Protective Effect of IgY Embedded in W/O/W Emulsion on LPS Enteritis-Induced Colonic Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of IgY-Embedded Double Emulsion
2.2. Stability Analysis of Double Emulsion Embedding
2.3. In Vitro Evaluation of IgY Embedded in W/O/W
2.3.1. Tolerance Test against Artificial Gastric Juice
2.3.2. Tolerance Test against Artificial Intestine Juice
2.4. Animals
2.5. Histological Analysis
2.6. Immunohistochemical
2.7. ELISA Detected TNF-α, IL-1β, IL-6, and IL-12
2.8. Detection of Redox Indicators
2.9. RT-qPCR
2.10. Stastical Analysis
3. Results
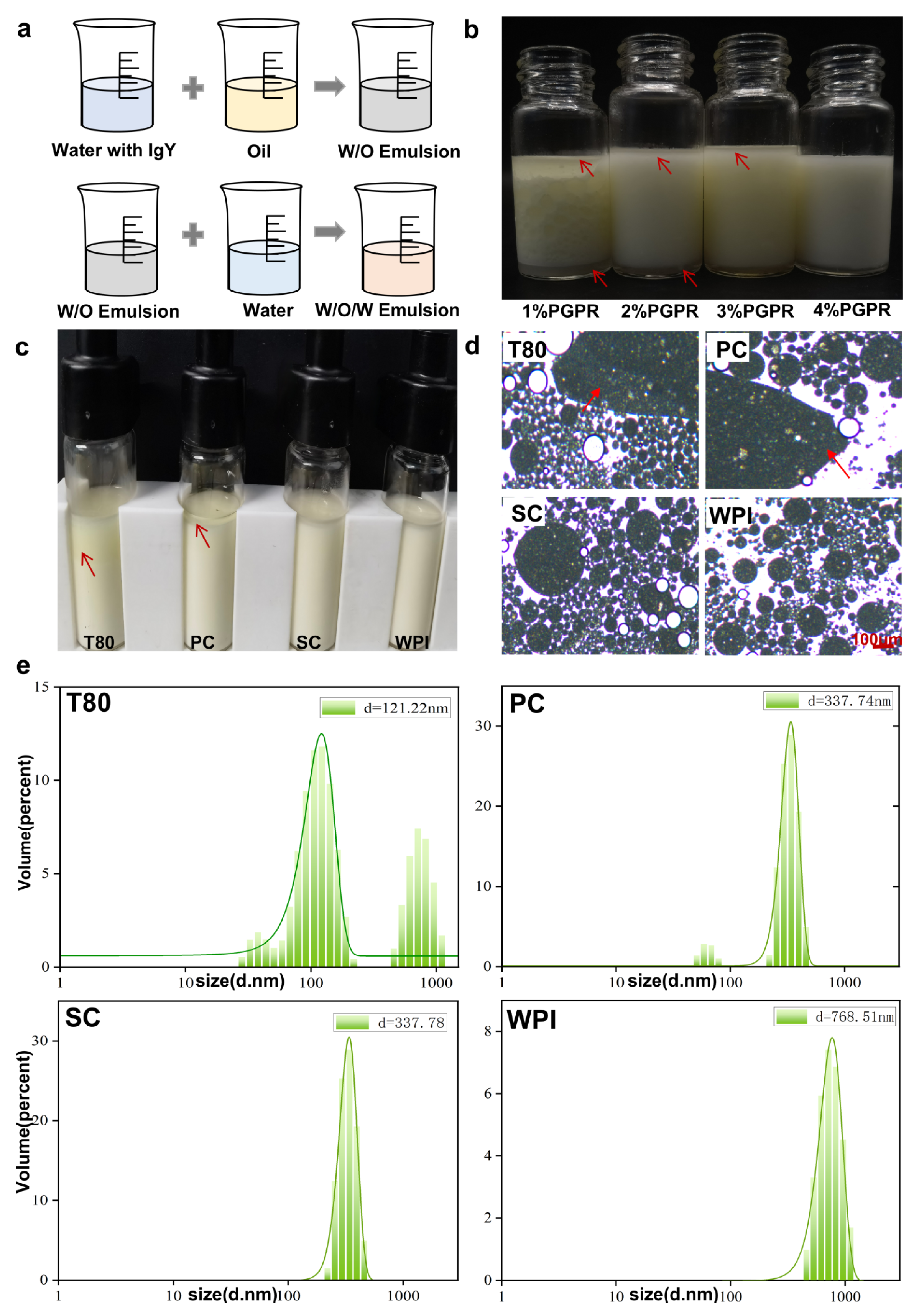
3.1. Characterize of IgY W/O/W Emulsion
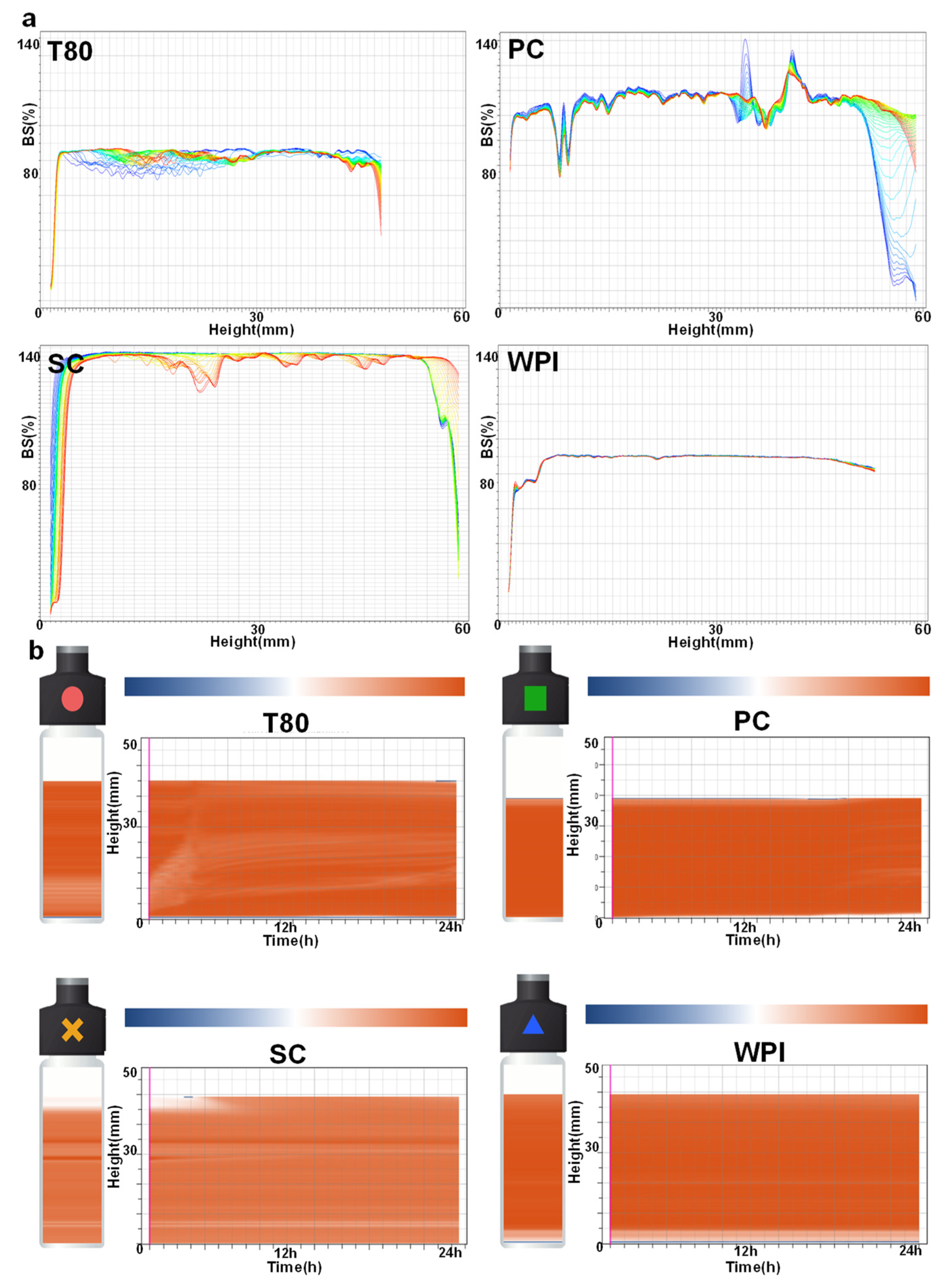
3.2. Stability Analysis of W/O/W Emulsions Prepared with Different Emulsifiers
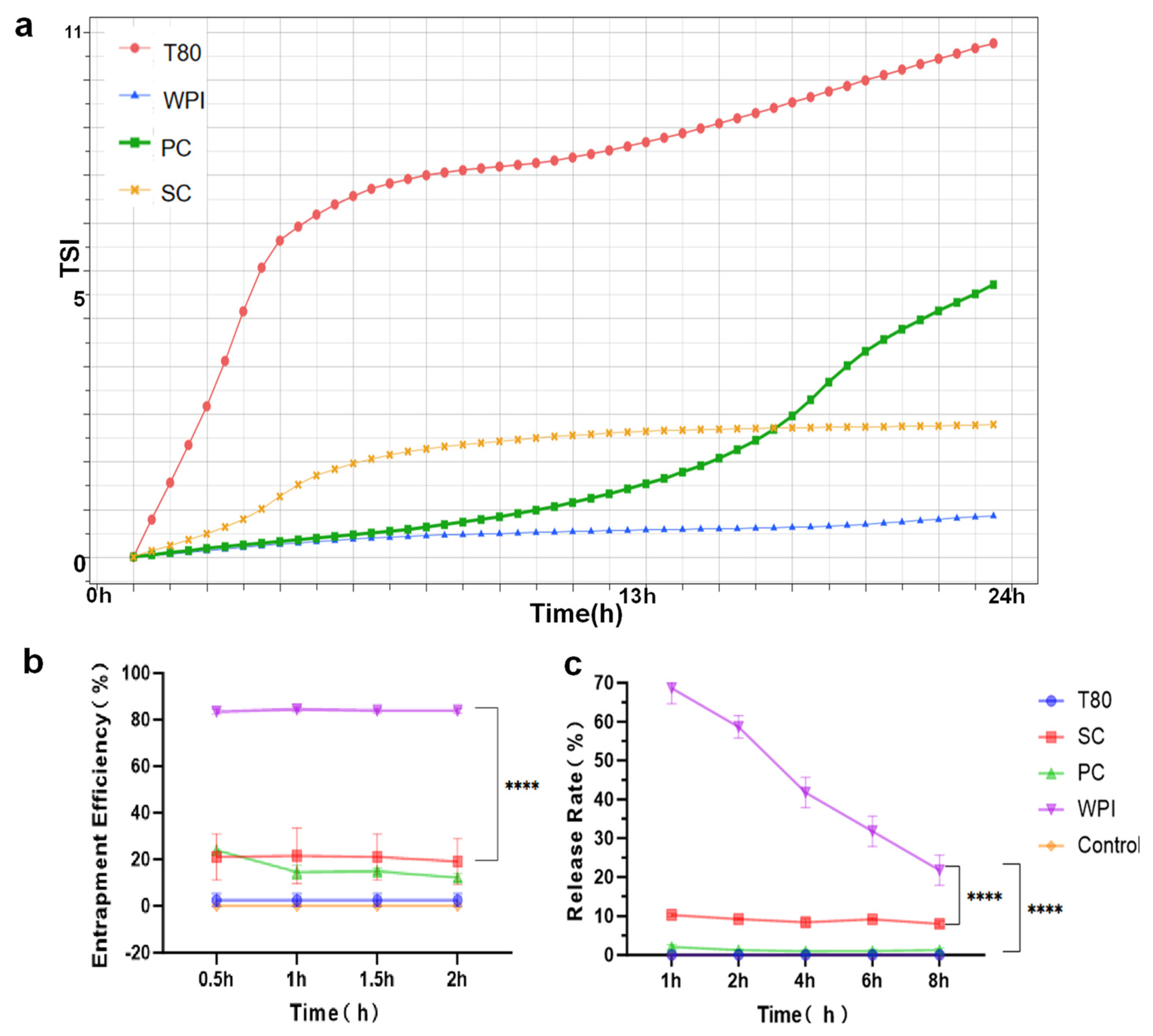
3.3. Stability Analysis and In Vitro Validation of Different W/O/W Emulsions
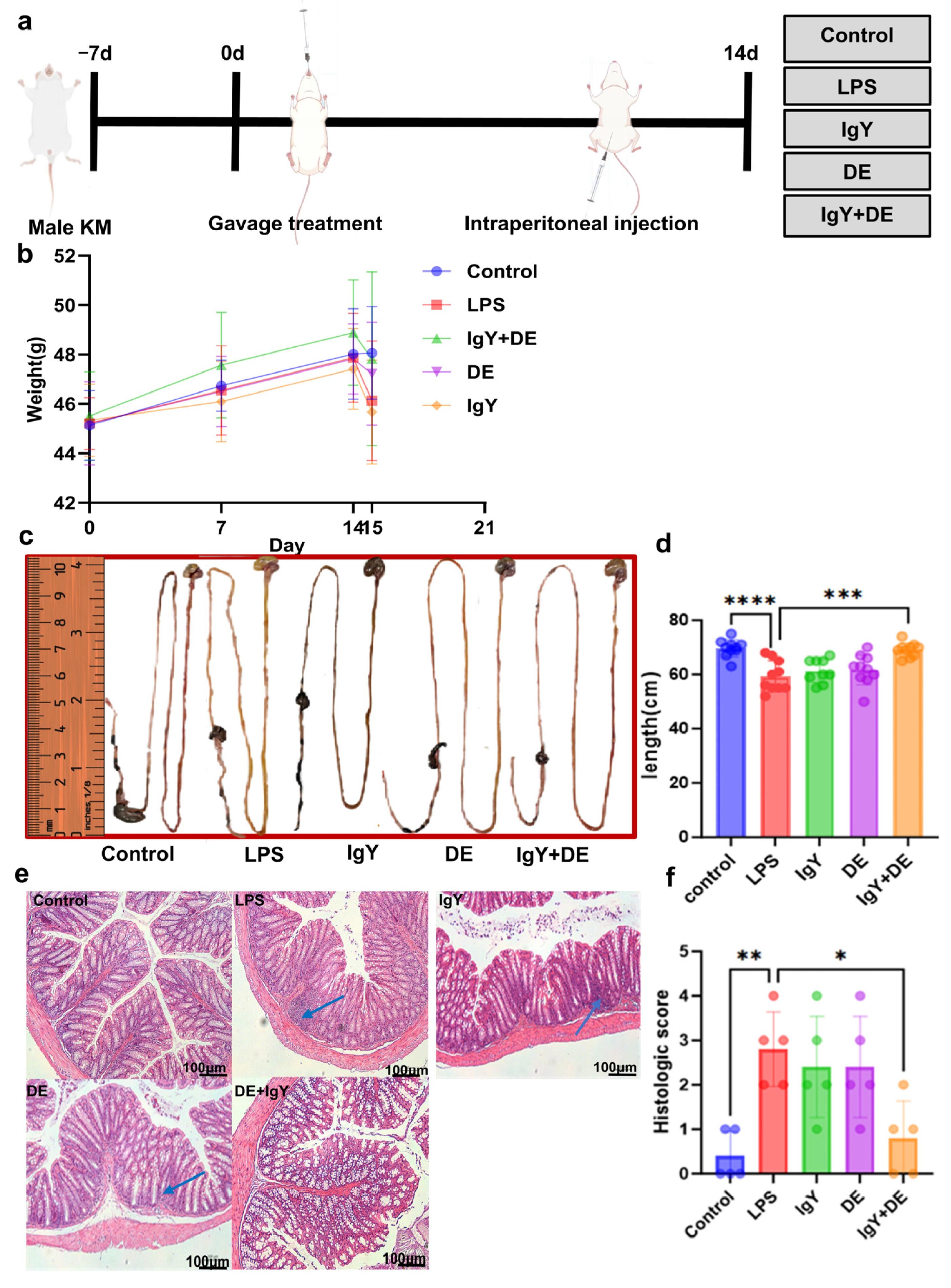
3.4. IgY + DE Ameliorates LPS-Induced Intestinal Damage
3.5. IgY + DE Alleviates LPS-Induced Colitis by Improving the Mucosal Barrier
3.6. IgY + DE Alleviates LPS-Induced Oxidative Stress in the Colon
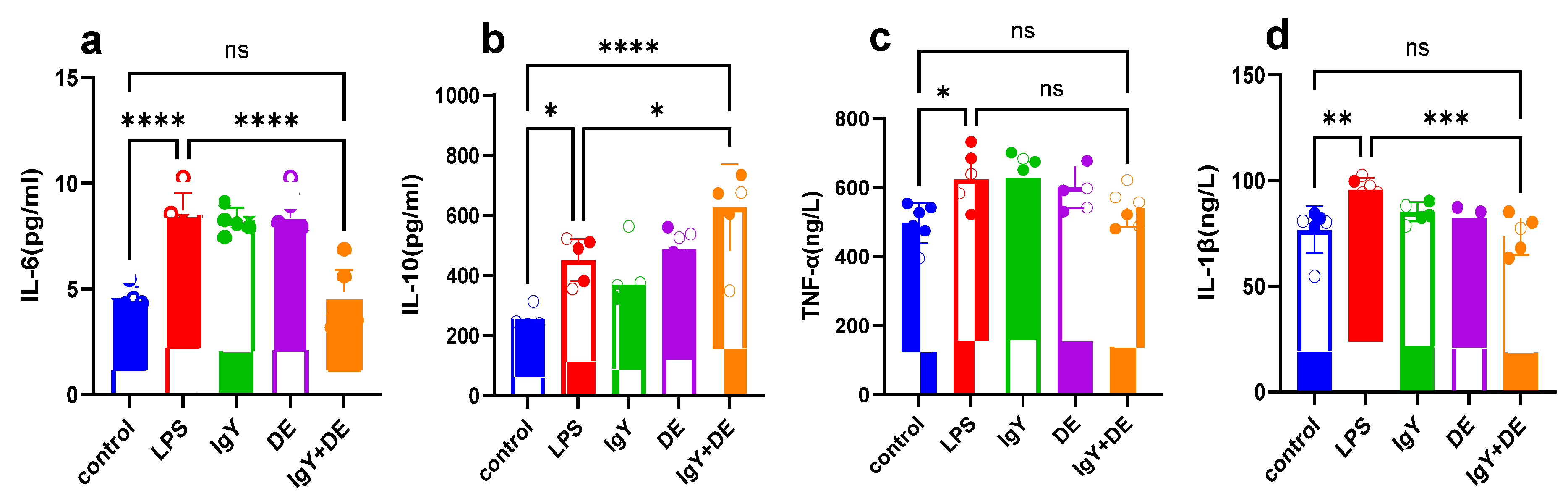
3.7. IgY + DE Reduces the Colonic Inflammatory Response Caused by LPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepat. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Lidar, M.; Langevitz, P.; Shoenfeld, Y. The role of infection in inflammatory bowel disease: Initiation, exacerbation and protection. ISR Med. Assoc. J. 2009, 11, 558–563. [Google Scholar] [PubMed]
- Cohen, J. The immunopathogenesis of sepsis. Nature 2002, 420, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Zhao, Y.; Shao, F. Innate immunity to intracellular LPS. Nat. Immunol. 2019, 20, 527–533. [Google Scholar] [CrossRef]
- Cox, C.M.; Lu, R.; Salcin, K.; Wilson, J.M. The Endosomal Protein Endotubin Is Required for Enterocyte Differentiation. Cell. Mol. Gastroenterol. 2018, 5, 145–156. [Google Scholar] [CrossRef]
- Xu, C.; Meng, S.; Pan, B. Drug therapy for ulcerative colitis. World J. Gastroenterol. 2004, 10, 2311–2317. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Jin, L.; Zhen, Y.; Lu, Y.; Li, S.; You, J.; Wang, L. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: A review. Biotechnol. Adv. 2011, 29, 860–868. [Google Scholar] [CrossRef]
- Abbas, A.T.; El-Kafrawy, S.A.; Sohrab, S.S.; Azhar, E. IgY antibodies for the immunoprophylaxis and therapy of respiratory infections. Hum. Vacc. Immunother. 2019, 15, 264–275. [Google Scholar] [CrossRef]
- Marcus, E.A.; Scott, D.R. Cell lysis is responsible for the appearance of extracellular urease in Helicobacter pylori. Helicobacter 2001, 6, 93–99. [Google Scholar] [CrossRef]
- Lee, E.N.; Sunwoo, H.H.; Menninen, K.; Sim, J.S. In vitro studies of chicken egg yolk antibody (IgY) against Salmonella enteritidis and Salmonella typhimurium. Poult. Sci. 2002, 81, 632–641. [Google Scholar] [CrossRef]
- Ratcliffe, M.J. Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Dev. Comp. Immunol. 2006, 30, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Thu, H.M.; Myat, T.W.; Win, M.M.; Thant, K.Z.; Rahman, S.; Umeda, K.; Nguyen, S.V.; Icatlo, F.J.; Higo-Moriguchi, K.; Taniguchi, K.; et al. Chicken Egg Yolk Antibodies (IgY) for Prophylaxis and Treatment of Rotavirus Diarrhea in Human and Animal Neonates: A Concise Review. Korean J. Food Sci. Anim. Resour. 2017, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Chen, Y.P.; Yang, M.X.; Zhang, L.L.; Lu, Z.X.; Zhou, Y.M.; Wang, T. Bacillus amyloliquefaciens supplementation alleviates immunological stress and intestinal damage in lipopolysaccharide—Challenged broilers. Anim. Feed Sci. Technol. 2015, 208, 119–131. [Google Scholar] [CrossRef]
- Mueller, S.; Schubert, A.; Zajac, J.; Dyck, T.; Oelkrug, C. IgY antibodies in human nutrition for disease prevention. Nutr. J. 2015, 14, 109. [Google Scholar] [CrossRef]
- Jin, L.Z.; Baidoo, S.K.; Marquardt, R.R.; Frohlich, A.A. In vitro inhibition of adhesion of enterotoxigenic Escherichia coli K88 to piglet intestinal mucus by egg-yolk antibodies. FEMS Immunol. Med. Microbiol. 1998, 21, 313–321. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, J.; Park, I.; Huh, C.; Baek, Y.; Park, J. Protective Effect of Microencapsulation Consisting of Multiple Emulsification and Heat Gelation Processes on Immunoglobulin in Yolk. J. Food Sci. 2005, 70, E148–E151. [Google Scholar] [CrossRef]
- Dickinson, E. Double emulsions stabilized by food biopolymers. Food Biophys. 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Garti, N. Progress in stabilization and transport phenomena of double emulsions in food applications. LWT-Food Sci. Technol. 1997, 30, 222–235. [Google Scholar] [CrossRef]
- Benichou, A.; Aserin, A.; Garti, N. W/O/W double emulsions stabilized with WPI–polysaccharide complexes. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 20–32. [Google Scholar] [CrossRef]
- Chevalier, R.C.; Gomes, A.; Cunha, R.L. Role of aqueous phase composition and hydrophilic emulsifier type on the stability of W/O/W emulsions. Food Res. Int. 2022, 156, 111123. [Google Scholar] [CrossRef]
- Bush, L.; Stevenson, L.; Lane, K.E. The oxidative stability of omega-3 oil-in-water nanoemulsion systems suitable for functional food enrichment: A systematic review of the literature. Crit. Rev. Food Sci. 2019, 59, 1154–1168. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Zhu, Y.; Teng, C.; Li, X. Effects of Several Natural Macromolecules on the Stability and Controlled Release Properties of Water-in-Oil-in-Water Emulsions. J. Agr. Food Chem. 2016, 64, 3873–3880. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, Y.; Tatsumi, E.; Saito, M.; Yin, L. The use of W/O/W controlled-release coagulants to improve the quality of bittern-solidified tofu. Food Hydrocolloid 2014, 35, 627–635. [Google Scholar] [CrossRef]
- Sul, O.J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.S.H.; Nickerson, M.T. Food proteins: A review on their emulsifying properties using a structure-function approach. Food Chem. 2013, 141, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, K.; Sun, F.; Tan, S.; Zhang, X.; Sheng, W.; Hao, W.; Liu, M.; Lv, W.; Han, W. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J. Transl. Med. 2021, 19, 96. [Google Scholar] [CrossRef]
- Dauphinee, S.M.; Karsan, A. Lipopolysaccharide signaling in endothelial cells. Lab Investig. 2006, 86, 9–22. [Google Scholar] [CrossRef]
- Sah, H.K.; Toddywala, R.; Chien, Y.W. Biodegradable microcapsules prepared by a w/o/w technique: Effects of shear force to make a primary w/o emulsion on their morphology and protein release. J. Microencapsul. 1995, 12, 59–69. [Google Scholar] [CrossRef]
- Song, Y.; Gao, H.; Zhang, S.; Zhang, Y.; Jin, X.; Sun, J. Prescription Optimization and Oral Bioavailability Study of Salvianolic Acid Extracts W/O/W Multiple Emulsion. Biol. Pharm. Bull. 2017, 40, 2081–2087. [Google Scholar] [CrossRef]
- Nafea, E.H.; El-Massik, M.A.; El-Khordagui, L.K.; Marei, M.K.; Khalafallah, N.M. Alendronate PLGA microspheres with high loading efficiency for dental applications. J. Microencapsul. 2007, 24, 525–538. [Google Scholar] [CrossRef]
- Biswal, A.K.; Hariprasad, P.; Saha, S. Efficient and prolonged antibacterial activity from porous PLGA microparticles and their application in food preservation. Mat. Sci. Eng. C-Mater. 2020, 108, 110496. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Leung, W.K.; Yeung, K.L.; Lau, P.S.; Kwan, J.K. A multilevel antimicrobial coating based on polymer-encapsulated ClO2. Langmuir 2009, 25, 13472–13480. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Samardzic, K.; Wallach, M.; Frumkin, L.R.; Mochly-Rosen, D. Immunoglobulin Y for Potential Diagnostic and Therapeutic Applications in Infectious Diseases. Front. Immunol. 2021, 12, 696003. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wu, Z.; Liu, C.; Han, D.; Feng, C.; Wang, S.; Wang, J. Milk Fat Globule Membrane Supplementation Promotes Neonatal Growth and Alleviates Inflammation in Low-Birth-Weight Mice Treated with Lipopolysaccharide. Biomed. Res. Int. 2019, 2019, 4876078. [Google Scholar] [CrossRef]
- Garti, N.; Aserin, A. Double emulsions stabilized by macromolecular surfactants. In Surfactants in Solution; CRC Press: Boca Raton, FL, USA, 2020; pp. 297–332. [Google Scholar]
- Xiao, Z.; Kong, B.; Fang, J.; Qin, T.; Dai, C.; Shuai, W.; Huang, H. Ferrostatin-1 alleviates lipopolysaccharide-induced cardiac dysfunction. Bioengineered 2021, 12, 9367–9376. [Google Scholar] [CrossRef]
- Ye, R.; Huang, J.; Wang, Z.; Chen, Y.; Dong, Y. Trace Element Selenium Effectively Alleviates Intestinal Diseases. Int. J. Mol. Sci. 2021, 22, 11708. [Google Scholar] [CrossRef]
- Huang, X.; Yang, X.; Zhang, M.; Li, T.; Zhu, K.; Dong, Y.; Lei, X.; Yu, Z.; Lv, C.; Huang, J. SELENOI Functions as a Key Modulator of Ferroptosis Pathway in Colitis and Colorectal Cancer. Adv. Sci. 2024, 11, e2404073. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Y.; Ren, F.; Lei, X.G. Novel role and mechanism of glutathione peroxidase-4 in nutritional pancreatic atrophy of chicks induced by dietary selenium deficiency. Redox Biol. 2022, 57, 102482. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.; Zhu, K.; Luo, Y.; Huang, X.; Dong, Y.; Huang, J. Biomimetic MicroRNAs-Selenium-Nanocomposites for Targeted and Combined Hyperlipidemia Therapy. Adv. Healthc. Mater. 2024, 13, 2400064. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, P.; Chen, Y.; Ma, S.Y. Intact anti-LPS IgY is found in the blood after intragastric administration in mice. FEBS Open Bio 2019, 9, 428–436. [Google Scholar] [CrossRef]
- Tomioka, S.; Seki, N.; Sugiura, Y.; Akiyama, M.; Uchiyama, J.; Yamaguchi, G.; Yakabe, K.; Eijima, R.; Hattori, K.; Kimizuka, T.; et al. Cooperative action of gut-microbiota-accessible carbohydrates improves host metabolic function. Cell Rep. 2022, 40, 111087. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, H.; Wang, S.; Tu, Z.; Zhang, L.; Wang, X.; Hou, Y.; Wang, C.; Chen, J.; Liu, Y. Flaxseed Oil Attenuates Intestinal Damage and Inflammation by Regulating Necroptosis and TLR4/NOD Signaling Pathways Following Lipopolysaccharide Challenge in a Piglet Model. Mol. Nutr. Food Res. 2018, 62, 1700814. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xu, L.; Zeng, Y.; Gong, F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021, 91, 107272. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, H.; Liu, J.X.; Li, T.; Liu, S.; Shi, W.; Sun, C.; Fan, M.; Xue, L.; Wang, Y.; et al. l-Arabinose Inhibits Colitis by Modulating Gut Microbiota in Mice. J. Agr. Food Chem. 2019, 67, 13299–13306. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Linden, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef]
- Levi, E.; Klimstra, D.S.; Andea, A.; Basturk, O.; Adsay, N.V. MUC1 and MUC2 in pancreatic neoplasia. J. Clin. Pathol. 2004, 57, 456–462. [Google Scholar] [CrossRef]
- Lu, S.; Xu, S.; Chen, L.; Deng, Y.; Feng, J. Periplaneta americana Extract Pretreatment Alleviates Oxidative Stress and Inflammation and Increases the Abundance of Gut Akkermansia muciniphila in Diquat-Induced Mice. Antioxidants 2022, 11, 1806. [Google Scholar] [CrossRef]
- Xing, C.; Yang, F.; Lin, Y.; Shan, J.; Yi, X.; Ali, F.; Zhu, Y.; Wang, C.; Zhang, C.; Zhuang, Y.; et al. Hexavalent Chromium Exposure Induces Intestinal Barrier Damage via Activation of the NF-kappaB Signaling Pathway and NLRP3 Inflammasome in Ducks. Front. Immunol. 2022, 13, 952639. [Google Scholar] [CrossRef]
- Sun, S.C. The non-canonical NF-kappaB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Majalekar, P.P.; Shirote, P.J. Fluoroquinolones: Blessings Or Curses. Curr. Drug Targets 2020, 21, 1354–1370. [Google Scholar] [CrossRef]
- O’Donnell, J.A.; Gelone, S.P. Fluoroquinolones. Infect. Dis. Clin. N. Am. 2000, 14, 489–513. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; Fung, H.B. Besifloxacin: A topical fluoroquinolone for the treatment of bacterial conjunctivitis. Clin. Ther. 2010, 32, 454–471. [Google Scholar] [CrossRef] [PubMed]
- Fintelmann, R.E.; Hoskins, E.N.; Lietman, T.M.; Keenan, J.D.; Gaynor, B.D.; Cevallos, V.; Acharya, N.R. Topical fluoroquinolone use as a risk factor for in vitro fluoroquinolone resistance in ocular cultures. Arch. Ophthalmol. 2011, 129, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Van Nguyen, S.; Icatlo, F.J.; Umeda, K.; Kodama, Y. Oral passive IgY-based immunotherapeutics: A novel solution for prevention and treatment of alimentary tract diseases. Hum. Vacc. Immunother. 2013, 9, 1039–1048. [Google Scholar] [CrossRef]
- Lee, W.; Atif, A.S.; Tan, S.C.; Leow, C.H. Insights into the chicken IgY with emphasis on the generation and applications of chicken recombinant monoclonal antibodies. J. Immunol. Methods 2017, 447, 71–85. [Google Scholar] [CrossRef]
- Davalos-Pantoja, L.; Ortega-Vinuesa, J.L.; Bastos-Gonzalez, D.; Hidalgo-Alvarez, R. A comparative study between the adsorption of IgY and IgG on latex particles. J. Biomat. Sci.-Polym. E 2000, 11, 657–673. [Google Scholar] [CrossRef]


| Gene Product | Primer Sequence (59–39) | ||
|---|---|---|---|
| Forward | Reverse | Source | |
| Muc2 | AGGGCTCGGAACTCCAGAAA | CCAGGGAATCGGTAGACATCG | NM_023566.4 |
| Tff3 | TTGCTGGGTCCTCTGGGATAG | TACACTGCTCCGATGTGACAG | NM_011575.2 |
| Klf3 | AAGCCCAACAAATATGGGGT | GGACGGGAACTTCAGAGAGG | XM_006503751.5 |
| Itln1 | TGACAATGGTCCAGCATTACC | ACGGGGTTACCTTCTGGGA | XM_029475723.1 |
| Retnlb | AAGCCTACACTGTGTTTCCTTT | GCTTCCTTGATCCTTTGATCCAC | XM_021185515.1 |
| Ang4 | GGTTGTGATTCCTCCAACTCTG | CTGAAGTTTTCTCCATAAGGGCT | XM_021154346.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Ye, R.; Xu, Z.; Zhang, S.; Liu, C.; Zhu, K.; Wang, P.; Huang, J. Protective Effect of IgY Embedded in W/O/W Emulsion on LPS Enteritis-Induced Colonic Injury in Mice. Nutrients 2024, 16, 3361. https://doi.org/10.3390/nu16193361
Wang Z, Ye R, Xu Z, Zhang S, Liu C, Zhu K, Wang P, Huang J. Protective Effect of IgY Embedded in W/O/W Emulsion on LPS Enteritis-Induced Colonic Injury in Mice. Nutrients. 2024; 16(19):3361. https://doi.org/10.3390/nu16193361
Chicago/Turabian StyleWang, Zhaohui, Ruihua Ye, Zijian Xu, Shidi Zhang, Chuanming Liu, Kongdi Zhu, Pengjie Wang, and Jiaqiang Huang. 2024. "Protective Effect of IgY Embedded in W/O/W Emulsion on LPS Enteritis-Induced Colonic Injury in Mice" Nutrients 16, no. 19: 3361. https://doi.org/10.3390/nu16193361
APA StyleWang, Z., Ye, R., Xu, Z., Zhang, S., Liu, C., Zhu, K., Wang, P., & Huang, J. (2024). Protective Effect of IgY Embedded in W/O/W Emulsion on LPS Enteritis-Induced Colonic Injury in Mice. Nutrients, 16(19), 3361. https://doi.org/10.3390/nu16193361







