; Hoess, C.; et al. Value of handgrip strength to predict clinical outcomes and therapeutic response in malnourished medical inpatients: Secondary analysis of a randomized controlled trial. Am. J. Clin. Nutr. 2021, 114, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Bargetzi, A.; Emmenegger, N.; Wildisen, S.; Nickler, M.; Bargetzi, L.; Hersberger, L.; Segerer, S.; Kaegi-Braun, N.; Tribolet, P.; Gomes, F.; et al. Admission kidney function is a strong predictor for the response to nutritional support in patients at nutritional risk. Clin. Nutr. 2021, 40, 2762–2771. [Google Scholar] [CrossRef]
- Keller, U. Nutritional Laboratory Markers in Malnutrition. J. Clin. Med. 2019, 8, 775. [Google Scholar] [CrossRef]
- Bendavid, I.; Lobo, D.N.; Barazzoni, R.; Cederholm, T.; Coeffier, M.; de van der Schueren, M.; Fontaine, E.; Hiesmayr, M.; Laviano, A.; Pichard, C.; et al. The centenary of the Harris-Benedict equations: How to assess energy requirements best? Recommendations from the ESPEN expert group. Clin. Nutr. 2021, 40, 690–701. [Google Scholar] [CrossRef]
- Weinberger, K.M. Metabolomics in diagnosing metabolic diseases. Ther. Umsch. 2008, 65, 487–491. [Google Scholar] [CrossRef]
- Yet, I.; Menni, C.; Shin, S.Y.; Mangino, M.; Soranzo, N.; Adamski, J.; Suhre, K.; Spector, T.D.; Kastenmüller, G.; Bell, J.T. Genetic Influences on Metabolite Levels: A Comparison across Metabolomic Platforms. PLoS ONE 2016, 11, e0153672. [Google Scholar] [CrossRef]
- Illig, T.; Gieger, C.; Zhai, G.; Römisch-Margl, W.; Wang-Sattler, R.; Prehn, C.; Altmaier, E.; Kastenmüller, G.; Kato, B.S.; Mewes, H.W.; et al. A genome-wide perspective of genetic variation in human metabolism. Nat. Genet. 2010, 42, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Trabado, S.; Al-Salameh, A.; Croixmarie, V.; Masson, P.; Corruble, E.; Fève, B.; Colle, R.; Ripoll, L.; Walther, B.; Boursier-Neyret, C.; et al. The human plasma-metabolome: Reference values in 800 French healthy volunteers; impact of cholesterol, gender and age. PLoS ONE 2017, 12, e0173615. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.E.; Siskos, A.P.; Maitre, L.; Robinson, O.; Athersuch, T.J.; Want, E.J.; Urquiza, J.; Casas, M.; Vafeiadi, M.; Roumeliotaki, T.; et al. Determinants of the urinary and serum metabolome in children from six European populations. BMC Med. 2018, 16, 202. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Lopata, A.L.; Dasouki, M.; Abdel Rahman, A.M. Metabolomics toward personalized medicine. Mass. Spectrom. Rev. 2019, 38, 221–238. [Google Scholar] [CrossRef]
- Liu, X. Classification accuracy and cut point selection. Stat. Med. 2012, 31, 2676–2686. [Google Scholar] [CrossRef]
- Zhang, X.; Yap, Y.; Wei, D.; Chen, G.; Chen, F. Novel omics technologies in nutrition research. Biotechnol. Adv. 2008, 26, 169–176. [Google Scholar] [CrossRef]
- Lee, J.W.; Su, Y.; Baloni, P.; Chen, D.; Pavlovitch-Bedzyk, A.J.; Yuan, D.; Duvvuri, V.R.; Ng, R.H.; Choi, J.; Xie, J.; et al. Integrated analysis of plasma and single immune cells uncovers metabolic changes in individuals with COVID-19. Nat. Biotechnol. 2022, 40, 110–120. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Shimizu, K.; Hata, N.; Takagi, T.; Uejima, E.; Ogura, H.; Wasa, M.; Shimazu, T. Both high and low plasma glutamine levels predict mortality in critically ill patients. Surg. Today 2017, 47, 1331–1338. [Google Scholar] [CrossRef]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef]
- Pencina, K.M.; Bhasin, S.; Luo, M.; Baggs, G.E.; Pereira, S.L.; Davis, G.J.; Deutz, N.E.; Travison, T.G. Predictor Biomarkers of Nonelective Hospital Readmission and Mortality in Malnourished Hospitalized Older Adults. J. Frailty Aging 2020, 9, 226–231. [Google Scholar] [CrossRef]
- Poeze, M.; Luiking, Y.C.; Breedveld, P.; Manders, S.; Deutz, N.E. Decreased plasma glutamate in early phases of septic shock with acute liver dysfunction is an independent predictor of survival. Clin. Nutr. 2008, 27, 523–530. [Google Scholar] [CrossRef]
- Parry-Billings, M.; Evans, J.; Calder, P.C.; Newsholme, E.A. Does glutamine contribute to immunosuppression after major burns? Lancet 1990, 336, 523–525. [Google Scholar] [CrossRef]
- McClave, S.A.; Martindale, R.G.; Vanek, V.W.; McCarthy, M.; Roberts, P.; Taylor, B.; Ochoa, J.B.; Napolitano, L.; Cresci, G. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J. Parenter. Enter. Nutr. 2009, 33, 277–316. [Google Scholar] [CrossRef] [PubMed]

| Overall (n = 234) | Low Glutamine (n = 159) | High Glutamine (n = 75) | p-Value | |
|---|---|---|---|---|
| Sociodemographic | ||||
| Male sex | 135 (57.7%) | 87 (54.7%) | 48 (64.0%) | 0.18 |
| Mean age in years (SD) | 73.6 (13.3) | 73.5 (13.6) | 73.9 (13) | 0.81 |
| Nutritional assessment | ||||
| Mean body mass index in kg/m2 (SD) | 24 (5) | 24 (5) | 25 (5) | 0.32 |
| Mean bodyweight in kg (SD) | 69 (15) | 68 (15) | 71 (14) | 0.11 |
| Mean height in cm (SD) | 168.1 (8.6) | 167.5 (8.6) | 169.3 (8.4) | 0.15 |
| NRS total score | 0.75 | |||
| 3 points | 60 (25.6%) | 39 (24.5%) | 21 (28.0%) | |
| 4 points | 79 (33.8%) | 56 (35.2%) | 23 (30.7%) | |
| ≥5 points | 95 (40.6%) | 64 (40.3%) | 31 (41.3%) | |
| CRP, day 1, mg/L | 84.1 (79.8) | 85.1 (78.2) | 82.0 (83.4) | 0.78 |
| Admission diagnosis | ||||
| Infection | 63 (26.9%) | 40 (25.2%) | 23 (30.7%) | 0.38 |
| Cancer | 74 (31.6%) | 58 (36.5%) | 16 (21.3%) | 0.020 |
| Cardiovascular disease | 24 (10.3%) | 15 (9.4%) | 9 (12.0%) | 0.55 |
| Frailty | 13 (5.6%) | 11 (6.9%) | 2 (2.7%) | 0.19 |
| Lung disease | 10 (4.3%) | 6 (3.8%) | 4 (5.3%) | 0.58 |
| Gastrointestinal disease | 13 (5.6%) | 9 (5.7%) | 4 (5.3%) | 0.92 |
| Renal disease | 15 (6.4%) | 9 (5.7%) | 6 (8.0%) | 0.50 |
| Comorbidity | ||||
| Charlson Comorbidity Index | 6.4 (2.8%) | 6.4 (2.9%) | 6.3 (2.7%) | 0.76 |
| Hypertension | 137 (58.5%) | 92 (57.9%) | 45 (60.0%) | 0.76 |
| Malignant disease | 110 (47.0%) | 77 (48.4%) | 33 (44.0%) | 0.53 |
| Chronic kidney disease | 81 (34.6%) | 51 (32.1%) | 30 (40.0%) | 0.23 |
| Coronary heart disease | 54 (23.1%) | 36 (22.6%) | 18 (24.0%) | 0.82 |
| Diabetes mellitus | 43 (18.4%) | 23 (14.5%) | 20 (26.7%) | 0.025 |
| Congestive heart failure | 45 (19.2%) | 33 (20.8%) | 12 (16.0%) | 0.39 |
| Chronic obstructive pulmonary disease | 26 (11.1%) | 20 (12.6%) | 6 (8.0%) | 0.30 |
| Peripheral arterial disease | 26 (11.1%) | 16 (10.1%) | 10 (13.3%) | 0.46 |
| Cerebrovascular disease | 27 (11.5%) | 17 (10.7%) | 10 (13.3%) | 0.56 |
| Dementia | 11 (4.7%) | 8 (5.0%) | 3 (4.0%) | 0.73 |
| Metabolites | ||||
| Mean plasma glutamine concentration (μmol/L) | 522.5 (196.2) | 423.4 (130.2) | 732.7 (138.3) | <0.001 |
| Mean plasma glutamate concentration (μmol/L) | 152.10 (110.35) | 166.34 (124.38) | 121.91 (62.87) | 0.004 |
| Unadjusted | Adjusted * | |
|---|---|---|
| Glutamine (decrease of 10 μmol/L) | Coef (95% CI) p-value | Coef (95% CI) p-value |
| Nutritional assessment | ||
| Bodyweight (kg) | 0.06 (−0.11 to 0.23) p = 0.459 | 0.06 (−0.11 to 0.24) p = 0.474 |
| Body mass index (kg/m2) | 0.13 (−0.38 to 0.64) p = 0.620 | 0.12 (−0.39 to 0.63) p = 0.640 |
| NRS total score | ||
| 3 points | reference | reference |
| 4 points | −4.56 (−11.15 to 2.04) p = 0.175 | −4.80 (−11.51 to 1.91) p = 0.160 |
| ≥5 points | −1.45 (−7.77 to 4.87) p = 0.651 | −2.47 (−8.96 to 4.01) p = 0.453 |
| CRP, day 1, mg/L | 0.00 (−0.03 to 0.03) p = 0.948 | −0.01 (−0.08 to 0.05) p = 0.659 |
| NRS score components | ||
| Loss of appetite ** | −3,99 (−11.66 to 3.67) p = 0.306 | −5.82 (−13.80 to 2.15) p = 0.152 |
| Bodyweight loss (kg) | ||
| <5% in 3 months | reference | reference |
| >5% in 3 months | 6.00 (−1.03 to 13.03) p = 0.094 | 6.07 (−0.99 to 13.14) p = 0.092 |
| >5% in 2 months | −3.83 (0.293 to −11.00) p = 0.293 | −4.25 (−11.44 to 2.93) p = 0.245 |
| >5% in 1 month | −3.94 (−10.52 to 2.64) p = 0.239 | −3.55 (−10.18 to 3.07) p = 0.292 |
| Reduced dietary intake ** | ||
| >75% | reference | reference |
| 50–75% | −5.54 (−14.72 to 3.65) p = 0.236 | −5.57 (−14.83 to 3.69) p = 0.237 |
| 25–50% | −7.27 (−16.00 to 1.47) p = 0.102 | −6.95 (−15.83 to 1.92) p = 0.124 |
| <25% | −0.14 (−9.77 to 9.49) p = 0.977 | −0.26 (−9.98 to 9.47) p = 0.958 |
| Severity of disease | ||
| 1 | reference | reference |
| 2 | −2.89 (−8.16 to 2.38) p = 0.281 | −3.29 (−8.82 to 2.25) p = 0.243 |
| 3 | −7.04 (−45.16 to 31.09) p = 0.716 | −7.69 (−46.53 to 31.14) p = 0.697 |
| Unadjusted | Adjusted * | |||
|---|---|---|---|---|
| Short- and Long-Term Mortality | n. of Event (%) | n. of Event (%) | HR (95% CI) p-Value | HR (95% CI) p-Value |
| 30-day all-cause mortality | high | low | ||
| Glutamine | 14/75 (19%) | 43/159 (27%) | 1.50 (0.82 to 2.74) p = 0.189 | 1.46 (0.79 to 2.69) p = 0.223 |
| Glutamate | 10/74 (14%) | 47/160 (29%) | 2.40 (1.21 to 4.75) p = 0.012 | 2.35 (1.18 to 4.67) p = 0.015 |
| 180-day all-cause mortality | ||||
| Glutamine | 25/75 (36%) | 73/159 (46%) | 1.37 (0.88 to 2.13) p = 0.163 | 1.27 (0.81 to 1.99) p = 0.289 |
| Glutamate | 25/74 (34%) | 75/160 (47%) | 1.64 (1.05 to 2.59) p = 0.031 | 1.67 (1.06 to 2.63) p = 0.028 |
| 1-year all-cause mortality | ||||
| Glutamine | 31/75 (41%) | 83/159 (52%) | 1.39 (0.92 to 2.10) p = 0.119 | 1.31 (0.86 to 1.99) p = 0.203 |
| Glutamate | 28/74(38%) | 86/160 (54%) | 1.68 (1.10 to 2.58) p = 0.017 | 1.69 (1.10 to 2.59) p = 0.017 |
| 2-year all-cause mortality | ||||
| Glutamine | 41/75 (55%) | 95/159 (60%) | 1.23 (0.85 to 1.77) p = 0.269 | 1.20 (0.83 to 1.74) p = 0.331 |
| Glutamate | 33/74 (45%) | 103/160 (64%) | 1.76 (1.19 to 2.60) p = 0.005 | 1.78 (1.20 to 2.64) p = 0.004 |
| 3-year all-cause mortality | ||||
| Glutamine | 41/75 (55%) | 99/159 (62%) | 1.28 (0.89 to 1.84) p = 0.191 | 1.25 (0.86 to 1.80) p = 0.238 |
| Glutamate | 38/74 (51%) | 102/160 (64%) | 1.53 (1.05 to 2.22) p = 0.025 | 1.57 (1.08 to 2.28) p = 0.019 |
| 5-year all-cause mortality | ||||
| Glutamine | 45/75 (60%) | 108/159 (68%) | 1.28 (0.90 to 1.81) p = 0.167 | 1.24 (0.87 to 1.77) p = 0.224 |
| Glutamate | 46/74 (62%) | 107/160 (67%) | 1.38 (0.98 to 1.96) p = 0.067 | 1.42 (1.00 to 2.01) p = 0.050 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wunderle, C.; von Arx, D.; Mueller, S.C.; Bernasconi, L.; Neyer, P.; Tribolet, P.; Stanga, Z.; Mueller, B.; Schuetz, P. Association of Glutamine and Glutamate Metabolism with Mortality among Patients at Nutritional Risk—A Secondary Analysis of the Randomized Clinical Trial EFFORT. Nutrients 2024, 16, 222. https://doi.org/10.3390/nu16020222
Wunderle C, von Arx D, Mueller SC, Bernasconi L, Neyer P, Tribolet P, Stanga Z, Mueller B, Schuetz P. Association of Glutamine and Glutamate Metabolism with Mortality among Patients at Nutritional Risk—A Secondary Analysis of the Randomized Clinical Trial EFFORT. Nutrients. 2024; 16(2):222. https://doi.org/10.3390/nu16020222
Chicago/Turabian StyleWunderle, Carla, Diana von Arx, Sydney Chiara Mueller, Luca Bernasconi, Peter Neyer, Pascal Tribolet, Zeno Stanga, Beat Mueller, and Philipp Schuetz. 2024. "Association of Glutamine and Glutamate Metabolism with Mortality among Patients at Nutritional Risk—A Secondary Analysis of the Randomized Clinical Trial EFFORT" Nutrients 16, no. 2: 222. https://doi.org/10.3390/nu16020222
APA StyleWunderle, C., von Arx, D., Mueller, S. C., Bernasconi, L., Neyer, P., Tribolet, P., Stanga, Z., Mueller, B., & Schuetz, P. (2024). Association of Glutamine and Glutamate Metabolism with Mortality among Patients at Nutritional Risk—A Secondary Analysis of the Randomized Clinical Trial EFFORT. Nutrients, 16(2), 222. https://doi.org/10.3390/nu16020222






