Chinese Sumac (Rhus chinensis Mill.) Fruits Prevent Hyperuricemia and Uric Acid Nephropathy in Mice Fed a High-Purine Yeast Diet
Abstract
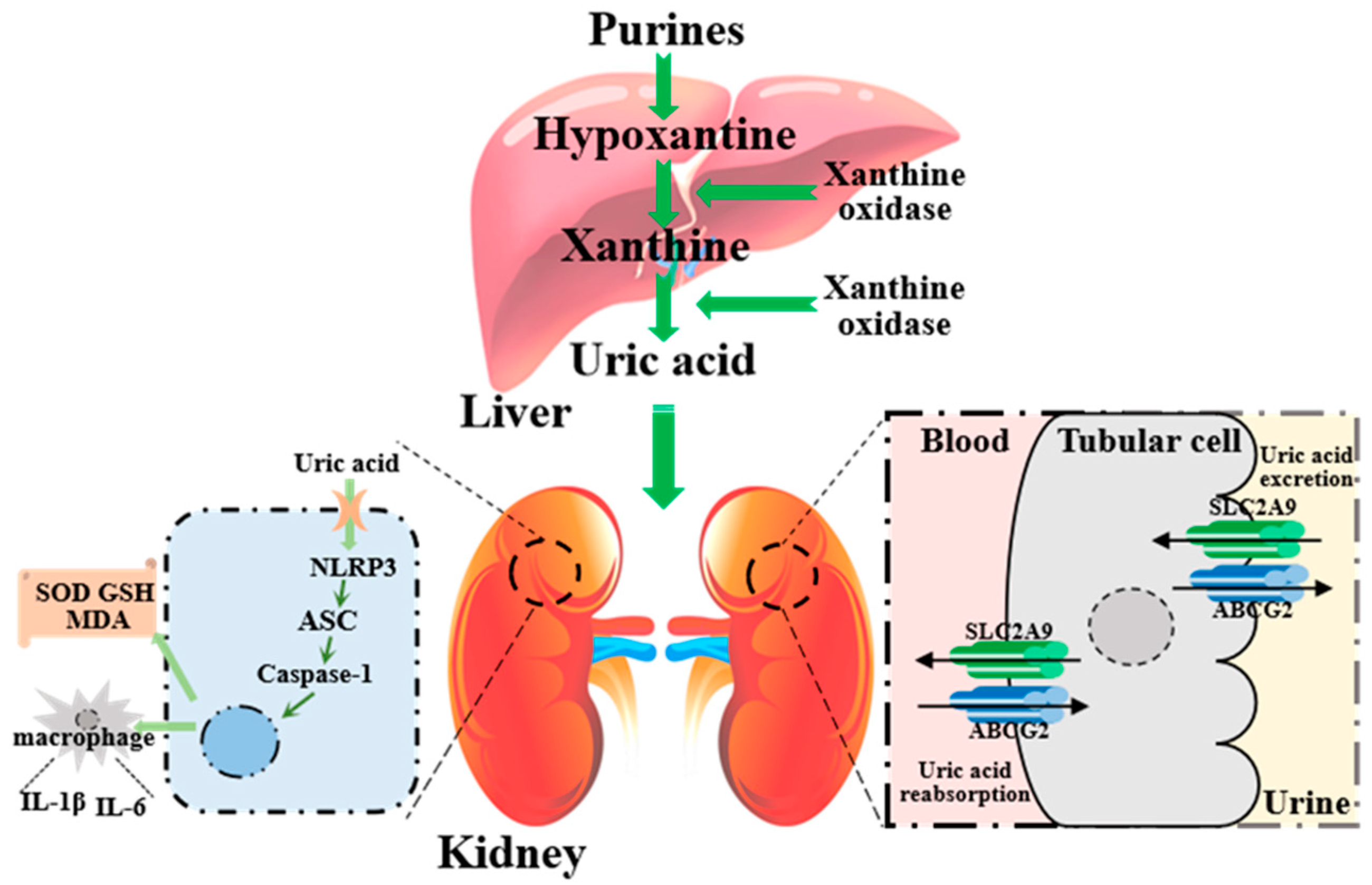
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation and Characterization of Chinese Sumac Fruit Extract
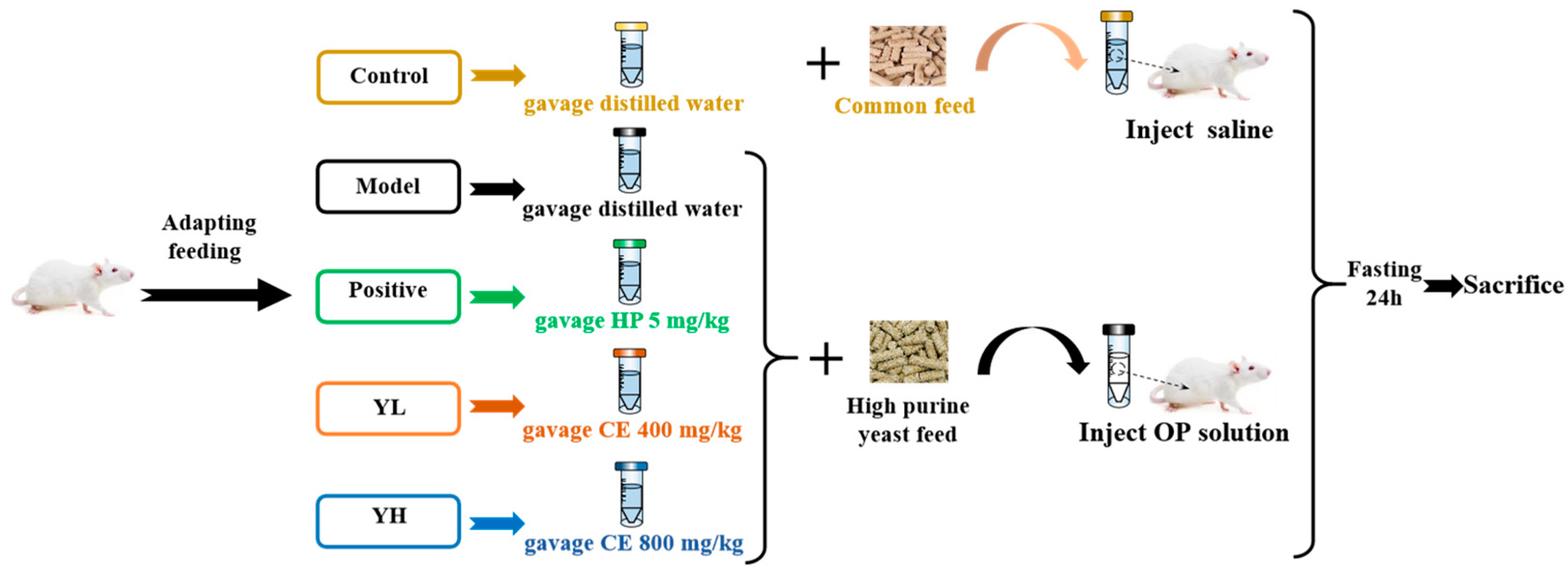
2.3. Animal Treatment
2.4. Measurement of Biochemical Indicators
2.5. Renal Histopathological Evaluation
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
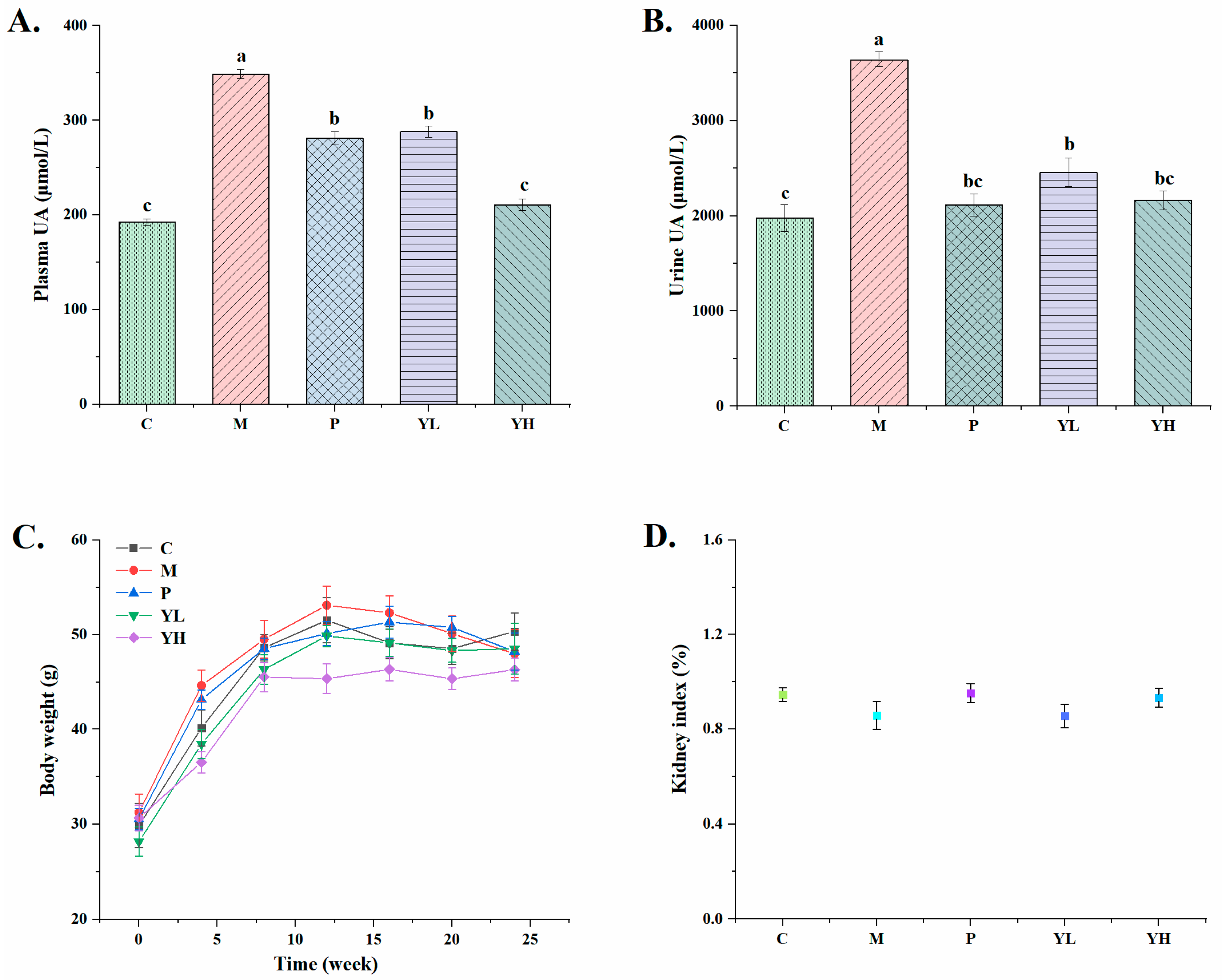
3.1. Effects of CE on the Levels of Plasma and Urinary Uric Acid in Hyperuricemia Mice Fed a High-Purine Yeast Diet
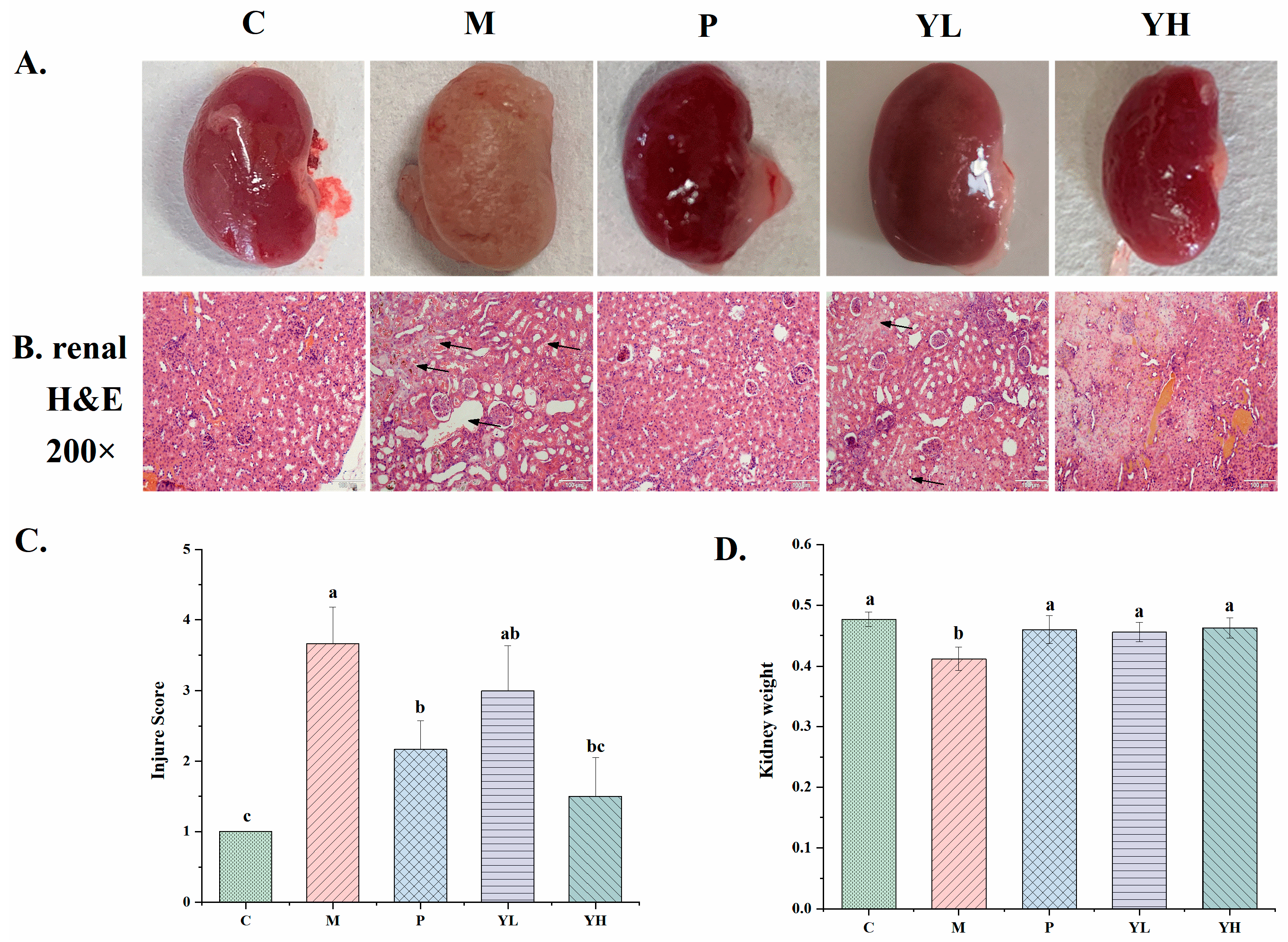
3.2. Effects of CE on Kidney Microstructure in Hyperuricemia Mice Fed a High-Purine Yeast Diet
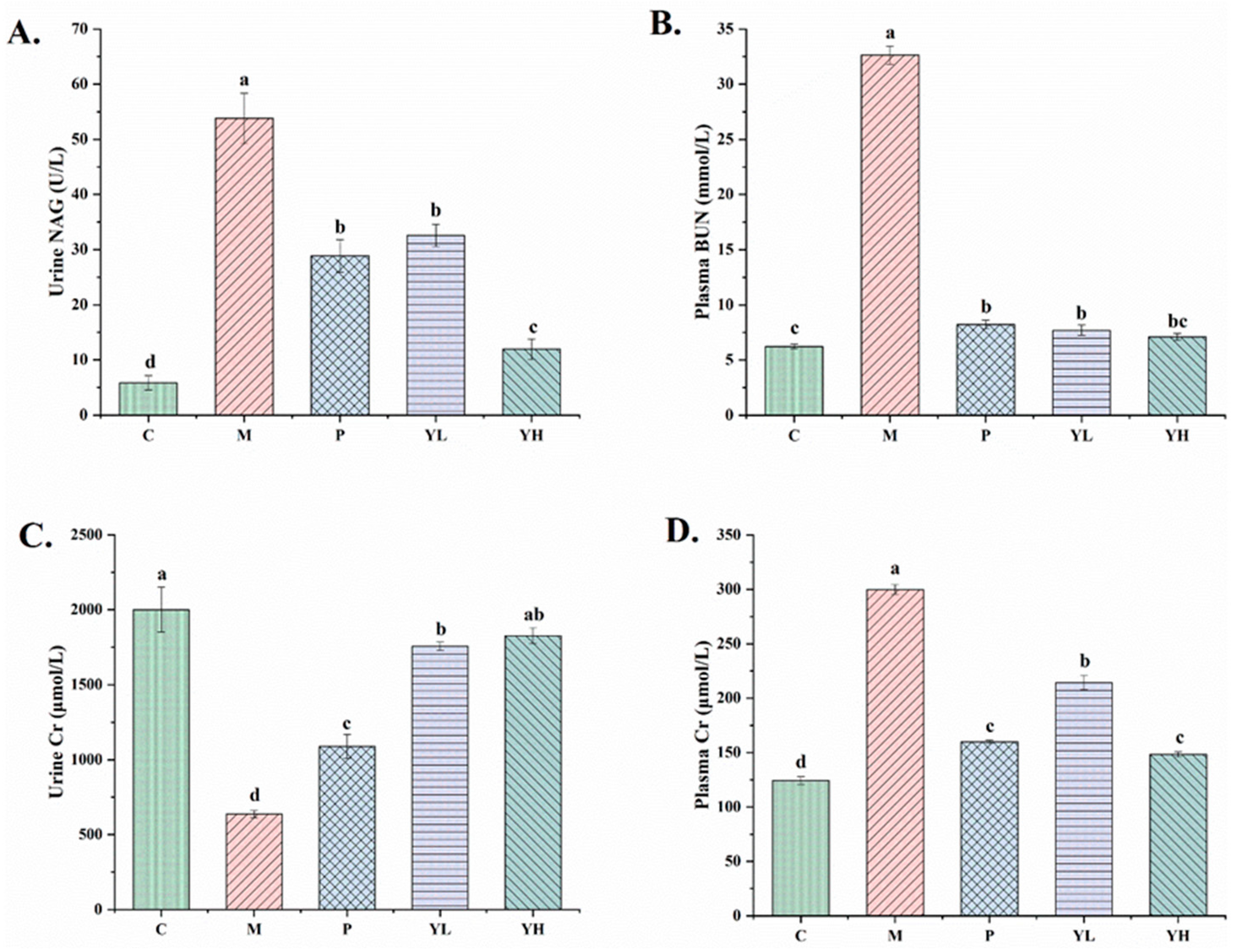
3.3. Effects of CE on Renal Biochemical Indicators in Mice with Hyperuricemia Fed a High-Purine Yeast Diet
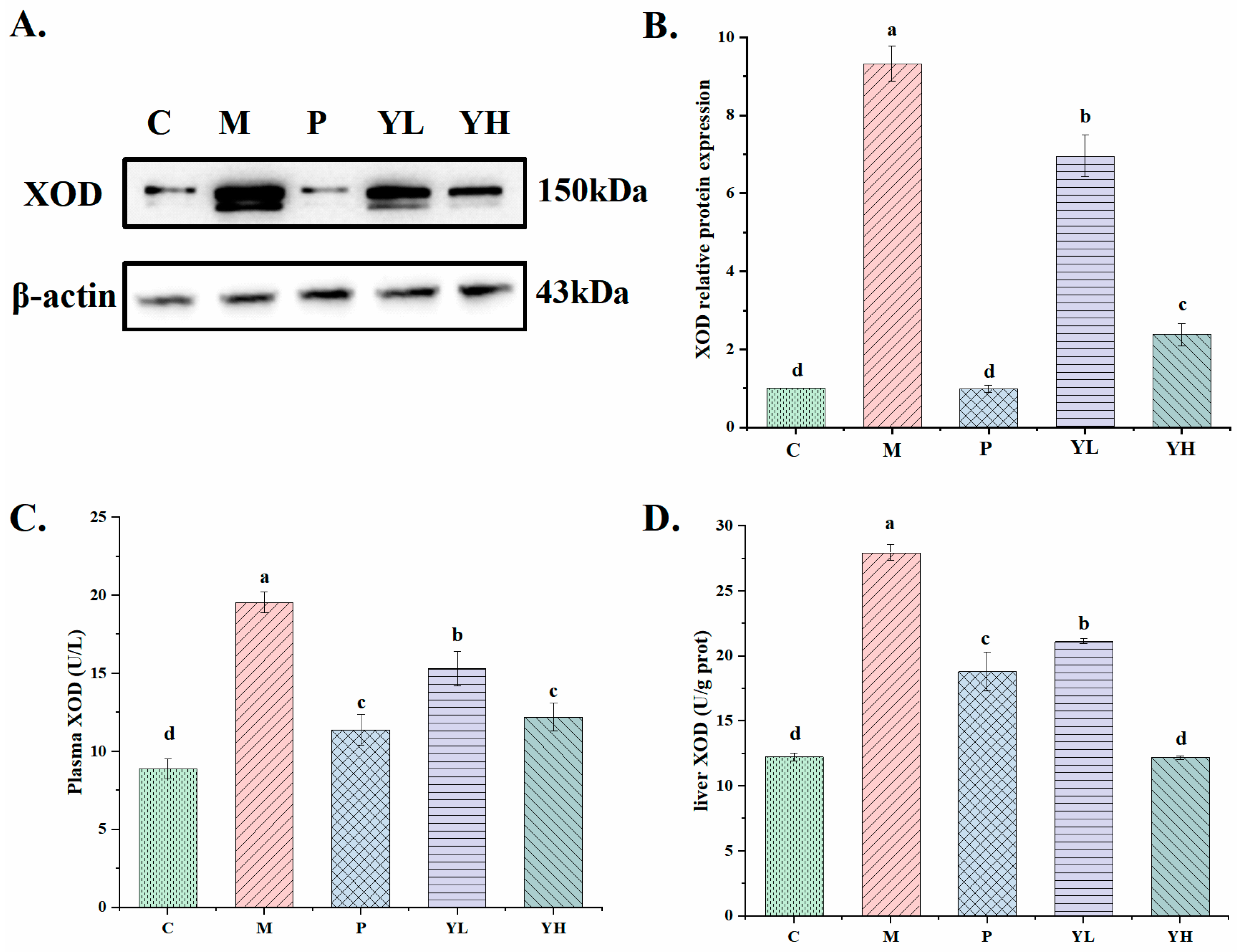
3.4. Effects of CE on the Activity and Expression of XOD in Hyperuricemia Mice Fed a High-Purine Yeast Diet
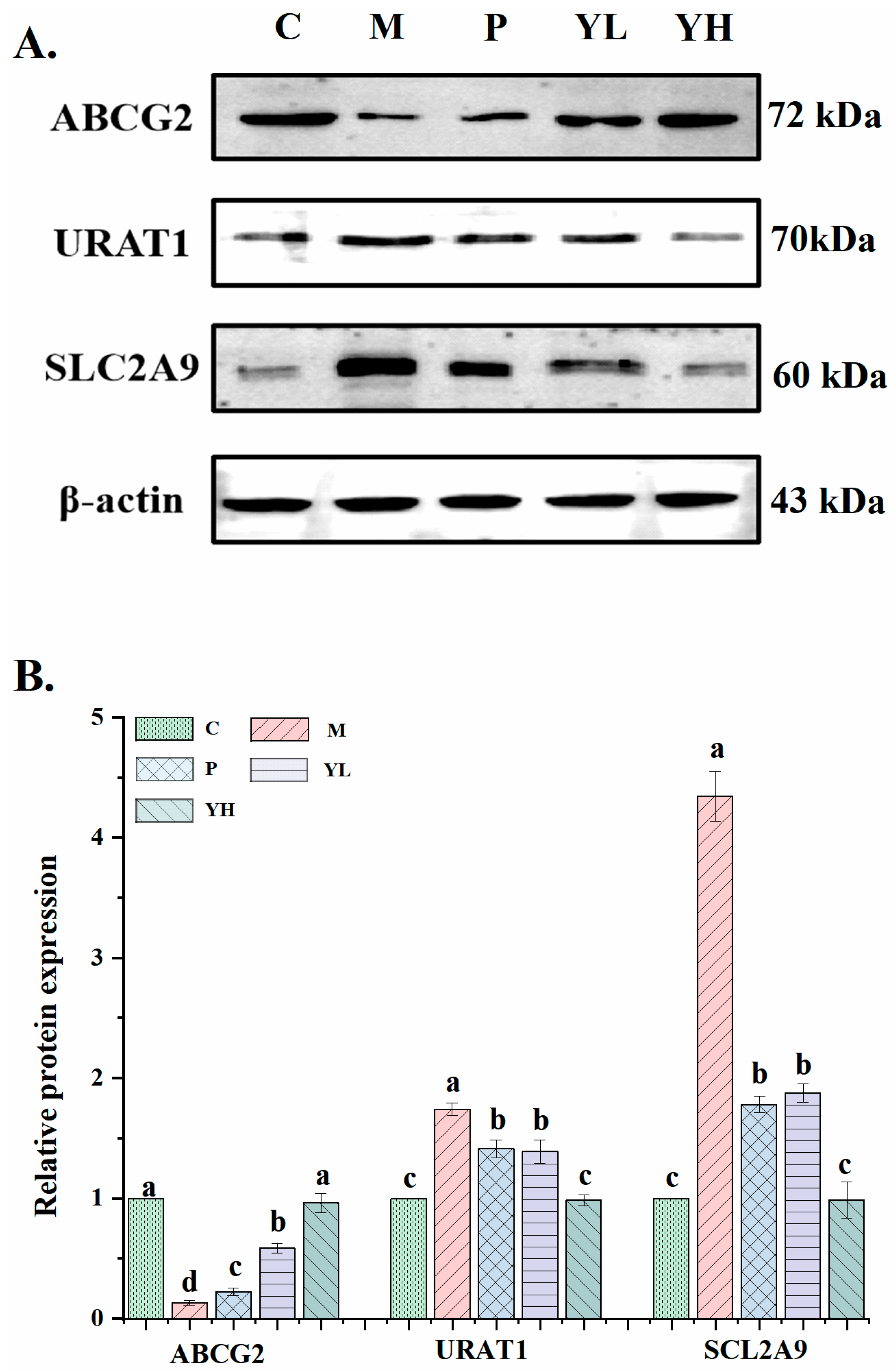
3.5. Effects of CE on the Expression of Uric Acid Transporter in the Kidney of Mice with Hyperuricemia Induced by a High-Purine Yeast Diet
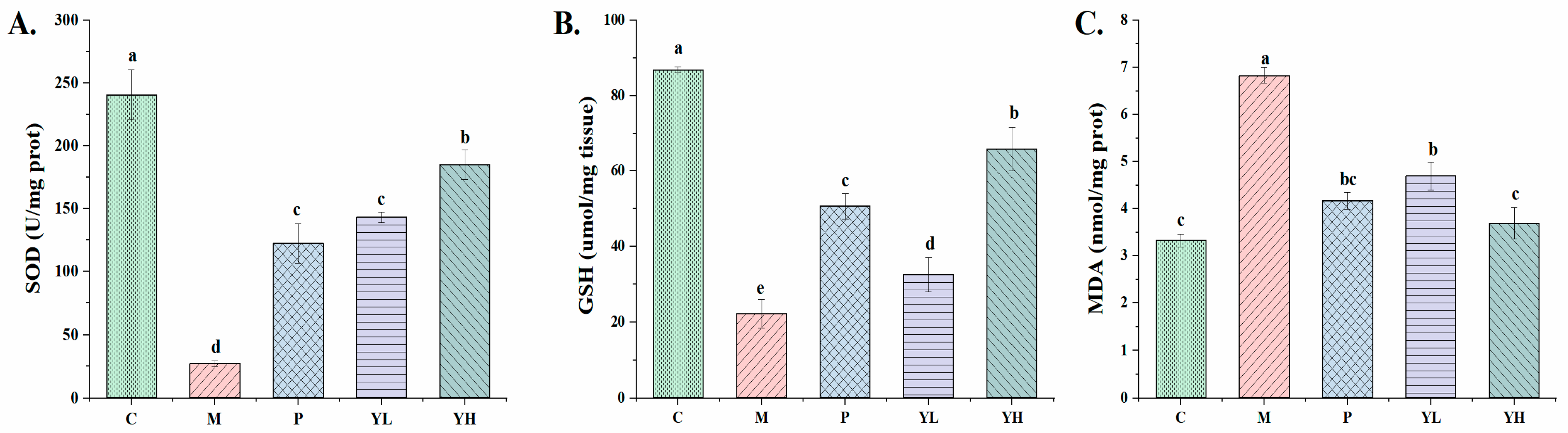
3.6. Effects of CE on Kidney Oxidative Stress in Hyperuricemia Mice Fed a High-Purine Yeast Diet
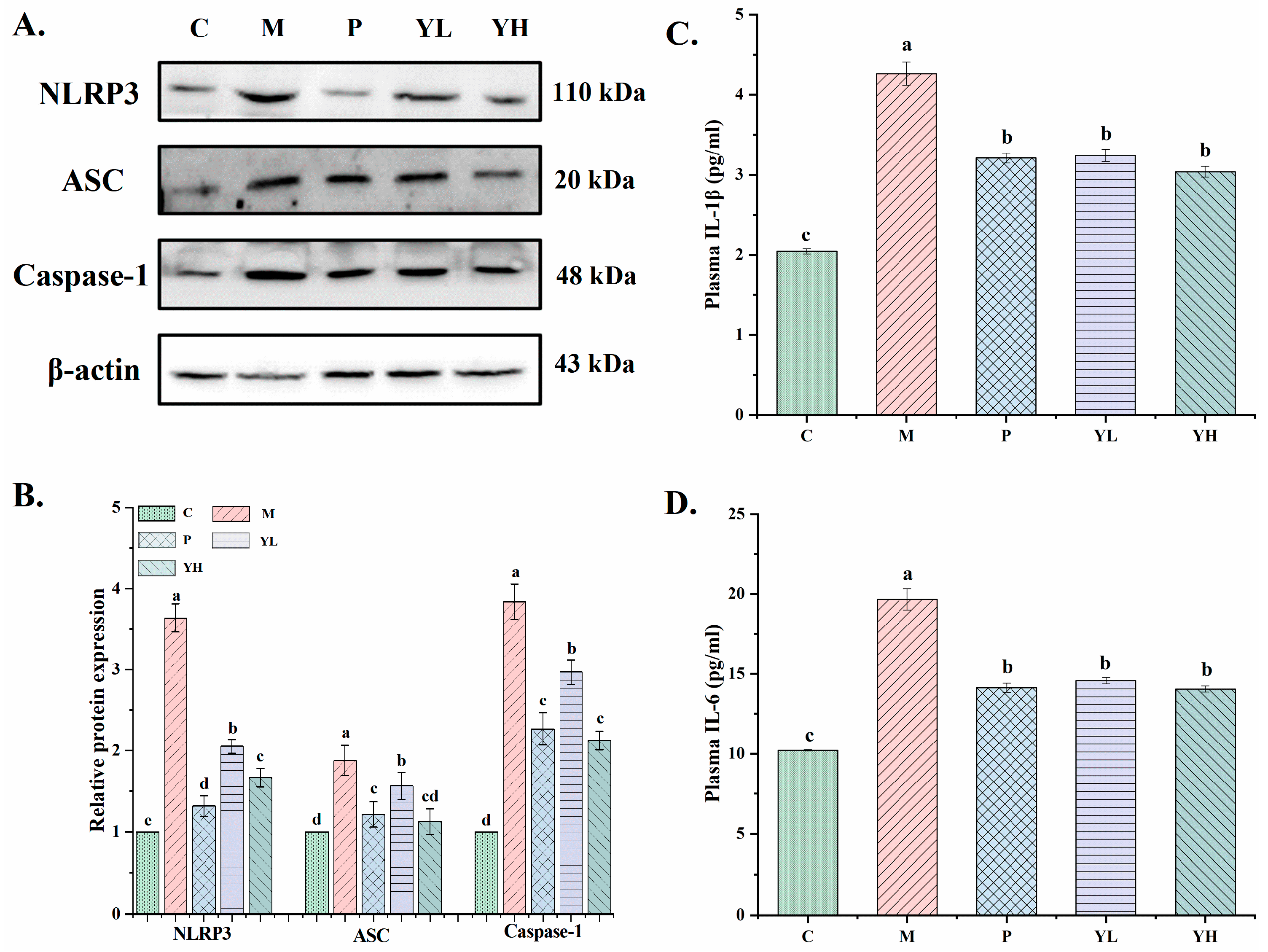
3.7. Effects of CE on Inflammatory Factors Levels and NLRP3 Inflammasome-Pathway-Related Proteins Expression in the Kidney of Hyperuricemia Mice Fed a High-Purine Yeast Diet
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, M.; Yang, F.; Yang, I.; Yin, Y.; Luo, J.J.; Wang, H.; Yang, X.-F. Uric acid, hyperuricemia and vascular diseases. Front. Biosci. 2012, 17, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Dong, B.; Geng, Z.; Xu, L. Excess uric acid induces gouty nephropathy through crystal formation: A review of recent insights. Front. Endocrinol. 2022, 13, 911968. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, H.; Maeda, N.; Shimomura, I. Impact of hyperuricemia on chronic kidney disease and atherosclerotic cardiovascular disease. Hypertens. Res. 2022, 45, 635–640. [Google Scholar] [CrossRef]
- Tien, Y.-Y.; Shih, M.-C.; Tien, C.-P.; Huang, H.-K.; Tu, Y.-K. To Treat or not to treat? Effect of urate-lowering therapy on renal function, blood pressure and safety in patients with asymptomatic hyperuricemia: A systematic review and network meta-analysis. J. Am. Board Fam. Med. 2022, 35, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Chen-Xu, M.; Yokose, C.; Rai, S.K.; Pillinger, M.H.; Choi, H.K. Contemporary Prevalence of Gout and Hyperuricemia in the United States and Decadal Trends: The National Health and Nutrition Examination Survey, 2007–2016. Arthritis Rheumatol. 2019, 71, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, M.; Kodama, T.; Ae, R.; Kanbay, M.; Andres-Hernando, A.; Borghi, C.; Hisatome, I.; Lanaspa, M.A. Update in uric acid, hypertension, and cardiovascular diseases. Hypertens. Res. 2023, 46, 1717–1726. [Google Scholar] [CrossRef]
- Feng, S.; Wu, S.; Xie, F.; Yang, C.S.; Shao, P. Natural compounds lower uric acid levels and hyperuricemia: Molecular mechanisms and prospective. Trends Food Sci. Technol. 2022, 123, 87–102. [Google Scholar] [CrossRef]
- Ishikawa, T.; Maeda, T.; Hashimoto, T.; Nakagawa, T.; Ichikawa, K.; Sato, Y.; Kanno, Y. Long-term safety and effectiveness of the xanthine oxidoreductase inhibitor, topiroxostat in Japanese hyperuricemic patients with or without gout: A 54-week open-label, multicenter, post-marketing observational study. Clin. Drug Investig. 2020, 40, 847–859. [Google Scholar] [CrossRef]
- Piani, F.; Agnoletti, D.; Borghi, C. Advances in pharmacotherapies for hyperuricemia. Expert Opin. Pharmacother. 2023, 24, 737–745. [Google Scholar] [CrossRef]
- Aihemaitijiang, S.; Zhang, Y.; Zhang, L.; Yang, J.; Ye, C.; Halimulati, M.; Zhang, W.; Zhang, Z. The association between purine-rich food intake and hyperuricemia: A cross-sectional study in Chinese adult residents. Nutrients 2020, 12, 3835. [Google Scholar] [CrossRef]
- Zhao, R.; Chen, D.; Wu, H. Pu-erh ripened tea resists to hyperuricemia through xanthine oxidase and renal urate transporters in hyperuricemic mice. J. Funct. Foods 2017, 29, 201–207. [Google Scholar] [CrossRef]
- Bortolotti, M.; Polito, L.; Battelli, M.G.; Bolognesi, A. Xanthine oxidoreductase: One enzyme for multiple physiological tasks. Redox Biol. 2021, 41, 101882. [Google Scholar] [CrossRef] [PubMed]
- Yiying, K.; Yongfang, L.; Husai, M.; Wangyu, L.; Ruilian, L.; Zhancui, D. Uric acid lowering effect of Tibetan Medicine RuPeng15 powder in animal models of hyperuricemia. J. Tradit. Chin. Med. 2016, 36, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.-T.; Chen, H.-Y.; Lin, P.-H.; Hsia, S.-M. Preventive effects of fucoidan and fucoxanthin on hyperuricemic rats induced by potassium oxonate, Preventive effects of fucoidan and fucoxanthin on hyperuricemic rats induced by potassium oxonate. Mar. Drugs 2019, 17, 343. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Zhao, L.; Ishaq, M.; Xin, W.; Zhao, L.; Wang, C.; Hossen, I.; Zhang, H.; Lian, Y.; Xu, M. Anti-hyperuricemic potential of stevia (Stevia rebaudiana Bertoni) residue extract in hyperuricemic mice. Food Funct. 2020, 11, 6387–6406. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, Y.; Gong, X.; Zhang, Y.; Zhao, L.; Cheng, G.; Cai, S. Rhus chinensis Mill. fruits prevent high-fat/ethanol diet-induced alcoholic fatty liver in rats via AMPK/SREBP-1/FAS signaling pathway. J. Funct. Foods 2019, 61, 103498. [Google Scholar] [CrossRef]
- Djakpo, O.; Yao, W. Rhus chinensis and Galla Chinensis–folklore to modern evidence. Phytother. Res. 2010, 24, 1739–1747. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Yi, J.; Cai, S. Phytochemical characteristics and biological activities of Rhus chinensis Mill.: A review. Curr. Opin. Food Sci. 2022, 48, 100925. [Google Scholar] [CrossRef]
- Li, M.; Wang, A.; Zhang, Y.; Han, T.; Guan, L.; Fan, D.; Liu, J.; Xu, Y. A comprehensive review on ethnobotanical, phytochemical and pharmacological aspects of Rhus chinensis Mill. J. Ethnopharmacol. 2022, 293, 115288. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Y.; Ma, Q.; Yi, J.; Cai, S. Anti-diabetic effects of different phenolic-rich fractions from Rhus chinensis Mill. fruits in vitro. eFood 2021, 2, 37–46. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, N.; Liu, X.; Yi, J.; Cai, S. Preventive effects of Chinese sumac fruits against acetaminophen-induced liver injury in mice via regulating oxidative stress, inflammation and apoptosis. J. Funct. Foods. 2021, 87, 104830. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, O.; Ma, N.; Yi, J.; Mi, H.; Cai, S. The preventive effect and underlying mechanism of Rhus chinensis Mill. fruits on dextran sulphate sodium-induced ulcerative colitis in mice. Food Funct. 2021, 12, 9965–9978. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Sun, Y.; Yi, J.; Zhou, L.; Cai, S. Chinese sumac (Rhus chinensis Mill.) fruits alleviate indomethacin-induced gastric ulcer in mice by improving oxidative stress, inflammation and apoptosis. J. Ethnopharmacol. 2022, 284, 114752. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhang, Y.; Wang, T.; Sun, Y.; Cai, S. The preventive effect of Chinese sumac fruit against monosodium urate-induced gouty arthritis in rats by regulating several inflammatory pathways. Food Funct. 2023, 14, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-H.; Xu, Y.-Y.; Zhu, L.-p.; Zheng, X.; Jiang, C.-H.; Liu, J.-J.; Zhang, J.; Yin, Z.-Q. Protective effects of Cyclocarya paliurus on hyperuricemia and urate-induced inflammation. J. Funct. Foods 2022, 94, 105130. [Google Scholar] [CrossRef]
- Jang, M.G.; Song, H.; Kim, J.H.; Oh, J.M.; Park, J.Y.; Ko, H.C.; Hur, S.-P.; Kim, S.-J. Prevention of hyperuricemia by Clerodendrum trichotomum leaf extract in potassium oxonate-induced mice. Dev. Reprod. 2020, 24, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhan, S.; Li, S.; Zhu, Z.; He, J.; Lorenzo, J.M.; Barba, F. Anti-hyperuricemic and nephroprotective effects of extracts from Chaenomeles sinensis (Thouin) Koehne in hyperuricemic mice. Food Funct. 2018, 9, 5778–5790. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Q.; Zhang, Y.; Xue, M.; Yan, H.; Qiu, X.; Tian, Y.; Zhang, H.; Liang, H. Fucoidan from Saccharina japonica Alleviates Hyperuricemia-Induced Renal Fibrosis through Inhibiting the JAK2/STAT3 Signaling Pathway. J. Agric. Food Chem. 2023, 71, 11454–11465. [Google Scholar] [CrossRef]
- Kaneko, K.; Aoyagi, Y.; Fukuuchi, T.; Inazawa, K.; Yamaoka, N. Total purine and purine base content of common foodstuffs for facilitating nutritional therapy for gout and hyperuricemia. Biol. Pharm. Bull. 2014, 37, 709–721. [Google Scholar] [CrossRef]
- Lu, J.; Dalbeth, N.; Yin, H.; Li, C.; Merriman, T.R.; Wei, W.-H. Mouse models for human hyperuricaemia: A critical review. Nat. Rev. Rheumatol. 2019, 15, 413–426. [Google Scholar] [CrossRef]
- Chen, L.; Li, M.; Wu, J.-L.; Li, J.-X.; Ma, Z.-C. Effect of lemon water soluble extract on hyperuricemia in a mouse model. Food Funct. 2019, 10, 6000–6008. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, L.; Liu, J.; Wang, W.; Yu, H.; Li, J.; Chen, Q.; Wang, T. Effect and mechanism of dioscin from Dioscorea spongiosa on uric acid excretion in animal model of hyperuricemia. J. Ethnopharmacol. 2018, 214, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, J.; Song, N.; Chen, R.; Zhang, T.; Ding, X. Hyperuricemia increases the risk of acute kidney injury: A systematic review and meta-analysis. BMC Nephrol. 2017, 18, 27. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Lozada, L.S.; Lanaspa, M.A.; Piani, F.; Borghi, C. Uric acid and chronic kidney disease: Still more to do. Kidney Int. Rep. 2022, 8, 229–23910. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, L.; Jiang, L.; Wu, Y.; Wei, L.; Wu, X.; Xiao, S.; Liu, Y.; Gao, C.; Cai, J.; et al. Sonneratia apetala seed oil attenuates potassium oxonate/hypoxanthine-induced hyperuricemia and renal injury in mice. Food Funct. 2021, 12, 9416–9431. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.; Liu, Z.; Sun, M.; Xie, Q.; Cai, X.; Wang, Y.; Dong, X.; Xu, Y. Anti-hyperuricemic effects of three theaflavins isolated from black tea in hyperuricemic mice. J. Funct. Foods 2020, 66, 103803. [Google Scholar] [CrossRef]
- Chen, M.; Ji, H.; Song, W.; Zhang, D.; Su, W.; Liu, S. Anserine beneficial effects in hyperuricemic rats by inhibiting XOD, regulating uric acid transporter and repairing hepatorenal injury. Food Funct. 2022, 13, 9434–9442. [Google Scholar] [CrossRef]
- Smereczański, N.M.; Brzóska, M.M.; Rogalska, J.; Hutsch, T. The Protective Potential of Aronia melanocarpa L. Berry Extract against Cadmium-Induced Kidney Damage: A Study in an Animal Model of Human Environmental Exposure to This Toxic Element. Int. J. Mol. Sci. 2023, 24, 11647. [Google Scholar] [CrossRef]
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef]
- Lin, S.; Meng, J.; Li, F.; Yu, H.; Lin, D.; Lin, S.; Li, M.; Zhou, H.; Yang, B. Ganoderma lucidum polysaccharide peptide alleviates hyperuricemia by regulating adenosine deaminase and urate transporters. Food Funct. 2022, 13, 12619–12631. [Google Scholar] [CrossRef]
- Shin, H.J.; Takeda, M.; Enomoto, A.; Fujimura, M.; Miyazaki, H.; Anzai, N.; Endou, H. Interactions of urate transporter URAT1 in human kidney with uricosuric drugs. Nephrology 2011, 16, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, A.; Kimura, H.; Chairoungdua, A.; Shigeta, Y.; Jutabha, P.; Ho Cha, S.; Hosoyamada, M.; Takeda, M.; Sekine, T.; Igarashi, T. Molecular identification of a renal urate–anion exchanger that regulates blood urate levels. Nature 2002, 417, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Döring, A.; Gieger, C.; Mehta, D.; Gohlke, H.; Prokisch, H.; Coassin, S.; Fischer, G.; Henke, K.; Klopp, N.; Kronenberg, F. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat. Genet. 2008, 40, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, C.; Zhou, P.; Jiang, T. Uric acid transporters hiding in the intestine. Pharm. Biol. 2016, 54, 3151–3155. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, H.; Nakayama, A.; Sakiyama, M.; Chiba, T.; Shimizu, S.; Kawamura, Y.; Nakashima, H.; Nakamura, T.; Takada, Y.; Oikawa, Y. ABCG2 dysfunction causes hyperuricemia due to both renal urate underexcretion and renal urate overload. Sci. Rep. 2014, 4, 3755. [Google Scholar] [CrossRef] [PubMed]
- So, A.; Thorens, B. Uric acid transport and disease. J. Clin. Investig. 2010, 120, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wu, Y.; Qu, C.; Lin, Y.; Yi, X.; Gao, C.; Cai, J.; Su, Z.; Zeng, H. Hypouricemic effect of gallic acid, a bioactive compound from Sonneratia apetala leaves and branches, on hyperuricemic mice. Food Funct. 2022, 13, 10275–10290. [Google Scholar] [CrossRef]
- Su, H.-y.; Yang, C.; Liang, D.; Liu, H.-f. Research advances in the mechanisms of hyperuricemia-induced renal injury. BioMed Res. Int. 2020, 2020, 5817348. [Google Scholar] [CrossRef]
- Kim, S.-M.; Lee, S.-H.; Kim, Y.-G.; Kim, S.-Y.; Seo, J.-W.; Choi, Y.-W.; Kim, D.-J.; Jeong, K.-H.; Lee, T.-W.; Ihm, C.-G. Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am. J. Physiol.-Renal. 2015, 308, F993–F1003. [Google Scholar] [CrossRef]
- So, A.K.; Martinon, F. Inflammation in gout: Mechanisms and therapeutic targets. Nat. Rev. Rheumatol. 2017, 13, 639–647. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, X.; Zhao, H.; Yang, E.; Wang, Y.; Cheng, N.; Cao, W. Impact of Camellia japonica bee pollen polyphenols on hyperuricemia and gut microbiota in potassium oxonate-induced mice. Nutrients 2021, 13, 2665. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, N.; Cai, S.; Sun, Y.; Chu, C. Chinese Sumac (Rhus chinensis Mill.) Fruits Prevent Hyperuricemia and Uric Acid Nephropathy in Mice Fed a High-Purine Yeast Diet. Nutrients 2024, 16, 184. https://doi.org/10.3390/nu16020184
Ma N, Cai S, Sun Y, Chu C. Chinese Sumac (Rhus chinensis Mill.) Fruits Prevent Hyperuricemia and Uric Acid Nephropathy in Mice Fed a High-Purine Yeast Diet. Nutrients. 2024; 16(2):184. https://doi.org/10.3390/nu16020184
Chicago/Turabian StyleMa, Nan, Shengbao Cai, Yilin Sun, and Chuanqi Chu. 2024. "Chinese Sumac (Rhus chinensis Mill.) Fruits Prevent Hyperuricemia and Uric Acid Nephropathy in Mice Fed a High-Purine Yeast Diet" Nutrients 16, no. 2: 184. https://doi.org/10.3390/nu16020184
APA StyleMa, N., Cai, S., Sun, Y., & Chu, C. (2024). Chinese Sumac (Rhus chinensis Mill.) Fruits Prevent Hyperuricemia and Uric Acid Nephropathy in Mice Fed a High-Purine Yeast Diet. Nutrients, 16(2), 184. https://doi.org/10.3390/nu16020184






