Exploring Associations and Mediating Factors between Multiple Trace Metals with Anemia in US Adults: Insight from NHANES 2017–2020
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Assessment of Trace Metals and Mediators
2.3. Definition of Anemia
2.4. Covariates
2.5. Statistical Analyses
2.5.1. Logistic Regression Model
2.5.2. Quantile G-Computation (QGC) Regression Model
2.5.3. Bayesian Kernel Machine Regression (BKMR) Model
2.5.4. Sex-Stratified Analysis
2.5.5. Mediation Analysis
3. Results
3.1. Population Characteristics
3.2. Trace Metals with Risk of Anemia in Logistic Regression Model
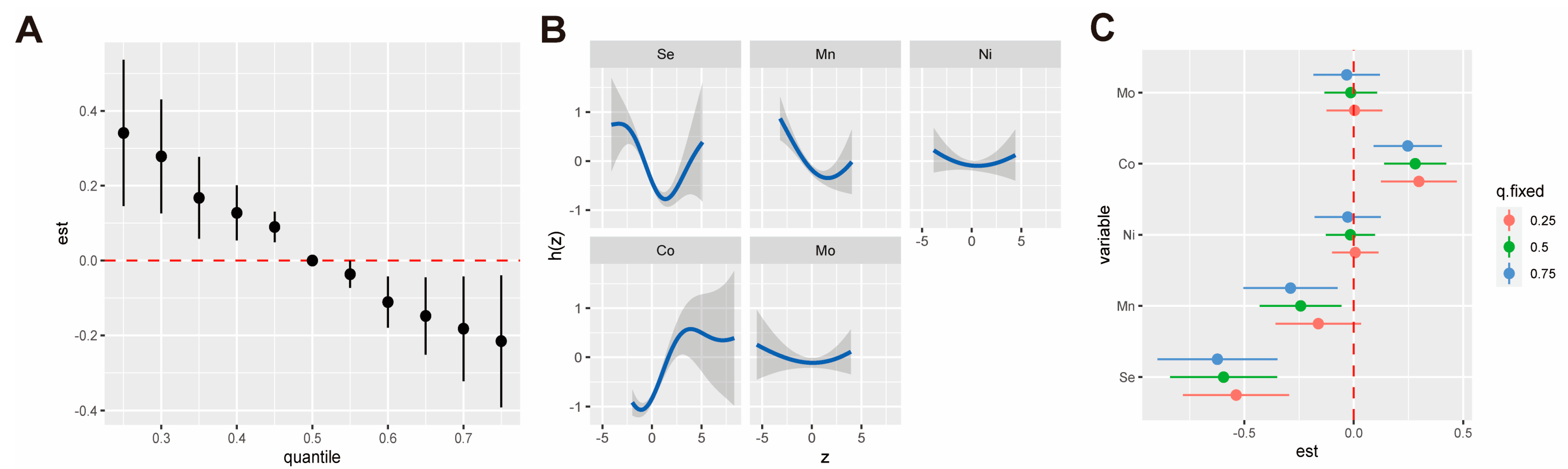
3.3. Trace Metals with Risk of Anemia in QGC Model
3.4. Trace Metal Exposure with Risk of Anemia in BKMR Model
3.5. The Mediating Effect of Iron Status and Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pasricha, S.-R.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron Deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Culleton, B.F.; Manns, B.J.; Zhang, J.; Tonelli, M.; Klarenbach, S.; Hemmelgarn, B.R. Impact of Anemia on Hospitalization and Mortality in Older Adults. Blood 2006, 107, 3841–3846. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Teng, F.; Branch, E.; Chu, S.; Joseph, K.S. Maternal and Perinatal Morbidity and Mortality Associated With Anemia in Pregnancy. Obstet. Gynecol. 2019, 134, 1234–1244. [Google Scholar] [CrossRef] [PubMed]
- Sarna, A.; Porwal, A.; Ramesh, S.; Agrawal, P.K.; Acharya, R.; Johnston, R.; Khan, N.; Sachdev, H.P.S.; Nair, K.M.; Ramakrishnan, L.; et al. Characterisation of the Types of Anaemia Prevalent among Children and Adolescents Aged 1–19 Years in India: A Population-Based Study. Lancet Child Adolesc. Health 2020, 4, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Paciorek, C.J.; Flores-Urrutia, M.C.; Borghi, E.; Namaste, S.; Wirth, J.P.; Suchdev, P.S.; Ezzati, M.; Rohner, F.; Flaxman, S.R.; et al. National, Regional, and Global Estimates of Anaemia by Severity in Women and Children for 2000–19: A Pooled Analysis of Population-Representative Data. Lancet Glob. Health 2022, 10, e627–e639. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.-A.; Noori, M.; Nejadghaderi, S.A.; Karamzad, N.; Bragazzi, N.L.; Sullman, M.J.M.; Abdollahi, M.; Collins, G.S.; Kaufman, J.S.; et al. Burden of Anemia and Its Underlying Causes in 204 Countries and Territories, 1990-2019: Results from the Global Burden of Disease Study 2019. J. Hematol. Oncol. 2021, 14, 185. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential Metals in Health and Disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, Y.; Wang, D.; Guo, Y.; Wang, B.; Xu, Y.; Chen, W. Associations between Essential Metals Exposure and Metabolic Syndrome (MetS): Exploring the Mediating Role of Systemic Inflammation in a General Chinese Population. Environ. Int. 2020, 140, 105802. [Google Scholar] [CrossRef]
- Xia, W.; Chen, L.; Deng, X.; Liang, G.; Giesy, J.P.; Rao, Q.; Wen, Z.; Wu, Y.; Chen, J.; Xie, P. Spatial and Interspecies Differences in Concentrations of Eight Trace Elements in Wild Freshwater Fishes at Different Trophic Levels from Middle and Eastern China. Sci. Total Environ. 2019, 672, 883–892. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- Amaral, A.F.S.; Arruda, M.; Cabral, S.; Rodrigues, A.S. Essential and Non-Essential Trace Metals in Scalp Hair of Men Chronically Exposed to Volcanogenic Metals in the Azores, Portugal. Environ. Int. 2008, 34, 1104–1108. [Google Scholar] [CrossRef] [PubMed]
- Nassef, Y.E.; Shady, M.A.; Mansour, M.; Hamed, M.A.A. Trace Elements, Heavy Metals and Vitamins in Egyptian School Children with Iron Deficiency Anemia. J. Pediatr. Biochem. 2014, 4, 171–179. [Google Scholar] [CrossRef]
- Qiao, G.; Shen, Z.; Duan, S.; Wang, R.; He, P.; Zhang, Z.; Dai, Y.; Li, M.; Chen, Y.; Li, X.; et al. Associations of Urinary Metal Concentrations with Anemia: A Cross-Sectional Study of Chinese Community-Dwelling Elderly. Ecotoxicol. Environ. Saf. 2024, 270, 115828. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zeng, Z.; Huang, J.; Tian, Q.; Cao, B.; Huo, X. Kindergarten Indoor Dust Metal(Loid) Exposure Associates with Elevated Risk of Anemia in Children. Sci. Total Environ. 2022, 851, 158227. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Hernández, L.A.; Boada, L.D.; Carranza, C.; Pérez-Arellano, J.L.; González-Antuña, A.; Camacho, M.; Almeida-González, M.; Zumbado, M.; Luzardo, O.P. Blood Levels of Toxic Metals and Rare Earth Elements Commonly Found in E-Waste May Exert Subtle Effects on Hemoglobin Concentration in Sub-Saharan Immigrants. Environ. Int. 2017, 109, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.-F.; Lin, C.-J.; Chen, S.-H.; Huang, C.-F.; Lee, C.-C. Association between Trace Element Concentrations and Anemia in Patients with Chronic Kidney Disease: A Cross-Sectional Population-Based Study. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2019, 67, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of Inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef]
- Camaschella, C. Iron Deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef]
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and Characterization of a Mammalian Proton-Coupled Metal-Ion Transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef]
- Chen, G.-D.; Pang, T.-T.; Li, P.-S.; Zhou, Z.-X.; Gou, X.-Y.; Wang, H.-Y.; Lin, D.-X.; Fan, D.-Z.; Li, H.-L.; Liu, Z.-P. Associations of Serum Concentrations of Metal Nutrients with Postpartum Anemia among Pregnant Chinese Women: A Large Retrospective Cohort Study. Front. Nutr. 2023, 10, 1086082. [Google Scholar] [CrossRef] [PubMed]
- Fort, M.; Grimalt, J.O.; Casas, M.; Sunyer, J. Interdependence between Urinary Cobalt Concentrations and Hemoglobin Levels in Pregnant Women. Environ. Res. 2015, 136, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hu, J.; Tang, R.; Guo, H.; Chen, Q.; Qiu, J.; Liu, Y.; Tan, R.; Zhong, X. Association between the Blood Manganese (Mn) and Hemoglobin in Patients Undergoing Maintenance Hemodialysis. J. Trace Elem. Med. Biol. Organ Soc. Miner. Trace Elem. GMS 2022, 71, 126947. [Google Scholar] [CrossRef] [PubMed]
- Schildroth, S.; Friedman, A.; Bauer, J.A.; Claus Henn, B. Associations of a Metal Mixture with Iron Status in U.S. Adolescents: Evidence from the National Health and Nutrition Examination Survey. New Dir. Child Adolesc. Dev. 2022, 2022, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Brucker, N.; Moro, A.; Charão, M.; Bubols, G.; Nascimento, S.; Goethel, G.; Barth, A.; Prohmann, A.C.; Rocha, R.; Moresco, R.; et al. Relationship between Blood Metals and Inflammation in Taxi Drivers. Clin. Chim. Acta 2015, 444, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.P.; McMillan, D.C.; Sattar, N. Effect of the Inflammatory Response on Trace Element and Vitamin Status. Ann. Clin. Biochem. 2000, 37, 289–297. [Google Scholar] [CrossRef]
- Biomonitoring Data Tables for Environmental Chemicals|CDC. Available online: https://www.cdc.gov/exposurereport/data_tables.html (accessed on 5 October 2024).
- Bakr, S.; Sayed, M.A.; Salem, K.M.; Morsi, E.M.; Masoud, M.; Ezzat, E.M. Lead (Pb) and Cadmium (Cd) Blood Levels and Potential Hematological Health Risk among Inhabitants of the Claimed Hazardous Region around Qaroun Lake in Egypt. BMC Public Health 2023, 23, 1071. [Google Scholar] [CrossRef]
- Caldwell, K.L.; Mortensen, M.E.; Jones, R.L.; Caudill, S.P.; Osterloh, J.D. Total Blood Mercury Concentrations in the U.S. Population: 1999–2006. Int. J. Hyg. Environ. Health 2009, 212, 588–598. [Google Scholar] [CrossRef]
- WHO Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Available online: https://iris.who.int/bitstream/handle/10665/85839/WHO_NMH_NHD_MNM_11.1_eng.pdf (accessed on 2 July 2024).
- Liang, J.; Yang, C.; Liu, T.; Tang, P.; Huang, H.; Wei, H.; Liao, Q.; Long, J.; Zeng, X.; Liu, S.; et al. Single and Mixed Effects of Prenatal Exposure to Multiple Bisphenols on Hemoglobin Levels and the Risk of Anemia in Pregnant Women. Environ. Res. 2022, 207, 112625. [Google Scholar] [CrossRef]
- Liao, Q.; Tang, P.; Pan, D.; Song, Y.; Lei, L.; Liang, J.; Liu, B.; Lin, M.; Huang, H.; Mo, M.; et al. Association of Serum Per- and Polyfluoroalkyl Substances and Gestational Anemia during Different Trimesters in Zhuang Ethnic Pregnancy Women of Guangxi, China. Chemosphere 2022, 309, 136798. [Google Scholar] [CrossRef]
- Tsai, J. Exposure to Secondhand Smoke among Nonsmokers—United States, 1988–2014. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1342–1346. [Google Scholar] [CrossRef]
- Nan, Y.; Yang, J.; Yang, J.; Wei, L.; Bai, Y. Associations between Individual and Combined Metal Exposures in Whole Blood and Kidney Function in U.S. Adults Aged 40 Years and Older. Biol. Trace Elem. Res. 2024, 202, 850–865. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, M.; Cheng, M.; Fan, L.; Wang, X.; Xu, T.; Wang, B.; Chen, W. Associations between Urinary Phthalate Metabolite Concentrations and Markers of Liver Injury in the US Adult Population. Environ. Int. 2021, 155, 106608. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.P.; Buckley, J.P.; O’Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020, 128, 047004. [Google Scholar] [CrossRef] [PubMed]
- Bobb, J.F.; Claus Henn, B.; Valeri, L.; Coull, B.A. Statistical Software for Analyzing the Health Effects of Multiple Concurrent Exposures via Bayesian Kernel Machine Regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef] [PubMed]
- Rushton, D.H.; Barth, J.H. What Is the Evidence for Gender Differences in Ferritin and Haemoglobin? Crit. Rev. Oncol. Hematol. 2010, 73, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Salive, M.E.; Cornoni-Huntley, J.; Guralnik, J.M.; Phillips, C.L.; Wallace, R.B.; Ostfeld, A.M.; Cohen, H.J. Anemia and Hemoglobin Levels in Older Persons: Relationship with Age, Gender, and Health Status. J. Am. Geriatr. Soc. 1992, 40, 489–496. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Office of Dietary Supplements—Selenium. Available online: https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/?os=app&ref=app (accessed on 27 September 2024).
- Jain, R.B.; Choi, Y.S. Normal Reference Ranges for and Variability in the Levels of Blood Manganese and Selenium by Gender, Age, and Race/Ethnicity for General U.S. Population. J. Trace Elem. Med. Biol. 2015, 30, 142–152. [Google Scholar] [CrossRef]
- Huang, J.; Xie, L.; Song, A.; Zhang, C. Selenium Status and Its Antioxidant Role in Metabolic Diseases. Oxid. Med. Cell. Longev. 2022, 2022, 7009863. [Google Scholar] [CrossRef]
- Lemire, M.; Philibert, A.; Fillion, M.; Passos, C.J.S.; Guimarães, J.R.D.; Barbosa, F.; Mergler, D. No Evidence of Selenosis from a Selenium-Rich Diet in the Brazilian Amazon. Environ. Int. 2012, 40, 128–136. [Google Scholar] [CrossRef]
- Semba, R.D.; Ferrucci, L.; Cappola, A.R.; Ricks, M.O.; Ray, A.L.; Xue, Q.-L.; Guralnik, J.M.; Fried, L.P. Low Serum Selenium Is Associated with Anemia among Older Women Living in the Community. Biol. Trace Elem. Res. 2006, 112, 97–107. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Semba, R.D.; Ricks, M.O.; Ferrucci, L.; Xue, Q.-L.; Guralnik, J.M.; Fried, L.P. Low Serum Selenium Is Associated with Anemia among Older Adults in the United States. Eur. J. Clin. Nutr. 2009, 63, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zhang, B.; Chen, X.; Chen, Q.; Hao, L. Association of Serum Selenium with Anemia-Related Indicators and Risk of Anemia. Food Sci. Nutr. 2021, 9, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide Dismutase Multigene Family: A Comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) Gene Structures, Evolution, and Expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, H.; Mawdesley, A.E.; Holland, J.P.; Kirby, J.A.; Deehan, D.J.; Tyson-Capper, A.J. Targeting Toll-like Receptor 4 Prevents Cobalt-Mediated Inflammation. Oncotarget 2016, 7, 7578–7585. [Google Scholar] [CrossRef][Green Version]
- Salloum, Z.; Lehoux, E.A.; Harper, M.-E.; Catelas, I. Effects of Cobalt and Chromium Ions on Oxidative Stress and Energy Metabolism in Macrophages in Vitro. J. Orthop. Res. 2018, 36, 3178–3187. [Google Scholar] [CrossRef]
- Lv, Y.; Wei, Y.; Zhou, J.; Xue, K.; Guo, Y.; Liu, Y.; Ju, A.; Wu, B.; Zhao, F.; Chen, C.; et al. Human Biomonitoring of Toxic and Essential Metals in Younger Elderly, Octogenarians, Nonagenarians and Centenarians: Analysis of the Healthy Ageing and Biomarkers Cohort Study (HABCS) in China. Environ. Int. 2021, 156, 106717. [Google Scholar] [CrossRef]
- Pieczyńska, J.; Płaczkowska, S.; Sozański, R.; Skórska, K.; Sołtysik, M. Effect of Nickel on Red Blood Cell Parameters and on Serum Vitamin B12, Folate and Homocysteine Concentrations during Pregnancy with and without Anemia. J. Trace Elem. Med. Biol. 2021, 68, 126839. [Google Scholar] [CrossRef]
- Yang, L.; Lin, Z.; Wang, Y.; Li, C.; Xu, W.; Li, Q.; Yao, W.; Song, Z.; Liu, G. Nickle(II) Ions Exacerbate Bleomycin-Induced Pulmonary Inflammation and Fibrosis by Activating the ROS/Akt Signaling Pathway. Environ. Sci. Pollut. Res. 2018, 25, 4406–4418. [Google Scholar] [CrossRef]
- Feng, J.; Chen, J.; Xing, C.; Huang, A.; Zhuang, Y.; Yang, F.; Zhang, C.; Hu, G.; Mao, Y.; Cao, H. Molybdenum Induces Mitochondrial Oxidative Damage in Kidney of Goats. Biol. Trace Elem. Res. 2020, 197, 167–174. [Google Scholar] [CrossRef]
- Camaschella, C. Iron-Deficiency Anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wu, Q.; Zhou, Y. Iron-Refractory Iron Deficiency Anemia: New Molecular Mechanisms. Kidney Int. 2009, 76, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Williet, N.; Cacoub, P. Guidelines on the Diagnosis and Treatment of Iron Deficiency across Indications: A Systematic Review. Am. J. Clin. Nutr. 2015, 102, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Schildroth, S.; Valeri, L.; Kordas, K.; Shi, B.; Friedman, A.; Smith, D.; Placidi, D.; Wright, R.O.; Lucchini, R.G.; White, R.F.; et al. Assessing the Mediating Role of Iron Status on Associations between an Industry-Relevant Metal Mixture and Verbal Learning and Memory in Italian Adolescents. Sci. Total Environ. 2024, 906, 167435. [Google Scholar] [CrossRef]
- Silvestre, O.M.; Gonçalves, A.; Nadruz, W., Jr.; Claggett, B.; Couper, D.; Eckfeldt, J.H.; Pankow, J.S.; Anker, S.D.; Solomon, S.D. Ferritin Levels and Risk of Heart Failure—The Atherosclerosis Risk in Communities Study. Eur. J. Heart Fail. 2017, 19, 340–347. [Google Scholar] [CrossRef]
- Nemeth, E.; Rivera, S.; Gabayan, V.; Keller, C.; Taudorf, S.; Pedersen, B.K.; Ganz, T. IL-6 Mediates Hypoferremia of Inflammation by Inducing the Synthesis of the Iron Regulatory Hormone Hepcidin. J. Clin. Investig. 2004, 113, 1271–1276. [Google Scholar] [CrossRef]
- Pergola, P.E.; Devalaraja, M.; Fishbane, S.; Chonchol, M.; Mathur, V.S.; Smith, M.T.; Lo, L.; Herzog, K.; Kakkar, R.; Davidson, M.H. Ziltivekimab for Treatment of Anemia of Inflammation in Patients on Hemodialysis: Results from a Phase 1/2 Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Soc. Nephrol. 2021, 32, 211. [Google Scholar] [CrossRef]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 Induces Hepcidin Expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef]
- Pietrangelo, A. Hepcidin in Human Iron Disorders: Therapeutic Implications. J. Hepatol. 2011, 54, 173–181. [Google Scholar] [CrossRef]
- Canny, S.P.; Orozco, S.L.; Thulin, N.K.; Hamerman, J.A. Immune Mechanisms in Inflammatory Anemia. Annu. Rev. Immunol. 2023, 41, 405–429. [Google Scholar] [CrossRef]
- Jelkmann, W. Proinflammatory Cytokines Lowering Erythropoietin Production. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 1998, 18, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Haroon, E.; Patel, T.A.; Goldsmith, D.R.; Wommack, E.C.; Woolwine, B.J.; Le, N.-A.; Feinberg, R.; Tansey, M.G.; Miller, A.H. What Does Plasma CRP Tell Us about Peripheral and Central Inflammation in Depression? Mol. Psychiatry 2020, 25, 1301–1311. [Google Scholar] [CrossRef]
- Tuttle, C.S.L.; Thang, L.A.N.; Maier, A.B. Markers of Inflammation and Their Association with Muscle Strength and Mass: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2020, 64, 101185. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M. Inflammatory Markers in Systemic Lupus Erythematosus. J. Autoimmun. 2020, 110, 102374. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, V.; García-Martinez, R.; Salvatella, X. Human Serum Albumin, Systemic Inflammation, and Cirrhosis. J. Hepatol. 2014, 61, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Akkiz, H.; Carr, B.I.; Bag, H.G.; Karaoğullarından, Ü.; Yalçın, K.; Ekin, N.; Özakyol, A.; Altıntaş, E.; Balaban, H.Y.; Şimşek, H.; et al. Serum Levels of Inflammatory Markers CRP, ESR and Albumin in Relation to Survival for Patients with Hepatocellular Carcinoma. Int. J. Clin. Pract. 2021, 75, e13593. [Google Scholar] [CrossRef]
- Alves, F.C.; Sun, J.; Qureshi, A.R.; Dai, L.; Snaedal, S.; Bárány, P.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P. The Higher Mortality Associated with Low Serum Albumin Is Dependent on Systemic Inflammation in End-Stage Kidney Disease. PLoS ONE 2018, 13, e0190410. [Google Scholar] [CrossRef]
- Keller, C.; Katz, R.; Sarnak, M.J.; Fried, L.F.; Kestenbaum, B.; Cushman, M.; Shlipak, M.G.; the CHS Study. Inflammatory Biomarkers and Decline in Kidney Function in the Elderly: The Cardiovascular Health Study. Nephrol. Dial. Transplant. 2010, 25, 119–124. [Google Scholar] [CrossRef]
- Sharon, B.; Nath, C. 443. Albumin Is a Reliable and Accurate Biomarker for Assessing Clinical Course of Multisystem Inflammatory Syndrome in Children. Open Forum Infect. Dis. 2023, 10, ofad500-513. [Google Scholar] [CrossRef]
- Röhrig, G.; Becker, I.; Polidori, M.C.; Schulz, R.-J.; Noreik, M. Association of Anemia and Hypoalbuminemia in German Geriatric Inpatients: Relationship to Nutritional Status and Comprehensive Geriatric Assessment. Z. Gerontol. Geriatr. 2015, 48, 619–624. [Google Scholar] [CrossRef]
- Agarwal, R.; Davis, J.L.; Smith, L. Serum Albumin Is Strongly Associated with Erythropoietin Sensitivity in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2008, 3, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Yazawa, H.; Nishikawa, R.; Tokoi, S.; Kayashima, R.; Kono, K.; Sakuma, M.; Abe, S.; Toyoda, S.; Nakajima, T. Physiological Role of Serum Growth Differentiation Factor-15 (GDF-15) Level and Iron Metabolism in Community-Dwelling Older Adults. Cureus 2024, 16, e60422. [Google Scholar] [CrossRef] [PubMed]
- Larvie, D.Y.; Doherty, J.L.; Donati, G.L.; Armah, S.M. Relationship between Selenium and Hematological Markers in Young Adults with Normal Weight or Overweight/Obesity. Antioxidants 2019, 8, 463. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and Selenoproteins: It’s Role in Regulation of Inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Carlson, B.A.; Paulson, R.F.; Prabhu, K.S. The Intricate Role of Selenium and Selenoproteins in Erythropoiesis. Free Radic. Biol. Med. 2018, 127, 165–171. [Google Scholar] [CrossRef]
- Junqué, E.; Grimalt, J.O.; Fernández-Somoano, A.; Tardón, A. Urinary Cobalt and Ferritin in Four-Years-Old Children. Environ. Res. 2020, 183, 109147. [Google Scholar] [CrossRef]
- Garrick, M.D.; Singleton, S.T.; Vargas, F.; Kuo, H.-C.; Zhao, L.; Knöpfel, M.; Davidson, T.; Costa, M.; Paradkar, P.; Roth, J.A.; et al. DMT1: Which Metals Does It Transport? Biol. Res. 2006, 39, 79–85. [Google Scholar] [CrossRef]
- Schildroth, S.; Kordas, K.; White, R.F.; Friedman, A.; Placidi, D.; Smith, D.; Lucchini, R.G.; Wright, R.O.; Horton, M.; Claus Henn, B. An Industry-Relevant Metal Mixture, Iron Status, and Reported Attention-Related Behaviors in Italian Adolescents. Environ. Health Perspect. 2024, 132, 027008. [Google Scholar] [CrossRef]
- Bachman, E.; Travison, T.G.; Basaria, S.; Davda, M.N.; Guo, W.; Li, M.; Connor Westfall, J.; Bae, H.; Gordeuk, V.; Bhasin, S. Testosterone Induces Erythrocytosis via Increased Erythropoietin and Suppressed Hepcidin: Evidence for a New Erythropoietin/Hemoglobin Set Point. J. Gerontol. Ser. A 2014, 69, 725–735. [Google Scholar] [CrossRef]
- Bajbouj, K.; Shafarin, J.; Allam, H.; Madkour, M.; Awadallah, S.; El-Serafy, A.; Sandeep, D.; Hamad, M. Elevated Levels of Estrogen Suppress Hepcidin Synthesis and Enhance Serum Iron Availability in Premenopausal Women. Exp. Clin. Endocrinol. Diabetes 2018, 126, 453–459. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, S.; Wang, L.; Li, J.; Qu, G.; He, J.; Rong, H.; Ji, H.; Liu, S. Estrogen Regulates Iron Homeostasis through Governing Hepatic Hepcidin Expression via an Estrogen Response Element. Gene 2012, 511, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Ashrap, P.; Sánchez, B.N.; Téllez-Rojo, M.M.; Basu, N.; Tamayo-Ortiz, M.; Peterson, K.E.; Meeker, J.D.; Watkins, D.J. In Utero and Peripubertal Metals Exposure in Relation to Reproductive Hormones and Sexual Maturation and Progression among Girls in Mexico City. Environ. Res. 2019, 177, 108630. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, R.A.; Krey, L.C. Cobalt-Protoporphyrin Suppresses Testosterone Secretion by Multiple Interactions within the Brain-Pituitary-Testicular Axis. Neuroendocrinology 2008, 49, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Sancini, A.; Pimpinella, B.; Scala, B.; Suppi, A.; Bonomi, S.; Samperi, I.; Rosati, M.V.; Tomei, G.; Tomei, F.; Caciari, T. Correlation between Urinary Nickel and Testosterone Plasma Values in Workers Occupationally Exposed to Urban Stressors. Ann Ig 2014, 26, 237–254. [Google Scholar] [PubMed]
- Luo, Q.; Zhao, H.; Jiang, Y.; Guo, J.; Lv, N.; Tang, J.; Li, S.; Zhang, D.; Bai, R.; Chen, G. Association of Blood Metal Exposure with Testosterone and Hemoglobin: A Cross-Sectional Study in Hangzhou Birth Cohort Study. Environ. Int. 2020, 136, 105451. [Google Scholar] [CrossRef]
- Gade, M.; Comfort, N.; Re, D.B. Sex-Specific Neurotoxic Effects of Heavy Metal Pollutants: Epidemiological, Experimental Evidence and Candidate Mechanisms. Environ. Res. 2021, 201, 111558. [Google Scholar] [CrossRef]

| Characteristics | Total | Anemic | Non-Anemic | p Value |
|---|---|---|---|---|
| (N = 1274) | (N = 155) | (N = 1119) | ||
| Age (year), mean ± SD | 59.87 ± 11.45 | 62.54 ± 12.87 | 59.50 ± 11.19 | 0.006 |
| Gender, n (%) | 0.078 | |||
| Male | 672 (52.74) | 71 (45.81) | 601 (53.71) | |
| Female | 602 (47.25) | 84 (54.19) | 518 (46.29) | |
| Race, n (%) | <0.001 | |||
| Mexican American | 123 (9.65%) | 14 (9.03%) | 109 (9.74%) | |
| Other Hispanic | 118 (9.26%) | 6 (3.87%) | 112 (10.01%) | |
| Non-Hispanic White | 535 (41.99%) | 48 (30.97%) | 487 (43.52%) | |
| Non-Hispanic Black | 328 (25.75%) | 78 (50.32%) | 250 (22.34%) | |
| Other Race | 170 (13.34%) | 9 (5.81%) | 161 (14.39%) | |
| BMI (kg/m2), mean ± SD | 30.57 ± 7.16 | 31.58 ± 8.56 | 30.43 ± 6.93 | 0.109 |
| Smoking, n (%) | 0.007 | |||
| Non-smoker | 697 (54.71%) | 90 (58.06%) | 607 (54.24%) | |
| Passive smoker | 276 (21.66%) | 43 (27.74%) | 233 (20.82%) | |
| Active smoker | 301 (23.63%) | 22 (14.19%) | 279 (24.93%) | |
| Drinking, n (%) | 0.008 | |||
| Non-drinker | 329 (25.82%) | 51 (32.90%) | 278 (24.84%) | |
| Moderate drinker | 584 (45.84%) | 75 (48.39%) | 509 (45.48%) | |
| Heavy drinker | 361 (28.34%) | 29 (18.71%) | 332 (29.67%) | |
| PIR, n (%) | 0.548 | |||
| <1 | 189 (14.84%) | 20 (12.90%) | 169 (15.10%) | |
| ≥1 | 1085 (85.16%) | 135 (87.10%) | 950 (84.90% | |
| Hypertension, n (%) | <0.001 | |||
| Yes | 770 (60.44%) | 116 (74.84%) | 654 (58.44%) | |
| No | 504 (39.56%) | 39 (25.16%) | 465 (41.55%) | |
| Diabetes, n (%) | <0.001 | |||
| Yes | 354 (27.79%) | 66 (42.58%) | 288 (25.73%) | |
| No | 920 (72.21%) | 89 (57.42%) | 831 (74.26%) | |
| Physical activity n (%) | 0.497 | |||
| Active | 579 (45.45%) | 66 (42.58%) | 513 (45.84%) | |
| Inactive | 695 (54.55%) | 89 (57.42%) | 606 (45.84%) | |
| Ferritin (μg/L), median (IQR) | 124.00 (64.03, 225.00) | 81.60 (19.25, 173.00) | 131.00 (69.95, 229.50) | <0.001 |
| Albumin (g/L), median (IQR) | 40.32 ± 3.16 | 38.26 ± 2.98 | 40.60 ± 3.07 | <0.001 |
| hsCRP(mg/L), median (IQR) | 2.17 (0.98, 4.64) | 2.94 (1.14, 6.35) | 2.14 (0.97, 4.37) | 0.002 |
| Trace metal concentrations, median (IQR) | ||||
| Se (μg/L) | 184.69 (169.08, 201.55) | 171.66 (168.16, 189.95) | 185.92 (171.00, 202.65) | <0.001 |
| Mn (μg/L) | 8.98 (7.10, 11.02) | 8.43 (6.61, 10.89) | 9.04 (7.20, 11.02) | 0.015 |
| Co (μg/L) | 0.14 (0.11, 0.18) | 0.16 (0.12, 0.27) | 0.14 (0.10, 0.17) | <0.001 |
| Ni (μg/g cre) | 1.15 (0.76, 1.83) | 1.33 (0.86, 2.25) | 1.12 (0.74, 1.79) | 0.004 |
| Mo (μg/g cre) | 33.87 (21.70, 50.14) | 30.80 (21.42, 49.47) | 33.93 (21.83, 50.36) | 0.405 |
| Metals | Crude Model | p Value | Model Ⅰ | p Value | Model Ⅱ | p Value | |
|---|---|---|---|---|---|---|---|
| Crude OR (95%CI) | Adjusted OR (95%CI) | Adjusted OR (95%CI) | |||||
| Ni | Q1 | Ref | Ref | Ref | |||
| Q2 | 1.11 (0.66, 1.88) | 0.691 | 1.14 (0.65, 1.99) | 0.634 | 1.11 (0.62, 1.97) | 0.742 | |
| Q3 | 1.27 (0.77, 2.12) | 0.352 | 1.46 (0.84, 2.57) | 0.183 | 1.18 (0.65, 2.14) | 0.595 | |
| Q4 | 2.01 (1.26, 3.26) | 0.004 | 2.34 (1.37, 4.06) | 0.002 | 1.72 (1.14, 3.12) | 0.006 | |
| Co | Q1 | Ref | Ref | Ref | |||
| Q2 | 1.12 (0.68, 1.84) | 0.658 | 1.23 (0.73, 2.09) | 0.435 | 1.55 (0.90, 2.69) | 0.112 | |
| Q3 | 0.94 (0.54, 1.60) | 0.814 | 1.06 (0.59, 1.86) | 0.848 | 1.40 (0.76, 2.55) | 0.272 | |
| Q4 | 2.49 (1.59, 3.86) | <0.001 | 2.97 (1.82, 4.92) | <0.001 | 3.34 (1.96, 5.81) | <0.001 | |
| Mn | Q1 | Ref | Ref | Ref | |||
| Q2 | 0.93 (0.59, 1.44) | 0.734 | 1.01 (0.63, 1.63) | 0.953 | 1.01 (0.61, 1.68) | 0.970 | |
| Q3 | 0.52 (0.31, 0.85) | 0.011 | 0.62 (0.36, 1.06) | 0.083 | 0.54 (0.30, 0.96) | 0.036 | |
| Q4 | 0.78 (0.49, 1.24) | 0.295 | 1.18 (0.70, 2.00) | 0.537 | 0.93 (0.52, 1.64) | 0.798 | |
| Se | Q1 | Ref | Ref | Ref | |||
| Q2 | 0.51 (0.33, 0.77) | 0.002 | 0.53 (0.34, 0.84) | 0.007 | 0.55 (0.34, 0.88) | 0.013 | |
| Q3 | 0.31 (0.19, 0.50) | <0.001 | 0.31 (0.18, 0.51) | <0.001 | 0.31 (0.18, 0.52) | <0.001 | |
| Q4 | 0.27 (0.16, 0.44) | <0.001 | 0.27 (0.15, 0.45) | <0.001 | 0.25 (0.14, 0.43) | <0.001 | |
| Mo | Q1 | Ref | Ref | Ref | |||
| Q2 | 0.98 (0.61, 1.56) | 0.917 | 1.22 (0.74, 2.00) | 0.440 | 1.07 (0.64, 1.81) | 0.772 | |
| Q3 | 0.87 (0.54, 1.39) | 0.553 | 1.01 (0.60, 1.68) | 0.978 | 0.87 (0.51, 1.50) | 0.630 | |
| Q4 | 0.92 (0.57, 1.47) | 0.718 | 1.06 (0.63, 1.77) | 0.835 | 1.01 (0.58, 1.75) | 0.987 | |
| Mediators | Metals | TE | ACME | Mediated Proportion (%) | p Value |
|---|---|---|---|---|---|
| Ln-ferritin | Ni | 3.81 × 10−2 (1.15 × 10−2, 0.06) | 2.22 × 10−2 (1.45 × 10−2, 0.03) | 58.18% | 0.004 |
| Co | 7.54 × 10−2 (4.85 × 10−2, 0.11) | 3.55 × 10−2 (2.49 × 10−2, 0.05) | 46.46% | <0.001 | |
| Mn | 1.85 × 10−2 (−3.44 × 10−2, 0.07) | 6.30 × 10−2 (4.57 × 10−2, 0.08) | |||
| Se | −3.59 × 10−1 (−4.92 × 10−1, −0.23) | −5.32 × 10−2 (−8.64 × 10−2, −0.03) | 14.72% | <0.001 | |
| Mo | −2.31 × 10−3 (−3.81 × 10−3, 0.01) | 7.99 × 10−4 (−2.80 × 10−2, 0.03) | |||
| Albumin | Ni | 3.69 × 10−2 (9.19 × 10−3, 0.06) | 6.21 × 10−3 (1.40 × 10−4, 0.01) | 15.83% | 0.042 |
| Co | 7.60 × 10−2 (4.78 × 10−2, 0.10) | 1.58 × 10−2 (9.73 × 10−3, 0.02) | 20.75% | <0.001 | |
| Mn | 1.96 × 10−2 (−4.05 × 10−2, 0.08) | −6.29 × 10−3 (−4.47 × 10−2, 0.08) | |||
| Se | −3.61 × 10−1 (−0.48, −0.23) | −8.42 × 10−2 (−1.24 × 10−1, −0.05) | 23.26% | <0.001 | |
| Mo | −2.61 × 10−5 (−2.62 × 10−2, 0.03) | 2.18 × 10−3 (−3.26 × 10−3, 0.01) | |||
| Ln-hsCRP | Ni | 3.66 × 10−2 (8.87 × 10−3, 0.06) | 3.87 × 10−3 (0.00, 7.99 × 10−3) | 10.19% | 0.004 |
| Co | 7.67 × 10−2 (4.80 × 10−2, 0.11) | −1.11 × 10−3 (−3.96 × 10−3, 0.01) | |||
| Mn | 1.83 × 10−2 (−3.94 × 10−2, 0.07) | −4.33 × 10−3 (−1.08 × 10−2, 0.01) | |||
| Se | −3.62 × 10−1 (−0.48, −0.25) | −4.87 × 10−2 (−7.67 × 10−2, −0.02) | 13.30% | <0.001 | |
| Mo | −3.62 × 10−4 (−2.66 × 10−2, 0.03) | −5.32 × 10−3 (9.80 × 10−3, 0.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Guan, X.; Zhou, Y.; He, Y.; Chen, S.; Xiao, W.; Yang, J.; Lu, J.; Hong, L.; Hu, Q.; et al. Exploring Associations and Mediating Factors between Multiple Trace Metals with Anemia in US Adults: Insight from NHANES 2017–2020. Nutrients 2024, 16, 3424. https://doi.org/10.3390/nu16193424
Xie L, Guan X, Zhou Y, He Y, Chen S, Xiao W, Yang J, Lu J, Hong L, Hu Q, et al. Exploring Associations and Mediating Factors between Multiple Trace Metals with Anemia in US Adults: Insight from NHANES 2017–2020. Nutrients. 2024; 16(19):3424. https://doi.org/10.3390/nu16193424
Chicago/Turabian StyleXie, Lijie, Xinchao Guan, Yixiang Zhou, Yujie He, Shilin Chen, Wanting Xiao, Jilong Yang, Jianyong Lu, Liecheng Hong, Qiansheng Hu, and et al. 2024. "Exploring Associations and Mediating Factors between Multiple Trace Metals with Anemia in US Adults: Insight from NHANES 2017–2020" Nutrients 16, no. 19: 3424. https://doi.org/10.3390/nu16193424
APA StyleXie, L., Guan, X., Zhou, Y., He, Y., Chen, S., Xiao, W., Yang, J., Lu, J., Hong, L., Hu, Q., Wang, Q., Li, C., & Wang, Q. (2024). Exploring Associations and Mediating Factors between Multiple Trace Metals with Anemia in US Adults: Insight from NHANES 2017–2020. Nutrients, 16(19), 3424. https://doi.org/10.3390/nu16193424







