Beat the Clock: Assessment of Night Eating Syndrome and Circadian Rhythm in a Sample of Greek Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Recruitment and Characteristics
2.2. Ethical Permission
2.3. Assessment of NES
2.4. Circadian Rhythm, Sleep and Mood
2.5. Translation of the Questionnaires in the Greek Language
2.6. Anthropometry
2.7. Statistical Analyses
3. Results
3.1. Translation and Validation of the Questionnaires
3.2. NEQ and SCRAM Results of the Sample
3.3. NEQ and SCRAM Results by Weight Strata
3.4. Relationship between NEQ and SCRAM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stunkard, A.J.; Grace, W.J.; Wolff, H.G. The night-eating syndrome; a pattern of food intake among certain obese patients. Am. J. Med. 1955, 19, 78–86. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Allison, K.C.; Lundgren, J.D.; O’Reardon, J.P.; Geliebter, A.; Gluck, M.E.; Vinai, P.; Mitchell, J.E.; Schenck, C.H.; Howell, M.J.; Crow, S.J.; et al. Proposed Diagnostic Criteria for Night Eating Syndrome. Int. J. Eat. Disord. 2010, 43, 241. [Google Scholar] [CrossRef]
- Beauchamp, M.T.; Allison, K.C.; Lundgren, J.D. The nature of night eating syndrome: Using network analysis to understand unique symptomological relationships. Int. J. Eat. Disord. 2021, 54, 733–744. [Google Scholar] [CrossRef]
- Goel, N.; Stunkard, A.J.; Rogers, N.L.; Van Dongen, H.P.A.; Allison, K.C.; O’Reardon, J.P.; Ahima, R.S.; Cummings, D.E.; Heo, M.; Dinges, D.F. Circadian Rhythm Profiles in Women with Night Eating Syndrome. J. Biol. Rhythms 2009, 24, 85. [Google Scholar] [CrossRef]
- Sutcu, C.; Pamuk, G.; Ongel, K. Evaluation of night eating syndrome in individuals with and without obesity. Endokrynol. Pol. 2021, 72, 539–544. [Google Scholar] [CrossRef]
- Mccuen-Wurst, C.; Ruggieri, M.; Allison, K.C. Disordered eating and obesity: Associations between binge eating-disorder, night-eating syndrome, and weight-related co-morbidities. Ann. N. Y Acad. Sci. 2018, 1411, 96–105. [Google Scholar] [CrossRef]
- Kaur, J.; Dang, A.B.; Gan, J.; An, Z.; Krug, I. Night Eating Syndrome in Patients with Obesity and Binge Eating Disorder: A Systematic Review. Front. Psychol. 2022, 12, 766827. [Google Scholar] [CrossRef]
- Riccobono, G.; Iannitelli, A.; Pompili, A.; Iorio, C.; Stratta, P.; Rossi, R.; Bersani, G.; Pacitti, F. Night Eating Syndrome, circadian rhythms and seasonality: A study in a population of Italian university students. Riv. Psichiatr. 2020, 55, 47–52. [Google Scholar] [CrossRef]
- Noh, J. The Effect of Circadian and Sleep Disruptions on Obesity Risk. J. Obes. Metab. Syndr. 2018, 27, 83. [Google Scholar] [CrossRef]
- Buijs, F.N.; León-Mercado, L.; Guzmán-Ruiz, M.; Guerrero-Vargas, N.N.; Romo-Nava, F.; Buijs, R.M. The Circadian System: A Regulatory Feedback Network of Periphery and Brain. Physiology 2016, 31, 170–181. [Google Scholar] [CrossRef]
- Buijs, R.M.; Soto Tinoco, E.C.; Hurtado Alvarado, G.; Escobar, C. The circadian system: From clocks to physiology. Handb. Clin. Neurol. 2021, 179, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Romo-Nava, F.; Guerdjikova, A.I.; Mori, N.N.; Scheer, F.A.J.L.; Burgess, H.J.; McNamara, R.K.; Welge, J.A.; Grilo, C.M.; McElroy, S.L. A matter of time: A systematic scoping review on a potential role of the circadian system in binge eating behavior. Front. Nutr. 2022, 9, 978412. [Google Scholar] [CrossRef] [PubMed]
- Roenneberg, T.; Daan, S.; Merrow, M. The Art of Entrainment. J. Biol. Rhythms 2003, 18, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Selvi, Y.; Aydin, A.; Atli, A.; Boysan, M.; Selvi, F.; Besiroglu, L. Chronotype differences in suicidal behavior and impulsivity among suicide attempters. Chronobiol. Int. 2011, 28, 170–175. [Google Scholar] [CrossRef]
- Kandeğer, A.; Eğilmez, Ü.; Selvi, Y. Feeding and Eating Disorders in the Context of Circadian Rhythms. Alpha Psychiatry 2021, 22, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, L.; Papadopoulos, M.R.; Antypa, N. Chronotype and Psychiatric Disorders. Curr. Sleep Med. Rep. 2018, 4, 94–103. [Google Scholar] [CrossRef]
- Adan, A.; Archer, S.N.; Hidalgo, M.P.; Di Milia, L.; Natale, V.; Randler, C. Circadian typology: A comprehensive review. Chronobiol. Int. 2012, 29, 1153–1175. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.J.; Golden, S.S.; O’Shea, E.K. Light-driven changes in energy metabolism directly entrain the cyanobacterial circadian oscillator. Science 2011, 331, 220–223. [Google Scholar] [CrossRef]
- Panda, S.; Antoch, M.P.; Miller, B.H.; Su, A.I.; Schook, A.B.; Straume, M.; Schultz, P.G.; Kay, S.A.; Takahashi, J.S.; Hogenesch, J.B. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 2002, 109, 307–320. [Google Scholar] [CrossRef]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004, 1, 210–217. [Google Scholar] [CrossRef]
- Nymo, S.; Kleppe, M.M.; Coutinho, S.R.; Rehfeld, J.F.; Kulseng, B.; Martins, C. Association between habitual sleep duration/quality and appetite markers in individuals with obesity. Physiol. Behav. 2021, 232, 113345. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; McHill, A.W.; Cox, R.C.; Broussard, J.L.; Dutil, C.; da Costa, B.G.G.; Sampasa-Kanyinga, H.; Wright, K.P. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 2022, 19, 82–97. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; McReynolds, A.; Trivedi, Z.B.; Roberts, A.L.; Sy, M.; Hirsch, J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am. J. Clin. Nutr. 2012, 95, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Greer, S.M.; Goldstein, A.N.; Walker, M.P. The impact of sleep deprivation on food desire in the human brain. Nat. Commun. 2013, 4, 2259. [Google Scholar] [CrossRef] [PubMed]
- Zerón-Rugerio, M.F.; Trinitat, C.; Izquierdo-Pulido, M. Sleep Restriction and Circadian Misalignment: Their Implications in Obesity. In Neurological Modulation of Sleep; Academic Press: Cambridge, MA, USA, 2020; pp. 131–143. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Tuccinardi, D.; Nicastro, V.; Barrea, L.; Colao, A.; Savastano, S., on behalf of Obesity Programs of nutrition, Education, Research and Assessment (OPERA) group. Sleep disturbances: One of the culprits of obesity-related cardiovascular risk? Int. J. Obes. Suppl. 2020, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Gordon, C.J.; Chapman, J.L.; Hoyos, C.M.; Marshall, N.S.; Miller, C.B.; Grunstein, R.R. The association of insomnia disorder characterised by objective short sleep duration with hypertension, diabetes and body mass index: A systematic review and meta-analysis. Sleep Med. Rev. 2021, 59, 101456. [Google Scholar] [CrossRef] [PubMed]
- Baron, K.G.; Reid, K.J. Circadian misalignment and health. Int. Rev. Psychiatry 2014, 26, 139–154. [Google Scholar] [CrossRef]
- Okoli, A.; Hanlon, E.C.; Brady, M.J. The relationship between sleep, obesity, and metabolic health in adolescents: A review. Curr. Opin. Endocr. Metab. Res. 2021, 17, 15–19. [Google Scholar] [CrossRef]
- Valladares, M.; Obregón, A.M.; Chaput, J.P. Association between genetic variants of the clock gene and obesity and sleep duration. J. Physiol. Biochem. 2015, 71, 855–860. [Google Scholar] [CrossRef]
- Dashti, H.S.; Follis, J.L.; Smith, C.E.; Tanaka, T.; Cade, B.E.; Gottlieb, D.J.; Hruby, A.; Jacques, P.F.; Lamon-Fava, S.; Richardson, K.; et al. Habitual sleep duration is associated with BMI and macronutrient intake and may be modified by CLOCK genetic variants. Am. J. Clin. Nutr. 2015, 101, 135–143. [Google Scholar] [CrossRef]
- Škrlec, I.; Talapko, J.; Džijan, S.; Cesar, V.; Lazić, N.; Lepeduš, H. The Association between Circadian Clock Gene Polymorphisms and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Biology 2021, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, J.; Yao, K.; Su, W.; Tan, B.; Wu, X.; Huang, X.; Li, T.; Yin, Y.; Tosini, G.; et al. Circadian rhythms and obesity: Timekeeping governs lipid metabolism. J. Pineal Res. 2020, 69, e12682. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, A.B.; Vitaterna, O.; Haugh, I.M.; Bavishi, A.A.; Zee, P.C.; Turek, F.W.; Sheldon, S.H.; Silverberg, J.I.; Paller, A.S. Nocturnal eczema: Review of sleep and circadian rhythms in children with atopic dermatitis and future research directions. J. Allergy Clin. Immunol. 2015, 136, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.C.; Lundgren, J.D.; O’Reardon, J.P.; Martino, N.S.; Sarwer, D.B.; Wadden, T.A.; Crosby, R.D.; Engel, S.G.; Stunkard, A.J. The Night Eating Questionnaire (NEQ): Psychometric properties of a measure of severity of the Night Eating Syndrome. Eat. Behav. 2008, 9, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.E.M.; Bullock, B.; Murray, G. Development of a Measure of Sleep, Circadian Rhythms, and Mood: The SCRAM Questionnaire. Front. Psychol. 2017, 8, 2105. [Google Scholar] [CrossRef]
- Byrne, J.E.M.; Bullock, B.; Brydon, A.; Murray, G. A psychometric investigation of the sleep, circadian rhythms, and mood (SCRAM) questionnaire. Chronobiol. Int. 2019, 36, 265–275. [Google Scholar] [CrossRef]
- Guillemin, F.; Bombardier, C.; Beaton, D. Cross-cultural adaptation of health-related quality of life measures: Literature review and proposed guidelines. J. Clin. Epidemiol. 1993, 46, 1417–1432. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- The Jamovi project Jamovi 2020. Available online: https://www.jamovi.org/ (accessed on 1 January 2024).
- R Core Team R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team R: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 1 January 2024).
- Tavakol, M.; Dennick, R. Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2011, 2, 55. [Google Scholar] [CrossRef]
- Rand, C.S.; Macgregor, A.M.; Stunkard, A.J. The night eating syndrome in the general population and among postoperative obesity surgery patients. Int. J. Eat. Disord. 1997, 22, 65–69. [Google Scholar] [CrossRef]
- De Zwaan, M.; Müller, A.; Allison, K.C.; Brähler, E.; Hilbert, A. Prevalence and correlates of night eating in the German general population. PLoS ONE 2014, 9, e97667. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Komada, Y.; Okajima, I.; Takaesu, Y.; Kuriyama, K.; Inoue, Y. A Cross-Sectional Study of Evening Hyperphagia and Nocturnal Ingestion: Core Constituents of Night Eating Syndrome with Different Background Factors. Nutrients 2021, 13, 4179. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.J.; Hay, P.; Touyz, S.; Currow, D.; Mannan, H. Association of participants who screened positive for night eating syndrome with physical health, sleep problems, and weight status in an Australian adult population. Eat. Weight Disord. 2023, 28, 77. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Ochani, R.K.; Memon, Z.A.A.; Shaikh, A.; Qureshi, N.E.; Bhimani, S.; Abbasi, M.K.; Farhan, A.; Qureshi, S.S.; Das, K. Relationship between Night Eating Syndrome and Self-esteem: A Cross-sectional Population-based Study in Karachi, Pakistan. Cureus 2019, 11, e5540. [Google Scholar] [CrossRef] [PubMed]
- Karamustafalioglu, O.K.; Cengiz, Y.; Gonenli, S.; Ozcelik, B.; Bakim, B. P0344—Prevalence of night eating syndrome in psychiatric outpatient population. Eur. Psychiatry 2008, 23, S182–S183. [Google Scholar] [CrossRef]
- Lavery, M.E.; Frum-Vassallo, D. An Updated Review of Night Eating Syndrome: An Under-Represented Eating Disorder. Curr. Obes. Rep. 2022, 11, 404. [Google Scholar] [CrossRef]
- Gallant, A.R.; Lundgren, J.; Drapeau, V. The night-eating syndrome and obesity. Obes. Rev. 2012, 13, 528–536. [Google Scholar] [CrossRef]
- Tholin, S.; Lindroos, A.K.; Tynelius, P.; Åkerstedt, T.; Stunkard, A.J.; Bulik, C.M.; Rasmussen, F. Prevalence of night eating in obese and nonobese twins. Obesity 2009, 17, 1050–1055. [Google Scholar] [CrossRef]
- Calugi, S.; Dalle Grave, R.; Marchesini, G. Night eating syndrome in class II–III obesity: Metabolic and psychopathological features. Int. J. Obes. 2009, 33, 899–904. [Google Scholar] [CrossRef][Green Version]
- Grilo, C.M.; Masheb, R.M. Night-time eating in men and women with binge eating disorder. Behav. Res. Ther. 2004, 42, 397–407. [Google Scholar] [CrossRef]
- Salman, E.J.; Kabir, R. Night Eating Syndrome; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Vander Wal, J.S. Night eating syndrome: A critical review of the literature. Clin. Psychol. Rev. 2012, 32, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Kucukgoncu, S.; Midura, M.; Tek, C. Optimal management of night eating syndrome: Challenges and solutions. Neuropsychiatr. Dis. Treat. 2015, 11, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.J.; Hay, P.; Mannan, H. A Scoping Review on the Association between Night Eating Syndrome and Physical Health, Health-Related Quality of Life, Sleep and Weight Status in Adults. Nutrients 2023, 15, 2791. [Google Scholar] [CrossRef] [PubMed]
- Striegel-Moore, R.H.; Franko, D.L.; Thompson, D.; Affenito, S.; Kraemer, H.C. Night eating: Prevalence and demographic correlates. Obesity 2006, 14, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Bruzas, M.B.; Allison, K.C. A Review of the Relationship between Night Eating Syndrome and Body Mass Index. Curr. Obes. Rep. 2019, 8, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lepley, T.; Schwager, Z.; Khalid, Z. Identification and Management of Night Eating Syndrome in the Adolescent and Young Adult Population. Prim. Care Companion CNS Disord. 2022, 24, 21r03062. [Google Scholar] [CrossRef]
- Lundgren, J.D.; Rempfer, M.V.; Brown, C.E.; Goetz, J.; Hamera, E. The prevalence of night eating syndrome and binge eating disorder among overweight and obese individuals with serious mental illness. Psychiatry Res. 2010, 175, 236. [Google Scholar] [CrossRef]
- Sevincer, G.M.; Ince, E.; Taymur, I.; Konuk, N. Night Eating Syndrome Frequency in University Students: Association with Impulsivity, Depression, and Anxiety. Klin. Psikofarmakol. Bülteni Bull. Clin. Psychopharmacol. 2016, 26, 238–247. [Google Scholar] [CrossRef]
- He, J.; Huang, F.; Yan, J.; Wu, W.; Cai, Z.; Fan, X. Prevalence, demographic correlates, and association with psychological distress of night eating syndrome among Chinese college students. Psychol. Health Med. 2018, 23, 578–584. [Google Scholar] [CrossRef]
- Runfola, C.D.; Allison, K.C.; Hardy, K.K.; Lock, J.; Peebles, R. Prevalence and clinical significance of night eating syndrome in university students. J. Adolesc. Health 2014, 55, 41–48. [Google Scholar] [CrossRef]
- Culbert, K.M.; Sisk, C.L.; Klump, K.L. A Narrative Review of Sex Differences in Eating Disorders: Is There a Biological Basis? Clin. Ther. 2021, 43, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulou, M.G.; Gkiouras, K.; Polychronidou, G.; Kaparounaki, C.; Gkouskou, K.K.; Magkos, F.; Donini, L.M.; Eliopoulos, A.G.; Goulis, D.G. Obsessed with Healthy Eating: A Systematic Review of Observational Studies Assessing Orthorexia Nervosa in Patients with Diabetes Mellitus. Nutrients 2021, 13, 3823. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, M.H.; De Amicis, R.; Galasso, L.; Cavallaro, R.; Mambrini, S.P.; Castelli, L.; Montaruli, A.; Roveda, E.; Esposito, F.; Leone, A.; et al. Sex Differences in the Relationship between Chronotype and Eating Behaviour: A Focus on Binge Eating and Food Addiction. Nutrients 2023, 15, 4580. [Google Scholar] [CrossRef]
- Hamdan, M.; Badrasawi, M.; Zidan, S.; Thawabteh, R.; Mohtaseb, R.; Arqoub, K.A. Night eating syndrome is associated with mental health issues among palestinian undergraduate students-cross sectional study. J. Eat. Disord. 2023, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.C.; Lundgren, J.D.; Stunkard, A.J.; Bulik, C.M.; Lindroos, A.K.; Thornton, L.M.; Rasmussen, F. Validation of screening questions and symptom coherence of night eating in the Swedish Twin Registry. Compr. Psychiatry 2014, 55, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Striegel-Moore, R.H.; Dohm, F.A.; Hook, J.M.; Schreiber, G.B.; Crawford, P.B.; Daniels, S.R. Night eating syndrome in young adult women: Prevalence and correlates. Int. J. Eat. Disord. 2005, 37, 200–206. [Google Scholar] [CrossRef]
- Miraj, M.; Kashoo, F.; Saleem, S.; Alzhrani, M.; Alanazi, A.; Alzahrani, H.; Shaphe, M.A.; Ahmad, M.; Ahmad, F.; Shaik, A.R.; et al. Prevalence of night eating syndrome associated with psychological disorders among university students: A metaanalysis. J. King Saud. Univ. Sci. 2022, 34, 102031. [Google Scholar] [CrossRef]
- Rogers, N.L.; Dinges, D.F.; Allison, K.C.; Maislin, G.; Martino, N.; O’Reardon, J.P.; Stunkard, A.J. Assessment of Sleep in Women With Night Eating Syndrome. Sleep 2006, 29, 814–819. [Google Scholar] [CrossRef]
- Prince, J. Depression, Emotional Eating and Food Choice; University of Maine: Orono, ME, USA, 2014. [Google Scholar]
- Konttinen, H.; Van Strien, T.; Männistö, S.; Jousilahti, P.; Haukkala, A. Depression, emotional eating and long-term weight changes: A population-based prospective study. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 1–11. [Google Scholar] [CrossRef]
- Dakanalis, A.; Mentzelou, M.; Papadopoulou, S.K.; Papandreou, D.; Spanoudaki, M.; Vasios, G.K.; Pavlidou, E.; Mantzorou, M.; Giaginis, C. The Association of Emotional Eating with Overweight/Obesity, Depression, Anxiety/Stress, and Dietary Patterns: A Review of the Current Clinical Evidence. Nutrients 2023, 15, 1173. [Google Scholar] [CrossRef]
- Echeverri, B.; Kozak, A.T.; Gildner, D.J.; Pickett, S.M. Night eating syndrome subtypes: Differences in binge eating and food addiction symptoms. Eat. Weight Disord. 2023, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- Plano, S.A.; Soneira, S.; Tortello, C.; Golombek, D.A. Is the binge-eating disorder a circadian disorder? Front. Nutr. 2022, 9, 964491. [Google Scholar] [CrossRef] [PubMed]
- Natale, V.; Adan, A.; Scapellato, P. Are seasonality of mood and eveningness closely associated? Psychiatry Res. 2005, 136, 51–60. [Google Scholar] [CrossRef]
- Hirata, F.C.; Lima, M.C.O.; De Bruin, V.M.S.; Nóbrega, P.R.; Wenceslau, G.P.; De Bruin, P.F.C. Depression in medical school: The influence of morningness-eveningness. Chronobiol. Int. 2007, 24, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Bersani, G.; Bersani, F.S.; Prinzivalli, E.; Limpido, L.; Marconi, D.; Valeriani, G.; Colletti, C.; Anastasia, A.; Pacitti, F. Premorbid circadian profile of patients with major depression and panic disorder. Riv. Psichiatr. 2012, 47, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Merikanto, I.; Partonen, T. Eveningness increases risks for depressive and anxiety symptoms and hospital treatments mediated by insufficient sleep in a population-based study of 18,039 adults. Depress. Anxiety 2021, 38, 1066–1077. [Google Scholar] [CrossRef]
- Chan, J.W.Y.; Lam, S.P.; Li, S.X.; Yu, M.W.M.; Chan, N.Y.; Zhang, J.; Wing, Y.-K. Eveningness and Insomnia: Independent Risk Factors of Nonremission in Major Depressive Disorder. Sleep 2014, 37, 917. [Google Scholar] [CrossRef]
- Alvaro, P.K.; Roberts, R.M.; Harris, J.K. The independent relationships between insomnia, depression, subtypes of anxiety, and chronotype during adolescence. Sleep Med. 2014, 15, 934–941. [Google Scholar] [CrossRef]
- Dolsen, E.A.; Harvey, A.G. Dim Light Melatonin Onset and Affect in Adolescents with an Evening Circadian Preference. J. Adolesc. Health 2018, 62, 94–99. [Google Scholar] [CrossRef]
- Natale, V.; Ballardini, D.; Schumann, R.; Mencarelli, C.; Magelli, V. Morningness–eveningness preference and eating disorders. Pers. Individ. Dif. 2008, 45, 549–553. [Google Scholar] [CrossRef]
- Yilmaz Yavuz, A.; Altinsoy, C. The relationship between chronotype, night eating behavior and fear of COVID-19 in academics. Chronobiol. Int. 2022, 39, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Riccobono, G.; Pompili, A.; Iannitelli, A.; Pacitti, F. The relationship between Night Eating Syndrome, depression and chronotype in a non-clinical adolescent population. Riv. Psichiatr. 2019, 54, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Maukonen, M.; Kanerva, N.; Partonen, T.; Kronholm, E.; Konttinen, H.; Wennman, H.; Männistö, S. The associations between chronotype, a healthy diet and obesity. Chronobiol. Int. 2016, 33, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Maukonen, M.; Kanerva, N.; Partonen, T.; Kronholm, E.; Tapanainen, H.; Kontto, J.; Männistö, S. Chronotype differences in timing of energy and macronutrient intakes: A population-based study in adults. Obesity 2017, 25, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, P.; Zawieja, P.; Ohayon, M.M. Associations between morningness/eveningness and psychopathology: An epidemiological survey in three in-patient psychiatric clinics. J. Psychiatr. Res. 2013, 47, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Sağlam, N.G.U.; Beştepe, E.E.; Sertler, İ.; Kurnaz, S.; Ayık, B.; Poyraz, C.A. The Relationship Among Seasonality, Night Eating, and Chronotype in Bipolar Disorder: Exploring the Mediating Role of Sleep Quality. J. Nerv. Ment. Dis. 2023. [Google Scholar] [CrossRef]
- Kirschbaum-Lesch, I.; Byrne, J.E.M.; Holtmann, M.; Murray, G.; Legenbauer, T. Translation and validation of the SCRAM questionnaire in a German adolescent inpatient sample. Chronobiol. Int. 2022, 39, 1027–1035. [Google Scholar] [CrossRef]
- Allison, K.C.; Spaeth, A.; Hopkins, C.M. Sleep and Eating Disorders. Curr. Psychiatry Rep. 2016, 18, 92. [Google Scholar] [CrossRef]
- Shoar, S.; Naderan, M.; Mahmoodzadeh, H.; Shoar, N.; Lotfi, D. Night eating syndrome: A psychiatric disease, a sleep disorder, a delayed circadian eating rhythm, and/or a metabolic condition? Expert Rev. Endocrinol. Metab. 2019, 14, 351–358. [Google Scholar] [CrossRef]
- Striegel-Moore, R.H.; Franko, D.L.; Garcia, J. The validity and clinical utility of night eating syndrome. Int. J. Eat. Disord. 2009, 42, 720–738. [Google Scholar] [CrossRef]
- Harb, A.; Levandovski, R.; Oliveira, C.; Caumo, W.; Allison, K.C.; Stunkard, A.; Hidalgo, M.P. Night eating patterns and chronotypes: A correlation with binge eating behaviors. Psychiatry Res. 2012, 200, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.T. Night Eating Syndrome and Network Analysis of Features. In Eating Disorders; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–27. [Google Scholar] [CrossRef]
- Fishbein, A.B.; Knutson, K.L.; Zee, P.C. Circadian disruption and human health. J. Clin. Investig. 2021, 131, e148286. [Google Scholar] [CrossRef] [PubMed]
- Vetter, C. Circadian disruption: What do we actually mean? Eur. J. Neurosci. 2020, 51, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.C.; Waterhouse, J.; De-Souza, D.A.; Rossato, L.T.; Silva, C.M.; Araújo, M.B.J.; Tufik, S.; de Mello, M.T.; Crispim, C.A. Association between chronotype, food intake and physical activity in medical residents. Chronobiol. Int. 2016, 33, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Gamsızkan, Z.; Önmez, A.; Sahip Karakaş, T. Chronobiological evaluation and an intervention study on timing of food intake in the treatment of obesity. Int. J. Clin. Pract. 2021, 75, e14502. [Google Scholar] [CrossRef]
- Pinto, T.F.; Da Silva, F.G.C.; De Bruin, V.M.S.; De Bruin, P.F.C. Night eating syndrome: How to treat it? Rev. Assoc. Med. Bras. 2016, 62, 701–707. [Google Scholar] [CrossRef]
- Martellini, M.; Barchiesi, M.; Oriani, M.G.; Nardi, B. Current and emerging drugs treatment for night eating syndrome. Eur. Psychiatry 2016, 33, S164–S165. [Google Scholar] [CrossRef]
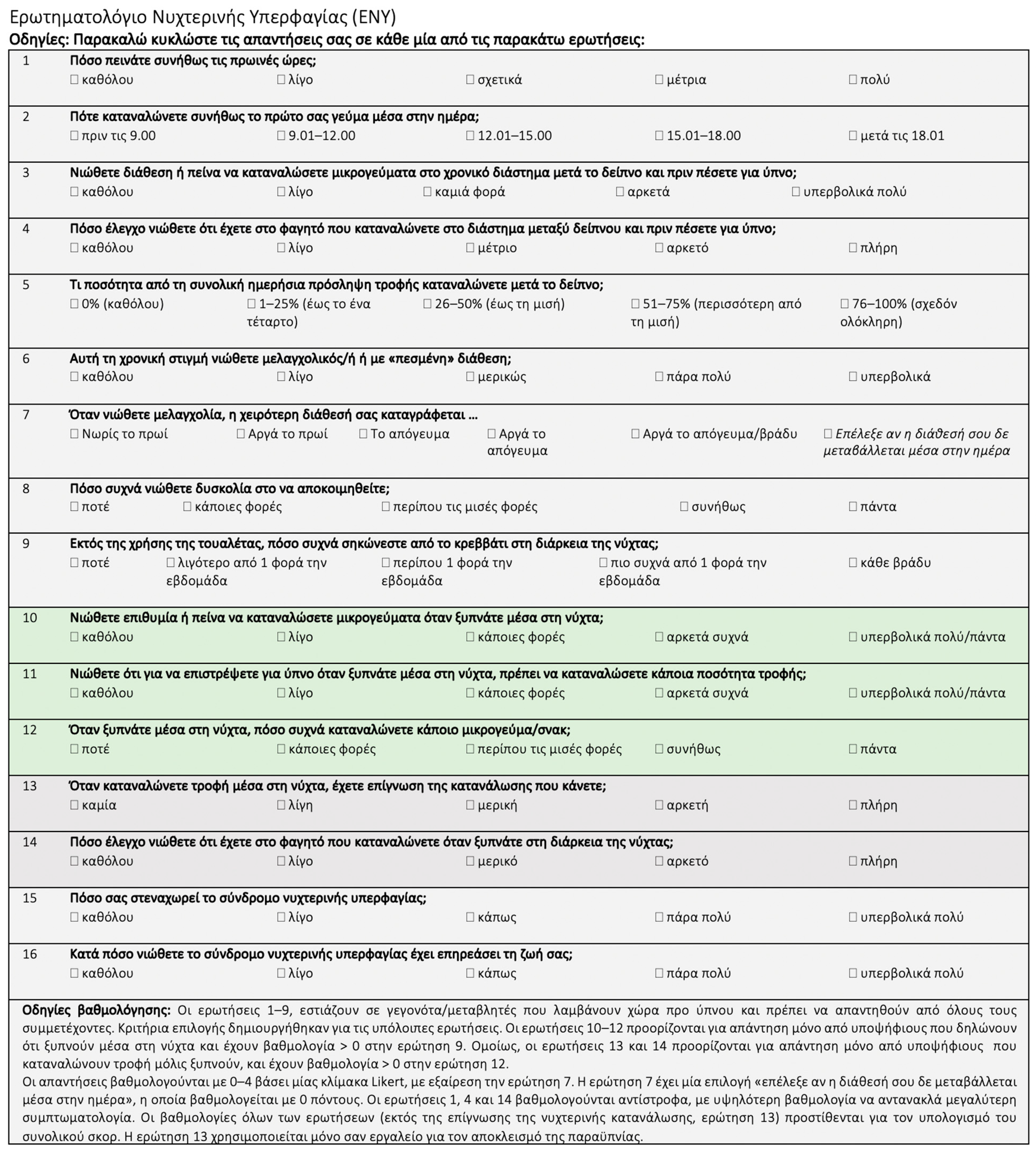
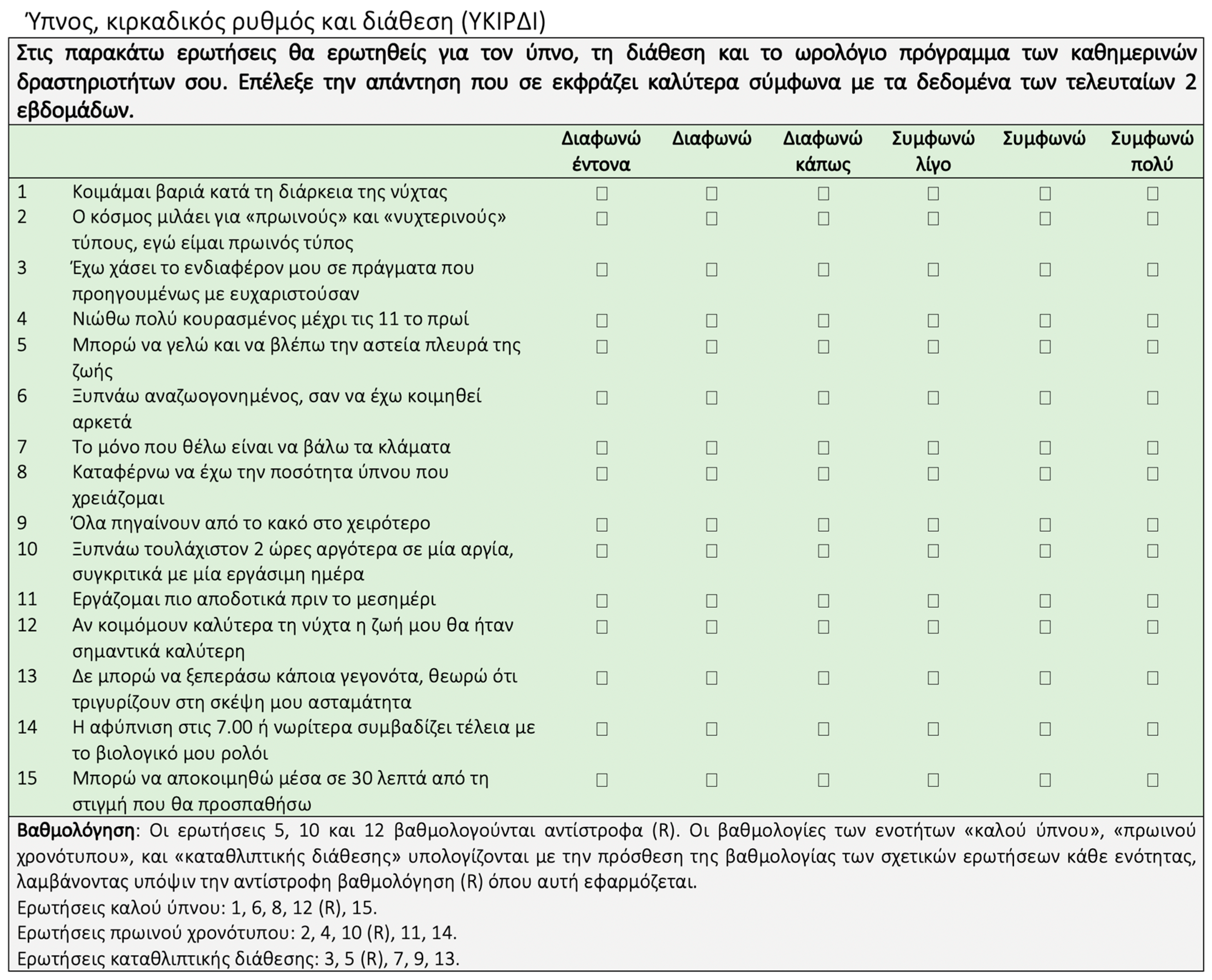
| Men (n = 177) | Women (n = 356) | Total (N = 533) | ||
|---|---|---|---|---|
| Age (years) | 26.5 ± 8.6 | 26.6 ± 9.7 | 26.6 ± 9.3 | |
| Educational level: | Primary/Secondary/Tertiary (%) | 1.7/31.6/59.9/6.8 | 1.7/18/66.9/13.5 | 1.7/22.5/75.8 |
| Marital status: | Single/in a relationship/married/ divorced/widowed (%) | 56.5/29.4/13/ 1.1/0 | 37.4/40.7/18.5/ 3.1/0.3 | 43.7/37.0/16.7/ 2.4/0.2 |
| Anthropometry: | BMI (kg/m2) | 26.1 ± 4.1 | 23.3 ± 4.7 | 23.4 (21.1, 26.5) |
| Underweight/normoweight/overweight/obese (%) | 1.1/44.6/37.3/16.9 | 9/64.6/19.4/7 | 6.4/58.0/25.3/10.3 |
| Men (n = 177) | Women (n = 356) | Total Sample (N = 533) | |
|---|---|---|---|
| Total NEQ (0–56) | 18.4 ± 7.9 | 17.8 ± 7.2 | 18.0 ± 7.4 |
| NEQ ≥ 25 (%) | 19.8 | 16.9 | 17.8 |
| NEQ ≥ 30 (%) | 8.5 | 7.9 | 8.1 |
| Total SCRAM (18–90) | 48.9 ± 7.7 | 50.2 ± 7.5 | 49.7 ± 7.9 |
| SCRAM Good Sleep (6–30) | 19.4 ± 4.5 | 19.3 ± 4.9 | 19.3 ± 4.8 |
| SCRAM Morningness (6–30) | 17.5 ± 5.1 | 19.4 ± 4.5 | 17.4 ± 5.1 |
| SCRAM Depression (6–30) | 11.9 ± 4.3 | 13.7 ± 5.0 *** | 13.1 ± 4.8 |
| Underweight (n = 34) | Normoweight (n = 309) | Overweight (n = 135) | Obesity (n = 55) | p | |
|---|---|---|---|---|---|
| Total NEQ | 18.5 ± 7.2 | 17.6 ± 7.2 | 18.6 ± 7.5 | 18.8 ± 8.4 | 0.449 |
| Total SCRAM | 50.6 ± 8.1 | 50.0 ± 7.8 | 49.2 ± 7.9 | 49.0 ± 8.4 | 0.631 |
| NEQ ≥ 25 (%) | 11.8 | 15.5 | 23.0 | 21.8 | 0.172 |
| NEQ ≥ 30 (%) | 11.8 | 6.1 | 9.6 | 12.7 | 0.239 |
| Model | OR | 95% CI | p | |
|---|---|---|---|---|
| NEQ score ≥ 25 vs. total SCRAM | Crude | 0.97 | 0.95–1.00 | 0.056 |
| Adjusted 1 | 0.97 | 0.95–1.00 | 0.060 | |
| Adjusted 2 | 0.97 | 0.95–1.00 | 0.070 | |
| NEQ score ≥ 25 vs. Good Sleep | Crude | 0.95 | 0.91–0.99 | 0.047 |
| Adjusted 1 | 0.95 | 0.91–0.99 | 0.047 | |
| Adjusted 2 | 0.96 | 0.91–1.00 | 0.073 | |
| NEQ score ≥ 25 vs. Morningness | Crude | 1.03 | 0.98–1.07 | 0.210 |
| Adjusted 1 | 1.03 | 0.98–1.08 | 0.243 | |
| Adjusted 2 | 1.03 | 0.98–1.07 | 0.287 | |
| NEQ score ≥ 25 vs. Depression | Crude | 1.05 | 1.01–1.10 | 0.021 |
| Adjusted 1 | 1.06 | 1.01–1.10 | 0.013 | |
| Adjusted 2 | 1.06 | 1.01–1.11 | 0.020 | |
| NEQ score ≥ 30 vs. total SCRAM | Crude | 0.99 | 0.96–1.04 | 0.960 |
| Adjusted 1 | 0.99 | 0.96–1.04 | 0.973 | |
| Adjusted 2 | 1.01 | 0.96–1.04 | 0.966 | |
| NEQ score ≥ 30 vs. Good Sleep | Crude | 0.95 | 0.89–1.02 | 0.198 |
| Adjusted 1 | 0.95 | 0.89–1.02 | 0.133 | |
| Adjusted 2 | 0.96 | 0.90–1.02 | 0.189 | |
| NEQ score ≥ 30 vs. Morningness | Crude | 1.08 | 1.02–1.15 | 0.012 |
| Adjusted 1 | 1.09 | 1.02–1.16 | 0.011 | |
| Adjusted 2 | 1.09 | 1.02–1.16 | 0.013 | |
| NEQ score ≥ 30 vs. Depression | Crude | 0.99 | 0.93–1.06 | 0.922 |
| Adjusted 1 | 0.99 | 0.93–1.07 | 0.957 | |
| Adjusted 2 | 0.99 | 0.93–1.06 | 0.844 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blouchou, A.; Chamou, V.; Eleftheriades, C.; Poulimeneas, D.; Kontouli, K.-M.; Gkiouras, K.; Bargiota, A.; Gkouskou, K.K.; Rigopoulou, E.; Bogdanos, D.P.; et al. Beat the Clock: Assessment of Night Eating Syndrome and Circadian Rhythm in a Sample of Greek Adults. Nutrients 2024, 16, 187. https://doi.org/10.3390/nu16020187
Blouchou A, Chamou V, Eleftheriades C, Poulimeneas D, Kontouli K-M, Gkiouras K, Bargiota A, Gkouskou KK, Rigopoulou E, Bogdanos DP, et al. Beat the Clock: Assessment of Night Eating Syndrome and Circadian Rhythm in a Sample of Greek Adults. Nutrients. 2024; 16(2):187. https://doi.org/10.3390/nu16020187
Chicago/Turabian StyleBlouchou, Anastasia, Vasiliki Chamou, Christos Eleftheriades, Dimitrios Poulimeneas, Katerina-Maria Kontouli, Konstantinos Gkiouras, Alexandra Bargiota, Kalliopi K. Gkouskou, Eirini Rigopoulou, Dimitrios P. Bogdanos, and et al. 2024. "Beat the Clock: Assessment of Night Eating Syndrome and Circadian Rhythm in a Sample of Greek Adults" Nutrients 16, no. 2: 187. https://doi.org/10.3390/nu16020187
APA StyleBlouchou, A., Chamou, V., Eleftheriades, C., Poulimeneas, D., Kontouli, K.-M., Gkiouras, K., Bargiota, A., Gkouskou, K. K., Rigopoulou, E., Bogdanos, D. P., Goulis, D. G., & Grammatikopoulou, M. G. (2024). Beat the Clock: Assessment of Night Eating Syndrome and Circadian Rhythm in a Sample of Greek Adults. Nutrients, 16(2), 187. https://doi.org/10.3390/nu16020187








