The Relationship between Bone Health Parameters, Vitamin D and Iron Status, and Dietary Calcium Intake in Young Males
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Procedures
2.2.1. DXA Measurements
2.2.2. Lumbar Spine BMAD and TBS Measurements
2.2.3. Blood Assays
2.2.4. Intake of Dietary Calcium
2.2.5. Body Composition
2.2.6. Bone Fracture
2.2.7. Statistical Analyses
3. Results
4. Discussion
4.1. Nutrients and Bone Mineral Density
4.2. Nutrients and Trabecular Bone Structure
4.3. Frequency of Fractures
4.4. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Staehelin, H.B.; Orav, J.E.; Stuck, A.E.; Theiler, R.; Wong, J.B.; Egli, A.; Kiel, D.P.; Henschkowski, J. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomised controlled trials. BMJ 2009, 339, b3692. [Google Scholar] [CrossRef]
- Laird, E.; Ward, M.; McSorley, E.; Strain, J.J.; Wallace, J. Vitamin D and bone health; Potential mechanisms. Nutrients 2010, 2, 693–724. [Google Scholar] [CrossRef] [PubMed]
- Ciosek, Ż.; Kot, K.; Kosik-Bogacka, D.; Łanocha-Arendarczyk, N.; Rotter, I. The effects of calcium, magnesium, phosphorus, fluoride, and lead on bone tissue. Biomolecules 2021, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef]
- Queiroz, J.R., Jr.; Cartaxo, M.F.; Paz, S.T.; Tenório, F.D.; Lemos, A.J.; Maia, C.S. Histomorphometry of bone microarchitecture in rats treated with vitamin d and bisphosphonate in the management of osteoporosis. Rev. Bras. Ortop. 2022, 57, 267–272. [Google Scholar]
- Lips, P.; Duong, T.; Oleksik, A.; Black, D.; Cummings, S.; Cox, D.; Nickelsen, T. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: Baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J. Clin. Endocrinol. Metab. 2001, 86, 1212–1221. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2015, 96, 365–408. [Google Scholar] [CrossRef]
- Bikle, D.; Bouillon, R.; Thadhani, R.; Schoenmakers, I. Vitamin D metabolites in captivity? Should we measure free or total 25(OH)D to assess vitamin D status? J. Steroid Biochem. Mol. Biol. 2017, 173, 105–116. [Google Scholar] [CrossRef]
- Bikle, D. Vitamin D: Production, Metabolism, and Mechanisms of Action; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Malczewska-Lenczowska, J.; Sitkowski, D.; Surała, O.; Orysiak, J.; Szczepańska, B.; Witek, K. The association between iron and vitamin D status in female elite athletes. Nutrients 2018, 10, 167. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Yakout, S.; Ghaleb, A.; Hussain, S.D.; Sabico, S. Iron and 25-hydroxyvitamin D in postmenopausal women with osteoporosis. Am. J. Transl. Res. 2022, 14, 1387–1405. [Google Scholar]
- Balogh, E.; Paragh, G.; Jeney, V. Influence of Iron on Bone Homeostasis. Pharmaceuticals 2018, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; An, J. Dietary iron intake and its impact on osteopenia/osteoporosis. BMC Endocr. Disord. 2023, 23, 154. [Google Scholar] [CrossRef] [PubMed]
- Valenti, L.; Varenna, M.; Fracanzani, A.L.; Rossi, V.; Fargion, S.; Sinigaglia, L. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos. Int. 2009, 20, 549–555. [Google Scholar] [CrossRef]
- Jeney, V. Clinical Impact and Cellular Mechanisms of Iron Overload-Associated Bone Loss. Front. Pharmacol. 2017, 8, 77. [Google Scholar] [CrossRef]
- Porthouse, J.; Cockayne, S.; Birks, Y.; Dumville, J.; Iglesias, C.; Puffer, S.; Watt, I.; Torgerson, D.J.; King, C.; Steele, E.; et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ 2005, 330, 1003. [Google Scholar] [CrossRef]
- Boonen, S.; Lips, P.; Bouillon, R.; Bischoff-Ferrari, H.A.; Vanderschueren, D.; Haentjens, P. Need for additional calcium to reduce the risk of hip fracture with vitamin D supplementation: Evidence from a comparative metaanalysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2007, 92, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Gleason, L.; Villareal, D.T. Vitamin K and bone health in older adults. J. Nutr. Gerontol. Geriatr. 2014, 33, 10–22. [Google Scholar] [CrossRef]
- Avenell, A.; Gillespie, W.J.; Gillespie, L.D.; O’Connell, D. Vitamin D and vitamin D analogues for preventing fractures associated with involutional and post-menopausal osteoporosis. Cochrane Database Syst. Rev. 2005, 20, CD000227. [Google Scholar]
- Allison, R.J.; Farooq, A.; Hamilton, B.; Close, G.L.; Wilson, M.G. No association between vitamin D deficiency and markers of bone health in athletes. Med. Sci. Sports Exerc. 2015, 47, 782–788. [Google Scholar] [CrossRef]
- Allison, R.J.; Farooq, A.; Cherif, A.; Hamilton, B.; Close, G.L.; Wilson, M.G. Why don’t serum vitamin D concentrations associate with BMD by DXA? A case of being “bound” to the wrong assay? Implications for vitamin D screening. Br. J. Sports Med. 2018, 52, 522–526. [Google Scholar] [CrossRef]
- Marwaha, R.K.; Tandon, N.; Garg, M.K.; Kanwar, R.; Narang, A.; Sastry, A.; Saberwal, A.; Bhadra, K.; Mithal, A. Bone health in healthy Indian population aged 50 years and above. Osteoporos. Int. 2011, 22, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Rao, S.D. Effect of serum 25-hydroxyvitamin D concentrations on skeletal mineralization in black and white women. J. Bone Miner. Metab. 2021, 39, 843–850. [Google Scholar] [CrossRef]
- Powe, C.E.; Ricciardi, C.; Berg, A.H.; Erdenesanaa, D.; Collerone, G.; Ankers, E.; Wenger, J.; Karumanchi, S.A.; Thadhani, R.; Bhan, I. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. J. Bone Miner. Res. 2011, 26, 1609–1616. [Google Scholar] [CrossRef]
- Malmstroem, S.; Rejnmark, L.; Imboden, J.B.; Shoback, D.M.; Bikle, D.D. Current assays to determine free 25-hydroxyvitamin D in serum. J. AOAC Int. 2017, 100, 1323–1327. [Google Scholar] [CrossRef] [PubMed]
- Tsuprykov, O.; Chen, X.; Hocher, C.F.; Skoblo, R.; Yin, L.; Hocher, B. Why should we measure free 25(OH) vitamin D? J. Steroid Biochem. Mol. Biol. 2018, 180, 87–104. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.M.; Baldock, P.A.; Thomas, G.P.; Sims, N.A.; Henderson, N.K.; Hollis, B.; White, C.P.; Sunn, K.L.; Morrison, N.A.; Walsh, W.R.; et al. Increased formation and decreased resorption of bone in mice with elevated vitamin D receptor in mature cells of the osteoblastic lineage. FASEB J. 2000, 14, 1908–1916. [Google Scholar] [CrossRef]
- Shiraishi, A.; Takeda, S.; Masaki, T.; Higuchi, Y.; Uchiyama, Y.; Kubodera, N.; Sato, K.; Ikeda, K.; Nakamura, T.; Matsumoto, T.; et al. Alfacalcidol inhibits bone resorption and stimulates formation in an ovariectomized rat model of osteoporosis: Distinct actions from estrogen. J. Bone Miner. Res. 2000, 15, 770–779. [Google Scholar] [CrossRef]
- Garrahan, M.; Gehman, S.; Rudolph, S.E.; Tenforde, A.S.; Ackerman, K.E.; Popp, K.L.; Bouxsein, M.L.; Sahni, S. Serum 25-Hydroxyvitamin D is Associated with Bone Microarchitecture and Strength in a Multiracial Cohort of Young Adults. J. Clin. Endocrinol. Metab. 2022, 107, E3679–E3688. [Google Scholar] [CrossRef]
- Viljakainen, H.T. Factors influencing bone mass accrual: Focus on nutritional aspects. Proc. Nutr. Soc. 2016, 75, 415–419. [Google Scholar] [CrossRef]
- Crabtree, N.J.; Arabi, A.; Bachrach, L.K.; Fewtrell, M.; El-Hajj Fuleihan, G.; Kecskemethy, H.H.; Jaworski, M.; Gordon, C.M. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: The revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 225–242. [Google Scholar] [CrossRef]
- Skeletal Health Assessment in Children from Infancy to Adolescence. Official Pediatric Positions of the ISCD updated in 2019. Available online: https://iscd.org/learn/official-positions/pediatric-positions/ (accessed on 1 September 2023).
- Cook, J.D.; Flowers, C.H.; Skikne, B.S. The quantitative assessment of body iron. Blood 2003, 101, 3359–3364. [Google Scholar] [CrossRef] [PubMed]
- Szymelfejnik, E.; Wądołowska, L.; Cichon, R.; Przysławski, J.; Bolesławska, I. Dairy products frequency questionnaire (ADOS-CA) calibration for calcium intake evaluation. Pol. J. Food Nutr. Sci. 2006, 56, 229–236. [Google Scholar]
- Cohen, B.H. Explaining Psychological Statistics; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 0470007184. [Google Scholar]
- King, B.M.; Rosopa, P.J.; Minium, E.W. Statistical Reasoning in the Behavioral Sciences; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 1119379733. [Google Scholar]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Normy Żywienia dla Populacji Polski i Ich Zastosowanie; Narodowy Instytut Zdrowia Publicznego-Państwowy Zakład Higieny: Warszawa, Poland, 2020; ISBN 8365870290. [Google Scholar]
- Pekkinen, M.; Viljakainen, H.; Saarnio, E.; Lamberg-Allardt, C.; Mäkitie, O. Vitamin D is a major determinant of bone mineral density at school age. PLoS ONE 2012, 7, e40090. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, M.S.; Grimnes, G.; Figenschau, Y.; Torjesen, P.A.; Almås, B.; Jorde, R. Serum free and bio-available 25-hydroxyvitamin D correlate better with bone density than serum total 25-hydroxyvitamin D. Scand. J. Clin. Lab. Investig. 2014, 74, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lieben, L.; Carmeliet, G. Vitamin D signaling in osteocytes: Effects on bone and mineral homeostasis. Bone 2013, 54, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zittermann, A.; Scheld, K.; Stehle, P. Seasonal variations in vitamin D status and calcium absorption do not influence bone turnover in young women. Eur. J. Clin. Nutr. 1998, 52, 501–506. [Google Scholar] [CrossRef]
- Chun, R.F.; Peercy, B.E.; Orwoll, E.S.; Nielson, C.M.; Adams, J.S.; Hewison, M. Vitamin D and DBP: The free hormone hypothesis revisited. J. Steroid Biochem. Mol. Biol. 2014, 144, 132–137. [Google Scholar] [CrossRef]
- Szabó, B.; Tabák, Á.G.; Toldy, E.; Szekeres, L.; Szili, B.; Bakos, B.; Balla, B.; Kósa, J.P.; Lakatos, P.; Takács, I. The role of serum total and free 25-hydroxyvitamin D and PTH values in defining vitamin D status at the end of winter: A representative survey. J. Bone Miner. Metab. 2017, 35, 83–90. [Google Scholar] [CrossRef]
- Waldron, J.L.; Ashby, H.L.; Cornes, M.P.; Bechervaise, J.; Razavi, C.; Thomas, O.L.; Chugh, S.; Deshpande, S.; Ford, C.; Gama, R. Vitamin D: A negative acute phase reactant. J. Clin. Pathol. 2013, 66, 620–622. [Google Scholar] [CrossRef]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise—A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef]
- Sun, X.; Cao, Z.B.; Taniguchi, H.; Tanisawa, K.; Higuchi, M. Effect of an acute bout of endurance exercise on serum 25(OH)D concentrations in young adults. J. Clin. Endocrinol. Metab. 2017, 102, 3937–3944. [Google Scholar] [CrossRef]
- Katsumata, S.; Katsumata-Tsuboi, R.; Uehara, M.; Suzuki, K. Severe iron deficiency decreases both bone formation and bone resorption in rats. J. Nutr. 2009, 139, 238–243. [Google Scholar] [CrossRef]
- Medeiros, D.M.; Stoecker, B.; Plattner, A.; Jennings, D.; Haub, M. Iron deficiency negatively affects vertebrae and femurs of rats independently of energy intake and body weight. J. Nutr. 2004, 134, 3061–3067. [Google Scholar] [CrossRef] [PubMed]
- Toxqui, L.; Perez-Granados, A.M.; Blanco-Rojo, R.; Wright, I.; de la Piedra, C.; Vaquero, M.P. Low iron status as a factor of increased bone resorption and effects of an iron and vitamin D-fortified skimmed milk on bone remodelling in young Spanish women. Eur. J. Nutr. 2014, 53, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Bijani, A.; Heidari, P.; Hosseini, S.R.; Heidari, B. Serum ferritin levels and bone mineral density in the elderly. Casp. J. Intern. Med. 2018, 9, 232–238. [Google Scholar]
- Peng, P.; Xiao, F.; Gao, S.; Fang, W.; Lin, T.; He, W.; Wei, Q. Association between serum ferritin and bone mineral density in US adults. J. Orthop. Surg. Res. 2022, 17, 494. [Google Scholar] [CrossRef]
- Kindler, J.M.; Lappe, J.M.; Gilsanz, V.; Oberfield, S.; Shepherd, J.A.; Kelly, A.; Winer, K.K.; Kalkwarf, H.J.; Zemel, B.S. Lumbar Spine Bone Mineral Apparent Density in Children: Results from the Bone Mineral Density in Childhood Study. J. Clin. Endocrinol. Metab. 2019, 104, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Greenfield, H.; Zhang, Q.; Du, X.; Ma, G.; Leng, H.F.; Cowell, C.T.; Fraser, D.R. Growth and bone mineral accretion during puberty in Chinese girls: A five-year longitudinal study. J. Bone Miner. Res. 2008, 23, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Hidayat, K.; Zhang, L.L.; Rizzoli, R.; Guo, Y.X.; Zhou, Y.; Shi, Y.J.; Su, H.W.; Liu, B.; Qin, L.Q. The Effects of Dairy Product Supplementation on Bone Health Indices in Children Aged 3 to 18 Years: A Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 1187–1196. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001, 22, 477–501. [Google Scholar] [CrossRef]
- Kuchuk, N.O.; Pluijm, S.M.F.; Van Schoor, N.M.; Looman, C.W.N.; Smit, J.H.; Lips, P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J. Clin. Endocrinol. Metab. 2009, 94, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Bischoff-Ferrari, H.A.; Dawson-Hughes, B.; Willett, W.C.; Staehelin, H.B.; Bazemore, M.G.; Zee, R.Y.; Wong, J.B. Effect of Vitamin D on falls: A meta-analysis. JAMA 2004, 291, 1999–2006. [Google Scholar] [CrossRef] [PubMed]
- Matkovic, V. Calcium metabolism and calcium requirements during skeletal modeling and consolidation of bone mass. Am. J. Clin. Nutr. 1991, 54, S245–S260. [Google Scholar] [CrossRef] [PubMed]
- Matkovic, V.; Heaney, R.P. Calcium balance during human growth: Evidence for threshold behavior. Am. J. Clin. Nutr. 1992, 55, 992–996. [Google Scholar] [CrossRef]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and Mechanobiology of Trabecular Bone: A Review. J. Biomech. Eng. 2015, 137, 0108021. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.I.; Choi, H.J.; Ohnaru, K.; Sone, T. Differential effects of jump versus running exercise on trabecular bone architecture and strength in rats. Phys. Act. Nutr. 2020, 24, 1–8. [Google Scholar] [CrossRef]
- Jones, I.E.; Taylor, R.W.; Williams, S.M.; Manning, P.J.; Goulding, A. Four-year gain in bone mineral in girls with and without past forearm fractures: A DXA study. J. Bone Miner. Res. 2002, 17, 1065–1072. [Google Scholar] [CrossRef]
- Tenforde, A.S.; Parziale, A.L.; Popp, K.L.; Ackerman, K.E. Low Bone Mineral Density in Male Athletes Is Associated with Bone Stress Injuries at Anatomic Sites With Greater Trabecular Composition. Am. J. Sports Med. 2018, 46, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Lassandro, G.; Chiarito, M.; Valente, F.; Ciaccia, L.; Giordano, P. How physical activity across the lifespan can reduce the impact of bone ageing: A literature review. Int. J. Environ. Res. Public Health 2020, 17, 1862. [Google Scholar] [CrossRef]
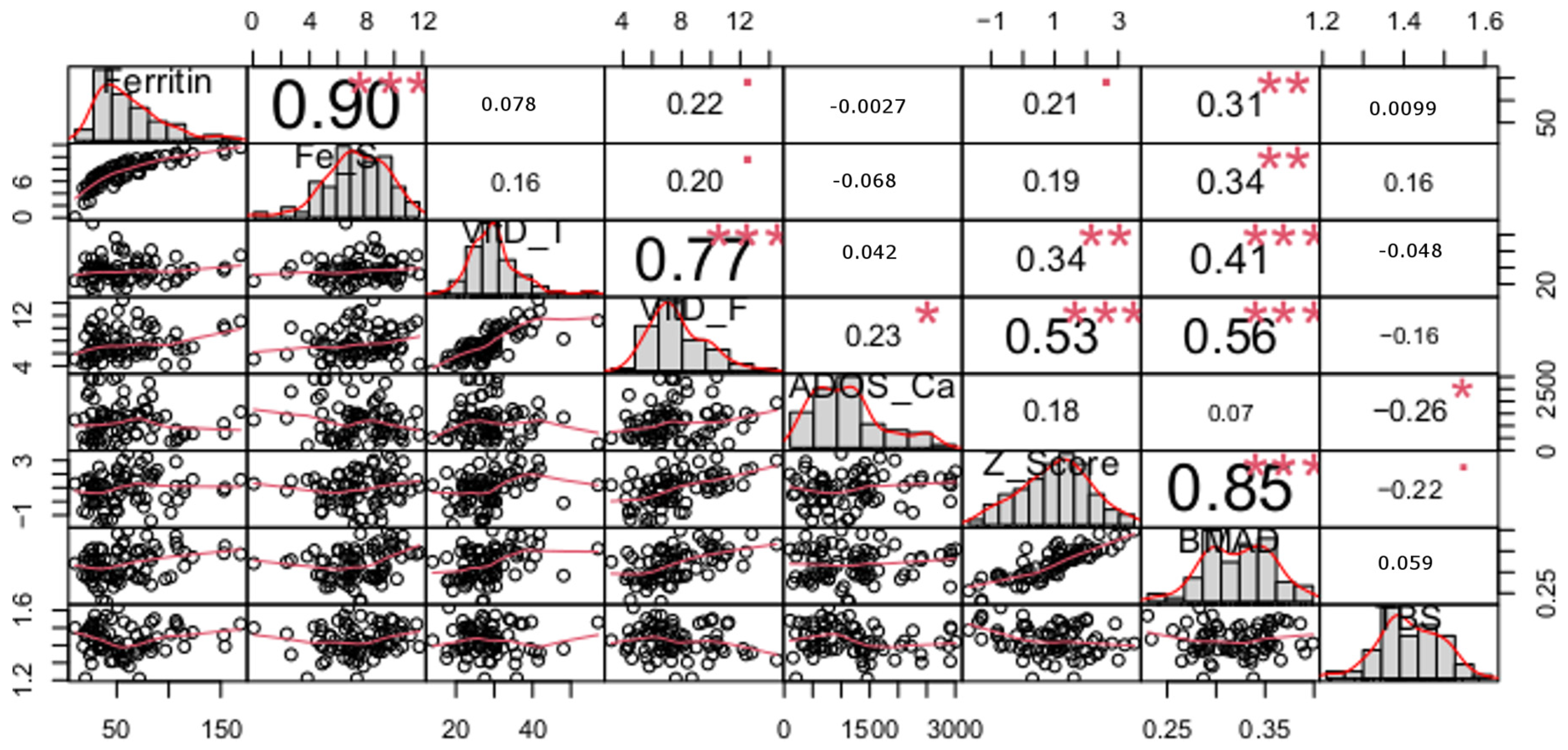
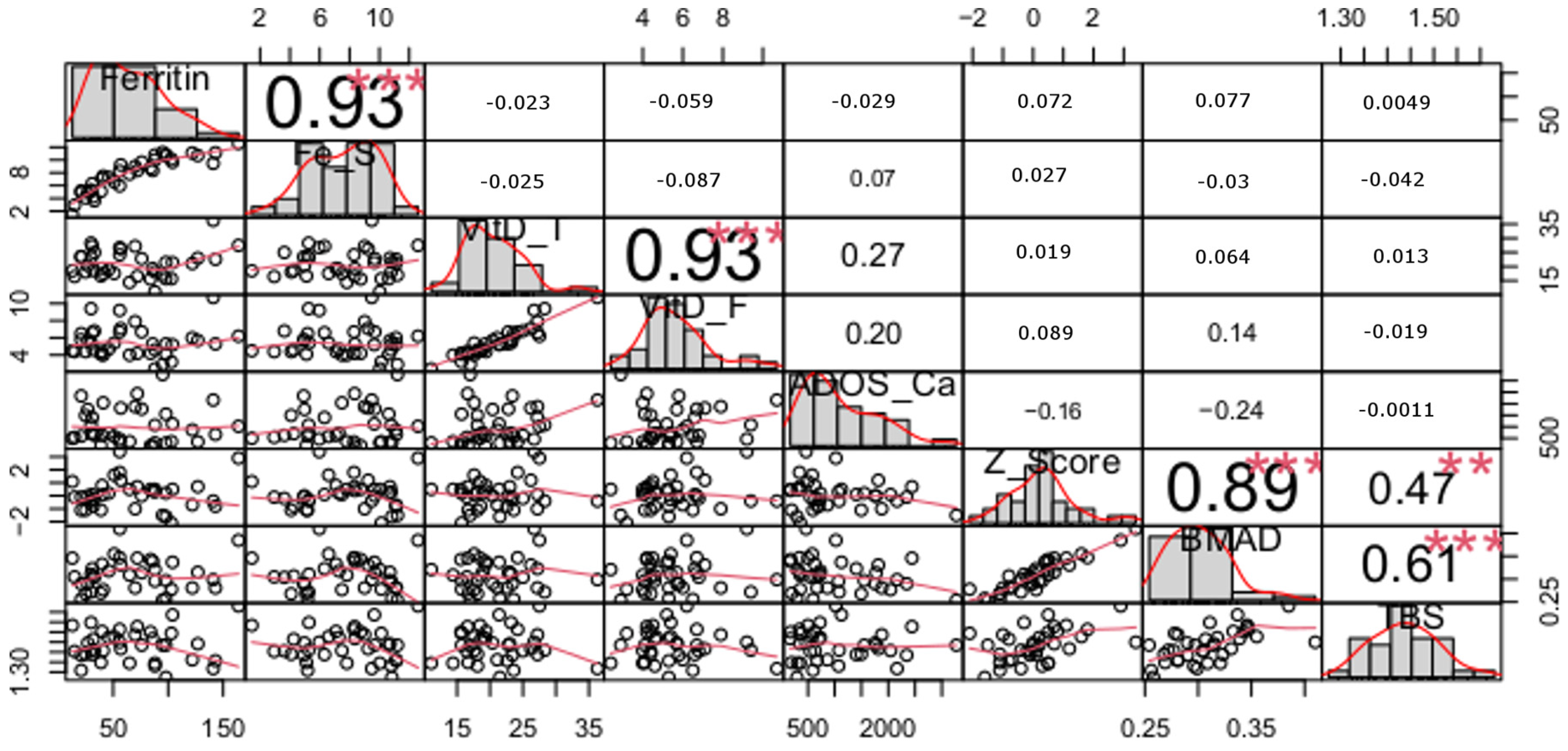
| Variables | Control n = 37 | Ski Jumping n = 28 | Volleyball n = 48 | p Value |
|---|---|---|---|---|
| Age (years) | 18.1 ± 1.4 * | 16.7 ± 1.2 | 17.3 ± 10.8 | ~0.000 |
| Body mass (kg) | 75.6 ± 12.0 ^ | 57.7 ± 7.0 ^ | 84.2 ± 9.2 ^ | ~0.000 |
| Height (m) | 1.81 ± 0.08 ^ | 1.74 ± 0.06 ^ | 1.96 ± 0.07 ^ | ~0.000 |
| BMI (kg/m2) | 23.8 ± 3.3 * | 20.0 ± 1.4 | 22.0 ± 2.0 | ~0.000 KW |
| Fat mass (%) | 15.2 ± 5.6 ^ | 7.18 ± 2.40 ^ | 10.8 ± 2.7 ^ | ~0.000 KW |
| (kg) | 12.4 ± 6.0 * | 4.26 ± 1.79 | 9.2 ± 2.8 | ~0.000 KW |
| Fat-free mass (kg) | 65.2 ± 7.1 ^ | 53.4 ± 5.6 ^ | 75.1 ± 7.6 ^ | ~0.000 |
| Muscle mass (kg) | 62.0 ± 6.8 ^ | 50.7 ± 5.4 ^ | 71.4 ± 7.3 ^ | ~0.000 |
| Training experience (years) | - | 7.37 ± 2.18 | 6.48 ± 2.86 | 0.09 MW |
| Group | ||
|---|---|---|
| Variable | Control | Sport (Ski Jumping + Volleyball) |
| n = 37 | n = 76 | |
| Ferritin (ng/mL) | 67.2 ± 39.9 | 58.3 ± 34.0 |
| Fe stores (g) | 7.72 ± 2.3 | 7.5 ± 2.2 |
| Total 25(OH)D (ng/mL) | 21.0 ± 5.0 | 28.5 ± 7.1 |
| Free 25(OH)D (ng/mL) | 5.50 ± 1.8 | 7.6 ± 2.3 |
| Calcium intake (mg/day) | 1039 ± 767 | 1207 ± 743 |
| Z-score | 0.11 ± 1.14 | 0.89 ± 1.16 |
| BMAD (g/cm3) | 0.31 ± 0.03 | 0.32 ± 0.04 |
| TBS | 1.433 ± 0.08 | 1.426 ± 0.08 |
| Fractures (%) | 22 | 34 |
| Control vs. Sport Group (Ski Jumping + Volleyball) | ||
|---|---|---|
| Variable | p | ES # |
| Ferritin | NS | |
| Fe stores | NS | |
| Total 25(OH)D | * | ε2 = 0.285; CI (0.161; 0.428) |
| Free 25(OH)D | * | ε2 = 0.206; CI (0.078; 0.348) |
| Calcium intake | NS | |
| Z-score | * | η2 = 0.09; CI (0.021; 0.161) |
| BMAD | NS | |
| TBS | NS | |
| Fractures | NS |
| Variable | Nonstandard Coefficients | Standard Coefficients | Unique Contribution | |||
|---|---|---|---|---|---|---|
| Beta | SE | Beta | t | p | U | |
| Intercept | 0.246 | 0.013 | 19.38 | <0.001 | ||
| Ferritin | 0.0002 | 0.0001 | 0.227 | 2.32 | <0.05 | 0.07 |
| Free 25(OH)D | 0.008 | 0.0015 | 0.486 | 4.97 | <0.001 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malczewska-Lenczowska, J.; Surała, O.; Granda, D.; Szczepańska, B.; Czaplicki, A.; Kubacki, R. The Relationship between Bone Health Parameters, Vitamin D and Iron Status, and Dietary Calcium Intake in Young Males. Nutrients 2024, 16, 215. https://doi.org/10.3390/nu16020215
Malczewska-Lenczowska J, Surała O, Granda D, Szczepańska B, Czaplicki A, Kubacki R. The Relationship between Bone Health Parameters, Vitamin D and Iron Status, and Dietary Calcium Intake in Young Males. Nutrients. 2024; 16(2):215. https://doi.org/10.3390/nu16020215
Chicago/Turabian StyleMalczewska-Lenczowska, Jadwiga, Olga Surała, Dominika Granda, Beata Szczepańska, Adam Czaplicki, and Rafał Kubacki. 2024. "The Relationship between Bone Health Parameters, Vitamin D and Iron Status, and Dietary Calcium Intake in Young Males" Nutrients 16, no. 2: 215. https://doi.org/10.3390/nu16020215
APA StyleMalczewska-Lenczowska, J., Surała, O., Granda, D., Szczepańska, B., Czaplicki, A., & Kubacki, R. (2024). The Relationship between Bone Health Parameters, Vitamin D and Iron Status, and Dietary Calcium Intake in Young Males. Nutrients, 16(2), 215. https://doi.org/10.3390/nu16020215







