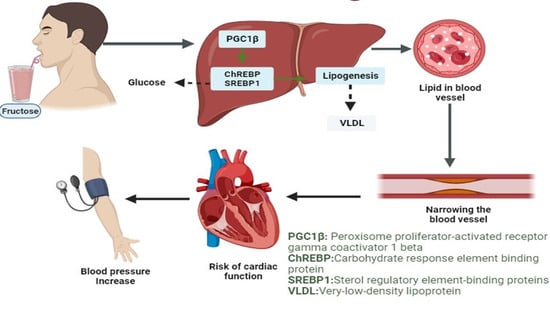
Acute Intake of Fructose Increases Arterial Pressure in Humans: A Meta-Analysis and Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
- Studies should include human subjects.
- Only studies evaluating acute fructose loads were included.
- The selected articles must have a normal diet group for comparison with the fructose diet group.
- The articles should have two parts: the starting and ending points of fructose in-take humans.
- The data addresses regulation of prediabetic and diabetic conditions as well as hypertension.
- All the selected data should be presented in either tabulated form or figure with the standard deviation (SD) or standard error (SE).
- The data of the selected articles must be well organized and described. Humans must be free from pre-existing metabolic and cardiovascular diseases.
- Subjects should be nonalcoholic.
- The selected articles must be published in an international peer-reviewed journal within the selected study period. This indicates that preprint or unpublished articles were not considered for this study. Moreover, all selected articles must be written in English.
2.3. Study Classification
2.4. Data Extraction
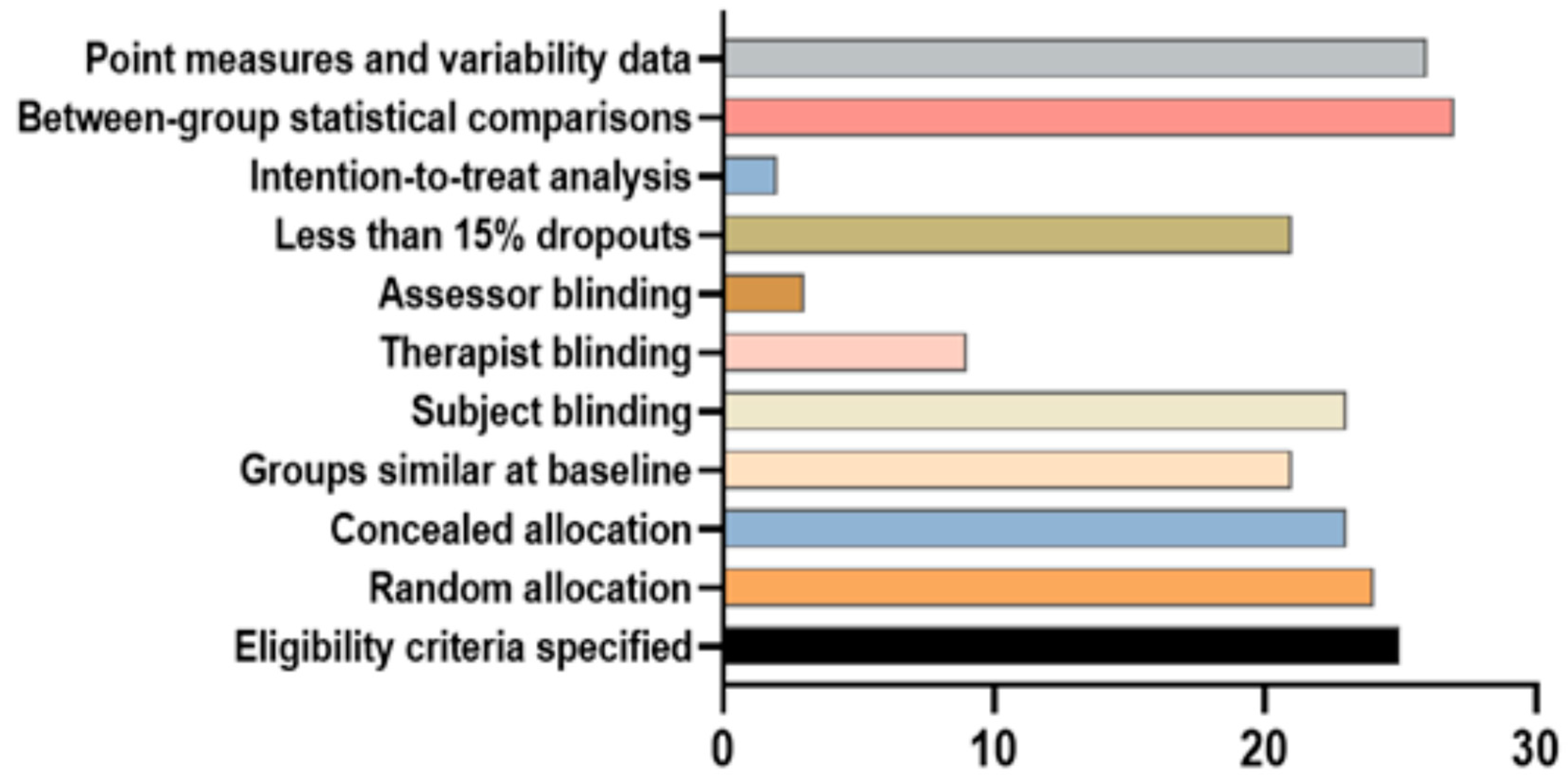
2.5. Study Quality Assessment
2.6. Statistical Analysis
3. Results
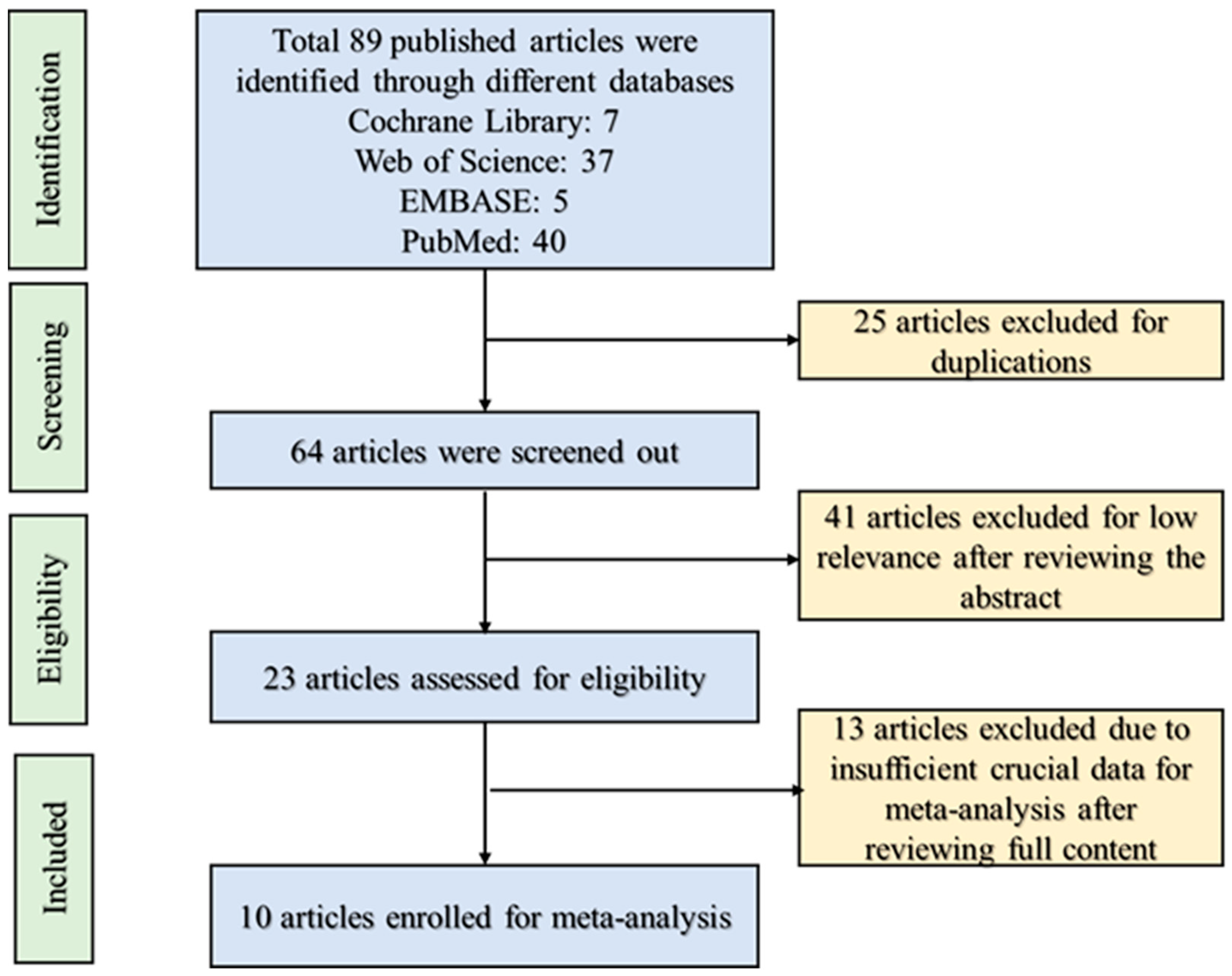
3.1. Study Retrieval and Selection
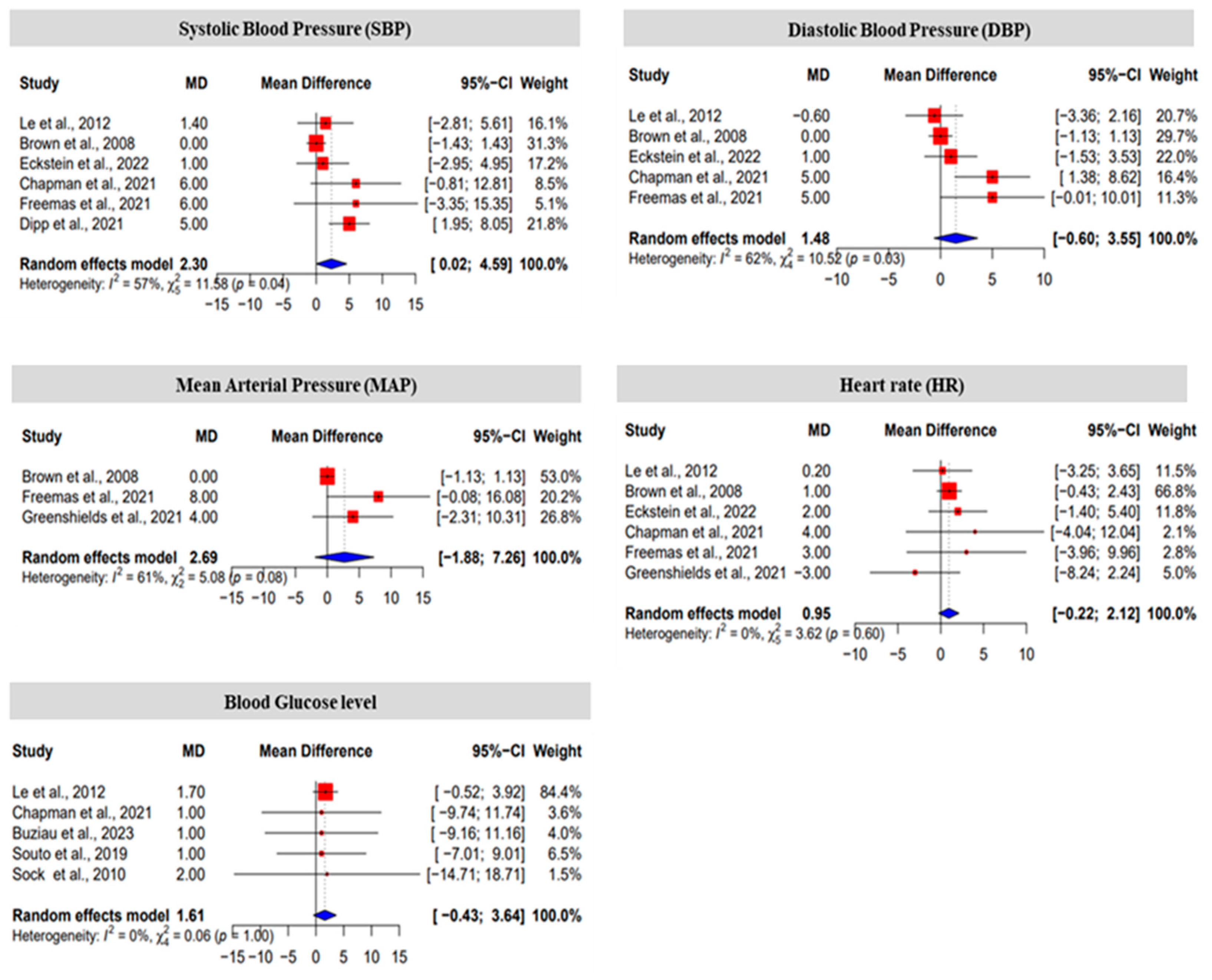
3.2. The Effect Size of Fructose on Blood Pressure, Heart Rate and Blood Glucose Level
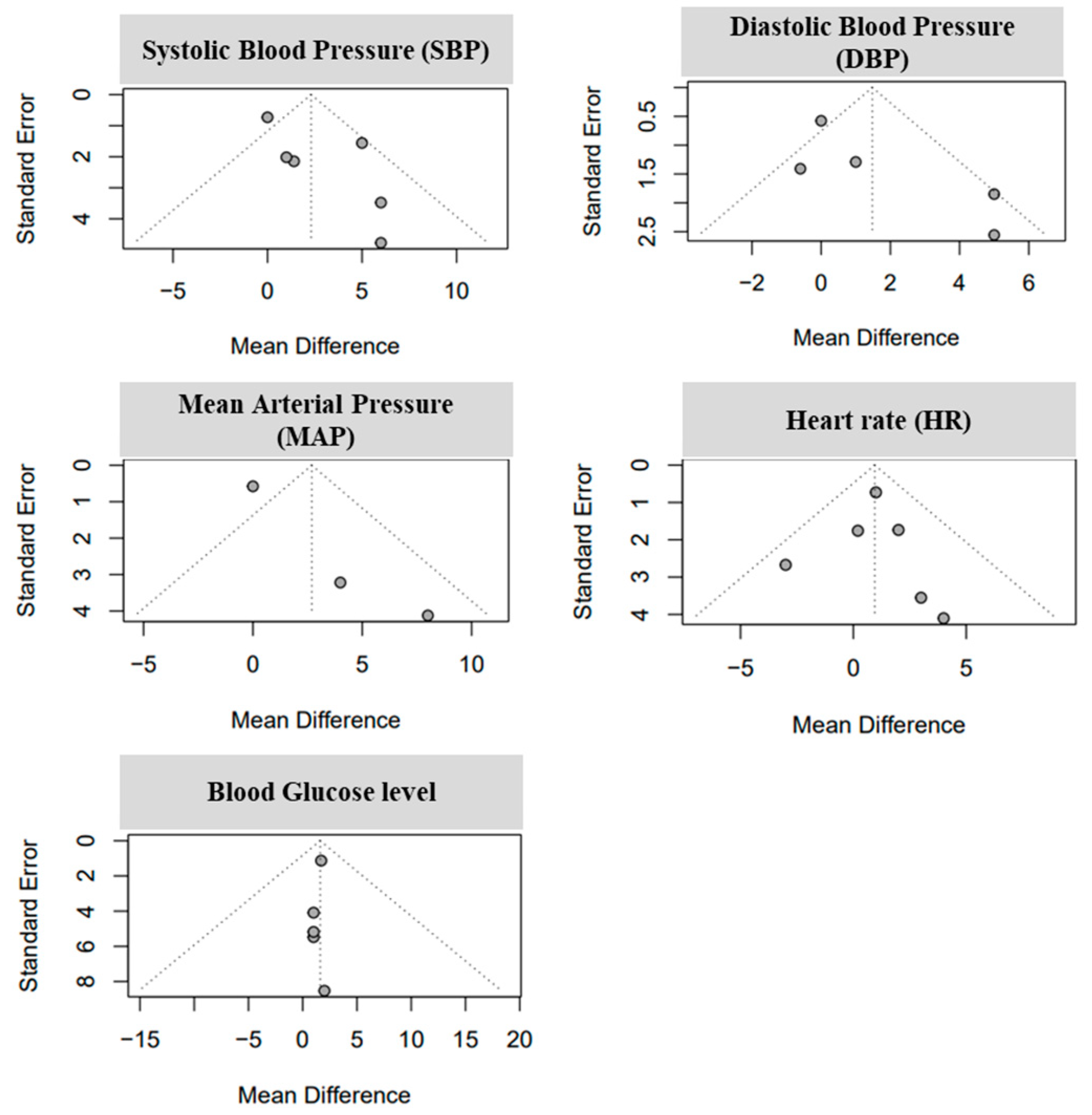
3.3. Analysis of Study Standariezed Mean Difference and Bias Levels
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kass, D.A.; Hare, J.M.; Georgakopoulos, D. Murine Cardiac Function. Circ. Res. 1998, 82, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.L.; Wolfe, L.A. Physiological adaptation in early human pregnancy: Adaptation to balance maternal-fetal demands. Appl. Physiol. Nutr. Metab. 2006, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, M.L.; Kassiri, Z.; Virag, J.A.; de Castro Brás, L.E.; Scherrer-Crosbie, M. Guidelines for measuring cardiac physiology in mice. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H733–H752. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, J.; Asselbergs, F.W.; Bakkers, J.; Batkai, S.; Bertrand, L.; Bezzina, C.R.; Bot, I.; Brundel, B.J.; Carrier, L.; Chamuleau, S. Animal models and animal-free innovations for cardiovascular research: Current status and routes to be explored. Consensus document of the ESC working group on myocardial function and the ESC Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 2022, 118, 3016–3051. [Google Scholar] [CrossRef] [PubMed]
- Côté, F.; Fligny, C.; Fromes, Y.; Mallet, J.; Vodjdani, G. Recent advances in understanding serotonin regulation of cardiovascular function. Trends Mol. Med. 2004, 10, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.C.; Davisson, R.L. Redox signaling in central neural regulation of cardiovascular function. Prog. Biophys. Mol. Biol. 2004, 84, 125–149. [Google Scholar] [CrossRef]
- Dampney, R.A. Central mechanisms regulating coordinated cardiovascular and respiratory function during stress and arousal. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2015, 309, R429–R443. [Google Scholar] [CrossRef]
- Yu, E.; Malik, V.S.; Hu, F.B. Cardiovascular disease prevention by diet modification: JACC Health Promotions Series. J. Am. Coll. Cardiol. 2018, 72, 2951–2963. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Klein, A.V.; Kiat, H. The mechanisms underlying fructose-induced hypertension: A review. J. Hypertens. 2015, 33, 912. [Google Scholar] [CrossRef]
- Levanovich, P.E.; Diaczok, A.; Rossi, N.F. Clinical and Molecular Perspectives of Monogenic Hypertension. Curr. Hypertens. Rev. 2020, 16, 91–107. [Google Scholar] [CrossRef]
- Gordish, K.L.; Kassem, K.M.; Ortiz, P.A.; Beierwaltes, W.H. Moderate (20%) fructose-enriched diet stimulates salt-sensitive hypertension with increased salt retention and decreased renal nitric oxide. Physiol. Rep. 2017, 5, e13162. [Google Scholar] [CrossRef] [PubMed]
- Garvin, J.L.; Herrera, M.; Ortiz, P.A. Regulation of renal NaCl transport by nitric oxide, endothelin, and ATP: Clinical implications. Annu. Rev. Physiol. 2011, 73, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Ha, V.; Sievenpiper, J.L.; de Souza, R.J.; Chiavaroli, L.; Wang, D.D.; Cozma, A.I.; Mirrahimi, A.; Yu, M.E.; Carleton, A.J.; Dibuono, M. Effect of fructose on blood pressure: A systematic review and meta-analysis of controlled feeding trials. Hypertension 2012, 59, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Jayalath, V.H.; Sievenpiper, J.L.; de Souza, R.J.; Ha, V.; Mirrahimi, A.; Santaren, I.D.; Blanco Mejia, S.; Di Buono, M.; Jenkins, A.L.; Leiter, L.A. Total fructose intake and risk of hypertension: A systematic review and meta-analysis of prospective cohorts. J. Am. Coll. Nutr. 2014, 33, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Mok, A.; Rangan, A.M.; Louie, J.C.Y. Association of free sugar intake with blood pressure and obesity measures in Australian adults. Eur. J. Nutr. 2020, 59, 651–659. [Google Scholar] [CrossRef]
- Zenner, Z.P.; Gordish, K.L.; Beierwaltes, W.H. Free radical scavenging reverses fructose-induced salt-sensitive hypertension. Integr. Blood Press. Control 2018, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Soncrant, T.; Komnenov, D.; Beierwaltes, W.H.; Chen, H.; Wu, M.; Rossi, N.F. Bilateral renal cryodenervation decreases arterial pressure and improves insulin sensitivity in fructose-fed Sprague-Dawley rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R529–R538. [Google Scholar] [CrossRef]
- Béghin, L.; Huybrechts, I.; Drumez, E.; Kersting, M.; Walker, R.W.; Kafatos, A.; Molnar, D.; Manios, Y.; Moreno, L.A.; De Henauw, S. High fructose intake contributes to elevated diastolic blood pressure in adolescent girls: Results from the HELENA study. Nutrients 2021, 13, 3608. [Google Scholar] [CrossRef]
- Ngo Sock, E.T.; Lê, K.A.; Ith, M.; Kreis, R.; Boesch, C.; Tappy, L. Effects of a short-term overfeeding with fructose or glucose in healthy young males. Br. J. Nutr. 2010, 103, 939–943. [Google Scholar] [CrossRef]
- Marriott, B.P.; Cole, N.; Lee, E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J. Nutr. 2009, 139, 1228s–1235s. [Google Scholar] [CrossRef] [PubMed]
- Giussani, M.; Lieti, G.; Orlando, A.; Parati, G.; Genovesi, S. Fructose Intake, Hypertension and Cardiometabolic Risk Factors in Children and Adolescents: From Pathophysiology to Clinical Aspects. A Narrative Review. Front. Med. 2022, 9, 792949. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Chun, B.Y.; Park, P.S.; Choi, B.Y.; Kim, M.K.; Shin, M.H.; Lee, Y.H.; Shin, D.H.; Kim, S.K. Higher consumption of sugar-sweetened soft drinks increases the risk of hyperuricemia in Korean population: The Korean Multi-Rural Communities Cohort Study. Semin. Arthritis Rheum. 2014, 43, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Song, E.K.; Moser, D.K.; Kang, S.M.; Lennie, T.A. Self-reported Adherence to a Low-Sodium Diet and Health Outcomes in Patients With Heart Failure. J. Cardiovasc. Nurs. 2016, 31, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.; Roem, J.; Ducharme-Smith, K.; Vizthum, D.; Lu, M.; Agrawal, P.; Urbina, E.M.; Brady, T.M. Association of Sodium and Sugar-Sweetened Beverage Intake with Cardiovascular Disease Risk Factors in Adolescents and Young Adults with Obesity. Clin. Pediatr. 2023, 2023, 00099228231186666. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, K.; Sun, Y.; Yu, B.; Tan, X.; Lu, Y.; Wang, Y.; Xia, F.; Wang, N. Sweetened beverages and incident heart failure. Eur. J. Prev. Cardiol. 2023, 30, 1361–1370. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Hossain, R.; Herrera-Bravo, J.; Islam, M.T.; Atolani, O.; Adeyemi, O.S.; Owolodun, O.A.; Kambizi, L.; Dastan, S.D.; Calina, D.; et al. Natural antioxidants from some fruits, seeds, foods, natural products, and associated health benefits: An update. Food Sci. Nutr. 2023, 11, 1657–1670. [Google Scholar] [CrossRef]
- Warrens, M.J. Kappa coefficients for dichotomous-nominal classifications. Adv. Data Anal. Classif. 2021, 15, 193–208. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Siddiqui, S.H.; Khan, M.; Choe, H.; Kang, D.; Shim, K. HSP expression depends on its molecular construction and different organs of the chicken: A meta-analysis. Sci. Rep. 2022, 12, 14901. [Google Scholar] [CrossRef]
- Moseley, A.M.; Herbert, R.D.; Sherrington, C.; Maher, C.G. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro). Aust. J. Physiother. 2002, 48, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.F.; Ferreira, P.H.; Micheletti, J.K.; de Almeida, A.C.; Lemes, Í.R.; Vanderlei, F.M.; Netto Junior, J.; Pastre, C.M. Can Water Temperature and Immersion Time Influence the Effect of Cold Water Immersion on Muscle Soreness? A Systematic Review and Meta-Analysis. Sports Med. 2016, 46, 503–514. [Google Scholar] [CrossRef] [PubMed]
- van Enst, W.A.; Ochodo, E.; Scholten, R.J.; Hooft, L.; Leeflang, M.M. Investigation of publication bias in meta-analyses of diagnostic test accuracy: A meta-epidemiological study. BMC Med. Res. Methodol. 2014, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Kacker, R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials 2007, 28, 105–114. [Google Scholar] [CrossRef]
- Le, M.T.; Frye, R.F.; Rivard, C.J.; Cheng, J.; McFann, K.K.; Segal, M.S.; Johnson, R.J.; Johnson, J.A. Effects of high-fructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects. Metab. Clin. Exp. 2012, 61, 641–651. [Google Scholar] [CrossRef]
- Brown, C.M.; Dulloo, A.G.; Yepuri, G.; Montani, J.P. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R730–R737. [Google Scholar] [CrossRef]
- Eckstein, M.L.; Brockfeld, A.; Haupt, S.; Schierbauer, J.R.; Zimmer, R.T.; Wachsmuth, N.B.; Zunner, B.E.M.; Zimmermann, P.; Erlmann, M.; Obermayer-Pietsch, B.; et al. Acute Changes in Heart Rate Variability to Glucose and Fructose Supplementation in Healthy Individuals: A Double-Blind Randomized Crossover Placebo-Controlled Trial. Biology 2022, 11, 338. [Google Scholar] [CrossRef]
- Chapman, C.L.; Reed, E.L.; Worley, M.L.; Pietrafesa, L.D.; Kueck, P.J.; Bloomfield, A.C.; Schlader, Z.J.; Johnson, B.D. Sugar-sweetened soft drink consumption acutely decreases spontaneous baroreflex sensitivity and heart rate variability. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R641–R652. [Google Scholar] [CrossRef]
- Freemas, J.A.; Greenshields, J.T.; Baker, T.; Carter, S.J.; Johnson, B.D.; Schlader, Z.J. Arterial stiffness is not acutely modified by consumption of a caffeinated soft drink sweetened with high-fructose corn syrup in young healthy adults. Physiol. Rep. 2021, 9, e14777. [Google Scholar] [CrossRef]
- Greenshields, J.T.; Keeler, J.M.; Freemas, J.A.; Baker, T.B.; Johnson, B.D.; Carter, S.J.; Schlader, Z.J. Cutaneous microvascular vasodilatory consequences of acute consumption of a caffeinated soft drink sweetened with high-fructose corn syrup. Physiol. Rep. 2021, 9, e15074. [Google Scholar] [CrossRef]
- Dipp, T.; Moraes-Silva, I.C.; Dos Santos, F.; Casali, K.R.; Morris, M.; Signori, L.U.; De Angelis, K.; Aletti, F.; Irigoyen, M.C.; Plentz, R.D.M.; et al. Acute ingestion of a high-fructose drink impairs vascular autonomic modulation and reflex control of blood pressure in first-degree relatives of diabetic patients. Diabetes Res. Clin. Pract. 2021, 177, 108793. [Google Scholar] [CrossRef] [PubMed]
- Buziau, A.M.; Scheijen, J.; Stehouwer, C.D.A.; Schalkwijk, C.G.; Brouwers, M. Effects of fructose added to an oral glucose tolerance test on plasma glucose excursions in healthy adults. Metabol. Open 2023, 18, 100245. [Google Scholar] [CrossRef] [PubMed]
- Souto, D.L.; Lima, É.D.S.; Dantas, J.R.; Zajdenverg, L.; Rodacki, M.; Rosado, E.L. Postprandial metabolic effects of fructose and glucose in type 1 diabetes patients: A pilot randomized crossover clinical trial. Arch. Endocrinol. Metab. 2019, 63, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Dianat, M.; Veisi, A.; Ahangarpour, A.; Fathi Moghaddam, H. The effect of hydro-alcoholic celery (Apiumgraveolens) leaf extract on cardiovascular parameters and lipid profile in animal model of hypertension induced by fructose. Avicenna J. Phytomed. 2015, 5, 203–209. [Google Scholar]
- Xing, S.S.; Bi, X.P.; Tan, H.W.; Zhang, Y.; Xing, Q.C.; Zhang, W. Overexpression of interleukin-18 aggravates cardiac fibrosis and diastolic dysfunction in fructose-fed rats. Mol. Med. 2010, 16, 465–470. [Google Scholar] [CrossRef]
- Komnenov, D.; Levanovich, P.E.; Perecki, N.; Chung, C.S.; Rossi, N.F. Aortic Stiffness and Diastolic Dysfunction in Sprague Dawley Rats Consuming Short-Term Fructose Plus High Salt Diet. Integr. Blood Press. Control 2020, 13, 111–124. [Google Scholar] [CrossRef]
- Komnenov, D.; Rossi, N.F. Fructose-induced salt-sensitive blood pressure differentially affects sympathetically mediated aortic stiffness in male and female Sprague-Dawley rats. Physiol. Rep. 2023, 11, e15687. [Google Scholar] [CrossRef]
- Busnatu, S.S.; Salmen, T.; Pana, M.A.; Rizzo, M.; Stallone, T.; Papanas, N.; Popovic, D.; Tanasescu, D.; Serban, D.; Stoian, A.P. The Role of Fructose as a Cardiovascular Risk Factor: An Update. Metabolites 2022, 12, 67. [Google Scholar] [CrossRef]
- Perret-Guillaume, C.; Joly, L.; Benetos, A. Heart rate as a risk factor for cardiovascular disease. Prog. Cardiovasc. Dis. 2009, 52, 6–10. [Google Scholar] [CrossRef]
- Buckley, L.F.; Baker, W.L.; Van Tassell, B.W.; Cohen, J.B.; Alkhezi, O.; Bress, A.P.; Dixon, D.L. Systolic blood pressure time in target range and major adverse kidney and cardiovascular events. Hypertension 2023, 80, 305–313. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Lazaridis, A.; Anyfanti, P.; Yiannaki, E.; Dolgyras, P.; Nikolaidou, B.; Vasileiadis, I.; Alexandrou, M.E.; Margouta, A.; Markala, D. Circulating microvesicles across a population with various degree of cardiovascular burden are associated with systolic blood pressure. J. Hum. Hypertens. 2023, 37, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Picone, D.S.; Stoneman, E.; Cremer, A.; Schultz, M.G.; Otahal, P.; Hughes, A.D.; Black, J.A.; Bos, W.J.; Chen, C.-H.; Cheng, H.-M. Sex differences in blood pressure and potential implications for cardiovascular risk management. Hypertension 2023, 80, 316–324. [Google Scholar] [CrossRef] [PubMed]
- DeMers, D.; Wachs, D. Physiology, Mean Arterial Pressure. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538226/ (accessed on 14 December 2023).
- Poznyak, A.V.; Litvinova, L.; Poggio, P.; Sukhorukov, V.N.; Orekhov, A.N. Effect of glucose levels on cardiovascular risk. Cells 2022, 11, 3034. [Google Scholar] [CrossRef] [PubMed]
- Depla, A.; De Wit, L.; Steenhuis, T.; Slieker, M.; Voormolen, D.; Scheffer, P.; De Heus, R.; Van Rijn, B.; Bekker, M. Effect of maternal diabetes on fetal heart function on echocardiography: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2021, 57, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, H.; Goto, T.; Wakami, K.; Kamiya, T.; Ohte, N. Effects of exercise training on cardiac function, exercise capacity, and quality of life in heart failure with preserved ejection fraction: A meta-analysis of randomized controlled trials. Heart Fail. Rev. 2019, 24, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Cañizo-Gómez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef]
- He, L. Metformin and Systemic Metabolism. Trends Pharmacol. Sci. 2020, 41, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Andreadi, A.; Muscoli, S.; Tajmir, R.; Meloni, M.; Muscoli, C.; Ilari, S.; Mollace, V.; Della Morte, D.; Bellia, A.; Di Daniele, N.; et al. Recent Pharmacological Options in Type 2 Diabetes and Synergic Mechanism in Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 1646. [Google Scholar] [CrossRef]
- Nyby, M.D.; Abedi, K.; Smutko, V.; Eslami, P.; Tuck, M.L. Vascular Angiotensin type 1 receptor expression is associated with vascular dysfunction, oxidative stress and inflammation in fructose-fed rats. Hypertens. Res. 2007, 30, 451–457. [Google Scholar] [CrossRef]
- Gatineau, E.; Savary-Auzeloux, I.; Migne, C.; Polakof, S.; Dardevet, D.; Mosoni, L. Chronic Intake of Sucrose Accelerates Sarcopenia in Older Male Rats through Alterations in Insulin Sensitivity and Muscle Protein Synthesis. J. Nutr. 2015, 145, 923–930. [Google Scholar] [CrossRef]
- Trommelen, J.; Fuchs, C.J.; Beelen, M.; Lenaerts, K.; Jeukendrup, A.E.; Cermak, N.M.; van Loon, L.J. Fructose and Sucrose Intake Increase Exogenous Carbohydrate Oxidation during Exercise. Nutrients 2017, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Behers, B.J.; Melchor, J.; Behers, B.M.; Meng, Z.; Swanson, P.J.; Paterson, H.I.; Mendez Araque, S.J.; Davis, J.L.; Gerhold, C.J.; Shah, R.S.; et al. Vitamins and Minerals for Blood Pressure Reduction in the General, Normotensive Population: A Systematic Review and Meta-Analysis of Six Supplements. Nutrients 2023, 15, 4223. [Google Scholar] [CrossRef]
- Hoogeveen, R.C.; Ballantyne, C.M. Residual cardiovascular risk at low LDL: Remnants, lipoprotein (a), and inflammation. Clin. Chem. 2021, 67, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.D. Complexity of Triglyceride-Rich Lipoproteins Remnant Cholesterol with Atherosclerotic Cardiovascular Disease Risk; Oxford University Press: Oxford, UK, 2023; p. zwad064. [Google Scholar]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxidat. Med. Cell. Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Havel, P.J. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup. Am. J. Clin. Nutr. 2008, 88, 1733S–1737S. [Google Scholar] [CrossRef]
- Taskinen, M.R.; Packard, C.J.; Boren, J. Dietary Fructose and the Metabolic Syndrome. Nutrients 2019, 11, 1987. [Google Scholar] [CrossRef]
- Hochuli, M.; Aeberli, I.; Weiss, A.; Hersberger, M.; Troxler, H.; Gerber, P.A.; Spinas, G.A.; Berneis, K. Sugar-sweetened beverages with moderate amounts of fructose, but not sucrose, induce Fatty Acid synthesis in healthy young men: A randomized crossover study. J. Clin. Endocrinol. Metab. 2014, 99, 2164–2172. [Google Scholar] [CrossRef]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef]
- Koivisto, V.A.; Yki-Järvinen, H. Fructose and insulin sensitivity in patients with type 2 diabetes. J. Intern. Med. 1993, 233, 145–153. [Google Scholar] [CrossRef]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and hepatic insulin resistance. Crit. Rev. Clin. Lab. Sci. 2020, 57, 308–322. [Google Scholar] [CrossRef]
- Zavaroni, I.; Chen, Y.D.; Reaven, G.M. Studies of the mechanism of fructose-induced hypertriglyceridemia in the rat. Metab. Clin. Exp. 1982, 31, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.; Botezelli, J.D.; da Cruz Rodrigues, K.C.; Mekary, R.A.; Cintra, D.E.; Pauli, J.R.; da Silva, A.S.R.; Ropelle, E.R.; de Moura, L.P. Fructose Consumption in the Development of Obesity and the Effects of Different Protocols of Physical Exercise on the Hepatic Metabolism. Nutrients 2017, 9, 405. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, C.; Ji, G.; Zhang, L. The Contribution of Dietary Fructose to Non-alcoholic Fatty Liver Disease. Front. Pharmacol. 2021, 12, 783393. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.S.; Neeland, I.J. Body Fat Distribution, Diabetes Mellitus, and Cardiovascular Disease: An Update. Curr. Cardiol. Rep. 2023, 25, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Mellor, K.M.; Wendt, I.R.; Ritchie, R.H.; Delbridge, L.M. Fructose diet treatment in mice induces fundamental disturbance of cardiomyocyte Ca2+ handling and myofilament responsiveness. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H964–H972. [Google Scholar] [CrossRef] [PubMed]
- Annandale, M.; Daniels, L.J.; Li, X.; Neale, J.P.H.; Chau, A.H.L.; Ambalawanar, H.A.; James, S.L.; Koutsifeli, P.; Delbridge, L.M.D.; Mellor, K.M. Fructose Metabolism and Cardiac Metabolic Stress. Front. Pharmacol. 2021, 12, 695486. [Google Scholar] [CrossRef]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef]
- Dai, S.; McNeill, J.H. Fructose-induced hypertension in rats is concentration- and duration-dependent. J. Pharmacol. Toxicol. Methods 1995, 33, 101–107. [Google Scholar] [CrossRef]
- Herrera, J.; Ferrebuz, A.; MacGregor, E.G.; Rodriguez-Iturbe, B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J. Am. Soc. Nephrol. 2006, 17, S218–S225. [Google Scholar] [CrossRef]
- Sanguesa, G.; Shaligram, S.; Akther, F.; Roglans, N.; Laguna, J.C.; Rahimian, R.; Alegret, M. Type of supplemented simple sugar, not merely calorie intake, determines adverse effects on metabolism and aortic function in female rats. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H289–H304. [Google Scholar] [CrossRef]
- Takagawa, Y.; Berger, M.E.; Hori, M.T.; Tuck, M.L.; Golub, M.S. Long-term fructose feeding impairs vascular relaxation in rat mesenteric arteries. Am. J. Hypertens. 2001, 14, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Levanovich, P.E.; Daugherty, A.M.; Komnenov, D.; Rossi, N.F. Dietary fructose and high salt in young male Sprague Dawley rats induces salt-sensitive changes in renal function in later life. Physiol. Rep. 2022, 10, e15456. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Kuwabara, M.; Andres-Hernando, A.; Li, N.; Cicerchi, C.; Jensen, T.; Orlicky, D.J.; Roncal-Jimenez, C.A.; Ishimoto, T.; Nakagawa, T.; et al. High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. USA 2018, 115, 3138–3143. [Google Scholar] [CrossRef]
- Schwarz, J.; Acheson, K.; Tappy, L.; Piolino, V.; Muller, M.; Felber, J.; Jéquier, E. Thermogenesis and fructose metabolism in humans. Am. J. Physiol.-Endocrinol. Metab. 1992, 262, E591–E598. [Google Scholar] [CrossRef]
- Martinez, F.J.; Rizza, R.A.; Romero, J.C. High-fructose feeding elicits insulin resistance, hyperinsulinism, and hypertension in normal mongrel dogs. Hypertension 1994, 23, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, Z.; Chang, R.; Zeng, C.; Zhao, Y. High-Fructose Diet Induces Cardiac Dysfunction via Macrophage Recruitment in Adult Mice. J. Cardiovasc. Pharmacol. Ther. 2023, 28, 10742484231162249. [Google Scholar] [CrossRef] [PubMed]
- Szűcs, G.; Sója, A.; Péter, M.; Sárközy, M.; Bruszel, B.; Siska, A.; Földesi, I.; Szabó, Z.; Janáky, T.; Vígh, L. Prediabetes induced by fructose-enriched diet influences cardiac lipidome and proteome and leads to deterioration of cardiac function prior to the development of excessive oxidative stress and cell damage. Oxidat. Med. Cell. Longev. 2019, 2019, 3218275. [Google Scholar] [CrossRef]
- Levanovich, P.E.; Chung, C.S.; Komnenov, D.; Rossi, N.F. Fructose plus High-Salt Diet in Early Life Results in Salt-Sensitive Cardiovascular Changes in Mature Male Sprague Dawley Rats. Nutrients 2021, 13, 3129. [Google Scholar] [CrossRef]
- Iizuka, K. Recent Progress on Fructose Metabolism—Chrebp, Fructolysis, and Polyol Pathway. Nutrients 2023, 15, 1778. [Google Scholar] [CrossRef]
- Li, C.; He, J.; Li, S.; Chen, W.; Bazzano, L.; Sun, X.; Shen, L.; Liang, L.; Shen, Y.; Gu, X.; et al. Novel Metabolites Are Associated with Augmentation Index and Pulse Wave Velocity: Findings from the Bogalusa Heart Study. Am. J. Hypertens. 2019, 32, 547–556. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Xuereb, R.A.; Magri, C.J.; Xuereb, R.G. Arterial Stiffness and its Impact on Cardiovascular Health. Curr. Cardiol. Rep. 2023, 25, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Noronha, J.C.; Khan, T.A.; McGlynn, N.; Back, S.; Grant, S.M.; Kendall, C.W.C.; Sievenpiper, J.L. The Effect of Non-Nutritive Sweetened Beverages on Postprandial Glycemic and Endocrine Responses: A Systematic Review and Network Meta-Analysis. Nutrients 2023, 15, 1050. [Google Scholar] [CrossRef]
- Brown, I.J.; Stamler, J.; Van Horn, L.; Robertson, C.E.; Chan, Q.; Dyer, A.R.; Huang, C.C.; Rodriguez, B.L.; Zhao, L.; Daviglus, M.L.; et al. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: International study of macro/micronutrients and blood pressure. Hypertension 2011, 57, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.K.; Bundy, V.; Kanto, W.; Davis, C.L.; Bernard, P.J.; Zhu, H.; Gutin, B.; Dong, Y. Greater fructose consumption is associated with cardiometabolic risk markers and visceral adiposity in adolescents. J. Nutr. 2012, 142, 251–257. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| SI | Authors [Ref] | Sample Size (n) | Experiment Duration | Treatment (Fructose) | Experimental Condition |
|---|---|---|---|---|---|
| 1 | Le et al., 2012 [35] | 40 | 6 h | 24 oz of cold, carbonated soft drinks sweetened with high fructose |
|
| 2 | Brown et al., 2008 [36] | 15 | 2 h | 60 g fructose (water) |
|
| 3 | Eckstein et al., 2022 [37] | 15 | 2 h | 50% fructose in drinking water |
|
| 4 | Chapman et al., 2021 [38] | 12 | 2 h | Soft drinks contain 50% fructose |
|
| 5 | Freemas et al., 2021 [39] | 13 | 2 h | 500 mL Mountain Dew which contains 59.5% fructose |
|
| 6 | Greenshields et al., 2021 [32,40] | 14 | 2 h | 500 mL Coca Cola |
|
| 7 | Dipp et al., 2021 [41] | 14 | 1 h | Drink contains 100 g fructose, 30 mL lemon juice in 300 mL water |
|
| 8 | Buziau et al., 2023 [42] | 13 | 2 h | 82.5 g dextrose monohydrate and 15 g fructose dissolved 250 mL water |
|
| 9 | Souto et al., 2019 [43] | 7 | 3 h | 75 g fructose dissolved with 200 mL water |
|
| 10 | Ngo Sock et al., 2010 [20] | 11 | 1 h | 3.5 g fructose per kg of meal |
|
| Parameter | 95% Confidence Interval (CI) | Cohen’s d | Effect Size * | |
|---|---|---|---|---|
| Lower | Upper | |||
| Systolic Blood pressure (SBP) | −0.726 | 1.901 | 0.587 | medium |
| Diastolic Blood Pressure (DBP) | −0.853 | 2.137 | 0.642 | medium |
| Mean Arterial Pressure (MAP) | −2.284 | 3.697 | 0.706 | medium |
| Heart Rate (HR) | −0.614 | 1.56 | 0.473 | small |
| Blood Glucose Level | −1.518 | 1.951 | 0.216 | small |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, S.H.; Rossi, N.F. Acute Intake of Fructose Increases Arterial Pressure in Humans: A Meta-Analysis and Systematic Review. Nutrients 2024, 16, 219. https://doi.org/10.3390/nu16020219
Siddiqui SH, Rossi NF. Acute Intake of Fructose Increases Arterial Pressure in Humans: A Meta-Analysis and Systematic Review. Nutrients. 2024; 16(2):219. https://doi.org/10.3390/nu16020219
Chicago/Turabian StyleSiddiqui, Sharif Hasan, and Noreen F. Rossi. 2024. "Acute Intake of Fructose Increases Arterial Pressure in Humans: A Meta-Analysis and Systematic Review" Nutrients 16, no. 2: 219. https://doi.org/10.3390/nu16020219
APA StyleSiddiqui, S. H., & Rossi, N. F. (2024). Acute Intake of Fructose Increases Arterial Pressure in Humans: A Meta-Analysis and Systematic Review. Nutrients, 16(2), 219. https://doi.org/10.3390/nu16020219